Abstract
Rat models of adjuvant arthritis were established, and anti-corticotropin release hormone serum injection in the lateral ventricles and electroacupuncture at right Jiaji (EX-B2) were performed. The pain threshold was decreased at 45 and 60 minutes after injection of the anti-corticotropin release hormone serum. Electroacupuncture at Jiaji can resist this effect. Immunohistochemical staining results showed that the expression of corticotropin release hormone in the hypothalamic paraventricular nucleus was greater in the electroacupuncture + anti-corticotropin release hormone serum group compared with the anti-corticotropin release hormone serum group. The expression of corticotropin release hormone was correlated with the pain threshold. The effect of endogenous corticotropin release hormone in pain modulation can be obstructed by anti-corticotropin release hormone serum. The analgesia of electroacupuncture can partially resist the depressed pain threshold caused by injection of anti-corticotropin release hormone serum. The analgesic effect of electroacupuncture is associated with the corticotropin release hormone content in the hypothalamus.
Keywords: endogenous corticotropin release hormone, electroacupuncture, adjuvant arthritis, analgesia, hypothalamus, neuron, neural regeneration
Research Highlights
(1) Endogenous corticotropin release hormone plays a certain role in pain modulation, but this effect can be obstructed by anti-corticotropin release hormone serum. (2) Electroacupuncture can delay the decrease in the pain threshold following anti-corticotropin release hormone serum injection. (3) The analgesic effect of electroacupuncture is associated with the corticotropin release hormone content in the hypothalamus.
INTRODUCTION
Corticotropin release hormone has a strong correlation with pain[1]. Corticotropin release hormone can accommodate nociceptive stimuli in each layer of the neural system as a neurohumor or neuromediator through activation of intercellular signals[2]. Corticotropin release hormone injection in release hormone[6,7,8]. Corticotropin release hormone is an the lateral ventricles can interrupt the physiologic reaction of locus coeruleus neurons from stimulating sciatic nerves of anesthetized rats[3,4]. Corticotropin release hormone inhibits electrophysiological activity of the thalamic and hypothalamic paraventricular nuclei, which are involved in pain[5]. Numerous studies have demonstrated the analgesic and anti-inflammatory effects of corticotropin important endogenous trigger point in inhibition of inflammatory pain[9].
Electroacupuncture exerts analgesic effects by activating the endogenous analgesia system, resulting in the release of many neurotransmitters such as noradrenaline, 5-serotonin, and endogenous opiate. Exploration of the association of electroacupuncture analgesia and corticotropin release hormone is important to treat inflammatory pain and to investigate the mechanism underlying electroacupuncture analgesia. Exogenous corticotropin release hormone strengthens the analgesic effect of electroacupuncture[10]. There are few studies regarding the analgesic effects of electroacupuncture on endogenous corticotropin release hormone levels. Acupuncture at Jiaji (EX-B2) exerts analgesic effects by regulating the endogenous analgesia system[11,12,13]. Pain was induced by nociceptive stimuli in the sciatic nerves of rats with adjuvant arthritis and transmitted to the L3-5 spinal cord from the dorsal root ganglia[14]. A good analgesic effect can be obtained by electroacupuncture at Jiaji.
This study observed the influence of anti-corticotropin release hormone serum injection on electroacupuncture analgesia and corticotropin release hormone expression in the hypothalamic paraventricular nucleus after electroacupuncture, explored the role of endogenous corticotropin release hormone during electroacupuncture analgesia and the effect of electroacupuncture on the corticotropin release hormone level, and further clarified the mechanism of action of electroacupuncture analgesia in inflammatory pain models of rats with adjuvant arthritis.
RESULTS
Quantitative analysis of experimental animals
A total of 30 rats with a stable pain threshold were chosen. Rat models of adjuvant arthritis were established by injecting Freund's adjuvant 3 days after burying the tube in the lateral ventricle. Three rats from each group had incorrect tube positions in the lateral ventricle and were replaced by new rats. The pain threshold results in three rats were not reliable and were eliminated upon analysis. The rat models were equally and randomly divided into three groups: the model group (injection of saline in the lateral ventricles), anti-corticotropin release hormone serum group (injection of anti-corticotropin release hormone serum in the lateral ventricles), and electroacupuncture + anti-corticotropin release hormone serum group (electroacupuncture at Jiaji + injection of anti-corticotropin release hormone serum in the lateral ventricles). Data of one rat from each group were used to select the concentration and temperature of primary antibodies during immunohistochemistry. A total of 24 rats were included in the final analysis.
Effects of anti-corticotropin release hormone serum injection and electroacupuncture on pain threshold
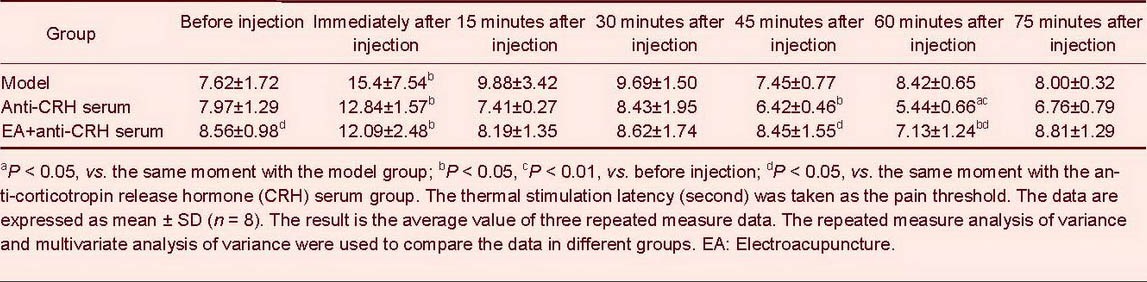
The pain threshold was significantly decreased at 60 minutes after injection of anti-corticotropin release hormone serum in the lateral ventricles (P < 0.05). The pain threshold was significantly increased after electroacupuncture at 45 and 60 minutes following injection of anti-corticotropin release hormone serum (P < 0.05; Table 1).
Table 1.
Comparison of pain threshold (second) at different time points in each group
Effects of anti-corticotropin release hormone serum injection and electroacupuncture on corticotropin release hormone expression in the hypothalamic paraventricular nucleus
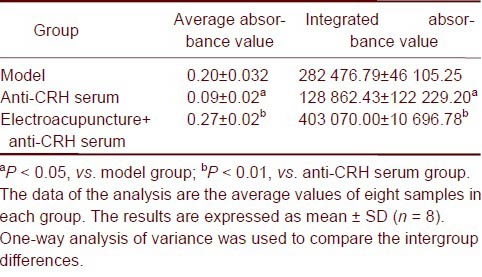
The expression of corticotropin release hormone neurons in the hypothalamic paraventricular nucleus was reduced after injection of anti-corticotropin release hormone serum (P < 0.05) and increased when electroacupuncture was performed (P < 0.01; Table 2).
Table 2.
Expression of corticotropin release hormone (CRH) in the hypothalamic paraventricular nucleus in different groups
Under light microscopy, there were numerous corticotropin release hormone-positive neurons in the hypothalamic paraventricular nucleus in the electroacupuncture + anti-corticotropin release hormone serum group (Figure 1).
Figure 1.
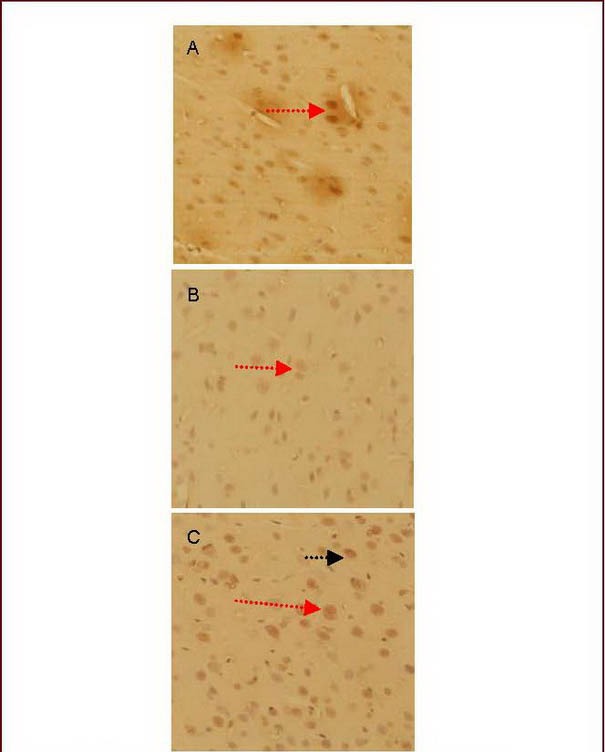
Morphology of the corticotropin release hormone (CRH)-positive neurons in the hypothalamic paraventricular nucleus (light microscopy, diaminobenzidine staining, ×400).
(A) Model group.
(B) Anti-CRH serum group.
(C) Electroacupuncture + anti-CRH serum group.
The CRH immunoreactive substances exhibited yellow granules in the hypothalamus (red arrow), gathering in the perinuclear cytoplasm.
The CRH-positive neurons exhibited spindle and spherical shapes. Some positive cell bodies had one or two dendrites (black arrow). The cell body and dendrites were stained yellow.
Pain threshold at 60 minutes after anti-corticotropin release hormone serum injection was positively correlated with corticotropin release hormone expression in the hypothalamic paraventricular nucleus
Linear correlation analysis showed that the partial correlation coefficients of the pain threshold and integral absorbance in the model group, anti-corticotropin release hormone serum group, and electroacupuncture + anti-corticotropin release hormone serum group were 0.738, 0.936, and 0.933, respectively. The partial correlation coefficients of the pain threshold and average absorbance were 0.768, 0.951, and 0.948 respectively. These results indicate that corticotropin release hormone expression is positively correlated with electroacupuncture and anti-corticotropin release hormone serum injection.
DISCUSSION
The analgesic effect of corticotropin release hormone was correlated with the dose[15,16]. The effective dose lies within a narrow range of micrograms when corticotropin release hormone exerts an analgesic effect from the intracalvarium[17,18]. Results from this study demonstrated that the pain threshold was decreased at 45 minutes and lowest at 60 minutes following anti-corticotropin release hormone serum injection. The analgesic effect of electroacupuncture was also reduced at 60 minutes after anti-corticotropin release hormone serum injection, suggesting that endogenous corticotropin release hormone participates in the pain modulation in rats with adjuvant arthritis and in the analgesic function of electroacupuncture. This effect can be obstructed by anti-corticotropin release hormone serum. The main mechanism is that the amount of endogenous corticotropin release hormone decreased when anti-corticotropin release hormone serum was injected, resulting in decreases in both the pain threshold of rats with adjuvant arthritis and the analgesic effect of electroacupuncture. The probable mechanism of participation of endogenous corticotropin release hormone in pain modulation and the analgesic effect of electroacupuncture is that the physiological dose of corticotropin release hormone is regulated by non-opioid peptide mechanisms[19,20,21,22], which are correlated with the hormone[23].
Electroacupuncture can exert an analgesic effect through opioid peptide release[24]. Electrical stimulation at a certain frequency can induce the release of thyroxine release factor[25]. Therefore, peripheral electric stimulation exerts an analgesic effect through central neuropeptide release[26]. In the present study, the pain threshold of the electroacupuncture + anti-corticotropin release hormone serum group decreased slowly and transiently, suggesting that electroacupuncture can slow down and delay the decrease in the pain threshold following anti-corticotropin release hormone serum injection. The mechanism is that electroacupuncture stimulates endogenous corticotropin release hormone release, and corticotropin release hormone expression then increases from activation of the endogenous analgesia system, resulting in neutralization of a small amount of anti-corticotropin release hormone serum. The hypothalamic paraventricular nucleus is a part of the endogenous analgesia system. Electroacupuncture exerts an analgesic effect through the neurons in hypothalamic paraventricular nucleus to activate opiate receptors and adjusts the transmission of nociceptive signals from endogenous analgesia descending inhibitory systems[28]. Corticotropin release hormone is extensively distributed in the central and peripheral nerve tissue, and corticotropin release hormone expression is highest in hypothalamic paraventricular nucleus cells. Electroacupuncture exerts an analgesic effect and simultaneously regulates corticotropin release hormone expression in inflammatory areas in rats with adjuvant arthritis[27]. What are the effects of electroacupuncture on corticotropin release hormone-positive neurons in the hypothalamic paraventricular nucleus?
In the present study, the pain threshold was decreased and the number of corticotropin release hormone-positive neurons was significantly reduced following anti-corticotropin release hormone serum injection in rats with adjuvant arthritis. Corticotropin release hormone expression in the hypothalamic paraventricular nucleus was increased when the pain threshold was increased in the electroacupuncture group. Statistical results confirmed that the pain threshold is correlated with corticotropin release hormone expression following electroacupuncture and anti-corticotropin release hormone serum injection. Numerous studies have suggested that electroacupuncture can exert an analgesic effect through positive feedback to increase corticotropin release hormone expression in the hypothalamic paraventricular nucleus[29,30,31]. Electroacupuncture exerts an analgesic effect by upregulating the number of corticotropin release hormone-positive neurons in the hypothalamic paraventricular nucleus[32] and increasing the endogenous corticotropin release hormone levels. Endogenous corticotropin release hormone is an important point of accommodation during electroacupuncture. There is another pathway of electroacupuncture analgesia other than the mechanism involving opioid peptides[33], and acupuncture analgesia has been characterized by many pathways and links. However, the precise action pathway of corticotropin release hormone after increasing corticotropin release hormone contents during acupuncture analgesia and the association of corticotropin release hormone with other neurotransmitters and opioid peptides require further investigation.
This research explored the relationship between electroacupuncture and endogenous corticotropin release hormone and indicated that electroacupuncture can increase the amounts of corticotropin release hormone in the body. These results indicate that we can use electroacupuncture to treat painful diseases with decreased corticotropin release hormone levels. Because electroacupuncture has the advantage of convenience and no side effects compared with other methods, it can be widely used in clinics.
MATERIALS AND METHODS
Design
This was a randomized controlled animal experiment.
Time and setting
This experiment was conducted at the Experimental Center of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine, China from March 2009 to July 2010.
Materials
A total of 30 specific pathogen-free male Wistar rats aged 2 months and weighing 200 ± 20 g were purchased from the Experimental Center of Shandong University, China (license No. SCXK (Lu) 20090001). The protocols were conducted in accordance with Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of the People's Republic of China[34].
Methods
Establishment of pain model of adjuvant arthritis rats
A total of 0.1 mL of Freund's adjuvant containing 1 g/L tubercle bacillus (Beijing Boaosen Biotechnology, Beijing, China) was subcutaneously injected into the right toe in a sterile manner. The finger pulp lightly touched the injection site for a moment and then relaxed. The right hock and toes become red and swollen at 4 hours after injection and peaked at 16 to 28 hours. The joint motion of the rats was limited; activities were obviously reduced, and the rats exhibited limping or leg-raising with an inability to touch the ground. Irritability and a decreased pain threshold were also displayed. The above-described symptoms indicated that the model of adjuvant arthritis rats was successfully established[35].
Burying the tube in the lateral ventricles and injections
The rats were intraperitoneally anesthetized with 10% chloral hydrate (300 mg/kg) and fixed on a ZH-B solid positioner (Anhui Huaibei Biological Equipment, Anhui Province, China). The tube (made with a No. 7 injection needle) was buried in the left lateral ventricle[36,37,38] (B: −0.8 mm, L: 1.6 mm, H: 4.0 mm) according to the stereotaxic atlas of rats[36]. When cerebrospinal fluid effused from the tube, the core was inserted (it was longer than the tube by about 0.5 mm) and fixed at the bone surface with glass ionomer cement and glass ionomer sticky solid-liquid. All surgical procedures were performed under sterile conditions, and penicillin G potassium was injected once a day (1 × 105 U) for 3 consecutive days to avoid infection. The pain threshold was detected, and microinjection into the lateral ventricles was then performed 7 days after model establishment. The core was pulled out during injection. The microinjector connected to the polyethylene pipe was inserted in a stainless steel tube of 0.3-mm external diameter for microinjection.
According to the standard dose in the literature[17,18], the anti-corticotropin release hormone serum was diluted with aqua pro injection at 1:100. The density of anti-corticotropin release hormone serum was 1.4 mg/mL, and the dose of injection was 5 μL. A total of 5 μL of saline was extracted with a microsyringe. The air bubble was extruded, and about 5 μL of anti-corticotropin release hormone serum (Beijing Boaosen Biotechnology) was extracted. The injection dose was 10 μL (5 μL medicine + 5 μL saline rinse solution). The injection was performed within 1 minute, and the needle was maintained for 1 minute.
Electroacupuncture method
A 15.0- × 0.3-mm Hua Tuo brand needle (Suzhou Medical Products Factory, China) was used for acupuncture at Jiaji (dissection position: the lateral side about 3 mm below the 3, 5 spinous process of the lumbar vertebra) 2 days after model induction. The standard of location and depth of needling was in accordance with Laboratory Animals Acupoint Icon by the Chinese Acupuncture Society of Experimental Acupuncture Research. Acupuncture was perpendicular to the muscular layer and then connected with a HANS Pain Treatment Instrument (Nanjing Joint Creation of the Economic Health Medical Technology, China). Electroacupuncture was performed with sparse waves at 2 Hz and dense waves at 100 Hz. The strength was 1 mA for 30 minutes once a day for 7 consecutive days, and then the pain threshold was detected.
Measurement of the pain threshold
The pain threshold was detected at the right hind feet at various time points including before injection of saline or anti-corticotropin release hormone serum; at the moment of injection of saline or anti-corticotropin release hormone serum; and at 15, 30, 45, 60, and 75 minutes after injection in each group. In accordance with the method of a previous study[39], a 35-W halogen projector lamp was used. The voltage was stable at 12 V. The distance of the lamp source and glass plate was adjusted to make the diameter of the radiation aperture 5 mm. The strength of the pyrogen was constant. The time (seconds) from shining to rats flinching was recorded and served as the thermal stimulation latency. The environment was kept quiet at 23 ± 2°C. The rats were placed in the bell glass (20 cm × 15 cm × 20 cm) of a radiant heat dolorimeter instrument (Institute of Biomedical Engineering, Chinese Academy of Medical Sciences, Beijing, China). The rats were able to freely run in the cage. The right hind feet of the rats were shined when the rats were quiet for 15 to 20 minutes in the cage. The response latency of thermal stimulation was detected three times, once every 6 minutes. The average response latency represented the pain threshold. The cut-off time of the response latency was 20 seconds to avoid tissue damage. The pain threshold was examined by one researcher at 8 a.m. to 12 a.m., and the results were handled by another person.
Immunohistochemical staining of corticotropin release hormone expression in the hypothalamic paraventricular nucleus
Heart perfusion was performed and hypothalamic tissues were obtained after the pain threshold was detected in each group. The above-described tissues were fixed in 4% paraformaldehyde for 20 hours, embedded with paraffin, sliced into coronal sections, and immunohistochemically stained. The sections were deparaffinized and washed in PBS, then underwent antigen retrieval using microwaves. After washing with PBS, the specimens were treated with 3% H2O2 for 10 minutes and washed again in PBS. The sections were blocked in goat serum at room temperature for 20 minutes, incubated in primary antibody rabbit anti-corticotropin release hormone antibody (1:100) at 4°C overnight, rinsed in PBS, and incubated with horseradish peroxidase-labeled goat anti-rabbit IgG (Wuhan Boster, China) for 20 minutes at 37°C. After washing with PBS, streptavidin-biotin complex was added for 20 minutes at 37°C. The specimens were washed with PBS, developed with diaminobenzidine, rinsed in distilled water, counterstained with hematoxylin, dehydrated, permeabilized, mounted, and photographed with a microscopic image system.
Statistical analysis
Measurement data were expressed as mean ± SD and analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). The results were compared using analysis of variance methods such as repeated measures and multivariate at different time points in different groups. The result of the repeated measures analysis of variance method differed according to the test of sphericity. If P < 0.05 in the test of sphericity, it was not content with H-F conditions, and multivariate analysis of variance could be used to analyze the effect of interaction within subjective variables. If P > 0.05, one-way analysis of variance could be used (a = 0.05).
Acknowledgments:
This project was completed at the Experimental Center of Affiliated Hospital of Shandong University of Traditional Chinese Medicine, China. Immunohistochemical staining was performed at Shanxi College of Traditional Chinese Medicine. We received help and support from a teacher during the laboratory experiment and appreciate the hard work and enthusiasm.
Footnotes
Conflicts of interest: None declared.
Ethical approval: Experimental protocols were permitted by the Animal Ethics Committee of the Shandong University of Traditional Chinese Medicine, China.
(Edited by Zhang L, Mei ZG/Qiu Y/Wang L)
REFERENCES
- [1].Lariviere WR, Fiorenzani P, Ceccarelli l, et al. Central CRH administration changes formalin pain responses in male and female rats. Brain Res. 2011;1383:128–134. doi: 10.1016/j.brainres.2011.01.106. [DOI] [PubMed] [Google Scholar]
- [2].Schafer M, Mousa SA, Zhang Q, et al. Expression of corticotropin-releasing factor in inflamed tissue is required for intrinsic peripheral opioid analgesia. Proc Natl Acad Sci U S A. 1996;93(12):6096–6100. doi: 10.1073/pnas.93.12.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45(1):28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- [4].Borsody MK, Weiss JM. Influence of corticotropin-releasing hormone on electrophysiological activity of locus coeruleus neurons. Brain Res. 1996;724(2):149–168. doi: 10.1016/0006-8993(96)00199-0. [DOI] [PubMed] [Google Scholar]
- [5].Siggins GR, Gruol D, Aldenhoff J, et al. Electrophysiological actions of corticotropin-releasing factor in the central nervous system. Fed Proc. 1985;44(1 Pt 2):237–242. [PubMed] [Google Scholar]
- [6].Cui XY, Yu LC. Adrenocorticotropic hormone-releasing hormone and its receptor in pain regulation in the role of research progress. Shengli Kexue Jinzhan. 2003;34(3):263–265. [Google Scholar]
- [7].Wang WC, Ge HJ. Antinociceptive efficacy of preemptive intrathecal administration of CRH in rats. Disan Junyi Daxue Xuebao. 2006;28(10):1072–1074. [Google Scholar]
- [8].Wang WC, Ge HJ, Liu S, et al. Antinociceptive effect of pre-emptive intrathecal administration of CRH in rats following formalin-induced information pain. Chongqing Yixue. 2008;37(17):1906–1910. [Google Scholar]
- [9].Machelska H, Schopohl JK, Mousa SA, et al. Different mechanisms of intrinsic pain inhibition in early and late inflammation. J Neuroimmunol. 2003;141(1-2):30–39. doi: 10.1016/s0165-5728(03)00213-3. [DOI] [PubMed] [Google Scholar]
- [10].Lv YL, Wang J, Zhang YC, et al. Effect of electroacupuncture of jiaji (EX-B2) on pain, paw-swelling and T-lymphocyte subsets in adjuvant arthritis rats. Zhenci Yanjiu. 2006;31(2):82–85. [Google Scholar]
- [11].Li Q, Han XZ. Approach The Jiaji clinical applications and mechanism. Shandong Zhongyiyao Daxue Xuebao. 2009;33(4):289–290. [Google Scholar]
- [12].Wang SX, Hong J, Lai XS. The adjustment of rats with adjuvant arthritis pain exception with electro-acupuncture on jiaji and local acupoints. Xinzhongyi. 2000;32(12):29–30. [Google Scholar]
- [13].Zhang HX, Huang GF, Zhang TF. Clinical study on analgesic effect influence on the level of serumβ-EP of electroacupuncture at jiaji acupoints in patients with lumber disc herniation. Anhui Zhongyi Xueyuan Xuebao. 2006;14(6):11–14. [Google Scholar]
- [14].Wang H, Huang H, Cai KY. Effects of electroacupuncture at jiaji Acupuncture on pain threshold in Arthritis Rats by phosphorylation p38 MAPK Signal Pathway. Anhui Zhongyi Xueyuan Xuebao. 2010;29(5):34–37. [Google Scholar]
- [15].Schafer M, Mousa SA, Stein C, et al. Corticotropin-releasing factor in antinociception and inflammation. Eur J Pharmacol. 1997;323(1):1–10. doi: 10.1016/s0014-2999(97)00057-5. [DOI] [PubMed] [Google Scholar]
- [16].La JH, Sung TS, Kim HJ, et al. Peripheral corticotropin releasing hormone mediates post-inflammatory visceral hypersensitivity in rats. World J Gastroenterol. 2008;14(5):731–736. doi: 10.3748/wjg.14.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Williams DW, Jr, Lipton JM, Giesecke AH., Jr Influence of centrally administered peptides on ear withdrawal from heat in the rabbit. Peptides. 1986;7(6):1095–1100. doi: 10.1016/0196-9781(86)90139-7. [DOI] [PubMed] [Google Scholar]
- [18].Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84(1):1–12. doi: 10.1016/S0304-3959(99)00193-1. [DOI] [PubMed] [Google Scholar]
- [19].Huang J, Qin HL, Huang XK, et al. Effect of CRH on phagocytosis of rat enterocoelia macrophage. Chuangshan Waike Zazhi. 2006;8(1):59–62. [Google Scholar]
- [20].Bogdanov AI, Yarushkina NI. The role of hormones of the hypothalamo-hypophyseal-adrenocortical system in the analgesic effect of corticotrophin-releasing hormone. Neurosci Behav Physiol. 2007;37(4):363–367. doi: 10.1007/s11055-007-0022-7. [DOI] [PubMed] [Google Scholar]
- [21].Larushkina NI. The role of hypothalamo-hypophyseal-adrenocortical system hormones in controlling pain sensitivity. Neurosci Behav Physiol. 2008;38(8):759–766. doi: 10.1007/s11055-008-9044-z. [DOI] [PubMed] [Google Scholar]
- [22].Larushkina NI. The role of hormones of hypothalamic-pituitary-adrenocortical system in regulation of pain sensitivity. Ross Fiziol Zh Im I M Sechenova. 2007;93(11):1252–1262. [PubMed] [Google Scholar]
- [23].Larushkina NI, Bagaeva TR. Mechanisms of corticotrophin-releasing factor-induced analgesic effect in conscious rats. Ross Fiziol Zh lm l M Sechenova. 2010;96(2):128–137. [PubMed] [Google Scholar]
- [24].Cheng RS, Pomeranz B. Electro-acupuncture analgesia could be mediated by at least two pain-relieving mechanisms: endorphin and non-endorphin systems. Life Sci. 1979;25(23):1957–1962. doi: 10.1016/0024-3205(79)90598-8. [DOI] [PubMed] [Google Scholar]
- [25].Iverfeldt K, Serfozo P, Arnesto LD, et al. Differential release of coexisting neurotransmitters: frequency dependence of the efflux of substance P, thyrotropin releasing hormone and [3H] serotonin from tissue slice of rat vent ral cord. Acta Physiol Scand. 1989;137(1):63–71. doi: 10.1111/j.1748-1716.1989.tb08721.x. [DOI] [PubMed] [Google Scholar]
- [26].Han JS. Induction of the release of central neuropeptides by peripheral electrical stimulation. Beijing Daxue Xuebao: Yixue Ban. 2002;34(5):408–413. [Google Scholar]
- [27].Xiao Y, Han Y, Yang ZD, et al. influence of electroacupuncture on the immunoreactive of CRH-positive cells in rats with adjuvant-induced arthritis. Zhongguo Zuzhi Huaxue yu Xibao Huaxue Zazhi. 2006;5(5):517–522. [Google Scholar]
- [28].Cui CD, Kang GY, Zhang H, et al. Investigation of the relations between PVN and analgesia via intrabrain stimulation. Binzhou Yixueyuan Xuebao. 1994;17(2):101–104. [Google Scholar]
- [29].Chen YF, Wang YH, Yin QZ. The role of paraventricular nucleus of hypothalamus in acupuncture analgesia in rats. Zhenci Yanjiu. 1991;(1):32–35. [PubMed] [Google Scholar]
- [30].Ono N, Samson WK, McDonald JK, et al. Effect of intravenous and in-traventricular injection of antisera directed against corticotropin-releasing factor on the scretion of anterior pituitary hormones. Proc Natl Acad Sci U S A. 1985;82(22):7787–7790. doi: 10.1073/pnas.82.22.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McCann SM, Antunes-Rodriques J, Franci CR, et al. Role of the hypothalamic pituitary adrenal axis in the control of the response to stress and infection. Braz J Med Biol Res. 2000;33(10):1121–1131. doi: 10.1590/s0100-879x2000001000001. [DOI] [PubMed] [Google Scholar]
- [32].Chen LZ, Xu MH, Zhou JH, et al. Corticotropin-releasing hormone regulates corticotropin-releasing hormone mRNA expression through PKA signal pathway in rat hypothalamic slices in vitro. Disan Junyi Daxue Xuebao. 2004;26(16):1459–1462. [Google Scholar]
- [33].Zhang H, Wang J, Chen XY. Electric acupuncture its analgesic effect on adjuvant-induced arthritis in rats and influence on β-endorphins. Qingdao Daxue Yixueyuan Xuebao. 2010;46(5):390–392. [Google Scholar]
- [34].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [35].Chen BS, Xu YD, Zhong SQ, et al. Establishment and evaluation of experimental animal model of CFA induced adjuvant arthritis in rats. Harbin Yike Daxue Xuebao. 2005;(6):489–491. [Google Scholar]
- [36].Bao XM, Shu SY. Beijing: People's Health Publishing House; 1991. The Stereotaxic Atlas of the Rat Brain. [Google Scholar]
- [37].Yu HM, Wang BR, Li GL, et al. A modified method of implanting cannulae into the lateral ventricle of the rat. Shenjing Jiepouxue Zazhi. 2005;21(2):207–211. [Google Scholar]
- [38].Zhang XH, Yin GX, Ni H. Effect of intracerebroventricular neurotensin on blood pressure in rat. Shengli Xuebao. 1999;51(2):140–146. [PubMed] [Google Scholar]
- [39].Xu SY, Bian RX, Chen X. Beijing: People's Health Publishing House; 1991. Methodology of Pharmacological Experiments. [Google Scholar]


