Abstract
Increased endogenous αB-crystallin protein levels have been shown to reduce cell apoptosis, although the effects of exogenous αB-crystallin protein remain poorly understood. The present study established an acute ocular hypertension model in the right eye of Sprague-Dawley rats. Fluorogold retrograde tracing and immunofluorescence methods showed that the number of retinal ganglion cells decreased in the right eyes and caspase-3 expression increased following acute ocular hypertension. Intravitreal injection of αB-crystallin in the right eye increased the number of retinal ganglion cells and reduced caspase-3 expression. Results demonstrated that exogenous αB-crystallin protein inhibited caspase-3 expression and improved retinal ganglion cell survival following acute ocular hypertension.
Keywords: αB-crystallin protein, acute ocular hypertension, caspase-3, retinal ganglion cells, neural regeneration
Research Highlights
Intravitreal injection of exogenous αB-crystallin improved survival of retinal ganglion cells and reduced caspase-3 expression in a rat model of acute ocular hypertension.
Abbreviations
RGCs, retinal ganglial cells
INTRODUCTION
αB-crystallin protein, the main protein in the vertebrate eye lens, is a micromolecule heat shock protein[1,2]. Its expression increases in response to inflammation, ischemia, heat injury, osmotic pressure changes, or other nociceptive stimuli[3]. Previous studies have shown that endogenous and exogenous αB-crystallin inhibits cell apoptosis[4,5,6], and endogenous αB-crystallin binds cytoskeletal proteins under stress to stabilize the cytoskeleton and inhibit apoptosis[7,8,9]. In addition, the anti-apoptotic mechanisms of αB-crystallin are associated with apoptosis-related protease caspase-3 activation[4,5]. To determine whether exogenous αB-crystallin exhibits anti-apoptotic effects, the present study analyzed the influence of exogenous αB-crystallin on caspase-3 expression and survival of retinal ganglial cells (RGCs) in a rat model of acute ocular hypertension.
RESULTS
Quantitative analysis of experimental animals
A total of 144 Sprague-Dawley rats were selected and acute ocular hypertension was induced in the right eye. Five rats died due to anesthesia, 10 were excluded due to lens injury, and 9 were excluded due to corneal leakage caused by puncture. In total, 120 rats were included in the final analysis. The rats were randomly assigned to model, normal saline, and αB-crystallin groups, with 40 rats in each group. The normal saline and αB-crystallin groups were intravitreally injected with normal saline and αB-crystallin, respectively, following model establishment. Five rats from each group were selected at 1, 2, 3, and 4 weeks following model establishment for fluorogold examination. An additional five rats from each group were selected at 1, 2, 3, and 7 days following model establishment for immunofluorescence examination.
αB-crystallin increased the number of surviving RGCs in a rat model of acute ocular hypertension
Fluorogold retrograde tracing revealed a decreased number of RGCs in the right eye of model rats, but intravitreal injection of αB-crystallin increased the number of RGCs (P < 0.01). Normal saline injection did not change the number of RGCs in the rat model of acute ocular hypertension (P > 0.05; Table 1, Figure 1).
Table 1.
Survival rate (%) of retinal ganglial cells (RGCs) in rats
Figure 1.
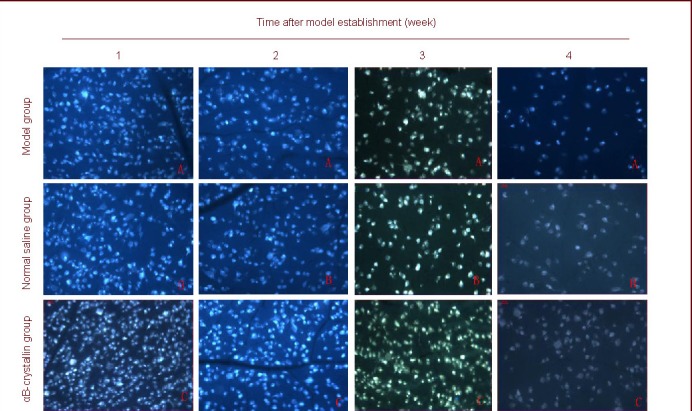
Survival of retinal ganglial cells (RGCs) in rats with acute ocular hypertension (fluorogold labeling, × 200).
Acute ocular hypertension decreases the number of RGCs, which gradually decrease with increasing time. Cells with fluorescence spots in the blue background are RGCs. Fluorogold mainly aggregates in the cytoplasm and is detected as a gold staining.
αB-crystallin decreased caspase-3 expression in a rat model of acute ocular hypertension
Immunofluorescence showed increased caspase-3 expression in RGCs from the right eye of model rats, but intravitreal injection of αB-crystallin inhibited the increase in caspase-3 expression (P < 0.01). Normal saline injection did not change caspase-3 expression in the rat model of acute ocular hypertension (P > 0.05; Figure 2, Table 2). Caspase-3 expression was not detected in the normal eye (left), which suggested that caspase-3 was expressed only in apoptotic cells.
Figure 2.
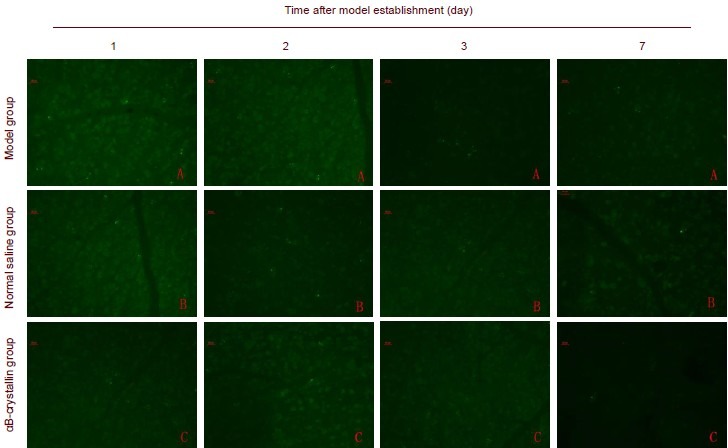
Caspase-3 expression in retinal ganglial cells (RGCs) in rats (immunofluorescence staining, × 200).
Following acute ocular hypertension, Caspase-3 positive expression is detected in the rat retina, mainly in the cytoplasm, and gradually decreased by 2 days. Green fluorescence spots represents caspase-3-positive cells. Fluorescein served as the fluorescent dye.
Table 2.
Caspase-3 expression (number of spots/200-fold field of view) in retinal ganglial cells in rats
DISCUSSION
The αB-crystallin used in the present study was of high purity, which was different than the mixed crystallin in a previous study[10]. Therefore, results helped to have a better understanding of the neuroprotective mechanisms specific to αB-crystallin. Acute ocular hypertension has been shown to induce retinal ischemic injury, which leads to RGC death[11]. In the present study, the number of RGCs decreased in a rat model of acute ocular hypertension. Intravitreal injection of αB-crystallin significantly increased RGC survival in these model rats, which suggested that exogenous αB-crystallin protected RGCs against acute ocular hypertension-induced apoptosis, consistent with previous results[12].
Caspase-3 is an apoptosis-related protease and an active component in the death receptor-mediated apoptosis pathway and apoptosis-related protease cascade reactions[12]. Immunofluorescence results from the present study showed that intravitreal αB-crystallin injection significantly reduced RGC caspase-3 expression in rats with acute ocular hypertension, which suggested that increased exogenous αB-crystallin inhibited caspase-3 expression during acute ocular hypertension and reduced cell apoptosis. However, results from the present study could not confirm an interaction between αB-crystallin and proteins from apoptosis-related pathways nor the precise mechanisms of αB-crystallin. Results provided neuroprotective evidence of αB-crystallin application in glaucoma.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The present study was performed at the Central Laboratory of College of Ophthalmology, Capital Medical University, China from October 2009 to March 2010.
Materials
A total of 120 male, Sprague-Dawley rats, aged 3 months and weighing 250 ± 20 g, were purchased from the Laboratory Animal Center, Academy of Military Medical Sciences (License No. SCXK (army) 2007-004). Experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[13].
Methods
Establishment of an acute ocular hypertension model
A model of acute ocular hypertension was established via anterior chamber perfusion[14]. The conjunctival sac was washed with 10% sodium sulfacetamide solution prior to experimentation. Following anesthesia by intraperitoneal injection of 10% chloral hydrate (4.5 mL/ 100 g), one drop of compound tropicamide and topical ophthalmic anesthetic was administered to the right eye. The anterior chamber was subsequently punctured from corneal limbus at an angle using a 4.5# transfusion needle connected to a normal saline infusion apparatus under an anatomical microscope (Leica, Wiesbaden, Germany). The puncture was carefully performed to prevent iris and crystal injury. The tubing was then opened and liquid pressure was slowly increased to 136 mm. The retinal vessel of the right eye was blocked and pale. After 60 minutes, the saline bottle was lowered, and the needle was removed. Retinal blood supply gradually recovered. The left eyes were considered normal controls and were not treated.
Vitreous puncture and injection
Vitreous puncture was conducted immediately after model establishment. The sclearotic wall, which is 2 mm from the discus opticus and vertical to the optic nerve, was punctured using a sterile microsyringe (Shenzhen RWD Life Science, Shenzhen, China)[15]. Normal saline and 5 μL αB-crystallin solution (0.001 g/L) were slowly intravitreally injected into the normal saline and αB-crystallin groups, respectively, and the needle was then removed after 1 minute. The cornea was gently pressed using a coverslip. Rats free of vitreous space hemorrhage, as well as iris or crystal injury, were included in the study.
Fluorogold retrograde tracing for RGC quantification
The rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (4.5 mL/100 g) and fixed to a stereotaxic apparatus (Shenzhen RWD Life Science). A 1.2-cm incision was made at the calvarium skin along the median line, and the scalp was opened using an eye speculum. The periosteum was dissected, and the bleeding was stopped. Sagittal suture and sutura interparietalis were exposed to identify bregma. Bilateral superior colliculi (6.8 ± 0.2 mm posterior to bregma, 1.6 ± 0.2 mm lateral to bregma) and the lateral geniculate body (4.6 ± 0.2 mm posterior to bregma, 4.0 ± 0.2 mm lateral to bregma) were confirmed according to rat brain stereotaxic coordinates[16]. Holes, 1 mm in diameter, were drilled at corresponding projection points and slowly injected with 2.5 μL fluorogold (prepared with 0.1 M; PBS, pH 7.4, 2%; Fluorogold, Denver, Colorado, USA) using a microsyringe at a depth of 4.0 ± 0.2 mm for the superior colliculus and 5.6 ± 0.2 mm for the lateral geniculate body[17]. The needle was held in place for 5 minutes and then slowly withdrawn. The fascia and skin were sutured layer-by-layer.
At 4 days after fluorogold labeling, the rats were sacrificed and both eyes were removed and fixed in 4% paraformaldehyde for 2 hours at room temperature. The retina was placed onto a glass slide, dried at room temperature, cleared, coverslipped in 70% glycerol, and observed by fluorescence microscopy (Leica). Four photos from four separate fields of view (200 ×) at 2 mm up, down, left, and right to the discus opticus, were collected. The number of fluorogold-labeled RGCs in each photo was quantified and the mean was calculated. The percent of RGCs in the right eye compared to the left eye was calculated and represented the survival rate of RGCs in the right eye.
Immunofluorescence of retinal caspase-3 expression
The rats were anesthetized and sacrificed. The right eyes were removed and fixed in 4% paraformaldehyde overnight at 4°C. The retina was harvested and routinely subjected to immunofluorescent staining. Briefly, the retina was blocked in 3% bovine serum albumin at 4°C for 1 hour, incubated with rabbit anti-rat caspase-3 monoclonal antibody (1:500; Wuhan Boster Biotechnology, Wuhan, China) at 4°C for 12 hours, followed by fluorescein-labeled goat anti-rabbit IgG (1:200; Wuhan Boster Biotechnology) at 4°C for 2 hours. The retina was then placed on a transparent glass slide and coverslipped with 70% glycerol. Four photos from four separate fields of view (200 ×) at 2 mm up, down, left, and right to the discus opticus, were collected. The number of fluorescence caspase-3 spots in each photo was quantified and the mean was calculated. The normal retina of the left eye served as the control (n = 5).
Statistical analysis
Data were analyzed using SPSS version 11.5 (SPSS, Chicago, IL, USA) and were expressed as mean ± SD. Differences were compared utilizing one-way analysis of variance (Student-Newman-Keuls test). A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Qingjun Lu, Kegao Liu and Qian Liu from the Central Laboratory of College of Ophthalmology, Capital Medical University, China for technical support. The authors also thank Ruihong Wang for help in drafting the manuscript.
Footnotes
Funding: This study was supported by the China Post-doctoral Foundation (The protective effect of αB-crystallin on retinal ganglial cells in glaucoma), No. 20080430444.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of General Hospital of Chinese People's Armed Police Forces, China.
(Edited by Yang JN, Hong J/Su LL/Song LP)
REFERENCES
- [1].Kim JY, Sohn HJ, Lee EY, et al. Expression of αβ-crystallin in the peripapillary glial cells of the developing chick retina. Neurochem Res. 2011;36(1):76–82. doi: 10.1007/s11064-010-0266-4. [DOI] [PubMed] [Google Scholar]
- [2].Tang G, Perng MD, Wilk S, et al. Oligomers of mutant glial fibrillary acidic protein (GFAP) inhibit the proteasome system in alexander disease astrocytes, and the small heat shock protein alphaB-crystallin reverses the inhibition. J Biol Chem. 2010;285(14):10527–10537. doi: 10.1074/jbc.M109.067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gabert BJ, Kültz D. Osmoprotective proteome adjustments in mouse kidney papilla. Biochim Biophys Acta. 2011;1814(3):435–448. doi: 10.1016/j.bbapap.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stegh AH, Kesari S, Mahoney JE, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci U S A. 2008;105(31):10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ousman SS, Tomooka BH, van Noort JM, et al. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448(7152):474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- [6].Goplen D, Bougnaud S, Rajcevic U, et al. αβ-crystallin is elevated in highly infiltrative apoptosis-resistant glioblastoma cells. Am J Pathol. 2010;177(4):1618–1628. doi: 10.2353/ajpath.2010.090063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Golenhofen N, Arbeiter A, Koob R, et al. Ischemia- induced association of the stress protein alpha B-crystallin with I-band portion of cardiac titin. J Mol Cell Cardiol. 2002;34(3):309–319. doi: 10.1006/jmcc.2001.1513. [DOI] [PubMed] [Google Scholar]
- [8].Wang X, Klevitsky R, Huang W, et al. AlphaB-crystallin modulates protein aggregation of abnormal desmin. Circ Res. 2003;93(10):998–1005. doi: 10.1161/01.RES.0000102401.77712.ED. [DOI] [PubMed] [Google Scholar]
- [9].Verschuure P, Croes Y, van den IJssel PR, et al. Translocation of small heat shock proteins to the actin cytoskeleton upon proteasomal inhibition. J Mol Cell Cardiol. 2002;34(2):117–128. doi: 10.1006/jmcc.2001.1493. [DOI] [PubMed] [Google Scholar]
- [10].Zhang L. Chongqing: Third Military Medical University; 2007. Effects of α-crystallin on axonal regeneration and survival of rats retinal ganglion cells in vivo following optic nerve injury. [Google Scholar]
- [11].Rosenbaum DM, Rosenbaum PS, Gupta A, et al. Retinal ischemia leads to apoptosis which is ameliorated by aurintricarboxylic acid. Vision Res. 1997;37(24):3445–3451. doi: 10.1016/S0042-6989(96)00328-8. [DOI] [PubMed] [Google Scholar]
- [12].Kamradt MC, Chen F, Sam S, et al. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277(41):38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- [13].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [14].Niu Y, Zhang R, Zhou Z, et al. An experimental study of therapeutic effect of basic fibroblast growth factor on experimental retinal ischemia/reperfusion injury. Zhonghua Yan Ke Za Zhi. 2002;38(9):530–534. [PubMed] [Google Scholar]
- [15].Liu ZM, Ren X, Wang Y, et al. Protective effects of mixed crystamn on injured retinal ganglion cells after optic nerve injury in Long-Evans rats. Zhonghua Chuangshang Zazhi. 2006;22(12):926–929. [Google Scholar]
- [16].Paxinos G, Waston C. 2nd ed. San Dieago: Academic Press; 1986. The Rat Brain in Stereotaxic Coordinate; pp. 54–56. [Google Scholar]
- [17].Duan XC, Qing GP, Li C. Quatitative analysis of retinal ganglion cells in animals. Guoji Yanke Zazhi. 2006;6(3):667–670. [Google Scholar]


