Abstract
New Zealand rabbits were randomly divided into an ischemia group (occlusion of the abdominal aorta for 60 minutes), an ischemia-reperfusion group (occlusion of the abdominal aorta for 60 minutes followed by 48 hours of reperfusion) and a sham-surgery group. Two-dimensional gel electrophoresis detected 49 differentially expressed proteins in spinal cord tissue from the ischemia and ischemia/ reperfusion groups and 23 of them were identified by mass spectrometry. In the ischemia group, the expression of eight proteins was up regulated, and that of the remaining four proteins was down regulated. In the ischemia/reperfusion group, the expression of four proteins was up regulated, and that of two proteins was down regulated. In the sham-surgery group, only one protein was detected. In the ischemia and ischemia/reperfusion groups, four proteins overlapped between groups with the same differential expression, including three that were up regulated and one down regulated. These proteins were related to energy metabolism, cell defense, inflammatory mechanism and cell signaling.
Keywords: spinal cord injury, ischemia/reperfusion, two-dimensional gel electrophoresis, mass spectrometry, rabbit, proteomics, neural regeneration
Research Highlights
Using two-dimensional gel electrophoresis and mass spectrometry, this study analyzed protein expression in spinal cord tissue of a rabbit model of spinal cord ischemia-reperfusion injury and identified 23 differentially expressed proteins related to energy metabolism, cellular defense, inflammatory reaction and cell signaling.
INTRODUCTION
Ischemia/reperfusion complicated by delayed neurological impairment is a dynamic pathological process, and the underlying mechanism is complex[1,2,3]. Several studies have shown that the mechanism may be related to microcirculation disturbance, inflammatory mechanisms, cellular necrosis and apoptosis, or biochemical self-destructive factors (such as calcium ion overloading, free radicals, excitatory amino acids)[3,4], however, the precise mechanism of ischemia/reperfusion injury is poorly understood. Therefore, this study used differential proteomics to analyze the change in expression of related proteins after spinal cord ischemia/reperfusion injury.
RESULTS
Quantitative analysis of experimental animals
Eighteen rabbits were randomly divided into a sham-surgery group (surgery only without occlusion of the abdominal aorta), an ischemia group (occlusion of the abdominal aorta for 60 minutes) and an ischemia/reperfusion group (occlusion of the abdominal aorta for 60 minutes followed by 48 hours of reperfusion), with six rabbits in each group. All 18 rabbits were included in the final analysis.
Fluorescent two-dimensional gel electrophoresis imaging analysis of differential protein expression during spinal cord ischemia/reperfusion injury
Spinal cord tissue proteins were separated using CyDye DIGE Fluor saturation dye-labeled two-dimensional gel electrophoresis and study of differentially expressed proteins was achieved by image analysis using DeCyder software system. Using the spinal cord protein expression spectrum from the sham-surgery group as a standard, protein expression spectra at two different time points, i.e., ischemia for 60 minutes and ischemia for 60 minutes followed by 48 hours of reperfusion were established (Figure 1). Protein spots from the ischemia group and ischemia/reperfusion group with a 2-fold expression difference compared to the sham surgery group, were included for further analysis. In total, 49 protein spots with obvious differential expression were screened. Compared with the sham-surgery group, 25 proteins were up regulated and five proteins were down regulated in the ischemia group, and 16 proteins were up regulated and three proteins were down regulated in the ischemia/reperfusion group.
Figure 1.
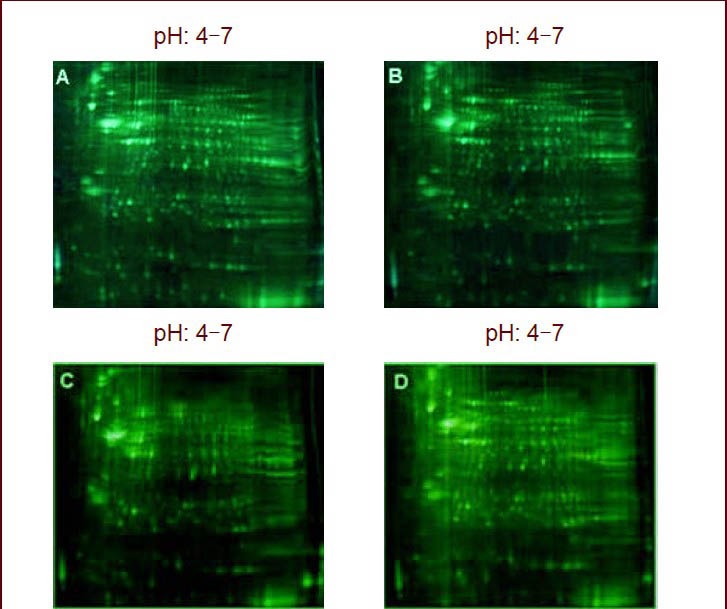
Fluorescent two-dimensional gel electrophoretogram of sham-surgery, ischemia, and ischemia/reperfusion groups.
Compared with sham-surgery group (A, C), there were 49 differentially expressed protein spots in the ischemia (B) group and ischemia/reperfusion group (D).
Mass spectrometry identification of differentially expressed protein spots in spinal cord ischemia/reperfusion injury
The primary and secondary structures of 49 differentially expressed proteins were analyzed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. A Mascot-based retrieval in NCBI was performed to reject repetitive results, and finally 23 proteins were identified (22 from the ischemia group and ischemia/reperfusion group, and one from the sham-surgery group).
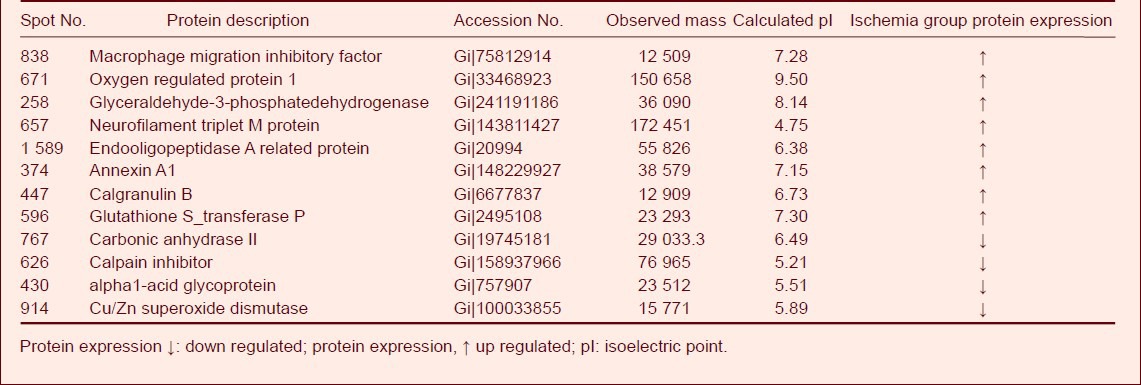
Compared to the sham-surgery group, the expression of eight proteins were significantly upregulated (P < 0.05) in spinal cord tissue from the ischemia group and included macrophage migration inhibitory factor, oxygen regulated protein 1, glyceraldehyde-3-phosphate dehydrogenase, neurofilament triplet M protein, endopeptidase A related protein, annexin A1, calgranulin B, and glutathione S-transferase P. In addition, the expression of carbonic anhydrase II, calpain inhibitor, alpha1-acid glycoprotein, and Cu/Zn superoxide dismutase tissue was significantly down regulated (P < 0.05) (Table 1).
Table 1.
Differentially expressed proteins in the ischemia and sham-surgery groups
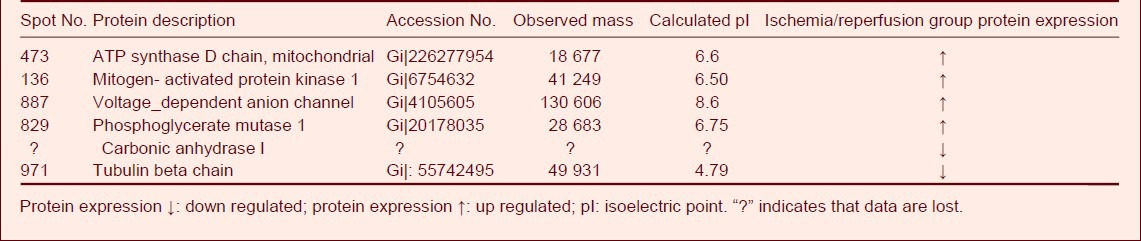
Compared to the sham-surgery group, the expression of five proteins was significantly up regulated (P < 0.05) in spinal cord tissue from the ischemia/reperfusion group and included ATP synthase D chain, mitochondrial mitogen-activated protein kinase 1, voltage-dependent anion channel, phosphoglycerate mutase 1, and hypothetical protein-10. In addition, the expression of tubulin beta chain and carbonic anhydrase was significantly down regulated (P < 0.05). Only calpastatin (spot No. 758,764; accession number: Giǀ126302556; Mr: 76965; calculated pI: 5.21) was detected in the sham-surgery group (Table 2).
Table 2.
Differentially expressed proteins in the ischemia/reperfusion and sham-surgery groups
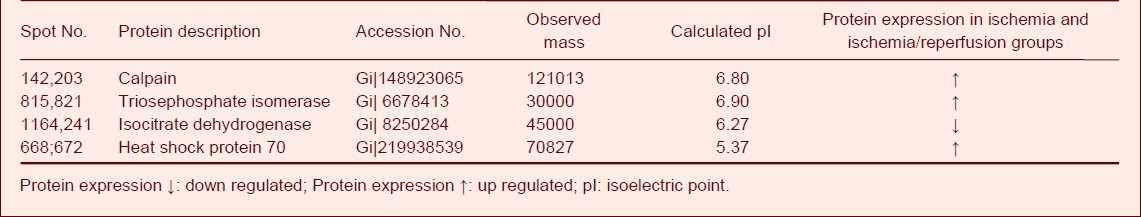
In the ischemia and ischemia/reperfusion group's the expression of triosephosphate isomerase, calpain and heat shock protein 70 in the spinal cord tissue was significantly up regulated (P < 0.05) and the expression of isocitrate dehydrogenase was significantly down regulated compared to the sham-surgery group (P < 0.05; Table 3).
Table 3.
Differentially expressed proteins in the ischemia and ischemia/reperfusion groups
DISCUSSION
Results demonstrated 23 differentially expressed proteins in the sham-surgery, ischemia and ischemia/reperfusion groups. The functions of these proteins are described below.
Proteins that regulate energy metabolism
Four proteins that demonstrated a significant and consistent change were detected in the ischemia and ischemia/reperfusion groups.
In the ischemia group, during the early stage of spinal cord ischemia and hypoxia, cells undergo glycolysis, presenting as lactic acid accumulation, which can inhibit the tricarboxylic acid cycle, thus, the expression of phosphotriose isomerase and lyceraldehyde-3-phosphatedehydrogenase, as key enzymes of glycolysis, was up regulated, while the expression of isocitrate dehydrogenase, a key enzyme of the tricarboxylic acid cycle, was down regulated.
This demonstrates that after spinal cord ischemia, cell energy metabolism and glycometabolism disorders[3,4,5,6] occur and are maintained throughout the pathological process of early spinal cord ischemia/reperfusion[4,5,6,7]. The functional changes in these three enzymes suggest they may be important factors in the repair and regeneration of spinal cord injuries. However, the expression of glycometabolism-related enzymes was also altered. For example, the expression of phosphoglyceromutase in the ischemia/reperfusion group was up regulated and the expression of carbonic anhydrase I and carbonic anhydrase II was down regulated. These enzymes participate in cellular glycolysis energy metabolism and balance and their expression was down regulated after reperfusion. This suggests that glucose oxidative and metabolic pathways are enhanced. Concurrently, expression levels of proteins related to energy metabolism, such as ATP synthase and its subunit, were also significantly up regulated. Severe energy supply disorders may occur in spinal injuries[7], and therefore cell metabolism may switch from glycolysis to the tricarboxylic acid cycle. The late-stage increase in ATP synthase also suggests this is occurring during spinal cord injury.
Proteins that participate in cell defense
Evidence exists that some anti-oxidation-related proteins, such as Cu/Zn superoxide dismutase, an enzyme with anti-oxidative effects, can reduce peroxide levels, regulate active oxygen, remove accumulated peroxide, and regulate cell signaling and cellular proliferation. In the ischemia group (at the early stage of spinal cord ischemia), Cu/Zn superoxide dismutase expression was down regulated. Heat shock protein 70, a stress protein, was up regulated during spinal cord ischemia and hypoxia, similar to the ischemia group. This may occur as heat shock protein 70 protects the spinal cord under many stress conditions[8,9]. For instance, heat shock protein 70 alleviates secondary injury to the spinal cord by alleviating H2O2-induced damage to mitochondria and inhibiting the mitochondrial-dependent cell apoptosis pathway. Studies have demonstrated that heat shock protein 70 expression is up regulated after peripheral nerve injury and protects neurons from injury, possibly by altering the cytoskeleton during axonal regeneration. Results from this study showed that heat shock protein 70 expression was up regulated in the spinal cord during ischemia/reperfusion injury, indicating heat shock protein 70 is likely to be a key protein in the repair of spinal cord ischemia/reperfusion injury.
Voltage-dependent anion channel 1, located in the outer mitochondrial membrane of all eukaryotic cells and cerebral presynaptic membranes, can regulate the current direction of a series of anion metabolites and strengthen outer membrane permeability, playing an important role in mitochondrial metabolism and cell apoptosis. Evidence exists that voltage-dependent anion channel 1 participates in the process of mitochondrial dysfunction and neuronal injury. Over-expression of voltage-dependent anion channel 1 can produce pathological lesions due to an imbalance of Ca2+ in the mitochondrial matrix and cytoplasm. After spinal cord injury, spinal cord blood flow progressively decreases, spinal cord ischemia and hypoxia is aggravated, and there is an influx of high Ca2+ levels. After a series of biochemical reactions, pathological free radicals are produced, leading to intracellular lipid peroxidation.
Results from this study demonstrated that upregulated voltage-dependent anion channel 1 expression possibly leads to the excessive accumulation of Ca2+ that aggravates spinal cord injury, therefore, voltage- dependent anion channel 1 may cause delayed spinal cord injury after ischemia/reperfusion[10,11].
Proteins that participate in inflammation
In the ischemia group, we observed up regulation of the calcium binding proteins calgranuliin B and annexin A1, which participate in inflammatory reactions to protect against pathological microorganisms. These proteins are primarily expressed in the cytoplasm of neutrophilic granulocytes and monocytes, which migrate to extracellular regions and produce high levels of calcium binding proteins during inflammation[12,13,14,15]. Calgranuliin B and annexin A1 are likely to be key proteins or target proteins that participate in inflammatory pathways[16,17]. In the ischemia/reperfusion group, we observed up regulation of macrophage migration inhibitory factor which functions as a pleiotropic protein and participates in inflammatory and immune responses, regulates the active defense functions of macrophages, and exerts anti-inflammatory effects by up regulating anti-inflammatory glucocorticoids. In addition, we observed down regulation of alpha1-acid glycoprotein, a stable acute-phase reactant that inhibits neutrophil migration by a nitric oxide-dependent process, although its biological functions remains poorly understood[18,19].
Proteins that participate in cell signaling
Calpain is a calcium-dependent, highly conserved protease. Recent studies demonstrated that calpain participates in cytoskeletal protein reforming, cell transformation and migration, and accelerates cell circulation. Calcium overloading and calmodulin complex formation in nerve cells is a pathological mechanism underlying secondary central nerve injury[3,20]. Results from this study demonstrated that after ischemia/reperfusion, up regulated calpain expression causes the opening of calcium channels in nerve cells resulting in an abnormal increase in intracytoplasmic free calcium. This severe Ca2+ overloading can directly influence the activity of many intracellular metabolic enzymes, causing abnormal cellular metabolism and injury to cell structures and functions. Under physiological conditions, calpain binds to Ca2+ to form a Ca2+ CaM complex. Following spinal cord injury, Ca2+ and calpain increase simultaneously resulting in high levels of the Ca2+ CaM complex. However, in the sham-surgery group, calpastatin expression was significantly down regulated, and this loss of calpain control may be an important pathological factor causing delayed neurological impairment following ischemia/reperfusion.
Taken together, this study identified 23 proteins with significant changes in expression levels during the process of spinal cord ischemia/reperfusion. The proteins were related to energy metabolism, cell defense, inflammatory mechanisms, cell signaling, ion channels and metabolic enzymes.
Following spinal cord ischemia/reperfusion injury, severe energy supply disorder can occur due to an imbalance of the energy metabolic pathway. This can inhibit cytoskeletal protein conformation, cause formation of abnormal intracellular structures, and block substance and information transfer, which contribute to the pathological progression of ischemia/reperfusion. The mechanism of delayed neurological impairment following spinal cord ischemia/reperfusion injury requires further study.
MATERIALS AND METHODS
Design
A proteomic study.
Time and setting
Spinal ischemia/reperfusion injury animal models were established at the Laboratory Animal Center of Jilin University in China between October and December 2009. Proteomic experiments were conducted at the Beijing Central Laboratory of Proteomics in China between May and September 2010.
Materials
Eighteen healthy New Zealand rabbits of either gender, aged 7 months and weighing 2.3 ± 0.4 kg were provided and raised at 20°C at the Laboratory Animal Center of Jilin University in China (license No. SCXK-(Ji) 2008-0005). All experimental protocols were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of China[21].
Methods
Establishment of a rabbit model of spinal cord ischemia injury
Following anesthesia by intraperitoneal injection of 10% chloral hydrate (0.3 mg/kg), the abdominal aorta was occluded below the bifurcation of the left renal artery to induce ischemia at the lumbar segment of the spinal cord. At the designated time points, the vascular clamp was closed or open. Under anesthesia, lumbar segments of the spinal cord were taken and immediately frozen at −70°C in liquid nitrogen[1].
Preparation of protein samples
Spinal cord tissue was shattered after pre-cooling with liquid nitrogen and then ground under liquid nitrogen flow. Subsequently, spinal cord tissue was processed with 20 μL/mL cocktail protease inhibitor. After addition of lysis buffer, the suspensions were homogenized by sonication using a Dounce homogenizer in iced water and then pure water, and centrifuged. The supernatant was used to determine the protein concentration and then stored at −80°C.
Protein labeling
The protein sample was adjusted to a final concentration of 5 mg/mL and adjusted to pH 8.0–9.0. Each sample (50 μg, 10 μL) was used for analysis. The gel electrophoresis dye was mixed for 30 seconds, centrifuged at 12 000 × g for 30 seconds and left in place at room temperature for 10 minutes. Then, 0.4 μL dye and 0.6 μL dimethylformamide were mixed together. Subsequently, 50 μg protein sample was added. Following addition of sample buffer, the mixture was fully mixed and a hydration solution was added till the final volume was 450 μL.
Fluorescent two-dimensional gel electrophoresis for isolation of differential proteins in rabbit spinal cord tissue
According to a previously described method[22] and following the instructions of Ettan IPGphor 3 Isoelectric Focusing System, an immobilized pH gradient strip (3–10 NL, 17 cm; Promega, Madison, WI, USA) was used for gel equilibration and sodium dodecyl sulfate polyacrylamide gel electrophoresis. Thereafter, fluorescence staining was performed.
Gel scanning and image analysis
Cy2, Cy3, Cy5 dye-labeled images were scanned using a Typhoon 9410 scanner (Amersham Pharmacia, Cambridge, England) at a wavelength of 488/520 nm, 532/580 nm, and 633/670 nm respectively. All gel images were analyzed using DeCyder software v5.02 (Amersham Pharmacia, NJ, USA). Changes in each protein spot were observed at each time point after ischemia/reperfusion. Differentially expressed protein spots were compared and screened.
Mass spectrometry identification and data analysis
Peptide spectra were prepared using in-gel digestion. Matrix-assisted laser desorption/ionization-time of flight mass spectrometry (ABI, Foster City, CA, USA) was performed with a laser power of MS 5000 and MSMS 5500. A GPS software (ABI) was used for retrieval in NCBI database with the relative accuracy of MS 0.12 Da and MSMS 0.3 Da. The proteins that were scored > 52 in differential expression were selected and the confidence level was set > 95%[2].
Statistical analysis
Statistical analysis was performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). A two sample t-test was used for comparison of differential protein expression between groups. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: the National Natural Science Foundation of China, No. 30872609, 30972153.
Conflicts of interest: None declared.
Ethical approval: This study received approval from the Animal Ethics Committee, China-Japan Friendship Hospital, Jilin University in China.
(Edited by Cheng B, Liu YP/Song LP)
REFERENCES
- [1].Yang XY, Zhu QS, Zhao LJ, et al. Expression of intercellular adhesion molecule-1 and interleukin-1 after spinal cord ischemia/reperfusion injury. Zhongguo Jizhu Jisui Zazhi. 2004;14(3):167–168. [Google Scholar]
- [2].Zvara D, Zboyovski JM, Deal DD. Spinal cord blood flow after ischemic preconditioning in a rat model of spinal cord ischemia. ScientificWorldJournal. 2004;22(4):892–898. doi: 10.1100/tsw.2004.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liang CL, Lu K, Liliang PC, et al. Ischemic preconditioning ameliorates spinal cord ischemia-reperfusion injury by triggering autoregulation. J Vasc Surg. 2012;55(4):1116–1123. doi: 10.1016/j.jvs.2011.09.096. [DOI] [PubMed] [Google Scholar]
- [4].Simon F, Scheuerle A, Gröger M, et al. Comparison of carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury. Intensive Care Med. 2011;37(9):1525–1533. doi: 10.1007/s00134-011-2303-4. [DOI] [PubMed] [Google Scholar]
- [5].Chen A, McEwen ML, Sun S, et al. Proteomic and phosphoproteomic analyses of the soluble fraction following acute spinal cord contusion in rats. J Neurotrauma. 2010;27(1):263–262. doi: 10.1089/neu.2009.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kang SK, So HH, Moon YS, et al. Proteomic analysis of injured spinal cord tissue proteins using 2-DE and MALDI-TOF MS. Proteomics. 2006;6(9):2797–2812. doi: 10.1002/pmic.200500621. [DOI] [PubMed] [Google Scholar]
- [7].Mautes AE, Liu J, Brandewiede J, et al. Regional energy metabolism following short-term neural stem cell transplantation into the injured spinal cord. J Mol Neurosci. 2004;24(2):227–236. doi: 10.1385/JMN:24:2:227. [DOI] [PubMed] [Google Scholar]
- [8].Gorg A, Obermaier C, Boguth G, et al. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;21(6):1037–1053. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1037::AID-ELPS1037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- [9].Arrigo AP, Firdaus WJ, Mellier G, et al. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. 2005;35(2):126–138. doi: 10.1016/j.ymeth.2004.08.003. [DOI] [PubMed] [Google Scholar]
- [10].Duilio C, Ambrosio G, KuPPusamy P, et al. Neutrophils are primary source of O2 radieals during reperfusion after prolonged myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280(6):H2649–2657. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- [11].Tanaka K, Weihrauch D, Kehl F, et al. Mechanism of preconditioning by isoflurane in rabbits: a direct role for reactive oxygen species. Anesthesiology. 2002;97(6):1485–1490. doi: 10.1097/00000542-200212000-00021. [DOI] [PubMed] [Google Scholar]
- [12].Tatar I, Chou PC, Desouki MM, et al. Evaluating regional blood spinal cord barrier dysfunction following spinal cord injury using longitudinal dynamic contrast-enhanced MRI. BMC Med Imaging. 2009;9:10. doi: 10.1186/1471-2342-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu SQ, Ma YG, Peng H, et al. Monocyte chemoattractant protein-1 level in serum of patients with acute spinal cord injury. Chin J Traumatol. 2005;8(4):216–219. [PubMed] [Google Scholar]
- [14].Gordh T, Sharma HS. Chronic spinal nerve ligation induces microvascular permeability disturbances, astrocytic reaction, and structural changes in the rat spinal cord. Acta Neurochir Suppl. 2006;96:335–340. doi: 10.1007/3-211-30714-1_70. [DOI] [PubMed] [Google Scholar]
- [15].Hirose K, Okajima K, Taoka Y, et al. Activated protein C reduces the ischemia/reperfusion-induced spinal cord injury in rats by inhibiting neutrophil activation. Ann Surg. 2000;232(2):272–280. doi: 10.1097/00000658-200008000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oz OE, Kardes O, Korkmaz A, et al. Effects of vascular endothelial growth factor on ischemic spinal cord injury caused by aortic cross-clamping in rabbits. J Surg Res. 2009;151(1):94–99. doi: 10.1016/j.jss.2008.01.006. [DOI] [PubMed] [Google Scholar]
- [17].Savas S, Savas C, Altuntas I, et al. The correlation between nitric oxide and vascular endothelial growth factor in spinal cord injury. Spinal Cord. 2008;46(2):113–117. doi: 10.1038/sj.sc.3102066. [DOI] [PubMed] [Google Scholar]
- [18].Conti A, Miscusi M, Cardali S, et al. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev. 2007;54(1):205–218. doi: 10.1016/j.brainresrev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [19].Marsala J, Orendácová J, Lukácová N, et al. Traumatic injury of the spinal cord and nitric oxide. Prog Brain Res. 2007;161:171–183. doi: 10.1016/S0079-6123(06)61011-X. [DOI] [PubMed] [Google Scholar]
- [20].McEwen ML, Sullivan PG, Springer JE. Pretreatment with the cyclosporin derivative, NIM811, improves the function of synaptic mitochondria following spinal cord contusion in rats. J Neurotrauma. 2007;24(4):613–624. doi: 10.1089/neu.2006.9969. [DOI] [PubMed] [Google Scholar]
- [21].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [22].Zhu BQ, Yang XY, Gu R. Difference in proteome Maps between spinal cord ischemia reperfusion injury (IR) and normal rabbit spinal cord. Zhongguo Shiyan Zhenduanxue. 2010;14(4):487–489. [Google Scholar]


