Abstract
Bone marrow mesenchymal stem cells were isolated, purified and cultured in vitro by Percoll density gradient centrifugation combined with the cell adherence method. Passages 3–5 bone marrow mesenchymal stem cells were transplanted into rats with traumatic spinal cord injury via the caudal vein. Basso-Beattie-Bresnahan scores indicate that neurological function of experimental rats was significantly improved over transplantation time (1–5 weeks). Expressions of choline acetyltransferase, glutamic acid decarboxylase and synapsins in the damaged spinal cord of rats was significantly increased after transplantation, determined by immunofluorescence staining and laser confocal scanning microscopy. Bone marrow mesenchymal stem cells that had migrated into the damaged area of rats in the experimental group began to express choline acetyltransferase, glutamic acid decarboxylase and synapsins, 3 weeks after transplantation. The Basso-Beattie- Bresnahan scores positively correlated with expression of choline acetyltransferase and synapsins. Experimental findings indicate that intravenously transplanted bone marrow mesenchymal stem cells traverse into the damaged spinal cord of rats, promote expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins, and improve nerve function in rats with spinal cord injury.
Keywords: bone marrow mesenchymal stem cells, spinal cord injury, choline acetyltransferase, glutamic acid decarboxylase, synapsins, neural regeneration
Research Highlights
Intravenously transplanted bone marrow mesenchymal stem cells traverse into the damaged spinal cord of rats, where they promote expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins, and improve nerve function in rats with spinal cord injury.
Abbreviations
BBB, Basso-Beattie-Bresnahan scale
INTRODUCTION
Bone marrow mesenchymal stem cells can be found in a wide variety of tissue sources, are easily isolated and demonstrate multipotential differentiation into a variety of tissue cells of mesodermal and ectodermal origin during long-term in vitro culture. Furthermore, bone marrow mesenchymal stem cells elicit low immunological reactions and no tumorigenicity after transplantation. Their clinical application could therefore overcome ethical and immunological rejection barriers. Consequently, bone marrow mesenchymal stem cells are considered an ideal cell source for tissue engineering and cell transplantation treatments[1,2,3]. Intravenous transplantation of bone marrow mesenchymal stem cells enables the cells to cross the blood-spinal cord barrier, where they survive and traverse into the injured spinal cord[4]. Bone marrow mesenchymal stem cells can differentiate into neuron-like cells and express specific neural markers including neuronal nuclei and glial fibrillary acidic protein in the injured spinal cord, thus replacing apoptotic neurons[5,6]. Bone marrow mesenchymal stem cells can also secrete the brain-derived neurotrophic factor, nerve growth factor, vascular endothelial growth factor, hepatocyte growth factor and interleukins (such as interleukin-6, -7, -8, -11, -12, -14, -15) and other nutritional factors[7,8]. In parallel, bone marrow mesenchymal stem cells induce in vivo neural stem cell differentiation into neurons and increase their differentiation and survival rates after differentiation[9,10]. A connection between the cell bundle and host neurons allows a wide range of afferent and efferent connections to rebuild neural pathways and promote the recovery of neural functions[11].
In the injured spinal cord, increasing levels of neurotransmitter and synaptophysin contribute to the functional recovery of the injured spinal nerve[12,13,14,15,16,17]. However, there is little evidence on the influence of bone marrow mesenchymal stem cell transplantation on neurotransmitters and synaptophysin following spinal cord injury. To understand the mechanism underlying bone marrow mesenchymal stem cell transplantation for treatment of spinal cord injury, this study aimed to explore the effect of bone marrow mesenchymal stem cell transplantation on the expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins in rats with spinal cord injury, and to analyze the correlation between functions of injured spinal nerve and altered expression levels of choline acetyltransferase and synapsins.
RESULTS
Quantitative analysis of experimental animals
A total of 50 Sprague-Dawley rats were randomly divided into three groups: sham-operation group (sham-operation + bone marrow mesenchymal stem cell transplantation, n = 10), model group (preparation of spinal cord injury models + PBS, n = 10) and experimental group (preparation of spinal cord injury models + bone marrow mesenchymal stem cell transplantation, n = 30). Specimens were observed in the experimental group at 1, 3 and 5 weeks after cell transplantation, and in the sham-operation and model groups 5 weeks after transplantation.
Morphology and phenotype of isolated bone marrow mesenchymal stem cells
Bone marrow mesenchymal stem cells began to adhere to the vessel wall 24 hours after culture. They displayed a macromonocyte and fusiform morphology, with polygonal or star-shaped processes. Cells rapidly proliferated as single or multiple cell clones. By days 12–14 after culture, cells reached more than 90% confluence and were predominantly spindle-shaped. There were 1–2 × 106 bone marrow mesenchymal stem cells after primary culture and 1 × 1011 bone marrow mesenchymal stem cells after five passages (Figure 1). Ten cell samples at passage 2 were characterized by flow cytometry and were found to negatively express hematopoietic markers, CD34 and CD45 and positively express mesenchymal markers, CD29 and CD90 (Figure 2).
Figure 1.
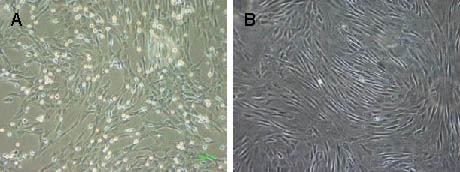
Morphology of bone marrow mesenchymal stem cells cultured in vitro (inverted phase contrast microscope, × 100).
(A) Primary cells; (B) passage 3 cells.
Figure 2.
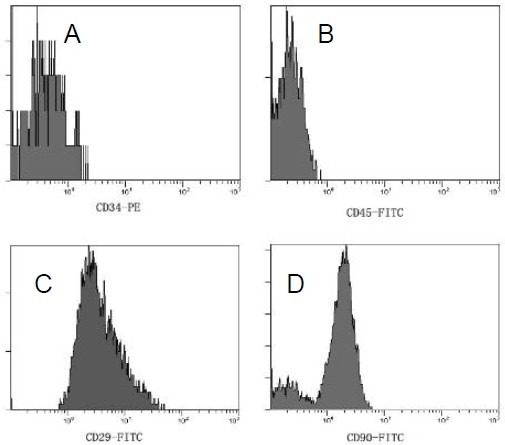
Phenotype of passage 2 bone marrow mesenchymal stem cells.
Flow cytometry analysis showed positive expression of CD34 (A), CD45 (B), CD29 (C) and CD90 (D) was 0.81%, 0.09%, 95.9% and 86.7% respectively. This indicated that the cells were bone marrow mesenchymal stem cells.
Bone marrow mesenchymal stem cell transplantation alleviated the pathological injury in spinal cord
Hematoxylin-eosin staining showed that spinal cord neurons from sham-operated rats had a normal morphology, with absence of swelling, round nuclei, visible nucleoli and an intact structure. Nissl staining demonstrated abundant Nissl bodies in these neurons. At week 1 after transplantation in the model rats, splinter hemorrhages and cyst formation, cellular edema, degeneration of neural cells, myelin swelling and a large number of vacuoles were visible in the spinal cord. Some nerve fibers disappeared and inflammatory cells appeared to infiltrate. Experimental rats showed clear spinal cord structures, with more surviving neurons, apoptotic and necrotic nerve cells, occasional small cavities in their gray matter and less lymphocyte infiltration (Figure 3).
Figure 3.
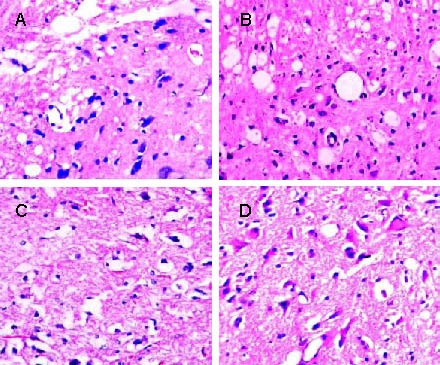
Pathological observations of the injured spinal cord of rats in each group (hematoxylin-eosin staining, light microscope, × 200).
(A) 1 week after cell transplantation, the spinal cord of model rats presented with cell edema, degeneration of neural cells, myelin swelling and large vacuoles.
(B) 1 week after cell transplantation, the spinal cord of experimental rats presented a clear gray matter structure and many surviving neurons.
(C) 3 weeks after cell transplantation, the spinal gray matter was destructed and large cavities were observed in model rats.
(D) 3 weeks after cell transplantation, the spinal gray matter displayed no necrosis and a clear structure in experimental rats.
In the model rats at weeks 3 and 5 after transplantation, the gray substance of the spinal cord had dissolved, glial scars had formed, large spaces were observed and Nissl bodies in the neurons were only slightly stained. In the experimental rats, necrosed areas became smaller, tissue structure was clearly visible, glial scar formation was limited and Nissl bodies were abundant (Figures 3, 4).
Figure 4.
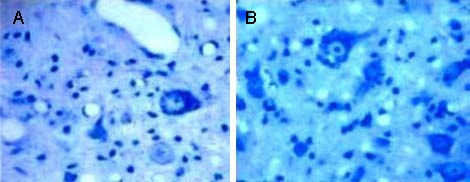
Nissl bodies in the injured spinal cord of rats in each group (Nissl staining, light microscope, × 400).
Nissl bodies in the model group (A) were significantly reduced, compared with the experimental group (B).
Expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins in the injured spinal cord of rats
Using inverted fluorescence microscopy, cells expressing choline acetyltransferase, glutamic acid decarboxylase and synapsins showed red fluorescence in the cytoplasm and were observed in the gray matter of rats from different groups (Figure 5).
Figure 5.
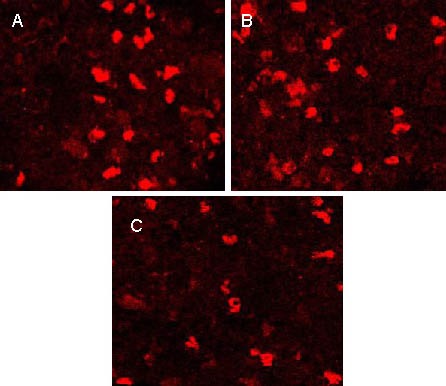
Expression of neurotransmitters and synapsins in the spinal gray matter of rats 3 weeks after transplantation (inverted fluorescence microscope, immunofluorescence staining, × 100).
Choline acetyltransferase (A), glutamic acid decarboxylase (B) and synapsins (C) were expressed in the spinal gray matter. Positive products are tetramethylrhodamine isothiocyanate-labeled for red fluorescence.
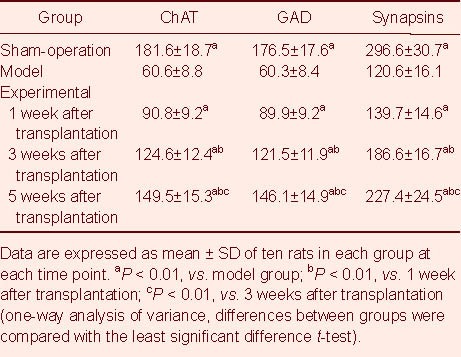
The number of choline acetyltransferase-, glutamic acid decarboxylase- and synapsins-positive cells and the fluorescence intensity in the injured spinal cord of the sham-operated and experimental rats were significantly higher than those in the model rats (P < 0.01). As post-transplantation time increased, the number of choline acetyltransferase-, glutamic acid decarboxylase- and synapsins-positive cells was also increased and the fluorescence intensity was enhanced in the experimental group (P < 0.01; Tables 1, 2).
Table 1.
Cell counts (n/0.16 mm2) for expression of choline acetyltransferase (ChAT), glutamic acid decarboxylase (GAD) and synapsins in the spinal cord of rats
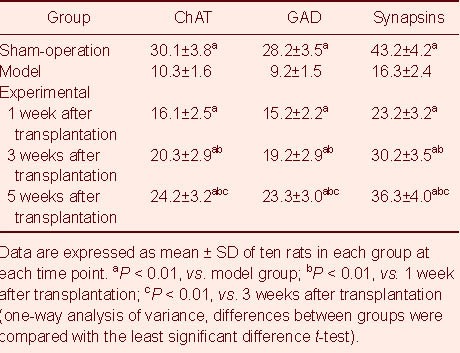
Table 2.
Fluorescence intensity of cells expressing choline acetyltransferase (ChAT), glutamic acid decarboxylase (GAD) and synapsins in the spinal cord of rats
Expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins in bone marrow mesenchymal stem cells migrating to the injured spinal cord of rats
Using laser scanning confocal microscopy, transplanted bone marrow mesenchymal stem cells (green fluorescence; carboxyfluorescein succinimidyl ester-labeled) and cells expressing choline acetyltransferase, glutamic acid decarboxylase and synapsins (red fluorescence; tetramethylrhodamine isothiocyanate-labeled) were observed. When the two signals overlapped, cells exhibited yellow fluorescence. At week 1 after transplantation, transplanted bone marrow mesenchymal stem cells with green fluorescence and nerve cells with red fluorescence were visible in the spinal cord of rats in the experimental group, but were not observed to overlap. At weeks 3 and 5 after transplantation, yellow fluorescence was visible in the gray matter of the spinal cord in the experimental rats, indicating that the transplanted bone marrow mesenchymal stem cells began to express choline acetyltransferase, glutamic acid decarboxylase and synapsins (Figures 6–8).
Figure 6.
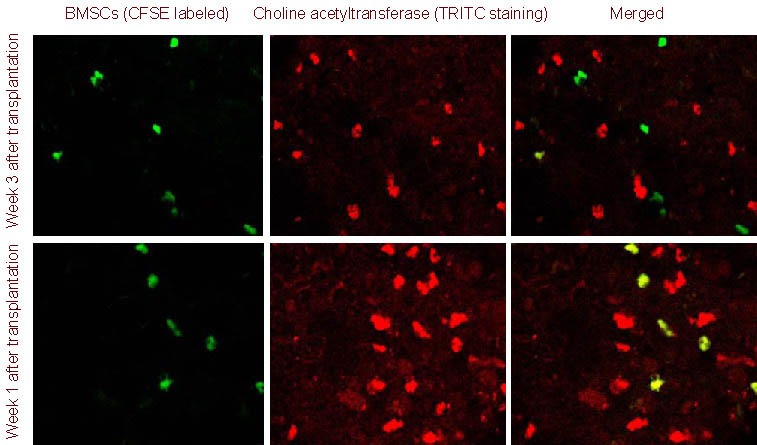
Choline acetyltransferase expression in bone marrow mesenchymal stem cells (BMSCs) migrating to the injured spinal cord (immunofluorescence double staining, laser confocal microscope, × 100).
At 1 week after transplantation, transplanted BMSCs [carboxyfluorescein succinimidyl ester (CFSE) marker; green] did not express choline acetyltransferase [tetramethylrhodamine isothiocyanate (TRITC) staining; red]; at 3 weeks after transplantation, BMSCs began to express choline acetyltransferase (yellow).
Figure 8.
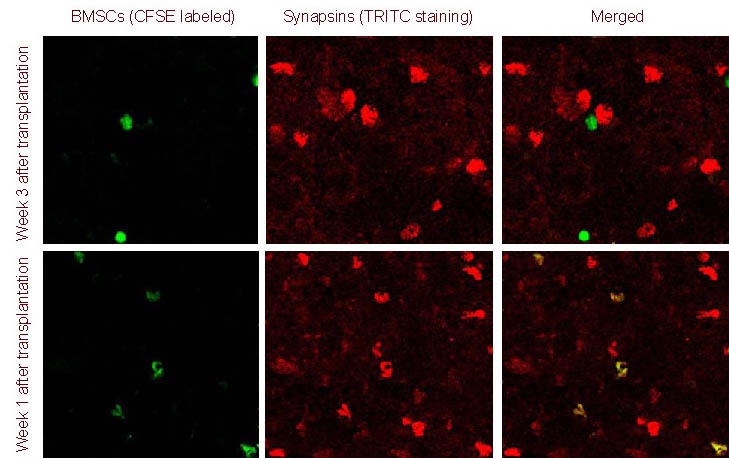
Synapsins expression in bone marrow mesenchymal stem cells (BMSCs) migrating to the injured spinal cord (immunofluorescence double staining, laser confocal microscope, × 100).
At 1 week after transplantation, transplanted BMSCs [carboxyfluorescein succinimidyl ester (CFSE) marker; green] did not express synapsins [tetramethylrhodamine isothiocyanate (TRITC) staining; red]; at 3 weeks after transplantation, BMSCs began to express synapsins (yellow).
Figure 7.
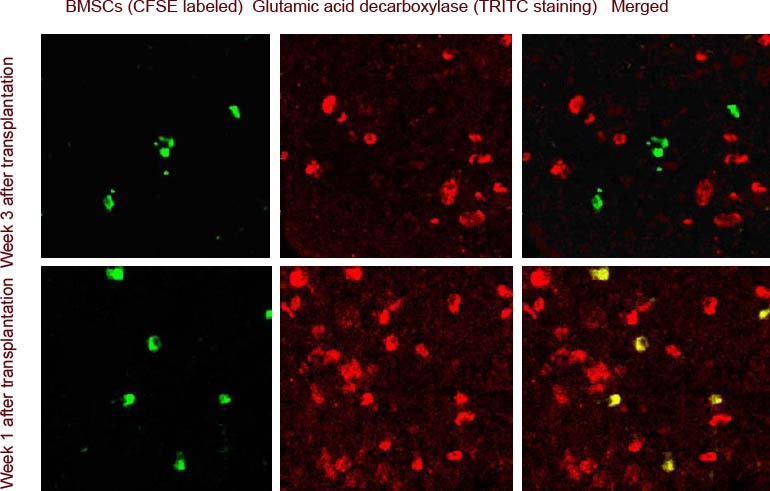
Glutamic acid decarboxylase expression in bone marrow mesenchymal stem cells (BMSCs) migrating to the injured spinal cord (immunofluorescence double staining, laser confocal microscope, × 100).
At 1 week after transplantation, transplanted BMSCs [carboxyfluorescein succinimidyl ester (CFSE) marker; green] did not express glutamic acid decarboxylase [tetramethylrhodamine isothiocyanate (TRITC) staining; red]; at 3 weeks after transplantation, BMSCs began to express glutamic acid decarboxylase (yellow).
Bone marrow mesenchymal stem cell transplantation improved neurological functions in rats with spinal cord injury
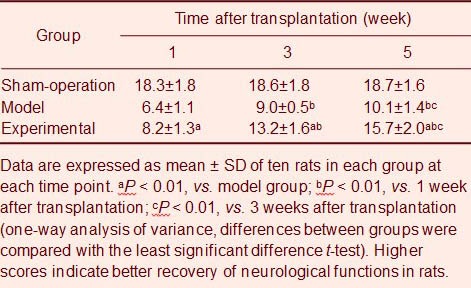
Basso-Beattie-Bresnahan (BBB) scale scores in the model and the experimental groups were decreased significantly compared with the sham-operation group (P < 0.01). Compared with the model group, experimental group rats had significantly increased scores (P < 0.01; Table 3).
Table 3.
Scores of Basso-Beattie-Bresnahan scale in different groups of rats at different time points
Correlation between neurological function scores and the number of cells expressing choline acetyltransferase, synapsins and fluorescence intensity
Pearson correlation analysis showed that the neurological function in rats was significantly and positively correlated with the number of cells expressing choline acetyltransferase and synapsins, as well as the fluorescence intensity in the experimental group (r = 0.825, 0.812, 0.856, 0.868; P < 0.05).
DISCUSSION
Previous studies in our research group have demonstrated that transplantation of bone marrow mesenchymal stem cells can significantly improve nerve functions of injured spinal cord and the effects may be associated with oligodendrocyte remyelination within the injured spinal cord[18]. This study aimed to further explore the mechanisms underlying bone marrow mesenchymal stem cell transplantation on improving neurological functions.
One primary physiological characteristic of neurons is to synthesize and release neurotransmitters, which are synthesized within cells under the action of neurotransmitter synthetase. Choline acetyltransferase is the rate-limiting enzyme in the process of neurotransmitter acetylcholine biosynthesis. It exists primarily in the cytoplasm of central cholinergic neurons and binds with acetyl coenzyme A and choline, functioning to transfer the acetyl of acetyl coenzyme A to choline to synthesize acetylcholine. Because anterior horn motor neurons within the gray matter of the spinal cord depend on acetylcholine as a neurotransmitter to enable normal function, choline acetyltransferase is the marker enzyme for anterior horn motor neurons in spinal cord gray matter, and choline acetyltransferase immunoreactive cells are commonly used to represent cholinergic neurons[19,20].
Glutamic acid decarboxylase is the rate-limiting enzyme to catalyze glutamate decarboxylation and to form the inhibitory neurotransmitter, γ-aminobutyric acid. Determination of glutamic acid decarboxylase immunoreactive cells can indicate γ-aminobutyric acid neurons[21,22]. Synapsins are a group of phosphoric acid proteins associated with the synapse, which are specific for neurons and locate mainly in the bodies of mature neurons and presynaptic vesicle membranes[23]. Synapsins modulate the release of neurotransmitters through their phosphorylation and dephosphorylation, maintaining the stability of synaptic vesicle storage pool and regulating synaptic vesicle mobility, neurotransmitter release and synaptic plasticity[24]. Because they are expressed prior to synaptic formation and are involved in synaptic formation and stabilization, they are considered as both synaptogenesis markers and indicators of synaptic transmission efficacy[25]. This study showed that, the number of cells expressing choline acetyltransferase, glutamic acid decarboxylase and synapsins, and the fluorescence intensity in the injured spinal cord, were significantly lower than those in the sham-operation group. This provides evidence that the number of injured spinal neurons was decreased and the ability of neurotransmitters synthesizing acetylcholine and γ-aminobutyric acid neurotransmitters was reduced. In parallel to this, the synaptic structure was destroyed and the number of synapses was reduced, affecting spinal nerve function. Histological results showed that, at week 1 after bone marrow mesenchymal stem cell transplantation, model rats presented with splinter hemorrhages, cyst formation, degeneration of neural cells, nerve fiber disappearance and inflammatory cell infiltration. In the model rats at 3 and 5 weeks, spinal gray matter was destructed, glial scars were formed and large lacuna were present. The BBB scale scores confirmed the decline in neurological functions of rats after spinal cord injury.
Following bone marrow mesenchymal stem cell transplantation, the number of cells expressing choline acetyltransferase, glutamic acid decarboxylase and synapsins in the injured spinal cord was increased and the fluorescence intensity was enhanced. This indicated that transplanted bone marrow mesenchymal stem cells can promote high expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins. At 1 week after transplantation, the rat spinal gray matter presented with transplanted green fluorescent bone marrow mesenchymal stem cells and red fluorescent choline acetyltransferase, glutamic acid decarboxylase and synapsins cells, however they were not overlapped. This provides evidence that the transplanted bone marrow mesenchymal stem cells did not express choline acetyltransferase, glutamic acid decarboxylase and synapsins and had no ability of secreting the neurotransmitters, synthetase and synapsins, nor displayed the physiological functions of mature neurons at 1 week. The high expression levels of choline acetyltransferase, glutamic acid decarboxylase and synapsins may be due to the secretion of neurotrophic factors, in vivo induced differentiation of neural stem cells and an increased number of spinal cord neurons and synapses[4,5,6,7]. At 3 and 5 weeks after transplantation, some cells overlapped in the spinal cord of rats in the experimental group, suggesting some transplanted bone marrow mesenchymal stem cells can secrete neurotransmitter synthetase and synapsins, and have physiological functions of mature neurons. At this time, up-regulated expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins may be associated with bone marrow mesenchymal stem cell differentiation into functional neurons in the injured spinal cord. Transplantation of bone marrow mesenchymal stem cells may promote expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins after spinal cord injury, which indicates that bone marrow mesenchymal stem cell transplantation can increase the number of spinal cord neurons, promote the synthesis of acetylcholine, γ-aminobutyric acid and other neurotransmitters and improve the synaptic structure and quantity in the injured spinal cord, thus facilitating the recovery of neural functions[12,13,14,15,16,17]. Histological observation in the experimental rats showed at 1 week after transplantation, spinal cord tissue structure was relatively clear, many neurons survived and neuronal apoptosis and necrosis were slightly visible. By 3 and 5 weeks, gray matter necrosis was not observed, tissue remained clear and glial scar formation was reduced. In parallel, the BBB scale score confirmed that neural functions of injured spinal cord were significantly improved.
Correlation analysis showed that the neurological functions of rats in the experimental group were positively correlated with expression of choline acetyltransferase and synapsins, suggesting that the neurological function of rats after spinal cord injury was associated with upregulation of choline acetyltransferase and synapsins by bone marrow mesenchymal stem cell transplantation.
In summary, intravenously transplanted bone marrow mesenchymal stem cells can migrate to the injured spinal cord, promote expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins and improve the neurological functions of rats with spinal cord injury. Increased expression levels of choline acetyltransferase, glutamic acid decarboxylase and synapsins may result from bone marrow mesenchymal stem cell differentiation into functional neurons in the injured spinal cord.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were performed from January 2008 to March 2009 in the Experimental Animal Center of Fujian Medical University, China.
Materials
A total of 55 healthy, male Sprague-Dawley rats, aged 2–3 months, weighing 150–250 g, were provided by the Experimental Animal Center of Fujian Medical University, China (license No. SCXK (Min) 2004-0008). Fifty rats were used for animal experiments and the other five rats were used for the preparation of bone marrow mesenchymal stem cells. Experimental disposals complied with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[26].
Methods
Isolation, culture and identification of bone marrow mesenchymal stem cells
The bilateral limb bone was excised under anesthesia, the metaphysic was removed and the bone marrow cavity was exposed and rinsed with 10 mL medium containing 10 000 U heparin. Bone marrow was added slowly into a tube containing 3 mL Ficoll-Paque solution and centrifuged at 2 000 r/min for 30 minutes. The granulocyte-abundant intermediate interface layer was collected and washed with PBS three times, then prepared into a single cell suspension for inoculation. Cells were incubated at 37°C in a relative humidity of 5% CO2 with minimum essential medium alpha (10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin). Culture medium was replenished every 3–4 days, non-adherent cells were discarded and adherent cells were collected when they reached confluence by digestion with trypsin. Cells were subcultured for passage and expression of CD34, CD45, CD29 and CD90 were determined with flow cytometry (Beckman counter Epics XL flow cytometry; BD, San Diego, CA, USA).
Establishment of spinal cord injury models
According to the Allen method[27] with some modifications, the T10 segment of the spinal cord was exposed for blast injury under the force of 50 g·cm. Spinal cord injury model success was based on the following performance: body tremble, rapid retraction and springing of bilateral lower limbs, tails cock and rapid falling, stasis purple color on the spinal cord surface, postoperative complete paralysis of bilateral lower limbs[27] (supplementary Figure 1 online). Sham-operated rats were treated with T10 exposure, in absence of injury.
Bone marrow mesenchymal stem cells labeling and intravenous injection
Prior to bone marrow mesenchymal stem cell injection, cells were incubated in culture medium containing 10 μM carboxyfluorescein succinimidyl ester-labeled (Sigma, St. Louis, MO, USA) at 37°C for 10 minutes. Upon injection, cells were washed with PBS three times and the density was adjusted to 1 × 106/mL. At 3 day after model establishment, 1 mL of single cell bone marrow mesenchymal stem cell suspension at passage 3, was injected via caudal veins into rats of the sham-operation and experimental groups, while 1 mL PBS was injected into the model group.
Assessment of neurological function
Neurological functions were double-blindly detected using the BBB scale[28] at 1, 3 and 5 weeks after transplantation. Higher scores indicated better neurological functions. Due to great variations in circadian activities, all animals were observed from 8:00 a.m. to 9:00 a.m. Prior to evaluation, all rats were forced to a complete micturition, thus avoiding the impact of bladder filling. Rats were allowed free activity in a 1-m-diameter smooth site for 5 minutes and their hind limb movement was recorded.
Histological observation
At 1, 3 and 5 weeks after bone marrow mesenchymal stem cell transplantation, rats were subjected to thoracotomy under anesthesia and fixed with 500 mL of 4% paraformaldehyde via left auricle perfusion. The T8-12 segments of the injured spinal cord were completely excised. The caudal spinal cord tissues were immersed in 10% neutral formalin for 24 hours, followed by conventional ethanol dehydration and embedded in paraffin. Spinal cord specimens were cut into 5 μm continuous sections for conventional hematoxylin-eosin and Nissl staining. The histopathological changes and Nissl body changes in the injured spinal cord were observed using optical microscopy (Olympus, Tokyo, Japan).
Immunofluorescence staining for expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins
The head-end spinal cord tissue was harvested after injury, fixed with 4% paraformaldehyde and soaked in 30% sucrose solution until it sank to the bottom of the bottle. Continuous transverse sections of the spinal cord were prepared on a freezing microtome, at a thickness of 20 μM. Every fifth slice was collected for immunofluorescence staining according to the manufacturer's instructions. Slices were incubated with rabbit anti-human choline acetyltransferase, glutamic acid decarboxylase and synapsins polyclonal antibodies (1:100; Fuzhou Maixin, Fuzhou, Fujian Province, China) at 4°C overnight, then with tetramethylrhodamine isothiocyanate-labeled goat anti-rabbit IgG (1:50; Beijing Zhongshan Biotechnology Company, Beijing, China) at room temperature for 30 minutes. PBS was used instead of primary antibody for negative controls. Expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins in the injured spinal cord was observed under an inverted fluorescence microscope (CoiCB5203; Olympus) and positive cells were counted using the grid test system (Xiamen Taijing Biotechnology Company Limited, Xiamen, China; four unit areas were defined on each slice of spinal cord tissue, each 0.16 mm2, and the total number of cells emitting fluorescence within each unit area was calculated). The immunohistochemical fluorescence intensity of choline acetyltransferase, glutamic acid decarboxylase and synapsins at 530 nm emission wavelength was determined with laser confocal microscopy (Leica, Solms, Germany).
Laser confocal microscopy
Specimens were scanned under a laser scanning confocal microscope, using the green laser channel (488 nm; carboxyfluorescein succinimidyl ester-labeled) and red laser channel (543 nm; tetramethylrhodamine isothiocyanate-labeled) tomography. Expression of choline acetyltransferase, glutamic acid decarboxylase and synapsins in bone marrow mesenchymal stem cells migrating to the injured spinal cord was detected.
Statistical analysis
Data were analyzed using one-way analysis of variance and SPSS 13.0 software (SPSS, Chicago, IL, USA). Differences between groups were compared with the least significant difference t-test. Data were expressed as mean ± SD. P < 0.05 indicated significant differences. Correlation analysis was performed using Pearson linear correlation analysis.
Footnotes
Funding: This study was supported by the Doctoral Fund of Ministry of Education of China, No. 20060392003 and Academic Development Foundation of Fujian Medical University, No. JS08004.
Conflicts of interest: None declared.
Ethical approval: This pilot was approved by the Animal Ethics Committee of Fujian Medical University in China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Zhao H, Yang XF/Yang Y/Song LP)
REFERENCES
- [1].Ayatollahi M, Salmani MK, Germizadeh B, et al. Conditions to improve expansion of human mesenchymal stem cells based on rat samples. World J Stem Cells. 2012;4(1):1–8. doi: 10.4252/wjsc.v4.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee Z, Dennis J, Caplan A. Imaging stem cell differentiation for cell-based tissue repair. Methods Enzymol. 2012;506(2):247–263. doi: 10.1016/B978-0-12-391856-7.00037-8. [DOI] [PubMed] [Google Scholar]
- [3].Tsuji O, Mura K, Okada Y, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107(28):12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ban DX, Ning GZ, Feng SQ, et al. Combination of activated Schwann cells with bone mesenchymal stem cells: the best cell strategy for repair after spinal cord injury in rats. Regen Med. 2011;6(6):707–720. doi: 10.2217/rme.11.32. [DOI] [PubMed] [Google Scholar]
- [5].Osaka M, Honmou O, Murakami T, et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010;1343(1):226–235. doi: 10.1016/j.brainres.2010.05.011. [DOI] [PubMed] [Google Scholar]
- [6].Alexanian AR, Fehlings MG, Zhang Z, et al. Transplanted neurally modified bone marrow-derived mesenchymal stem cells promote tissue protection and locomotor recovery in spinal cord injured rats. Neurorehabil Neural Repair. 2011;25(9):873–880. doi: 10.1177/1545968311416823. [DOI] [PubMed] [Google Scholar]
- [7].Zhang YJ, Zhang W, Lin CG, et al. Neurotrophin-3 gene modified mesenchymal stem cells promote remyelination and functional recovery in the demyelinated spinal cord of rats. J Neurol Sci. 2012;313(1-2):64–74. doi: 10.1016/j.jns.2011.09.027. [DOI] [PubMed] [Google Scholar]
- [8].Liu WG, Wang ZY, Huang ZS. Bone marrow-derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res. 2011;33(7):686–693. doi: 10.1179/1743132810Y.0000000031. [DOI] [PubMed] [Google Scholar]
- [9].Kishk NA, Gabr H, Hamdy S, et al. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010;24(8):702–708. doi: 10.1177/1545968310369801. [DOI] [PubMed] [Google Scholar]
- [10].Momin EN, Mohyeldin A, Zaidi HA, et al. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther. 2010;5(4):326–344. doi: 10.2174/157488810793351631. [DOI] [PubMed] [Google Scholar]
- [11].Karaoz E, Kabatas S, Duruksu G, et al. Reduction of lesion in injured rat spinal cord and partial functional recovery of motility after bone marrow derived mesenchymal stem cell transplantation. Turk Neurosurg. 2012;22(2):207–217. doi: 10.5137/1019-5149.JTN.5412-11.1. [DOI] [PubMed] [Google Scholar]
- [12].Liu T, Xu J, Chan BP, et al. Sustained release of neurotrophin-3 and chondroitinase ABC from electrospun collagen nanofiber scaffold for spinal cord injury repair. J Biomed Mater Res. 2012;100(1):236–242. doi: 10.1002/jbm.a.33271. [DOI] [PubMed] [Google Scholar]
- [13].Hwang IK, Yoo DY, Yoo KY, et al. Microtubule associated protein 2 and choline acetyltransferase immunoreactivity in the lumbar spinal cord of young adult and aged dogs. J Neurophysiol. 2011;106(2):731–740. doi: 10.1016/j.rvsc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- [14].Pedersen NP, Vaughan CW, Christie MJ. Opioid receptor modulation of GABAergic and serotonergic spinally projecting neurons of the rostral ventromedial medulla in mice. BMC Neurosci. 2011;7(1):12–16. doi: 10.1152/jn.01062.2010. [DOI] [PubMed] [Google Scholar]
- [15].Fukushima T, Tsuda M, Kofuji T, et al. Physiological properties of enkephalin-containing neurons in the spinal dorsal horn visualized by expression of green fluorescent protein in BAC transgenic mice. J Neurosci. 2012;32(16):5362–5373. doi: 10.1186/1471-2202-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu J, Huang T, Li T, et al. c-Maf is required for the development of dorsal horn laminae III/IV neurons and mechanoreceptive DRG axon projections. Tidsskr Nor Laegeforen. 2012;132(7):831–837. doi: 10.1523/JNEUROSCI.6239-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kanchiku T, Kato Y, Suzuki H, et al. Development of less invasive neuromuscular electrical stimulation model for motor therapy in rodents. J Spinal Cord Med. 2012;35(3):162–169. doi: 10.1179/2045772312Y.0000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin JH, Lei SM, Kang DZ, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after spinal cord injury. Zhonghua Guke Zazhi. 2005;25(9):556–559. [Google Scholar]
- [19].Johann S, Dahm M, Kipp M, et al. Regulation of choline acetyltransferase expression by 17 β-oestradiol in NSC-34 cells and in the spinal cord. J Neuroendocrinol. 2011;23(9):839–848. doi: 10.1111/j.1365-2826.2011.02192.x. [DOI] [PubMed] [Google Scholar]
- [20].Hwang IK, Yoo DY, Yoo KY, et al. Microtubule associated protein 2 and choline acetyltransferase immunoreactivity in the lumbar spinal cord of young adult and aged dogs. Res Vet Sci. 2011;91(3):1–5. doi: 10.1016/j.rvsc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- [21].Lee HJ, Choi JH, Ahn JH, et al. Comparison of GAD65 and 67 immunoreactivity in the lumbar spinal cord between young adult and aged dogs. Neurochem Res. 2011;36(3):435–442. doi: 10.1007/s11064-010-0361-6. [DOI] [PubMed] [Google Scholar]
- [22].Miyazato M, Sugaya K, Saito S, et al. Suppression of detrusor-sphincter dyssynergia by herpes simplex virus vector mediated gene delivery of glutamic acid decarboxylase in spinal cord injured rats. J Urol. 2010;184(3):1204–1210. doi: 10.1016/j.juro.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qin ZF, Hou DY, Fang YQ, et al. Interferon-alpha enhances excitatory transmission in substantia gelatinosa neurons of rat spinal cord. Neuroimmunomodulation. 2012;19(4):235–240. doi: 10.1159/000335167. [DOI] [PubMed] [Google Scholar]
- [24].Yoshida J, Kubo T, Tamashita T. Inhibition of branching and spine maturation by repulsive guidance molecule in cultured cortical neurons. Biochem Biophys Res Commun. 2008;372(4):725–729. doi: 10.1016/j.bbrc.2008.05.124. [DOI] [PubMed] [Google Scholar]
- [25].Gulino R, Dimaitino M, Casabona A, et al. Synaptic plasticity modulates the spontaneous recovery of locomotion after spinal cord hemisection. Neurosci Res. 2007;57(1):148–156. doi: 10.1016/j.neures.2006.10.001. [DOI] [PubMed] [Google Scholar]
- [26].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [27].Allen AR, MacPhail RC. Surgery of experimental lesion of spinal cord equivalent to crush injury of fractured is location of spinal column. Pharmacol Biochem Behav. 1991;57(7):878–880. [Google Scholar]
- [28].Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomtor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):112–115. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]


