Abstract
In the present study, we examined the analgesic effect of repeated electroacupuncture at bilateral Zusanli (ST36) and Yanglingquan (GB34) once a day for 14 consecutive days in a rat model of chronic sciatic nerve constriction injury-induced neuropathic pain. In addition, concomitant changes in calcium/calmodulin-dependent protein kinase II expression and synaptic ultrastructure of neurons in the hippocampal CA3 region were examined. The thermal pain threshold (paw withdrawal latency) was increased significantly in both groups at 2 weeks after electroacupuncture intervention compared with 2 days of electroacupuncture. In ovariectomized rats with chronic constriction injury, the analgesic effect was significantly reduced. Electroacupuncture for 2 weeks significantly diminished the injury-induced increase in synaptic cleft width and thinning of the postsynaptic density, and it significantly suppressed the down-regulation of intracellular calcium/calmodulin-dependent protein kinase II expression in the hippocampal CA3 region. Repeated electroacupuncture intervention had a cumulative analgesic effect on injury-induced neuropathic pain reactions, and it led to synaptic remodeling of hippocampal neurons and upregulated calcium/calmodulin-dependent protein kinase II expression in the hippocampal CA3 region.
Keywords: chronic neuropathic pain, acupuncture analgesia, cumulative effect, synaptic plasticity, hippocampal CA3 region, calcium/calmodulin-dependent protein kinase II, neurobiology, electroacupuncture, neural regeneration
Abbreviations
CaMKII, calcium/calmodulin-dependent kinase II; CCI, chronic constrictive injury; OVX, ovariectomized; PWL, paw withdrawal latency
INTRODUCTION
Functional changes in pain states have been a focus of many studies[1,2,3], but little attention has been paid to the fact that tremendous potential for plasticity exists in the pathways for pain at the structural and representational levels. The hippocampus is a target of stress-induced hormones, such as adrenal glucocorticoids, which modulate changes in synapse formation and dendritic structure, acutely and over the long-term[4,5,6].
Clinical studies revealed that the hippocampus is involved in processing physiological pain and also in the development of pain syndromes[7,8]. Furthermore, experimental studies have demonstrated that hippocampal synaptic plasticity is associated with learning, memory and adaptive processes, and is accompanied by changes in expression of a variety of genes and proteins[9,10]. For example, calcium/calmodulin-dependent kinase II (CaMKII) is a multifunctional serine/threonine protein kinase in neurons and functions in regulating neurotransmission, memory and synaptic plasticity in response to calcium signaling produced by neuronal activity[11,12,13]. Hippocampal CaMKII is also involved in the development of persistent pain in both mice and rats[14,15].
It has been demonstrated that repeated electroacupuncture at Zusanli (ST36) and Baihui (GV20) significantly increases the number of well-developed (tertiary) dendrites in the subgranular zone of the dentate gyrus and promotes neuroblast differentiation via phosphorylated cAMP response element binding protein and brain-derived neurotrophic factor activation in the dentate gyrus in normal rats[16,17]. Electroacupuncture also mitigates the cerebral ischemia-induced decrease in amplitude and slope rate of population spikes in the hippocampal CA1 region in rats with diabetes mellitus[18]. However, no study has determined whether hippocampal CaMKII plays a role in acupuncture analgesia, particularly as Zusanli and Yanglingquan (GB34) are clinically frequently used to treat sciatica[19,20].
Our previous studies[21,22,23] demonstrated that, in chronic constrictive injury (CCI) of the sciatic nerve in rats, repeated electroacupuncture intervention effectively relieved neuropathic pain. Concomitantly, upregulation of plasma cortisol, beta-endorphin and adrenocorticotropin hormone levels, and increased expression of hippocampal intracellular protein kinase A were found. In ovariectomized (OVX) + CCI rats, the analgesic effect of repeated electroacupuncture was significantly weakened, suggesting a close association between analgesic effect and estrogen levels. Thus, repeated electroacupuncture-induced pain relief may involve modulation of hippocampal neuronal plasticity by various hormones and neurotransmitters.
Biologically, functional activity and structure are closely related. Thus, functional changes are often accompanied by structural alterations. However, the relationship between the analgesic effects of electroacupuncture and changes in neuronal plasticity in the hippocampus remains poorly understood. The present study was designed to analyze the relationship between the analgesic effect of repeated electroacupuncture and hippocampal synaptic plasticity in ovariectomized rats with CCI of the sciatic nerve.
RESULTS
Quantitative analysis of experimental animals
A total of 98 female Wistar rats were randomized into normal control, CCI (ligation of the sciatic nerve), CCI + electroacupuncture 2 days, CCI + electroacupuncture 2 weeks, OVX + CCI, OVX + CCI + electroacupuncture 2 days and OVX + CCI + electroacupuncture 2 weeks groups, with 14 animals in each group. Ten rats from each group were used for behavioral and immunohistochemistry experiments, and four were used for electron microscopy.
The CCI model of chronic neuropathic pain was employed in the CCI, CCI + electroacupuncture 2 days, CCI + electroacupuncture 2 weeks, CCI + OVX, CCI + OVX + electroacupuncture 2 days and the CCI + OVX + electroacupuncture 2 weeks groups. The OVX model of learning and memory impairment was applied in the CCI + OVX, CCI + OVX + electroacupuncture 2 days and the CCI + OVX + electroacupuncture 2 weeks groups before establishment of chronic neuropathic pain. Rats in the electroacupuncture 2 days and electroacupuncture 2 weeks groups were subjected to electroacupuncture at Zusanli and Yanglingquan from the 16th day and 4th day after surgery, respectively (i.e. electroacupuncture lasted for 2 days and 2 weeks, respectively). Three rats in the CCI + OVX group died during ovariectomization. Therefore, 98 rats were included in the final analysis following supplementation.
Electroacupuncture decreases pain reaction after CCI
The pain threshold was measured as paw withdrawal latency (PWL; PWL of the healthy limb - PWL of the affected limb). Pain scores were significantly greater in the CCI and OVX + CCI rat groups compared with the normal control group (P < 0.05), suggesting a decreased pain threshold. The pain scores were significantly less in the CCI + electroacupuncture 2 weeks group and OVX + CCI + electroacupuncture 2 weeks group compared with the CCI and OVX + CCI groups (P < 0.05). The pain score was significantly increased in the OVX + CCI + electroacupuncture 2 weeks group compared with the CCI + electroacupuncture 2 weeks group (P < 0.05; Table 1).
Table 1.
Comparison of hyperalgesia scores among groups

Electroacupuncture reduces synaptic cleft width between hippocampal neurons
Compared with the normal control group, the synaptic cleft width was significantly increased in the CCI and OVX + CCI groups (P < 0.05). The synaptic cleft width was significantly decreased in the CCI + electroacupuncture 2 weeks group compared with the CCI group (P < 0.05), but the width remained unchanged in the CCI + electroacupuncture 2 days group. Compared with the OVX + CCI group, synaptic cleft width was reduced remarkably in the OVX + CCI + electroacupuncture 2 weeks group (P < 0.05). The synaptic cleft width was significantly increased in the OVX + CCI + electroacupuncture 2 weeks group compared with the CCI + electroacupuncture 2 weeks group (P < 0.05; Figure 1).
Figure 1.
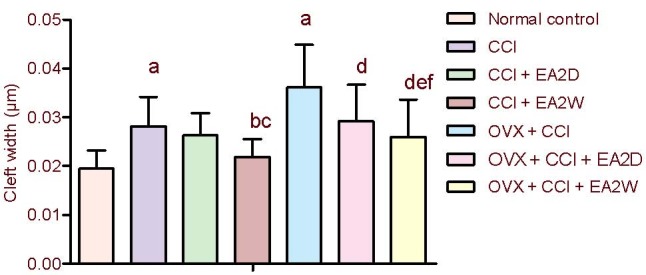
Comparison of synaptic cleft width of neurons in the hippocampal CA3 region among the various groups.
Data were expressed as mean ± SD (n = 4) and analyzed by one-way analysis of variance and least significant difference-t test.
aP < 0.05, vs. normal control group; bP < 0.05, vs. CCI group; cP < 0.05, vs. CCI + EA2D group; dP < 0.05, vs. OVX + CCI group; eP < 0.05, vs. OVX + CCI + EA2D group; fP < 0.05, vs. CCI + EA2W group.
CCI: Chronic constrictive injury; OVX: ovariectomy; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks.
Electroacupuncture increases postsynaptic density of hippocampal neurons
Postsynaptic density values were significantly decreased in the CCI and OVX + CCI groups compared with the normal control group (P < 0.05), suggesting deterioration of excitatory signal transmission. The postsynaptic density value was significantly increased in the CCI + electroacupuncture 2 weeks group compared with the CCI group (P < 0.05), and it was significantly increased in the OVX + CCI + electroacupuncture 2 weeks group compared with the OVX + CCI group (P < 0.05). No significant differences were found between the CCI and CCI + electroacupuncture 2 days groups or between the OVX + CCI and OVX + CCI + electroacupuncture 2 days groups (P > 0.05). The postsynaptic density value was significantly less in the OVX + CCI + electroacupuncture 2 weeks group than in the CCI + postsynaptic 2 weeks group (P < 0.05; Figure 2).
Figure 2.
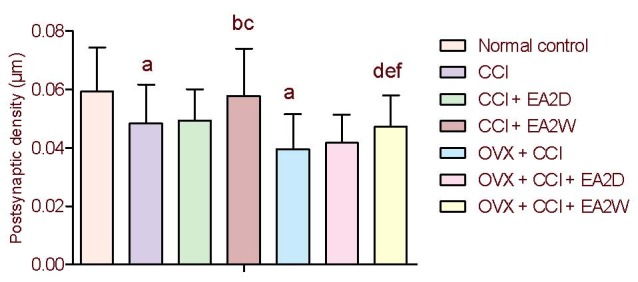
Comparison of synaptic cleft width of neurons in the hippocampal CA3 region among the various groups.
Data were expressed as mean ± SD (n = 4) and analyzed by one-way analysis of variance and least significant difference-t test.
aP < 0.05, vs. normal control group; bP < 0.05, vs. CCI group; cP < 0.05, vs. CCI + EA2D group; dP < 0.05, vs. OVX + CCI group; eP < 0.05, vs. OVX + CCI + EA2D group; fP < 0.05, vs. CCI + EA2W group.
CCI: Chronic constrictive injury; OVX: ovariectomy; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks.
Electroacupuncture increases synaptic active zone length in hippocampal neurons
Compared with the control group, the active zone length values for the CCI and OVX + CCI groups were significantly decreased (P < 0.05). The synaptic active zone length was significantly longer in the CCI + electroacupuncture 2 weeks group compared with the CCI group, and in the OVX + CCI + electroacupuncture 2 weeks group compared with the OVX + CCI group (both P < 0.05), suggesting an improvement of synaptic efficacy after repeated electroacupuncture. The active zone length was significantly greater in the CCI + electroacupuncture 2 weeks group compared with the OVX + CCI + electroacupuncture 2 weeks group (P < 0.05). No significant differences were found between the CCI and CCI + electroacupuncture 2 days groups or between the OVX + CCI and OVX + CCI + electroacupuncture 2 days groups in active zone length (both P > 0.05; Figure 3).
Figure 3.
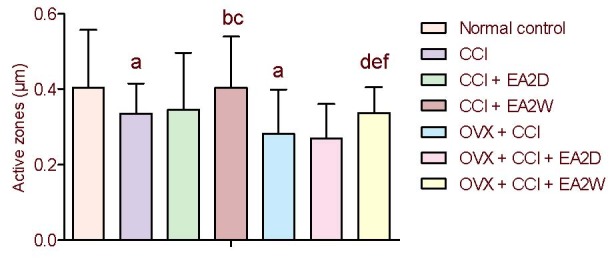
Comparison of synaptic active zone values of neurons in the hippocampal CA3 region among the various groups.
Data were expressed as mean ± SD (n = 4) and analyzed by one-way analysis of variance and least significant difference-t test.
aP < 0.05, vs. normal control group; bP < 0.05, vs. CCI group; cP < 0.05, vs. CCI + EA2D group; dP < 0.05, vs. OVX + CCI group; eP < 0.05, vs. OVX + CCI + EA2D group.
CCI: Chronic constrictive injury; OVX: ovariectomy; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks.
Electroacupuncture upregulates CaMKII expression in the hippocampal CA3 region
CaMKII is an important calcium messenger regulating hippocampal synaptic plasticity and learning. The integral grey values of CaMKII expression were significantly increased in the CCI and OVX + CCI groups compared with the normal control group (P < 0.05), a downregulation of this protein in CCI and OVX + CCI rats. Compared with the CCI group, the integral grey value was significantly lower in the CCI + electroacupuncture 2 weeks group compared with the CCI group (P < 0.05), but remained unchanged in the CCI + electroacupuncture 2 days group. The change in CaMKII expression in CCI rats was similar to that in OVX + CCI rats. The integral grey value of CaMKII immunoreactivity was significantly higher in the OVX + CCI + electroacupuncture 2 weeks group compared with the CCI + electroacupuncture 2 weeks group (P < 0.05). Collectively, these results indicate that electroacupuncture upregulates CaMKII expression in the hippocampal CA3 region (Figure 4).
Figure 4.
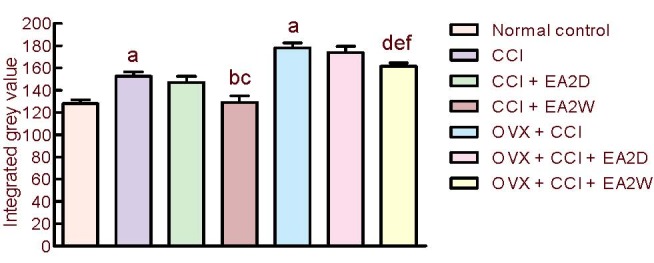
Comparison of calcium/calmodulin-dependent kinase II expression (integrated grey value) in hippocampal CA3 region among the various groups.
Data were expressed as mean ± SD (n = 4) and analyzed by one-way analysis of variance and least significant difference-t test.
aP < 0.05, vs. normal control group; bP < 0.05, vs. CCI group; cP < 0.05, vs. CCI + EA2D group; dP < 0.05, vs. OVX + CCI group; eP < 0.05, vs. OVX + CCI + EA2D group; fP < 0.05, vs. CCI + EA2W group.
CCI: Chronic constrictive injury; OVX: ovariectomy; EA: electroacupuncture; 2D: 2 days; 2W: 2 weeks.
DISCUSSION
Synaptic plasticity, including changes in synapse number, structure and transmission efficacy, is the basis of neural plasticity. Sensory neurons undergo functional, chemical and structural changes (activity and signaling molecule-dependent) in response to changes in their environment (including chronic nociceptive pain) that modify transduction, conduction and transmission efficiency[24]. McEwen[25] observed that after chronic constriction injury of the sciatic nerve in rats, the hippocampus exhibits structural plasticity, involving ongoing neurogenesis of the dentate gyrus, synaptogenesis under control of estrogens in the CA1 region and dendritic remodeling caused by repeated stress or elevated levels of exogenous glucocorticoids in the CA3 region. Peripheral nerve injury (e.g. CCI) may also increase hippocampal postsynaptic density and synaptic cleft area[26] or decrease hippocampal synaptic interface curvature in the rat[27]. Chronic pain is also a type of chronic stress. Under chronic stress conditions, significant decreases in hippocampal synaptic density and surface density were found[28].
At the spinal level, peripheral nerve injury may result in higher synaptic density or number and increase the percentage of positively curved synapses[29,30]. It may also lead to synaptic interface curvature and synaptic perforation in the dorsal horn of the spinal cord[31]. This morphological rearrangement may alter the organism's capacity for pain perception or result in abnormal sensory processing (as in tactile allodynia) as an adaptive and protective response[25,32,33]. CaMKII plays an important role in peripheral stimulation-induced neural plasticity in the hippocampus[34,35].
The synaptic cleft (a space between the presynaptic terminals and postsynaptic membrane) is a space for transmitting action potentials and chemical transmitters. The postsynaptic density is a protein-dense specialization attached to the postsynaptic membrane, serving as a signaling apparatus. The synaptic active zone is a unique presynaptic membrane specialization that is believed to be the site of neurotransmitter release. Our present results revealed that, in chronic neuropathic pain rats, the pain threshold was decreased significantly. Concomitantly, hippocampal synaptic cleft area was increased significantly, while synaptic postsynaptic density and active zone length were significantly decreased. These results are indicative of abnormal changes in plasticity and disruption of normal synaptic transmission. Moreover, hippocampal CaMKII expression was significantly downregulated in CCI rats. Acupuncture can effectively alleviate both acute and chronic pain. Generally, one acupuncture treatment session for acute disease may produce a substantial and immediate therapeutic effect. In order to obtain robust and reliable clinical efficacy, repeated treatment is necessary, particularly for chronic conditions. Our present results revealed that compared with simple CCI rats, the pain threshold after electroacupuncture for 2 weeks was significantly increased and its effect was markedly superior to that of electroacupuncture for 2 days. This outcome is similar to previous results that electroacupuncture at Huantiao (GB30) and Weizhong (BL40) for one week alleviates neuropathic pain in CCI rats[36].
Acupuncture is an effective method for stimulating the central nervous system. Acupuncture plays an important role in brain functional reorganization and compensation. Shou et al[37] observed that repeated electroacupuncture could upregulate the expression of cannabinoid receptor-1 mRNA and dopamine 1 receptor mRNA in the nucleus accumbens-caudate nucleus region in rats with inflammatory pain. Xing et al[38] found that, in rats with neuropathic pain, electroacupuncture treatment had a modulatory effect on long-term synaptic plasticity in the spinal dorsal horn. In addition, acupuncture could reduce the decline in the amplitude and slope rate of population spikes induced by diabetes mellitus and cerebral ischemia, which may help improve memory by regulating hippocampal synaptic plasticity[18]. The mechanism of action of acupuncture was hypothesized to involve dendritic or synaptic plasticity in the ipsilateral hippocampal CA3 region.
Our present results indicate that the cumulative analgesic effect of electroacupuncture is associated with the following: (1) significant increases in CaMKII expression, postsynaptic density area and active zone length in the hippocampal CA3 region; and (2) a significant reduction in synaptic cleft width. In OVX rats, the synaptic plasticity of neurons in the hippocampal CA3 region and the cumulative effect of acupuncture analgesia were diminished. These results suggest that repeated electroacupuncture-induced pain relief is accompanied by improvement of hippocampal neural synaptic plasticity.
A number of studies have examined the effects of repeated electroacupuncture[39,40], but a systematic study of the underlying mechanisms is needed. Our present study is the first attempt to reveal the mechanisms behind the analgesic effect of repeated electroacupuncture on hippocampal synaptic plasticity and CaMKII protein expression. It is possible that electroacupuncture intervention induces synaptic changes associated with pain-relief which are gradually consolidated with repeated treatment, eventually establishing the observed cumulative analgesic effect. In conclusion, repeated electroacupuncture at Zusanli and Yanglingquan has a cumulative analgesic effect on chronic neuropathic pain in rats. This effect is closely associated with its ability to suppress the CCI-induced down-regulation of CaMKII protein expression and the increase in synaptic cleft width and thinning of the postsynaptic density in the hippocampal CA3 region.
MATERIALS AND METHODS
Design
A randomized and controlled animal experiment.
Time and setting
The study was performed at the Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, China, from November 2007 to April 2009.
Materials
A total of 98 clean-grade, female Wistar rats, aged 3-4 months, weighing 240-250 g, were purchased from the Experimental Animal Center of Chinese Academy of Medical Sciences (License No. SCXX-Army 2007-004). Animal care and experimental process were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals, formulated in 2005 by our institute and the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[41].
Methods
Establishment of the CCI model
A model of chronic neuropathic pain was established by ligation of the sciatic nerve, based on a modification of the Bennett and Xie method[42]. Under anesthesia by intraperitoneal injection with a solution of 28 mg/100 g urethane (Beijing Chemistry Reagent, Beijing, China) and 3.3 mg/100 g chloralose (Sigma-Aldrich, St. Louis, MO, USA), followed by routine sterilization, the rat's left sciatic nerve was exposed at the mid-thigh level by blunt dissection through the biceps femoris muscle. Four constrictive ligatures (4-0 surgical suture) were tied around the nerve at the distal end close to the nerve bifurcation at spaces about 1.0 mm apart. The ligature was considered to be suitable if local and moderate muscular contraction of the leg was clearly observed. Following local application of antibiotics (sodium penicillin, 9 000–10 000 U/rat), the muscle and skin tissues were sutured in layers. In order to reduce experimental variability, all surgeries were performed by the same operator. For OVX + CCI rats, CCI surgery was performed following Morris water maze testing.
Establishment of memory impairment model
After anesthesia, rats in the CCI + OVX, CCI + OVX + electroacupuncture 2 days, and CCI + OVX + electroacupuncture 2 weeks groups underwent ovariectomy (OVX). Briefly, bilateral mid-abdominal dorsolateral incisions (about 2 cm long) were made, and both ovaries were removed. Four weeks after OVX, four animals from each group (CCI, CCI + electroacupuncture 2 days, CCI + electroacupuncture 2 weeks, CCI + OVX, CCI + OVX + electroacupuncture 2 days and CCI + OVX + electroacupuncture 2 weeks) were subjected to a vaginal smear test for verifying OVX success. Forty-five days after OVX, the rats’ learning-memory ability was analyzed by escape latency (place navigation test), swimming distance in the target quadrant and target quadrant crossing times (spatial probe test) in the Morris water maze test for 7 days using a Morris water maze apparatus (DigBehv-MWM Morris Water Maze Video Analysis System, Shanghai Jiliang Company Ltd, China) with reference to Nunez's article [Nunez J. Morris Water Maze Experiment. http://www.jove.com/index/details.stp?ID=897.].
Electroacupuncture
Bilateral Zusanli (5 mm beneath the capitulum fibulae and lateral posterior to the knee-joint) and Yanglingquan (about 5 mm superior-lateral to Zusanli) were punctured with filiform needles (Gauge 28), respectively, and electrically stimulated using a HANS Electroacupuncture Apparatus (LH202, Beijing Huawei Industrial Developing Company, Beijing, China). Electroacupuncture (2/15 Hz, 1 mA) intervention was administered for 30 minutes, once daily from the 4th day on after CCI surgery for 2 weeks for rats of the CCI + electroacupuncture 2 weeks and OVX + CCI + electroacupuncture 2 weeks groups, and from the 16th day on after CCI surgery for two sessions for rats of the CCI + electroacupuncture 2 days and OVX + CCI + electroacupuncture 2 days groups.
Thermal pain threshold detection
Each rat was placed into a black cloth bag with the hindlimbs and tail exposed for mobility. A mobile radiant heat source (high-intensity light beam with radiant heat dolorimeter) was focused on the plantar surface of the hind paw. Paw withdrawal latency (i.e. pain threshold) of the bilateral footplates was measured three times with an interval of 3-5 minutes between detections. To avoid potential tissue damage, the cutoff time of the radiant heat was set to 20 seconds. Thermal pain threshold detection was conducted prior to, as well as 4, 8, 12, 16 and 18 days after CCI. Pain thresholds were measured in rats from the CCI + electroacupuncture and CCI + OVX + electroacupuncture groups on the following day morning.
Transmission electron microscopy observation of the morphology of the hippocampal CA3 region
At the end of each experiment, under deep anesthesia, rats were perfused transcardially with a solution of 2% paraformaldehyde + 2% glutaraldehyde. Then, brain tissue containing the hippocampal CA3 region was cut into small cubes (about 1 mm3), fixed with 3% glutaraldehyde (EMS Company, Shanghai, China) and 1% osmium tetroxide, respectively, for 2 hours, dehydrated with ethanol, infused in acetone, embedded in 812 Epon-Araldite, sectioned into 250-nm-thick slices, and stained using uranyl acetate and lead citrate. The brain sections were then examined under a JEM-1230 transmission electron microscope (JEOL Ltd., Tokyo, Japan) and areas of interest which contained synapses were imaged using a NIS-Elements BR2.30 (Nikon, Tokyo, Japan) operated at 200 KeV and a Gatan 2K × 2K CCD camera at a magnification of 50 000×. The average synaptic cleft width, the thickness of the postsynaptic densities and the lengths of active zones in 10 random visual fields of sections of each brain (4 rats/group) were examined according to a previously described method[43]. The length and thickness of the postsynaptic density and the synaptic cleft were assessed separately as previously described[43].
Immunohistochemical staining for CaMKII
The animals were deeply anesthetized with the same anesthetics mentioned above and transcardially perfused with saline, followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The brain (without the cerebellum) was removed and post-fixed in 30% sucrose solution (containing 4% paraformaldehyde) at 4°C. Serial coronal sections (60 μm) were cut on a cryostat (whole body slicing microtome Leitz 400 with a chest mobile freezer; Leitz OM, Leica, Germany) and stored in 0.01 M PBS solution at 4°C.
The brain sections containing the hippocampal CA3 region were mounted onto glass slides, immersed in PBS for 5 minutes, and incubated in 3% H2O2/deionized water for 15 minutes. Excess fluid was removed and the sections were incubated in 5% normal goat serum at room temperature for 15 minutes, followed by rabbit anti-CaM kinase II polyclonal antibody (1:1 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 37°C for 24 hours. The sections were then washed three times in PBS for 5 minutes each, incubated with goat anti-rabbit IgG (1:300; Zhongshan Golden Bridge Biotechnology, Beijing, China) at room temperature for 2 hours, washed three times in PBS, incubated in avidin-biotin-horseradish peroxidase (1:300; Zhongshan Golden Bridge Biotechnology) at room temperature for 2 hours, washed three times with PBS for 5 minutes each, and stained with 3,3’-diaminobenzidine. The sections were then washed in running water, counterstained with 0.01% cresyl fast violet at 37°C for 15 minutes, incubated in a mixture of 100% alcohol:ether:chloroform (1:1:1) for 10 minutes, rinsed 2 minutes in water, dehydrated in alcohol, and then immersed twice in dimethyl benzene for 10 minutes. The slides were sealed with neutral gel and dried at room temperature. The immunostained structure (grey scale values) of the CA3 region was analyzed using NIS-Elements BR2.30 image analysis system (Nikon). Two sections were selected from each rat, and three areas of the hippocampal CA3 region of each slice were selected for determining the average grey scale value.
Statistical analysis
Data were expressed as mean ± SD. Differences in paw withdrawal latency were assessed using one-way analysis of variance with repeated measures when appropriate. Least significant difference-t test was used to compare data between two different groups. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank Yuanshen Wang and Qing Cai from the Capital Medical University for their technical assistance.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 30472241, 90709031 and 30973796, and the Ministry of Science and Technology of China (“973” Project), No. 2007CB512505. Funding was also provided by the Foundation of Hainan Province, No. 310054 and the Health Department of Hainan Province, QiongWei-45.
Conflicts of interest: None declared.
Ethical approval: All procedures were approved by the Institute of Acupuncture and Moxibustion of China Academy of Chinese Medical Sciences.
(Edited by Song XG, Wei JZ/Su LL/Wang L)
REFERENCES
- [1].Zimmerman ME, Pan JW, Hetherington HP, et al. Hippocampal correlates of pain in healthy elderly adults: a pilot study. Neurology. 2009;73(19):1567–1570. doi: 10.1212/WNL.0b013e3181c0d454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Panigada T, Gosselin RD. Behavioural alteration in chronic pain: Are brain glia involved? Med Hypotheses. 2011;77(4):584–588. doi: 10.1016/j.mehy.2011.06.036. [DOI] [PubMed] [Google Scholar]
- [3].Ren WJ, Liu Y, Zhou LJ, et al. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011;36(5):979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- [5].McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- [6].Zhao XY, Liu MG, Yuan DL, et al. Nociception-induced spatial and temporal plasticity of synaptic connection and function in the hippocampal formation of rats: a multi-electrode array recording. Mol Pain. 2009;5:55. doi: 10.1186/1744-8069-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leong MS, Solvason HB. Case Report: Limbic system activation by intravenous lidocaine in a patient with a complex regional pain syndrome and major depression. Pain Med. 2000;1(4):358–361. doi: 10.1046/j.1526-4637.2000.00038.x. [DOI] [PubMed] [Google Scholar]
- [8].Emad Y, Ragab Y, Zeinhom F, et al. Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome. a study with single-voxel magnetic resonance spectroscopy. J Rheumatol. 2008;35(7):1371–1377. [PubMed] [Google Scholar]
- [9].Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- [10].Jian YX, Chen RQ, Zhang J, et al. Endocannabinoids differentially modulate synaptic plasticity in rat hippocampal CA1 pyramidal neurons. PLoS One. 2010;5(4):e10306. doi: 10.1371/journal.pone.0010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sun CY, Qi SS, Lou XF, et al. Changes of learning, memory and levels of CaMKII, CaMmRNA, CREB mRNA in the hippocampus of chronic multiple-stressed rats. Chin Med J (Engl) 2006;119(2):140–147. [PubMed] [Google Scholar]
- [12].Ashpole NM, Song W, Brustovetsky T, et al. Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J Biol Chem. 2012 doi: 10.1074/jbc.M111.323915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Savina TA, Shchipakina TG, Godukhin OV. Effect of seizure activity on subunit composition of Ca2+/calmodulin-dependent protein kinase II in hippocampus of Krushinskii-Molodkina rats. Ross Fiziol Zh Im I M Sechenova. 2011;97(6):590–600. [PubMed] [Google Scholar]
- [14].Seo YJ, Kwon MS, Choi HW, et al. Differential expression of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse hippocampus induced by various nociceptive stimuli. Neuroscience. 2008;156(3):436–49. doi: 10.1016/j.neuroscience.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [15].Zeitz KP, Giese KP, Silva AJ, et al. The contribution of autophosphorylated alpha-calcium-calmodulin kinase II to injury-induced persistent pain. Neuroscience. 2004;128:889–898. doi: 10.1016/j.neuroscience.2004.07.029. [DOI] [PubMed] [Google Scholar]
- [16].Hwang IK, Chung JY, Yoo DY, et al. Effects of electroacupuncture at Zusanli and Bahui on brain-derived neurotrophic factor and cyclic AMP response element-binding protein in the hippocampal dentate gyrus. J Vet Med Sci. 2010;72(11):1431–1436. doi: 10.1292/jvms.09-0527. [DOI] [PubMed] [Google Scholar]
- [17].Hwang IK, Chung JY, Yoo DY, et al. Comparing the effects of acupuncture and electroacupuncture at Zusanli and Bahui on cell proliferation and neuroblast differentiation in the rat hippocampus. J Vet Med Sci. 2010;72(3):279–284. doi: 10.1292/jvms.09-0374. [DOI] [PubMed] [Google Scholar]
- [18].Jing XH, Shi H, Cai H, et al. Effect of acupuncture on long-term potentiation of hippocampal CA 1 area in diabetic rats with concurrent cerebral ischemia. Zhen Ci Yan Jiu. 2008;33(6):397–401. [PubMed] [Google Scholar]
- [19].Meng FY. Observation on therapeutic effect of electroacupuncture for lumbar intervertebral disc protrusion induced sciatica in patients. Zhong Guo Yi Yao Dao Bao. 2011;8(6):78. [Google Scholar]
- [20].Zhang CM, Yin GH. Observation on therapeutic effect of acupuncture treatment of sciatica. Liaoning Zhongyi Zazhi. 2004;31(8):684. [Google Scholar]
- [21].Liu JL, Chen SP, Gao YH, et al. Correlation between cumulative analgesic effect of electroacupuncture and plasma β-EP, ACTH, COR. Zhen Ci Yan Jiu. 2007;32(5):306–312. [PubMed] [Google Scholar]
- [22].Liu JL, Chen SP, Gao YH, et al. Effects of repeated electroacupuncture on β-endorphin and ACTH levels in hypothalamus and pituitary in rats with chronic pain and ovariectomy. Chin J Integr Med. 2010;16(4):315–323. doi: 10.1007/s11655-010-0503-3. [DOI] [PubMed] [Google Scholar]
- [23].Wang JY, Chen SP, Li YH, et al. Effect of repeated electroacupuncture on hypothalamus and hippocampal PKA expression in neuropathic pain rats. Zhen Ci Yan Jiu. 2008;33(2):80–87. [PubMed] [Google Scholar]
- [24].Dubner R, Gold M. The neurobiology of pain. Proc Natl Acad Sci U S A. 1999;96(14):7627–7630. doi: 10.1073/pnas.96.14.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- [26].Liu H, Shi XS, Zhang XY, et al. Effects of neuropathic pain on the abilities of learning and memory and synaptic ultrastructure of hippocampus in aged rats. Zhong Guo Kang Fu Yi Xue Za Zhi. 2010;25(3):211–214. [Google Scholar]
- [27].Li YL. Fujian: Fujian Traditional Chinese Medicine College; 2008. To observe the influence of the electroacupuncture to aim directly at the morphous of the hippocampal synaptic curvature of the rat model of focal cerebral ischemia under electron microscope. [Google Scholar]
- [28].Ao HQ, Xu ZW, Yan C, et al. Effect of Xiaoyao Powder on synaptic structural plasticity of hippocampal CA3 region about rats under multi-stress model. Zhong Cheng Yao. 2006;28(5):697–700. [Google Scholar]
- [29].Bakkum BW, Henderson CN, Hong SP, et al. Preliminary morphological evidence that vertebral hypomobility induces synaptic plasticity in the spinal cord. J Manipulative Physiol Ther. 2007;30(5):336–342. doi: 10.1016/j.jmpt.2007.04.007. [DOI] [PubMed] [Google Scholar]
- [30].Peng B, Yang ZW, Min S. Number of synapses increased in the rat spinal dorsal horn after sciatic nerve transection: a stereological study. Brain Res Bull. 2011;84(6):430–433. doi: 10.1016/j.brainresbull.2011.01.007. [DOI] [PubMed] [Google Scholar]
- [31].Peng ZG, Wang YJ, Wu XY, et al. Changes in synaptic structural plasticity in lamine II of spinal dorsal horn of rats with neuropathic pain. Shen Jing Sun Shang Yu Gong Neng Chong Jian. 2011;6(3):161–165. [Google Scholar]
- [32].Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hama AT, Pappas GD, Sagen J. Adrenal medullary implants reduce transsynaptic degeneration in the spinal cord of rats following chronic constriction nerve injury. Exp Neurol. 1996;137(1):81–93. doi: 10.1006/exnr.1996.0009. [DOI] [PubMed] [Google Scholar]
- [34].Zhang N, Pu XN, Fang L, et al. Role of CaMKII signaling pathways in peripheral and central sensory of chronic pain. Zhongguo Linchuang Jiepuoxue Zazhi. 2008;26(3):344–347. [Google Scholar]
- [35].Borgesius NZ, van Woerden GM, Buitendijk GH, et al. βCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting αCaMKII to synapses. J Neurosci. 2011;31(28):10141–10148. doi: 10.1523/JNEUROSCI.5105-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yan LP, Wu XT, Yin ZY, et al. Effect of electroacupuncture on the levels of amino acid neurotransmitters in the spinal cord in rats with chronic constrictive injury. Zhen Ci Yan Jiu. 2011;36(5):353–356. 379. [PubMed] [Google Scholar]
- [37].Shou Y, Zhao YQ, Xu MS, et al. Effects of repeated electroacupuncture on gene expression of cannabinoid receptor-1 and dopamine 1 receptor in nucleus accumbens-caudate nucleus region in inflammatory-pain rats. Zhen Ci Yan Jiu. 2011;36(1):18–22. [PubMed] [Google Scholar]
- [38].Xing GG, Liu FY, Qu XX, et al. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol. 2007;208(2):323–332. doi: 10.1016/j.expneurol.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [39].Dong ZQ, Ma F, Xuan CT, et al. Cumulative electroacupuncture enhances expression of GDNF mRNA in dorsal root ganglions of neuropathic pain rats. Shanghai Zhen Jiu Za Zhi. 2005;24(2):33–36. [Google Scholar]
- [40].Takahashi H. Effects of scalp acupuncture and auricular therapy on acute herpetic pain and postherpetic neuralgia: a case series. Acupunct Med. 2007;19(2):113–120. [Google Scholar]
- [41].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [42].Bennett GJ, Xie YK. A peripheralmononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- [43].Güldner FH, Ingham CA. Increase in postsynaptic density material in optic target neuron of the rat suprachiasmatic necleus after bilateral enucleation. Neurosci Lett. 1980;17(1-2):27–31. doi: 10.1016/0304-3940(80)90056-7. [DOI] [PubMed] [Google Scholar]


