Abstract
In this study, we determined the expression levels of matrix metalloproteinase-2 and -9 and matrix metalloproteinase tissue inhibitor-1 and -2 in brain tissues and blood plasma of patients undergoing surgery for cerebellar arteriovenous malformations or primary epilepsy (control group). Immunohistochemistry and enzyme-linked immunosorbent assay revealed that the expression of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 was significantly higher in patients with cerebellar arteriovenous malformations than in patients with primary epilepsy. The ratio of matrix metalloproteinase-9 to matrix metalloproteinase tissue inhibitor-1 was significantly higher in patients with hemorrhagic cerebellar arteriovenous malformations compared with those with non-hemorrhagic malformations. Matrix metalloproteinase-2 and matrix metalloproteinase tissue inhibitor-2 levels were not significantly changed. These findings indicate that an imbalance of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1, resulting in a relative overabundance of matrix metalloproteinase-9, might be the underlying mechanism of hemorrhage of cerebellar arteriovenous malformations.
Keywords: cerebellar arteriovenous malformations, hemorrhage, matrix metalloproteinase-2, matrix metalloproteinase-9, tissue matrix metalloproteinase inhibitor-1, tissue matrix metalloproteinase inhibitor-2, immunohistochemistry, enzyme-linked immunosorbent assay, neural regeneration
Research Highlights
(1) Matrix metalloproteinase-9 is the leading cause of cerebral arteriovenous malformations.
(2) An imbalance of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 might be the underlying mechanism of hemorrhage of cerebral arteriovenous malformations.
INTRODUCTION
Cerebral arteriovenous malformations result from abnormal vascular remodeling after the appearance of a malformed vascular mass[1]. Matrix metalloproteinases are Zn2+- dependent proteolytic enzymes that function to degrade and remodel extracellular matrix. Matrix metalloproteinase-2 and matrix metalloproteinase-9 are important members of the matrix metalloproteinase family, and they can be inhibited by endogenous specific tissue inhibitors of matrix metalloproteinases (matrix metalloproteinase tissue inhibitor-2, matrix metalloproteinase tissue inhibitor-1). matrix metalloproteinase-9 mRNA levels were higher in cerebellar arteriovenous malformations vascular endotheliocytes than that in normal vessels, and were especially concentrated in adventitia. The increase in matrix metalloproteinase-9 expression was proportional to the extent of collagen fiber damage. It has been hypothesized that some risk factors induce focal up-regulation of matrix metalloproteinase-9 mRNA, causing rupture of elastic collagenous fiber and even whole layers of structural damage, leading to vascular dilatation and vascular malformation[2]. Despite extensive research on the subject, the mechanisms of cerebellar arteriovenous malformations progress are still not clear. Previous studies of cerebellar arteriovenous malformations-related hemorrhage have mainly focused on vascular structural factors. However, the relationship between matrix metalloproteinase-2/matrix metalloproteinase-9 and the mechanisms of cerebellar arteriovenous malformations progress and hemorrhage have rarely been studied.
In this study, we determined the expression levels of matrix metalloproteinase-2, matrix metalloproteinase-9, matrix metalloproteinase tissue inhibitor-1 and matrix metalloproteinase tissue inhibitor-2 in brain tissues and blood plasma of patients with cerebellar arteriovenous malformations or primary epilepsy receiving surgery treatment. We used immunohistochemistry and enzyme-linked immunosorbent assay, and compared cerebellar arteriovenous malformations patients with epileptic patients without malformations.
RESULTS
Quantitative analysis of subjects
A total of 72 patients were recruited, including 24 cerebellar arteriovenous malformations patients with hemorrhage (cerebellar arteriovenous malformations-H group), 24 cerebellar arteriovenous malformations patients with no hemorrhage (cerebellar arteriovenous malformations-N group), and 24 patients with primary epilepsy (control group). All patients were receiving surgery. Tissue samples were collected from all patients, and no subjects dropped out.
Baseline analysis of subjects
There was no significant difference in age and gender distributions between the groups (P > 0.05; Table 1).
Table 1.
Age and gender distributions in each group (n = 24)

Matrix metalloproteinase-2/9 and matrix metalloproteinase tissue inhibitor-1/2 levels in brain tissue
Only trace amounts of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 protein were found in the control group, whereas expression was significantly higher in cerebellar arteriovenous malformations patients (P < 0.01). The matrix metalloproteinase-9/matrix metalloproteinase tissue inhibitor-1 ratio was significantly higher in the cerebellar arteriovenous malformations-H group than in the cerebellar arteriovenous malformations-N group (P < 0.05). There were no significant group differences in matrix metalloproteinase-2 or matrix metalloproteinase tissue inhibitor-2 expression, or in the matrix metalloproteinase-2/matrix metalloproteinase tissue inhibitor-2 ratio (P > 0.05; Tables 2, 3, Figure 1).
Table 2.
The number (n/200-fold field) of positive expression cells (MMP-9, TIMP-1, MMP-9/TIMP-1) in each group

Table 3.
The number (n/200-fold field) of positive expression cells (MMP-2, TIMP-2, MMP-2/TIMP-2) in each group

Figure 1.
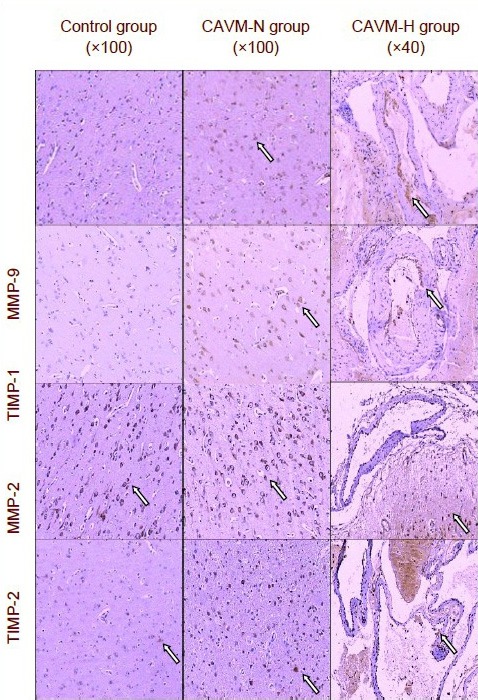
Expression of MMP-2, MMP-9, TIMP-1, and TIMP-2 in brain tissue in each group (immunohistochemistry).
Cells positive for MMP-2/9 and TIMP-1/2 (arrows refer to immunopositive cells with brown-red granules) were located in the endochylema in the control and CAVM-N groups, and in vascular endothelial cells and vascular outer layer in the CAVM-H group.
CAVM-H: Hemorrhagic patients with cerebral arteriovenous malformation; CAVM-N: non-hemorrhagic patients with cerebral arteriovenous malformation; MMP: matrix metalloproteinase; TIMP: matrix metalloproteinase tissue inhibitor.
Content of matrix metalloproteinase-2/9 and matrix metalloproteinase tissue inhibitor-1/2 in blood plasma
Enzyme-linked immunosorbent assay results are shown in Table 4.
Table 4.
Comparison of plasma concentrations (μg/L) of MMP-9, TIMP-1, MMP-2, TIMP-2, MMP-9/TIMP-1, and MMP-2/TIMP-2 among all groups

Comparison of the cerebellar arteriovenous malformations-H, cerebellar arteriovenous malfor-mations-N, and control groups at different time points
Matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 expression levels were significantly higher in both the cerebellar arteriovenous malformations-H and cerebellar arteriovenous malformations-N groups before surgery and on day 3 and day 7 after surgery compared with the control group (P < 0.01). The matrix metalloproteinase-9/matrix metalloproteinase tissue inhibitor-1 ratio in the cerebellar arteriovenous mal-formations-H group was significantly higher than that in the cerebellar arteriovenous malformations-N group (P < 0.05; Table 4).
Changes in protein expression over time
The matrix metalloproteinase-9 and matrix metallopro-teinase tissue inhibitor-1 expression levels progressively decreased after surgery in the cerebellar arteriovenous malformations-H and cerebellar arteriovenous malformations-N groups. Plasma matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 levels were significantly higher before surgery than on day 3 and day 7 post-surgery (P < 0.05). The values reached the lowest levels on day 7, although they were still significantly higher than in the control group (P < 0.05; Table 4).
The matrix metalloproteinase-2 levels, matrix metalloproteinase tissue inhibitor-2 levels and matrix metalloproteinase-2/matrix metalloproteinase tissue inhibitor-2 ratio were not significantly changed during the treatment period in any of the groups (P > 0.05; Table 4).
DISCUSSION
The relationship of matrix metalloproteinase-2/9 and matrix metalloproteinase tissue inhibitor-1/2 expressions with cerebellar arteriovenous malformations progress
In this present study, no or little matrix metalloproteinase- 9 and matrix metalloproteinase tissue inhibitor-1 expression was found in the control group, whereas substantial expression was seen in tissue and plasma from cerebellar arteriovenous malformations patients. This indicates that matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 play crucial roles in the progress of cerebellar arteriovenous malformations. Overabundance of matrix metalloproteinase-9 probably promotes overdegradation of vascular endothelial matrix, destabilization of vascular structures, and remodeling of malformed vessels. This triggers a chronic change of the vascular internal diameter and structure of arterial walls, thus causing deterioration of vascular malformation. Studies of the plasma matrix metalloproteinase-9 levels in patients with arteriovenous malformations, cavernous hemangioma, venous angioma or saccular aneurysm have demonstrated that plasma matrix metalloproteinase-9 was significantly higher than in control subjects[3]. Matrix metalloproteinase-9 can be used as an important indicator for diagnosis and assessment of the disease process in patients with cerebral vascular malformations[3]. Kranzhöfer et al[4] reported that matrix metalloproteinase tissue inhibitors and matrix metalloproteinases are synergistically expressed during remodeling in many physiological tissues. In the present study, we observed synergistic expression of matrix metalloproteinase tissue inhibitor-1 and matrix metalloproteinase-9, indicating that matrix metalloproteinase-9 upregulation may be a factor that promotes matrix metalloproteinase tissue inhibitor-1 synthesis and secretion.
The substrate of matrix metalloproteinase-2 is similar to that of matrix metalloproteinase-9, and matrix metalloproteinase-2 is highly expressed in patients with hypoxic-ischemic encephalopathy, tumor, hypertension, pre-eclampsia, pregnancy-induced hypertension and aneurysm[5,6,7,8,9]. In this study, however, the expression of matrix metalloproteinase-2 and matrix metalloproteinase tissue inhibitor-2 were not different from control. This suggests that matrix metalloproteinase-2 and matrix metalloproteinase tissue inhibitor-2 play less important roles in the development and progress of cerebellar arteriovenous malformations.
Relationship of matrix metalloproteinase-2/9 and matrix metalloproteinase tissue inhibitor-1/2 expression with cerebellar arteriovenous malformations hemorrhage
Risk factors for rupture and hemorrhage can be classified into vascular structural factors and tissue pathological factors. Previous studies mainly focused on the former, and it has been commonly accepted that the following factors are risk factors of cerebellar arteriovenous malformations hemorrhage: blood supply from multiple arteries, blood supply from perforator arteries and the vertebrobasilar artery system, deep cerebellar arteriovenous malformations, large volume of malformed vessels mass, large size of nidus, drainage of deep veins, single venous drainage, and concurrent aneurysm[10]. Apart from vascular structural factors, cerebellar arteriovenous malformations hemorrhage is directly related to pathological changes of vascular structures. Ling et al[11] reported that hemorrhage is associated with an increased thickness of malformed vessels. Sun et al[12] found that pathological properties of hemorrhagic cerebellar arteriovenous malformations include heterogenous vascular internal diameters, defluvium of endotheliocytes, attenuation of vascular walls, damage of internal elastic lamina, degeneration of intima and media, necrosis of smooth muscle cells, and heterogeneous thickness of internal elastic lamina. According to the studies of Hademenos et al[13], vascular intima damage could induce thrombosis and sclerosis of vascular walls, which further cause stenosis, occlusion, and increased obstruction of drainage veins, and finally cerebellar arteriovenous malformations rupture and hemorrhage.
A variety of substances, especially matrix metalloproteinase-9 and -2, are associated with the pathophysiological process of cerebral hemorrhage[14]. The basilar membrane of cerebellar capillary walls is mainly composed of type IV collagen, laminin and fibronectin. The above constituents are substrates of matrix metalloproteinase-9, which can cause hydrocephalus and hemorrhage through matrix destruction and opening of the blood-brain barrier. In addition, treatment with a monoclonal anti-matrix metalloproteinase-9 antibody can alleviate ischemic cerebellar damage[15].
The present study showed that there was no significant difference in matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 expression between the cerebellar arteriovenous malformations-H and cerebellar arteriovenous malformations-N groups, while the matrix metalloproteinase-9/matrix metalloproteinase tissue inhibitor-1 ratio was significantly different. We therefore propose that matrix metalloproteinase-9 promotes the hemorrhage progress in cerebellar arteriovenous malformations patients. Matrix metalloproteinase-9 may over-degrade the vessel endothelium extracellular matrix and accelerate destabilization of vascular walls, migration of neutrophil granulocytes, and focal inflammatory reactions. This in turn can cause structural damage of the blood-brain barrier, destruction of vascular wall integrity, weakening of vascular walls, rupture, and hemorrhage. This may lead to promotion of immune cell migration to the brain. matrix metalloproteinase tissue inhibitor-1, as an endogenous specific inhibitor of matrix metalloproteinase-9, may protect from cerebellar arteriovenous malformations hemorrhage. An imbalance of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1, resulting in the relative overabundance of matrix metalloproteinase-9, might be the underlying mechanism of hemorrhage of cerebellar arteriovenous malformations. Expression patterns of matrix metalloproteinase-2 and matrix metalloproteinase tissue inhibitor-2 do on the other hand not seem to be associated with hemorrhage.
Reason for the concurrent changes of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 in blood plasma and brain tissue
It has been suggested that elevated serum matrix metalloproteinase could be a marker of cerebellar arteriovenous malformations[16]. Starke et al[17] observed that the plasma matrix metalloproteinase-9 levels in cerebellar arteriovenous malformations patients were significantly higher than in the control group. We believe that one of the reasons for the concurrent change of plasma and brain matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 is a hemodynamic change in cerebellar arteriovenous malformations that increases permeability of the blood-brain barrier due to hypoxia and ischemia.
Because of the “steal phenomenon”[18], blood supply is reduced in brain tissues surrounding cerebellar arteriovenous malformations, causing hypoxic and ischemic conditions, and the blood-brain barrier is damaged due to loss of integrity and impaired function. Damage of the blood-brain barrier results not only in the release of matrix metalloproteinase-2/9 and matrix metalloproteinase tissue inhibitor-1/2 into the blood stream, but also in the production of large amounts of cytokines such as interleukin-1 and -6, tumor necrotic factor α, epidermal growth factor, platelet-derived growth factor, fibroblast growth factor, and TgT-β. These in turn increase the activity of matrix metalloproteinases through transcriptional regulation[19].
We found that the plasma levels of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 showed a declining trend after surgery in cerebellar arteriovenous malformations patients. This supports that the observed elevation of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 in plasma before surgery was caused by release of matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1 from the cerebellar arteriovenous malformations nidus and surrounding brain tissues.
Although matrix metalloproteinase-2 has great significance for diagnosis, disease monitoring, and prognosis in patients with acute cerebral infarction[20], this research suggests that matrix metalloproteinase-2 and matrix metalloproteinase tissue inhibitor-2 have no close relationship with cerebellar arteriovenous malformations. matrix metalloproteinase-9 and matrix metalloproteinase tissue inhibitor-1, however, are closely linked with cerebellar arteriovenous malformations occurrence, development, bleeding, and outcome.
SUBJECTS AND METHODS
Design
A controlled observational experiment.
Time and setting
Experiments were performed from December 2010 to February 2011 at the Department of Pathology and Department of Laboratory Medicine in Beijing Tiantan Hospital, Capital Medical University, China.
Subjects
A total of 48 tissue samples were collected from cerebellar arteriovenous malformations patients receiving surgery treatment (malformation resections) from July 2009 to November 2010 in Beijing Tiantan Hospital, China.
There were 29 males and 19 females, aged 12–62 years. The cerebellar arteriovenous malformations cases were classified according to the Spetzler-Martin grading scale (higher scores indicate a more difficult operation): Grade I, 0 case; II, 27 cases; III, 13 cases; IV, 8 cases; V, 0 cases. Because it is impossible to collect normal cerebral tissues as controls, we obtained control tissue from 24 patients with primary epilepsy undergoing resective surgery.
According to the Administrative Regulations on Medical Institution issued by the State Council of the People's Republic of China[21], the patients were informed of the experimental program and risks before the experiment, and provided informed consent.
Inclusion criteria of cerebellar arteriovenous malformations-H group
The inclusion criteria for the cerebellar arteriovenous malformations-H group included obvious symptoms of hemorrhagic spasm, and detection of the characteristic malformed vessel mass within the brain by CT, MRI, and DSA. The malformed vessel mass of enrolled subjects had to meet the following criteria: no concurrent aneurysm; medium size mass (3–6 cm); superficial, localized in epithelial lamina; less than three drainage veins.
Inclusion criteria of cerebellar arteriovenous malformations-N group
The inclusion criteria were the same as for the cerebellar arteriovenous malformations-H group, except for the absence of hemorrhagic symptoms.
Inclusion criteria of control group
Patients were diagnosed with primary epilepsy, and had characteristic clinical and electroencephalography manifestations of seizures. CT, MRI, and other imaging studies were used to exclude tumor, infarction, congenital vascular malformations, traumatic scars, or other diseases causing symptomatic epilepsy.
Exclusion criteria
Exclusion criteria for the three groups were acute inflammation, other cardiac and cerebrovascular diseases, cancer, and hypertension.
Methods
Pre-surgery examination
Cerebellar arteriovenous malformations patients admitted to hospital underwent routine preoperative examinations, followed by craniotomy under general anesthesia for cerebellar arteriovenous malformations resection. Admitted epilepsy patients underwent examinations to further localize the epileptogenic zone, and routine preoperative examination for elective resective surgery under general anesthesia.
Matrix metalloproteinase-2/9 and matrix metalloproteinase tissue inhibitor-1/2 expression in brain tissue detected by immunohistochemical staining
In the cerebellar arteriovenous malformations group, the malformed blood vessel mass and some surrounding brain tissues were removed during surgery, while control tissue was surgically obtained from epilepsy patients. Tissues were fixed in formalin for 24–48 hours after removal, embedded in paraffin, and cut into 4-μm thick continuous sections. An immunohistochemical kit was purchased from Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China. Paraffin-embedded sections were routinely deparaffinized, and treated with 3% H2O2 to inactivate endogenous peroxidases. After microwave-induced epitope retrieval and blocking, slides were incubated in rabbit anti-human primary antibody (1:400; R&D Systems Inc., Minneapolis, MN, USA) at 4°C overnight. The slides were soaked with a biotinylated goat anti-rabbit IgG antibody for 30 minutes, then incubated in horseradish peroxidase-labeled streptavidin, and developed using diaminobenzidine. The slides were counterstained with hematoxylin. After dehydration, slides were sealed with Canada Balsam. Specimens were observed under the light microscope. Five visual fields were selected randomly on each slide. Under 200 × magnification, immunopositive cells were observed as brown-red granules. The average cell count of the five chosen fields was used as the output measure.
Matrix metalloproteinase-2/9 and matrix metalloproteinase tissue inhibitor-1/2 content in brain tissue detected by enzyme-linked immunosorbent assay
Venous blood was collected in the fasting state on day 1 (before surgery), and days 3 and 7 after surgery. The sera were separated by centrifugation at 3 000 r/min for 10 minutes, and then stored at −80°C before use. Kits for quantitative analysis of matrix metalloproteinase-2, matrix metalloproteinase-9, matrix metalloproteinase tissue inhibitor-1 and matrix metalloproteinase tissue inhibitor-2 were purchased from R&D systems.
The matrix metalloproteinase-2/9 and matrix metalloproteinase tissue inhibitor-1/2 concentrations in serum were detected by double-antibody sandwich avidin-biotin-peroxidase complex enzyme-linked immunosorbent assay. In brief, rabbit anti-human monoclonal antibodies against matrix metalloproteinase-2, matrix metalloproteinase-9, matrix metalloproteinase tissue inhibitor-1, and matrix metalloproteinase tissue inhibitor-2 were packaged on a microtiter plate and incubated with standard substance and sample (matrix metalloproteinase-2/9, matrix metalloproteinase tissue inhibitor-1/2). Biotinylated rabbit anti-human (matrix metalloproteinase-2/9, matrix metalloproteinase tissue inhibitor-1/2) antibodies were added to form immune complexes attached to the plate. When horseradish peroxidase-labeled streptavidin was added to the biotinylated rabbit anti-human antibody as a substrate, a yellow color emerged, which then became darker after adding liquid sulfuric acid to terminate the reaction. The absorbance value was read at 492 nm. The concentrations of matrix metalloproteinase-2, matrix metalloproteinase-9, matrix metalloproteinase tissue inhibitor-1, and matrix metalloproteinase tissue inhibitor- 2 were positively related to the absorbance value, so they could be calculated using a standard curve.
Statistical analysis
Data were analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA) and represented as mean ± SD. One-way analysis of variance was used to compare group means. The Student-Newman-Keuls test was used for multiple comparisons of data between different groups. P < 0.05 was considered a significant difference.
Acknowledgments:
We would like to thank all the staff from the Operating Room, the Fifth and Eighth Wards of the Neurosurgery Department, Department of Clinical Laboratory and Pathology in Beijing Tiantan Hospital, Capital Medical University, China, for the guidance and cooperation in the specimen collection and data analysis.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This pilot study was approved by the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University, China.
(Edited by Liu PN, Yang LP/Yang Y/Song LP)
REFERENCES
- [1].Yasargil MG. New York: Georg Thieme Verlag; 1987. Microneurosurgery. IIIA: AVM of the brain, history, embryology, pathological considerations, hemodynamics, diagnostic studies, microsurgical anatomy. [Google Scholar]
- [2].Hashimoto T, Wen G, Lawton MT, et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34(4):925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- [3].Liu ZK, Lin ZM, Xu HZ, et al. Relationship between matrix metalloproteinase 9 and cerebral vascular malformations. Jilin Yixue. 2011;32(16):3175–3177. [Google Scholar]
- [4].Kranzhöfer A, Baker AH, George SJ, et al. Expression of tissue inhibitor of metalloproteinase-1, -2, and -3 during neointima formation in organ cultures of human saphenous vein. Arterioscler Thromb Vasc Biol. 1999;19(2):255–265. doi: 10.1161/01.atv.19.2.255. [DOI] [PubMed] [Google Scholar]
- [5].Liu ZK, Lin ZM, Xu HZ, et al. Relation between matrix metalloproteinase 2 and cerebral vascular malformations. Jilin Yixue. 2011;32(19):3887–3888. [Google Scholar]
- [6].Cui XY, Li JF, Niu CC, et al. Effect of repetitive hypoxia on MMP-2/9 expression and activation in murine brain. Zhongguo Yingyong Shenglixue Zazhi. 2006;22(3):298–301. [PubMed] [Google Scholar]
- [7].Feng QX, Song LJ, You C, et al. Expression of MMP-9 and TIMP-1 in human meningiomas tissue. Zhengzhou Daxue Xuebao: Yixue Ban. 2005;40(1):616–619. [Google Scholar]
- [8].Niu GM, Wan F, Li J, et al. Expressions of MMP-2 and TIMP-2 in human gliomas and their significance. Zhongguo Linchuang Shenjing Waike Zazhi. 2004;9(4):259–261. [Google Scholar]
- [9].Palei AC, Sandrim VC, Amaral LM, et al. Association between matrix metalloproteinase (MMP)-2 polymorphisms and MMP-2 levels in hypertensive disorders of pregnancy. Exp Mol Pathol. 2012;92(2):217–221. doi: 10.1016/j.yexmp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- [10].Zhong CL, Luo QZ, Wang Y, et al. Analysis of risk factors in predicting hemorrhage of cerebral arteriovenous malformations. Shanghai Yixue. 1998;21(7):376–378. [Google Scholar]
- [11].Ling F, Liu SS, Huang KW, et al. Investigation on the relationship between vessel wall measurement and hemorrhage in avm of brain. Zhonhua Fangshexue Zazhi. 1993;28(4):233–235. [Google Scholar]
- [12].Sun YL, Wang ZC, Qu BQ, et al. Relationship of ultrapathology and clinical manifestation in cerebral arteriovenous mal formation. Beijing Yixue. 1997;19(1):3–6. [Google Scholar]
- [13].Hademenos GJ, Massoud TF. Risk of intracranial arteriovenous malformation rupture due to venous drainage impairment. A theoretical analysis. Stroke. 1996;27(6):1072–1083. doi: 10.1161/01.str.27.6.1072. [DOI] [PubMed] [Google Scholar]
- [14].Castellazzi M, Tamborino C, De Santis G, et al. Timing of serum active MMP-9 and MMP-2 levels in acute and subacute phases after spontaneous intracerebral hemorrhage. Acta Neurochir Suppl. 2010;106:137–140. doi: 10.1007/978-3-211-98811-4_24. [DOI] [PubMed] [Google Scholar]
- [15].Heo JH, Lucero J, Abumiya T, et al. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19(6):624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- [16].Gaetani P, Rodriguez Y, Baena R, et al. Metalloproteases and intracranial vascular lesions. Neurol Res. 1999;21(4):385–390. doi: 10.1080/01616412.1999.11740948. [DOI] [PubMed] [Google Scholar]
- [17].Starke RM, Komotar RJ, Hwang BY, et al. Systemic expression of matrix metalloproteinase-9 in patients with cerebral arteriovenous malformations. Neurosurgery. 2010;66(2):343–348. doi: 10.1227/01.NEU.0000363599.72318.BA. [DOI] [PubMed] [Google Scholar]
- [18].Lo EH. A theoretical analysis of hemodynamic and biomechanical alterations in intracranial AVMs after radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27(2):353–361. doi: 10.1016/0360-3016(93)90247-s. [DOI] [PubMed] [Google Scholar]
- [19].Johnson C, Sung HJ, Lessner SM, et al. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res. 2004;94(2):262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tao J, Li Z, Liu HP. The clinical significance of serum matrix metallporotelnases-9 in patients with acute cerebral infarction. Shiyong Yiji Zazhi. 2011;18(12):1243–1245. [Google Scholar]
- [21].State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994-09-01 [Google Scholar]


