Abstract
An experiment was carried out in the key laboratory for Technique Diagnosis and Function Assessment of Winter Sports of China to investigate the differences in gait characteristics between healthy children and children with spastic hemiplegic cerebral palsy. With permission of their parents, 200 healthy children aged 3 to 6 years in the kindergarten of Northeastern University were enrolled in this experiment. Twenty children aged 3 to 6 years with spastic hemiplegic cerebral palsy from Shengjing Hospital, China were also enrolled in this experiment. Standard data were collected by simultaneously recording gait information from two digital cameras. DVracker was used to analyze the standard data. The children with hemiplegic cerebral palsy had a longer gait cycle, slower walking speed, and longer support phase than did the healthy children. The support phase was longer than the swing phase in the children with hemiplegic cerebral palsy. There were significant differences in the angles of the hip, knee, and ankle joint between children with cerebral palsy and healthy children at the moment of touching the ground and buffering, and during pedal extension. Children with hemiplegic cerebral palsy had poor motor coordination during walking, which basically resulted in a short stride, high stride frequency to maintain speed, more obvious swing, and poor stability.
Keywords: gait analysis, children, spastic hemiplegic cerebral palsy, walking, hip, knee, ankle, neural regeneration
Research Highlights
(1) Children with spastic hemiplegic cerebral palsy had a longer gait cycle, slower walking speed, and longer support phase than did healthy children. The support phase was longer than the swing phase in children with spastic hemiplegic cerebral palsy. (2) There were significant differences in the angles of the hip, knee, and ankle joint between children with spastic hemiplegic cerebral palsy and healthy children at the moment of touching the ground and buffering, and during pedal extension. (3) Children with spastic hemiplegic cerebral palsy had poor motor coordination during walking, which resulted in a short stride, high stride frequency to maintain speed, more obvious swing, and poor stability.
INTRODUCTION
Cerebral palsy can be divided into four types based on motor dysfunction: spastic, ataxia, athetotic, and mixed[1,2,3,4,5,6,7,8,9]. Rehabilitation through voluntary movement is mostly suitable for children with spastic cerebral palsy[10,11,12]; thus, this study focused on the spastic gait. A major goal of rehabilitation in children with cerebral palsy is to help establish and improve walking ability. Gait analysis, one part of sports function assessment, plays an important role in rehabilitation assessment of children with cerebral palsy[13,14]. A motion analysis system was adopted in this study to analyze the differences in gait characteristics between children with cerebral palsy and healthy children of various ages.
From characteristic analyses of healthy children's gaits, we know that space-time parameters and kinematic parameters of the gait of 3-year-old children are stable[15,16,17]. However, children with cerebral palsy present abnormalities in their gait when they are older than 3 years. By taking photos and performing video analysis, we aimed to comparatively analyze the gait of healthy children and children with cerebral palsy at the age of 3 to 6 years.
RESULTS
Quantitative analysis and basic data of participants
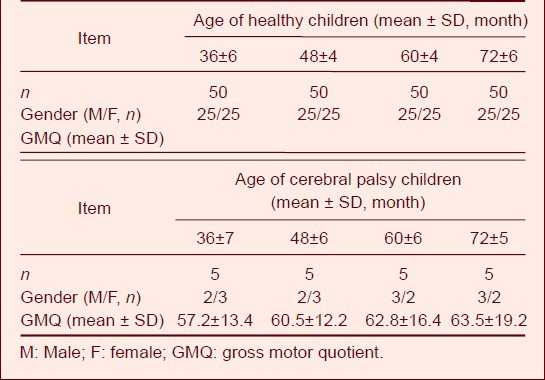
A total of 200 healthy children and 20 children with cerebral palsy were included in the study. Healthy children were divided into four age groups with 50 children in each group, and children with cerebral palsy were divided into four age groups with five children in each group. The basic data of the subjects are shown in Table 1.
Table 1.
General data of healthy children and children with cerebral palsy
Walking time parameters
Table 2 shows that with increased age, the single-leg support time increased. The single-leg support cycle of 3-year-old children accounted for 38%; this ratio stabilized at 40% after 3 years of age, which is consistent with the ratio in adults. However, the swing ratio of children with cerebral palsy was about 30%, which was significantly lower than that of healthy children and the 35% to 40% seen in adults.
Table 2.
Comparison of walking time parameters
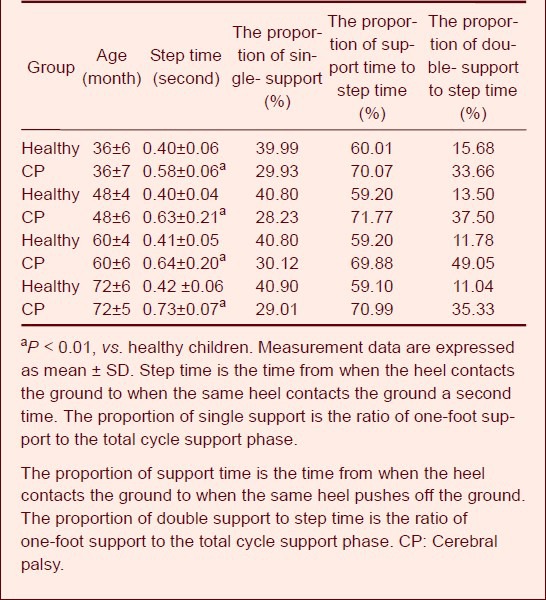
The support phase ratio of normal children was approximately 63% at < 3 years of age, which is slightly higher than the adult level. However, this ratio stabilized at 60% in normal children of > 3 years of age, which is consistent with the adult level. The ratio of the support phase of children with cerebral palsy was 70%, which is obviously longer than the normal level. The bipedal stance phase ratio decreased with the growth of children and was close to the adult level at > 5 years of age. The support ratio of 3-year-old children with cerebral palsy was 34%, which is close to that of normal 1-year-old children, whose bipedal stance phase ratio was 28.47%; however, there was still a gap of about 5%. The bipedal stance phase ratio of children with cerebral palsy was 13.5% and 10% higher than that of healthy children and adults, respectively.
Step length and step width
The ratio of step length and height can better describe the walking ability of children with cerebral palsy. Figure 1 shows that the average ratio of step length to height in children with cerebral palsy was 0.16, which is much lower than that of healthy children of the same age (0.41–0.61).
Figure 1.
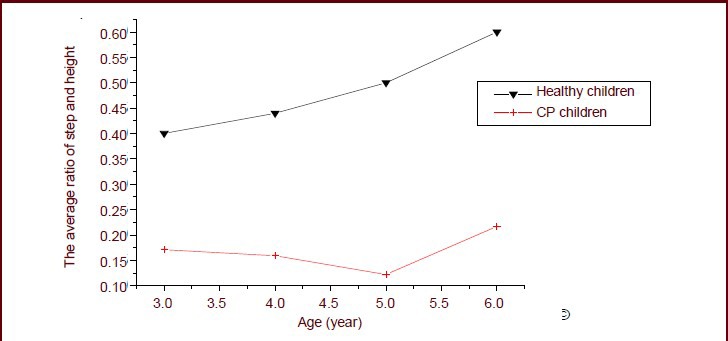
Ratio of step length to height of 3- to 6-year-old children during walking.
The average ratio of step length to height of children with cerebral palsy (CP) was 0.16, which was much lower than that of healthy children.
The step width is the distance between the medial arch of the two feet while walking, which is an important factor in the measurement of transverse stability. The normal step width is about 5 to 8 cm and decreases with increasing age[15,16,17,18,19]. The average step width of children with cerebral palsy was 11.69 cm, which approximates that of healthy 1-year-old children (data not shown).
Joint angle changes
Hip joint angle
Table 3 shows that there were significant differences in the hip angle between children with cerebral palsy and healthy children at the moment of touching the ground and buffering. The angle was so large that the entire pedal extension phase angle did not change. Figure 2 shows that the hip angle on the affected side increased slightly from the time of swing to pedal extension. The strength of the right hip flexor muscle was not powerful enough, so the flexion was not obvious.
Table 3.
Characteristics of hip angle values (°) during walking
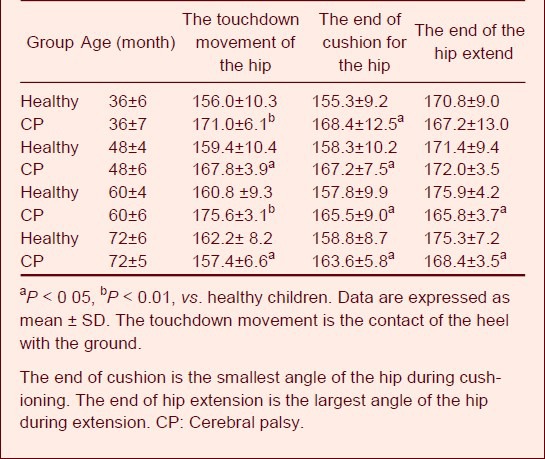
Figure 2.
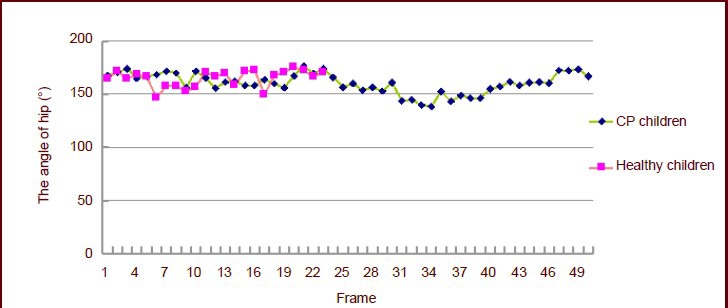
Angle changes in both hips of 3-year-old children.
Each frame interval time was 0.02 seconds. CP: Cerebral palsy.
They had to bend their upper body backward to compensate for their decreased hip flexor strength and impaired leg-lift. Based on the comparison of healthy children and children with cerebral palsy, at some time point, the hip angle of children with cerebral palsy was significantly lower than the lowest value of healthy children. The presence of spasm causes the proprioceptive disorder, which results in poor motor coordination and position sense.
Knee joint angle
Table 4 shows that the knee muscle in children with cerebral palsy had no buffering capacity, and the knee angle on the affected side remained unchanged from the stage of landing on the ground to the stage of buffering. The angle in the healthy children differed at all stages, which had great statistical significance. Especially at the end of stretching, without strong extension of the knee, the children with cerebral palsy began to extend their knees when the ankle ended its extension. There was a great difference in the knee angle between the children with cerebral palsy and the healthy children.
Table 4.
Characteristics of knee joint angle values (°) during walking
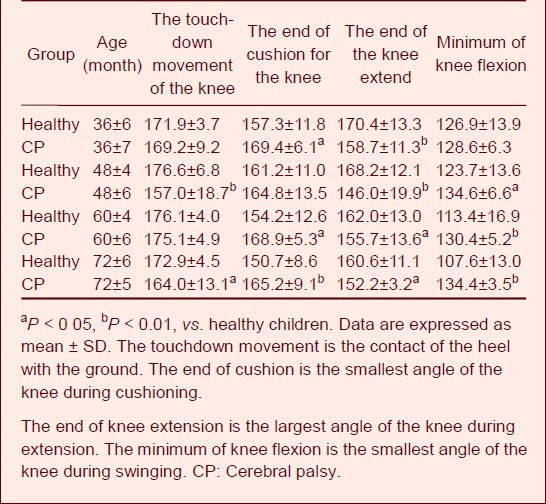
Figure 3 shows that the changes in the angle of the knee joint of children with cerebral palsy were stable during the swing phase of the right leg and during support of the left leg; when the right leg landed, the left knee began to buckle. Children with cerebral palsy could not fully walk forward, so they had to transfer their gravity center only by flexion and extension of the supporting leg. The knee angle increased when the right leg extended. After entering the stage of left leg flexion, children walked forward. The knee joint angle increased during the landing and support phases.
Figure 3.
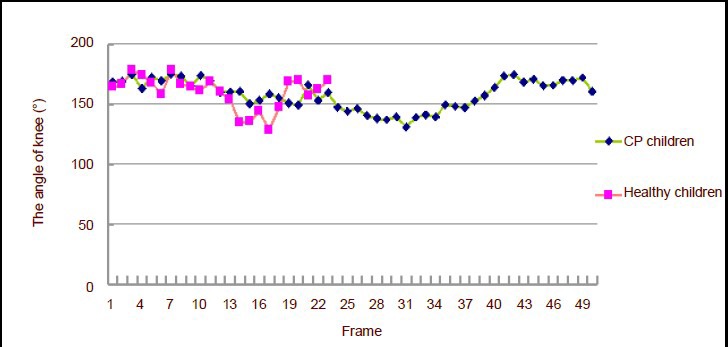
Knee joint angle changes in 4-year-old children.
Each frame interval time was 0.02 seconds. CP: Cerebral palsy.
Ankle joint angle
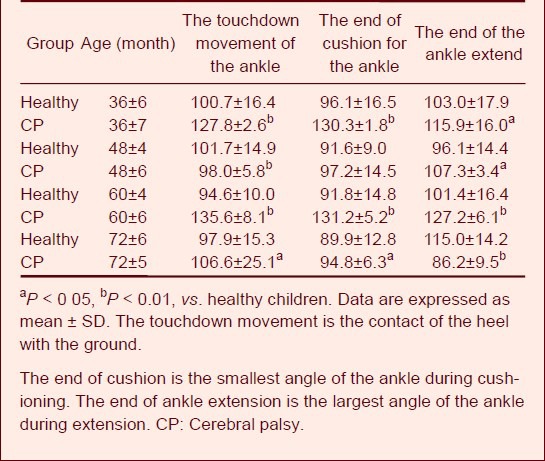
Table 5 shows that the ankle athletic ability of children with cerebral palsy was weaker. The angle did not significantly change during the entire walking cycle, and it differed from that of healthy children. This indicates that cerebral palsy greatly influences the athletic ability of the end portion of the walking cycle.
Table 5.
Values of ankle angle (°) during walking
Changes in trunk pitch angle
Figure 4 shows that children with cerebral palsy had difficulty in hip flexion because of muscle tension and extreme nervousness.
Figure 4.
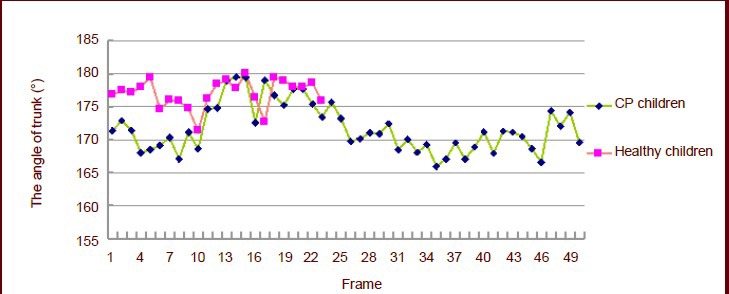
Trunk pitch angle changes in 4-year-old children.
Each frame interval time was 0.02 seconds. CP: Cerebral palsy.
The children contracted their abdominal muscles to compensate for their inability to flex the hip, and their truck leaned forward. The backward motion of the upper body then drove continuous flexion of the hip joints, and the trunk pitch angle changed in the same way as they raised their opposite leg.
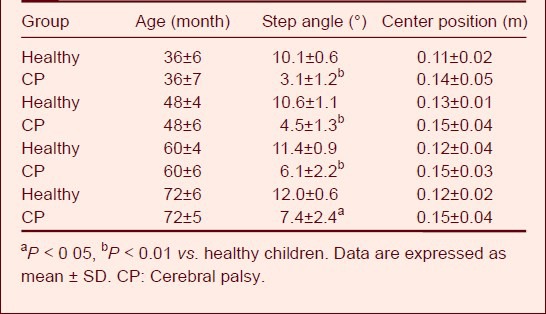
Step angle
The step angle, also known as the foot angle, is the angle between the feet and the center line while walking on a road. The angle is measured in the left and right foot separately, and the normal angle is about 15°[15,16]. This angle is caused by outward rotation of the lower limb joint, which occurs secondary to forward flexion of the femur and twisting of the shank bones. If the femur and shank bones twist abnormally, the heading angle will change obviously[2,3,4]. Table 6 shows that the step angle of children with cerebral palsy was nearly 5°. There was a great difference between the children with cerebral palsy and the healthy children.
Table 6.
Values of step angle and change in center posi-tion during walking in healthy children and children with cerebral palsy
Change of the center position
Figure 5 shows that the vertical gravity center fluctuation of children with cerebral palsy was about 0.15 meter. However, the vertical gravity center fluctuation of healthy children was about 0.13 meter. There was no significant difference in the center position between the children with cerebral palsy and the healthy children.
Figure 5.
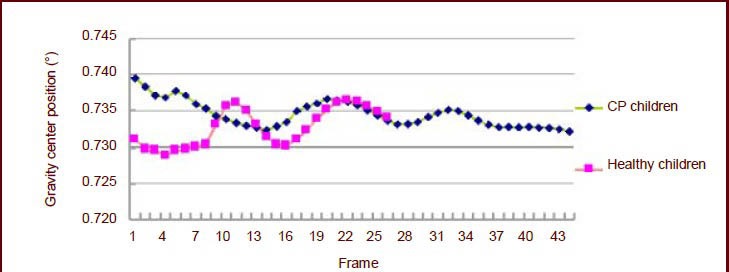
Change in vertical gravity center position of 4-year-old children.
Each frame interval time was 0.02 seconds. CP: Cerebral palsy.
DISCUSSION
The step time comprises the time from initial placement of the supporting heel on the ground to the time at which the same heel contacts the ground a second time. The step time comprises the support phase and swing phase. The support phase comprises the single-support phase and double-support phase. In gait analysis, gait characteristics can be reflected by the swing rate, the one-foot support rate, the total cycle support phase ratio, and the two-foot support ratio[15,16]. These measurements can be used to reflect the differences in time parameters between children with cerebral palsy and healthy children or even adults. As seen from this study, children with cerebral palsy have abnormal muscle extension and poor balance ability, and they must slow down to improve their stability. Therefore, the step length of children with cerebral palsy is obviously longer than that of healthy children.
With increased age, the single-leg support time of healthy children is increased because the motor system function of children strengthens with increased age. Their motor system functions are not so developed at younger ages, and their muscle strength, stability, coordination, and posture control are poor. The swing ratio of children with cerebral palsy is significantly lower than that of healthy children for the following reasons. First, the muscle tension of children with cerebral palsy is abnormal because the central nervous system is damaged and the leg swing is thus hampered. Second, the swing phase is not adequately addressed and precedes support phase because of poor balance.
Because balance and coordination are poor due to abnormal muscle tension, the support phase ratio in children with cerebral palsy is obviously longer than the normal level. The bipedal stance phase ratio of children with cerebral palsy is higher than that of healthy children. This is because the muscle extension of children with cerebral palsy is high, their limb control and balance ability are weak, their changing speed and posture accuracy are low, and they must maintain bodily balance by a compensatory mechanism (by decreasing the single-foot support time and increasing the two-foot support time). In this way, they can reduce the risk of falling down and ensure their next step. The average step width of children with cerebral palsy approximates that of normal 1-year-old children, but is higher than that of healthy children of other ages. The stability of children with cerebral palsy is poor, so they must improve their walking stability by increasing the step width and support surface.
There are significant differences between the hip angle on the affected side in children with cerebral palsy and healthy children at the moment of touching the ground and buffering. This is because the angle is so large that the entire pedal extension phase angle does not change, indicating that the hip extensor group plays no role during the buffering and treading stages; it only affects the fastness of the hip joint angle.
The change in the knee joint angle of children with cerebral palsy is stable during the swing phase of the right leg and support of the left leg; when the right leg lands, the left knee begins to buckle. This process is mainly caused by the poor stability of children with cerebral palsy. They cannot fully walk forward, so they must transfer their gravity center by only flexion and extension of the supporting leg. The knee angle increases when the right leg extends. After entering the left leg flexion stage, the children walk forward. The knee joint angle increases during the landing and support phases.
Because of the abnormal muscle tension of children with cerebral palsy, their coordination and balance are poor and there are more unusual fluctuations during the process of walking. The backward motion of the upper body drives continuous flexion of the hip joints, and the trunk pitch angle also changes in the same way as they raise their opposite leg. Because children with spastic cerebral palsy often have unbalanced muscle tension between the two sides, the trunk pitch angle on both body sides will vary when they walk. Their gravity center fluctuation is also larger than that of normal children. If their walking stability decreases, they are prone to falling down. The unusual fluctuation of the gravity center in the process of walking can also waste energy. In the course of walking, children with cerebral palsy will perform abnormal actions to compensate for gravity center changes caused by abnormal muscle movement. The step angle of children with cerebral palsy is larger than that of healthy children, which is mainly because these children's adductor muscle tension is so high and the hip rotation angle is so large that the step angle decreases. However, children with cerebral palsy who have normal adductor muscle tension with a normal step angle cannot be excluded from this study.
Conclusions
-
(1)
The step length of children with cerebral palsy is long, the proportion of the support phase greatly exceeds that of healthy children, and the support phase is obviously longer than the swing phase.
-
(2)
The swing ratio of children with cerebral palsy is significantly lower than that of healthy children. The swing phase is not adequately addressed and precedes the support phase because of poor balance.
-
(3)
The hip angle on the affected side increases slightly from the time of swing to pedal extension. Children with cerebral palsy must bend their upper body backward to compensate for their decreased hip flexor strength and inability to leg-lift.
-
(4)
At the end of stretching, without strong extension of the knee, children with cerebral palsy begin to extend their knees when the ankle ends its extension. There is a great difference between children with cerebral palsy and healthy children.
-
(5)
Children with cerebral palsy will contract their abdominal muscles to compensate for their inability in hip flexion, and their truck will lean forward. The trunk pitch angle of both body sides will vary when they walk.
MATERIALS AND METHODS
Design
A case-controlled study.
Time and setting
The experiment was carried out in Shengjing Hospital, China from December 2009 to June 2010.
Subjects
A total of 200 healthy children and 20 children with hemiplegic cerebral palsy aged 3 to 6 years were included in the study, and their parents allowed them to participate in the experiment. The children with cerebral palsy were from Shengjing Hospital, China Medical University and the healthy children were from the kindergarten of Northeastern University.
The children with hemiplegic cerebral palsy included in the study met the diagnostic criteria developed by the 2006 Second National Conference on Rehabilitation of Children and the Ninth National Conference on Infantile Cerebral Palsy[20], and they could walk independently for more than 10 meters. However, children with cerebral palsy with severe heart, liver, kidney, or other organic diseases or who had mental retardation, mental illness, or severe epilepsy were ineligible because of difficulty completing the experiment.
All healthy children had no pathological findings and their gross motor quotients were > 100. Hence, all children were allocated correctly, and the consistency of the two groups was considered to be reliable.
Children with cerebral palsy were selected according to the gross motor developmental quotient[21]: higher values referred to good gross motor function, and lower values referred to poor gross motor function. A quotient of 131 to 165 meant that the motor ability was very good, 121 to 130 was good, 91 to 120 was upper middle, 80 to 89 was middle, 70 to 79 was lower middle, 60 to 69 was poor, and 35 to 68 was very poor. We selected sick children whose gross motor developmental quotient was < 70.
Methods
Two Sony video cameras were used to obtain front and side views of children's gaits (the sampling frequency was 50 Hz). The main axis angle of the two cameras was close to 90°, and the height of the two cameras was 0.8 m. The camera used a 1-m2 scale for calibration, and the calibration error was < 0.5 mm. The author analyzed videos taken in this experiment with the DVracker motion picture analyzing system. Matsui model 15 was taken as the human model[22], with 21 track points to calculate the gravity center. The five-point smoothing method was used to process the data[22].
Analysis items included step length, step time, step width, hip joint angle, knee joint angle, ankle joint angle, trunk pitch angle, step angle, and center position change. The step length was the distance of the same-side heel or toe after a step. The time from initial placement of the supporting heel on the ground to when the same heel contacted the ground a second time was the step time. The step width was the distance between the medial arches of the two feet during walking. The joint angle was the angle of two adjacent segments. The trunk pitch angle was the angle of the trunk to the vertical axis. The step angle was the angle between the feet and the center line in during walking. The vertical gravity center fluctuation was the change in the vertical gravity center in one step time, and the horizontal fluctuation was the change in the horizontal gravity center in one step time.
Statistical analysis
The Statistical Software (SPSS 13.0) (SPSS, Chicago, IL, USA) was used to process the data. The data were presented as mean ± SD, and differences between the two groups were tested by an independent-samples t-test. A P value of < 0.05 represented a significant difference.
Footnotes
Funding: Financial support was provided by the Educational Bureau of Liaoning Province, No. 2009A671.
Conflicts of interest: None declared.
Ethical approval: This research was approved by the Ethics Committee of Shenyang Sport University, China.
(Edited by Feng J, Gong QH/Wang L)
REFERENCES
- [1].Chang CF, Wang TM, Lo WC. Balance control during level working in children with spastic diplegic cerebral palsy. BME. 2011;23:509–517. [Google Scholar]
- [2].Bland DC, Prosser LA, Bellini LA, et al. Tibialis anterior architecture, strength, and gait in individuals with cerebral palsy. Muscle Nerve. 2011;44:509–517. doi: 10.1002/mus.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen LW, Wang J, Wang ZM, et al. Biomechanical analysis of gait in children with spastic cerebral palsy. Zhongguo Yundong Yixue Zazhi. 2009;6:644–646. [Google Scholar]
- [4].Kwak EE. Effect of rhythmic auditory stimulation on gait performance in children with spastic cerebral palsy. J Music Ther. 2007;44:198–216. doi: 10.1093/jmt/44.3.198. [DOI] [PubMed] [Google Scholar]
- [5].Galli M, Cimolin V, Valente EM, et al. Computerized gait analysis of botulinum toxin treatment in children with cerebral palsy. Disabil Rehabil. 2007;29:659–664. doi: 10.1080/09638280600948136. [DOI] [PubMed] [Google Scholar]
- [6].Romkes J, Peeters W, Oosterom AM, et al. Evaluating upper body movements during gait in healthy children and children with diplegic cerebral palsy. J Pediatr Orthop B. 2007;16:175–180. doi: 10.1097/BPB.0b013e32801405bf. [DOI] [PubMed] [Google Scholar]
- [7].Yan HG, Wang X. Shenyang: Teaching Affairs Division of Shenyang Sport University; 2007. The Basic Action Principle and Analysis of Sports. [Google Scholar]
- [8].Li K. Research advancement of children's gait. Tiyu Kexue. 2009;10:72–75. [Google Scholar]
- [9].Zhou AY, Li H, Huang DF. Gait analysis in normal children of pre-school age with visual press gait analysis system. Zhongguo Linchuang Kangfu. 2005;9:115–117. [Google Scholar]
- [10].Qian JG. The biomechanics principle of walking and analysis on gaits. Nanjing Tiyu Xueyuan Xuebao. 2006;5:4. [Google Scholar]
- [11].Ying H, Wang ZZ, Yu WX, et al. Characteristic index of motor analysis system in children. Zhongguo Zuzhi Gongcheng Yanju yu Linchuang Kangfu. 2007;44:8882–8886. [Google Scholar]
- [12].Van de Walle P, Hallemans A, Schwartz M, et al. Mechanical energy estimation during walking: validity and sensitivity in typical gait and in children with cerebral palsy. Gait Posture. 2012;35:231–237. doi: 10.1016/j.gaitpost.2011.09.012. [DOI] [PubMed] [Google Scholar]
- [13].Williams SE, Gibbs S, Meadows CB, et al. Classification of the reduced vertical component of the ground reaction force in late stance in cerebral palsy gait. Gait Posture. 2011;34:370–373. doi: 10.1016/j.gaitpost.2011.06.003. [DOI] [PubMed] [Google Scholar]
- [14].Bojanic DM, Petrovacki-Balj BD, Jorgovanovic ND, et al. Quantification of dynamic EMG patterns during gait in children with cerebral palsy. J Neurosci Methods. 2011;198:325–331. doi: 10.1016/j.jneumeth.2011.04.030. [DOI] [PubMed] [Google Scholar]
- [15].Benedetti MG, D’Apote G, Faccioli S, et al. Equinus foot classification in cerebral palsy: an agreement study between clinical and gait analysis assessment. Eur J Phys Rehabil Med. 2011;47(2):213–221. [PubMed] [Google Scholar]
- [16].Russell S, Bennett B, Sheth P, et al. The gait of children with and without cerebral palsy: work, energy, and angular momentum. J Appl Biomech. 2011;27(2):99–107. doi: 10.1123/jab.27.2.99. [DOI] [PubMed] [Google Scholar]
- [17].Kwon JY, Chang HJ, Lee JY, et al. Effects of hippotherapy on gait parameters in children with bilateral spastic cerebral palsy. Arch Phys Med Rehabil. 2011;92(5):774–779. doi: 10.1016/j.apmr.2010.11.031. [DOI] [PubMed] [Google Scholar]
- [18].Salazar-Torres JJ, McDowell BC, Kerr C, et al. Pelvic kinematics and their relationship to gait type in hemiplegic cerebral palsy. Gait Posture. 2011;33(4):620–624. doi: 10.1016/j.gaitpost.2011.02.004. [DOI] [PubMed] [Google Scholar]
- [19].Kim WH, Park EY. Causal relation between spasticity, strength, gross motor function, and functional outcome in children with cerebral palsy: a path analysis. Dev Med Child Neurol. 2011;53(1):68–73. doi: 10.1111/j.1469-8749.2010.03777.x. [DOI] [PubMed] [Google Scholar]
- [20].Chen XJ, Li SC. Definition, typing and diagnostic criteria for cerebral palsy. Zhonghua Wuli Yixue yu Kangfu Zazhi. 2007;29(5):309. [Google Scholar]
- [21].Fewell RR, Folio MR. 2nd ed. Austin: Pro-Ed; 2000. Peabody Developmental Motor Scales. [Google Scholar]
- [22].Lu DM, Wang YD, Yan BT. Beijing: Beijing Sports University Press; 2001. Sports Biomechanics methods of measurement. [Google Scholar]


