Abstract
The default mode network is associated with senior cognitive functions in humans. In this study, we performed independent component analysis of blood oxygenation signals from 14 heroin users and 13 matched normal controls in the resting state through functional MRI scans. Results showed that the default mode network was significantly activated in the prefrontal lobe, posterior cingulated cortex and hippocampus of heroin users, and an enhanced activation signal was observed in the right inferior parietal lobule (P < 0.05, corrected for false discovery rate). Experimental findings indicate that the default mode network is altered in heroin users.
Keywords: heroin user, independent component analysis, functional MRI, resting state, default mode network, neural regeneration
Abbreviations
fMRI, functional MRI; DMN, default mode network
INTRODUCTION
Heroin addiction, a serious social and health problem, is defined as a chronic mental disorder characterized by enhanced and permanent saliency value for the drug, craving, loss of inhibitory control and compulsive drug intake[1,2]. It is a disorder that involves many systems in the brain, such as the memory and reward systems[3,4]. Studies using functional MRI (fMRI) have demonstrated that certain brain regions, including the posterior cingulate cortex and the ventral anterior cingulate cortex, consistently show greater activity during a resting state than during a tasking state. This network is commonly referred as the “default mode network” (DMN)[5,6]. According to leading hypotheses, deactivation of these areas could signal a reduction in inhibitory processes but also in the ability to redirect attentional processes from self-reflection to goal-directed behavior[7]. There is a vivid discussion concerning the role of DMN dysfunction in normal aging and in diseases, such as dementia, depression, or schizophrenia[8,9,10]. In heroin addiction, the DMN has been poorly investigated, although the functionality of the DMN might be specifically perturbed in this disease. Dysfunction of the reward system is a hallmark of heroin addiction. It is widely accepted that this leads to a relative functional disconnection of the limbic system loop, ultimately resulting in impaired modulation of reward system activity[11]. We predicted impaired deactivation of the DMN in heroin users relative to controls. To test this hypothesis, we used fMRI to examine brain activity in a group of 27 subjects under resting state. Independent component analysis was applied to compare the resting state data collected in heroin users and normal controls.
RESULTS
Quantitative analysis and baseline data of subjects
A total of 15 heroin users and 15 normal controls were selected in the experiment. One heroin user and two normal controls were lost because of head motion during MRI scanning. Thus, 14 heroin users and 13 normal controls were involved in the analysis of results. The clinical information of the heroin users and normal controls is summarized in Table 1.
Table 1.
Clinical information of heroin users and normal controls
DMN of heroin users and normal controls
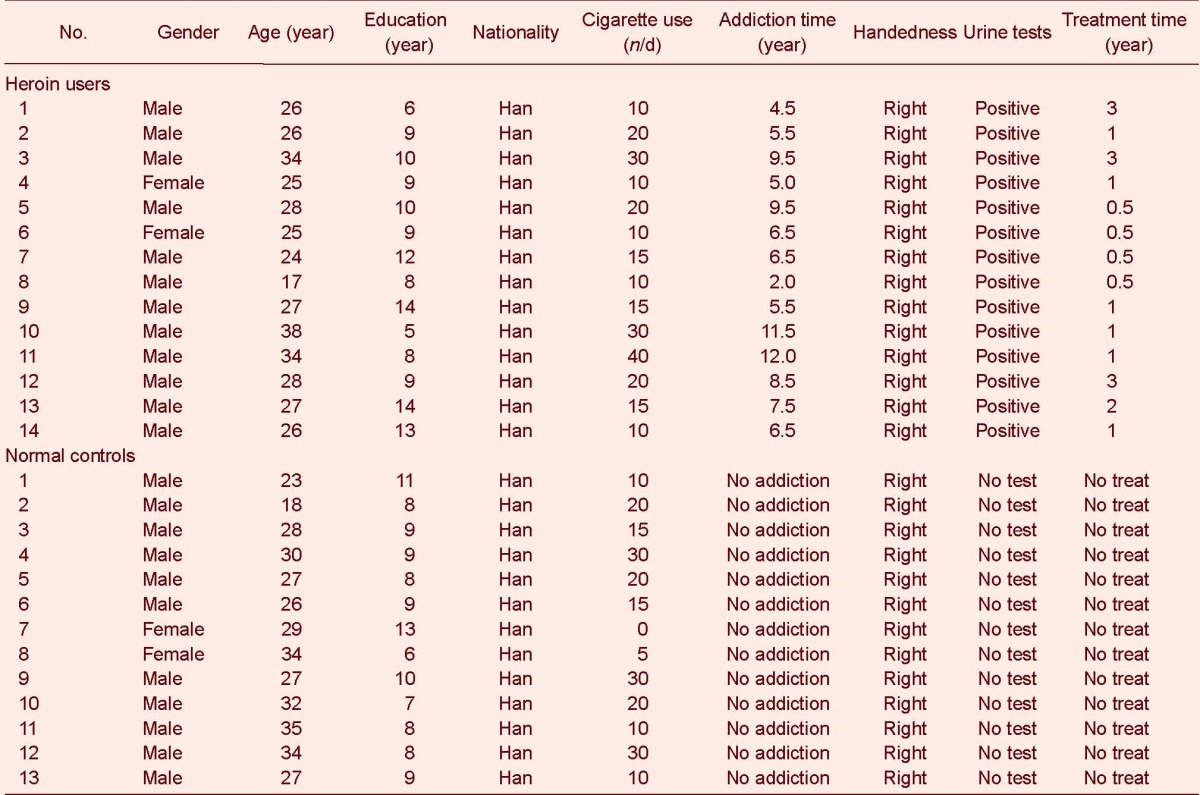
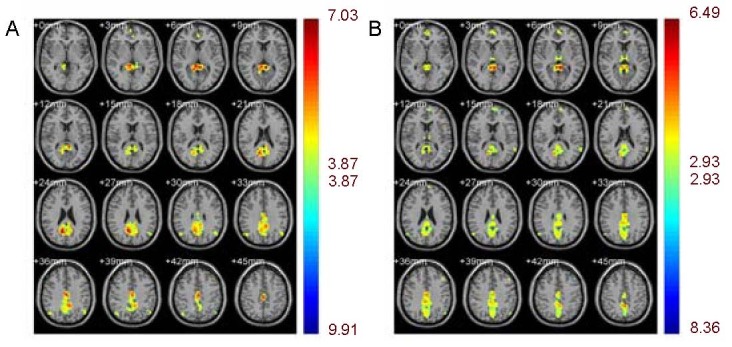
In the DMN, both heroin users and normal controls showed activation in the prefrontal cortex, posterior cingulated cortex, precuneus, lateral parietal lobe and medial temporal cortices (P < 0.05, corrected for false discovery rate; Figure 1).
Figure 1.
Default mode network of heroin users and normal controls.
Different colors represent varying degrees of activation. A cold color means negative activation while a warm color means positive activation.
(A) Normal controls showed activation in the prefrontal cortex, posterior cingulated cortex, precuneus, lateral parietal lobe and medial temporal cortices.
(B) Heroin users showed activation in the prefrontal cortex, posterior cingulated cortex, precuneus, lateral parietal lobe and medial temporal cortices.
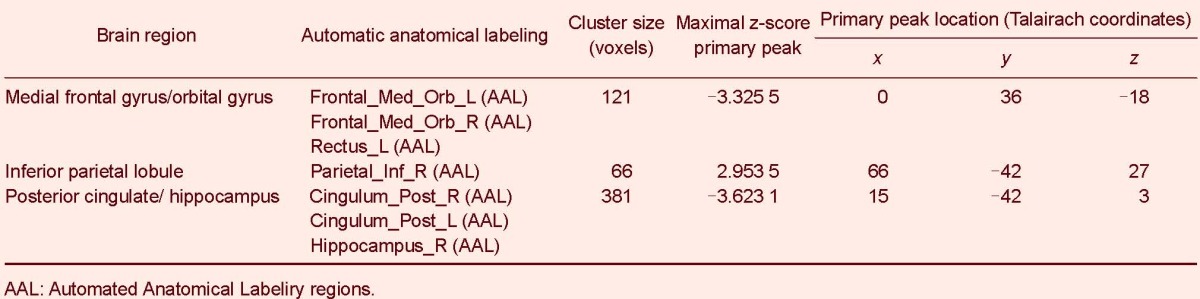
Heroin users, compared to normal controls, showed a decrease in the blood-oxygen level-dependent signal in the prefrontal cortex, posterior cingulated cortex and hippocampus. Concurrently, heroin users showed a significantly increased activation signal in the inferior parietal lobule (P < 0.05, corrected for false discovery rate; Figure 2 and Table 2).
Figure 2.
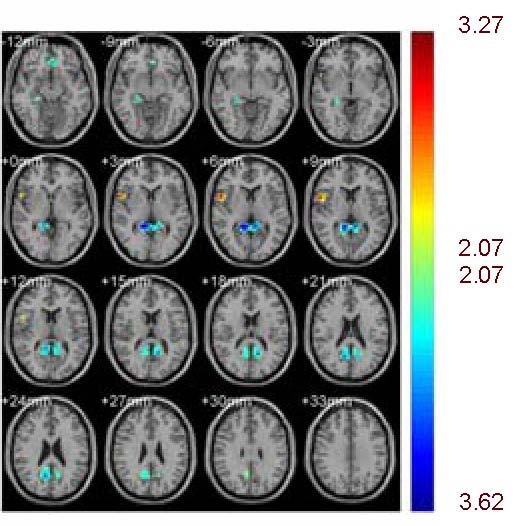
Heroin users showed a decrease in blood-oxygen level-dependent signal in the prefrontal cortex, posterior cingulated cortex, and hippocampus compared with normal controls.
Concurrently, heroin users showed a significantly increased activation signal in the inferior parietal lobule (P < 0.05, corrected for false discovery rate).
Different colors represent varying degrees of activation. A cold color means negative activation while a warm color means positive activation.
Table 2.
Changes in the default mode network in heroin users and normal controls
DISCUSSION
Following the observation that specific regions of the brain are consistently activated during the resting state and deactivated during engagement with a task, the DMN has been hypothesized to be an important system in the human brain. This network includes the posterior cingulate cortices, anterior cingulate cortices, medial and inferior parietal lobules, ventro-medial prefrontal cortex, and lateral temporal areas, which are all consistently deactivated during tasks relative to rest. Functionally, the DMN has been thought to govern spontaneous, stimulus-independent, self-referential, and social cognitive functions[12]. Similar analyses have also identified activity in the DMN in the anesthetized state, in coma, and even in slow-wave sleep[13]. Heroin use is associated with cognitive deficits and altered stimulus-independent performance[14]. There is little evidence regarding whether heroin users are also impaired in regional brain activation of the DMN. Thus, we used independent component analysis to identify differences in DMN activity between heroin users and normal controls. Heroin users showed a decrease in blood-oxygen level-dependent signal in the prefrontal lobe, posterior cingulated cortex and hippocampus compared to normal controls, as well as a significantly increased activation signal in the right inferior parietal lobule.
The function of the prefrontal lobe is complex, and contributes to cognitive processes such as inhibitory and attentional control, behavior monitoring and memory[15]. Drug dependence is commonly characterized as an affective or emotional phenomenon, given the central roles of psychological functions such as reward, reinforcement, craving, and stress[16]. Hedonic processes of liking and craving may be at the core of the motivation to consume drugs. However, certain cognitive processes such as memory likely contribute to these drives whereas others, such as impulse control, contribute to the individual's efforts to resist these drives[17,18]. Results of this study found dysfunction in the prefrontal lobe in the heroin users at rest. This means that the prefrontal lobe may be recruited at a lower level by DMN activity in drug addicts during the resting state, which may explain addiction-related weakened cognitive processes. The hippocampus plays an important role in learning and memory functions in the brain. Behavioral studies proposed that heroin addiction is a learning process, and the brain of heroin users creates new circuits in response to signaling induced by heroin[19,20]. In the DMN, hippocampal activity was less in heroin users than in normal controls. This result supported the conclusion of the behavioral studies. In this study, heroin users showed an increased activation signal in the inferior parietal lobule. The parietal lobule is part of the DMN[21].
In previous cognitive studies, reports about the function of parietal lobule were limited; most of the studies focused on memory and executive functions. The heroin users showed different activation signals in the parietal lobule, suggesting that DMN activity had changed with regard to memory and executive functions. However, future research is necessary to explore the exact reason for these changes[22].
In conclusion, heroin dependence is associated with DMN dysfunction in the cerebral cortex, and the changes in DMN activity in heroin users indicate disorders in several brain functions, especially in memory and cognitive processing.
SUBJECTS AND METHODS
Design
A functional neuroimaging, clinical research study.
Time and setting
The study was performed at the Anhui Provincial Hospital Affiliated to Anhui Medical University, China from September 2010 to July 2011.
Subjects
Heroin users
Heroin users were chronic heroin addicts recruited from the Anhui Detoxification and Rehabilitation Center (Hefei, Anhui Province, China). All participants filled in the survey questionnaires for baseline information of heroin addicts (supplementary Table 1 online), scale of heroin addiction (supplementary Table 2 online) and opiate withdrawal scale (Chinese version; supplementary Table 3 online).
Criteria for inclusion in the study: (1) Cases had a diagnosis of heroin dependence or abuse according to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); (2) Urine tests were positive for heroin before enrolling in the treatment program; (3) Cases aged from 16-45 years; (4) Chinese Han population; (5) Right-handed cases; (6) No history of head injury or psychiatric disorders; (7) No history of substance dependence (other than cigarette smoking and heroin); (8) The time of addiction was at least 2 years; (9) No contraindication for MR exam; (10) Under methadone treatment every day; (11) No illicit drug use during the treatment; (12) History of smoking.
Fifteen chronic heroin users were included in the study, including 13 males and 2 females; their age ranged from 18-35 years, with a mean age of 27.5 ± 3.2 years. The length of heroin use (from the time of their initial heroin use to the time of scanning) was 7.11 ± 2.82 years (ranging from 2-10 years), and the length of their education was 9.71 ± 2.7 years (ranging from 5-14 years).
Normal controls
Normal controls were volunteers recruited through advertisements in Anhui Medical University or Anhui Provincial Hospital, China.
Criteria for inclusion: (1) Aged from 16-45 years; (2) Chinese Han population; (3) Right handed; (4) No history of head injury; (5) No psychiatric disorders or history of substance dependence (other than cigarette smoking); (6) No contraindication for MR exam; (7) History of smoking.
Fifteen normal controls were included in the study, including 12 males and 3 females. Their ages ranged from 17–38 years, with a mean age of 28.6 ± 4.3 years, and the length of their education was 10.8 ± 1.6 years (ranging from 8–13 years).
The heroin users and normal controls were well matched in age and years of education (P > 0.05). Informed consent was obtained from all participants.
Methods
fMRI scanning and image preprocessing
The fMRI scan was performed on a Siemens MAGNETOM Trio 3T MRI scanner (Munich, Germany) within 24 hours after the subjects’ last methadone use. blood-oxygen level-dependent responses were measured with a T2-weighted single-shot gradient-echo echo planar imaging sequence (echo delay time/repetition time = 20/3 000 ms, 4 mm slice thickness, no gap, typically 33 coronal slices, 20 cm field of view, 64 × 64 matrix size, 90° flip angle, 200 kHz bandwidth with ramp sampling, 90 time points, 4 dummy scans). For the scan, all subjects were instructed to keep their eyes closed and think of nothing in particular. Padding was used to minimize head motion, which was inside the accepted threshold of 1 mm maximum displacement and 1.5° rotation as determined immediately after each run. A T1-weighted three- dimensional-modified driven equilibrium Fourier transform sequence was used for structural imaging; all MRI images were inspected to rule out gross morphological brain abnormalities (supplementary Figure 1 online).
Data analysis
Data were analyzed with statistical parametric mapping (SPM5; Wellcome Department of Imaging Neuroscience, University College, London, UK). First, images from the first ten TRs at the beginning of each trial were discarded for the signal to achieve steady-state equilibrium between radio frequency pulsing and relaxation. Images of each individual subject were realigned (motion-corrected) and corrected for slice timing. Second, a mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were co-registered with the high resolution structural image and then segmented for normalization to an Montreal Neurological Institute EPI template with affine registration followed by nonlinear transformation[23,24]. Finally, images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum, and band pass temporal filtering (0.01 Hz to 0.08 Hz) was performed on the residual signals. We used the Group Internal Carotid Artery fMRI Toolbox, version 2.0a (http://icatb.sourceforge.net) to analyze the fMRI data[25,26]. We first used a minimum description length algorithm to find the optimal number of spatially independent components[27]. The data were then further compressed with principal component analysis. Spatial internal carotid artery analysis was applied to this reduced data-set and performed on all of the subjects at once with the Infomax algorithm[28]. The internal carotid artery output consisted of series of spatial maps and their respective time courses.
Component selection
We were interested in the DMN. First, we discarded the components that were obvious artifacts. Second, we set a DMN model; this model includes the posterior and anterior cingulate cortices (Automated Anatomical Labeling regions 31, 32, 35, 36), medial and inferior parietal lobules (Automated Anatomical Labeling regions 61, 62), ventro-medial prefrontal cortex (Automated Anatomical Labeling regions 7, 8) and lateral temporal areas (Automated Anatomical Labeling regions 89, 90), which are all main regions in the DMN. Third, we compared each component spatial map with this model. Components that had a higher correlation with this model were considered meaningful activations and were kept. We qualitatively selected the component that best matched the model for future analysis.
Statistical analysis
Data were statistically analyzed with SPM5 software. In the first analysis, we constructed a one-sample t test each for normal controls and heroin users, and in the second analysis, we used a two-sample t test to compare normal controls and heroin users. The P value was set as 0.05, corrected for false discovery rate[29]. Brain regions were identified using Automated Anatomical Labeling regions[30]. All voxel activations are presented in MNI coordinates, and the functional results were shown on a CH2 structure model.
Acknowledgments
We would like to express our sincere thanks to Hao Zhang from Anhui Detoxification and Rehabilitation Center, Hefei, China for help with sample and data collection.
Footnotes
Funding: This study was financially sponsored by a grant from the National Natural Science Foundation of China, No. 30973084-C160801, C010604 and the Natural Science Foundation of Anhui Province, No. 11040606M167.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Ethics Committee of Provincial Hospital Affiliated to Anhui Medical University, Hefei, China
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Jia SW, He YS, Miao YW/Yang Y/Wang L)
REFERENCES
- [1].Sell LA, Morris J, Bearn J, et al. Activation of reward circuitry in human opiate addicts. Eur J Neurosci. 1999;11(3):1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- [2].Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- [3].Wrase J, Schlagenhauf F, Kienast T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- [4].Wang ZX, Zhang JX, Wu QL, et al. Alterations in the processing of non-drug-related affective stimuli in abstinent heroin addicts. Neuroimage. 2010;49(1):971–976. doi: 10.1016/j.neuroimage.2009.08.020. [DOI] [PubMed] [Google Scholar]
- [5].Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- [7].Mazoyer B, Zago L, Mellet E, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- [8].Sorg C, Riedl V, Mühlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Calhoun VD, Sui J, Kiehl K, et al. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2011;2:75. doi: 10.3389/fpsyt.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- [12].Mason MF, Norton MI, Van Horn JD, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- [14].Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162(8):1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- [15].D’Esposito M, Aguirre GK, Zarahn E, et al. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- [16].Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32(3):581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Van den Oever MC, Spijker S, Smit AB, et al. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35(2):276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- [18].Goldstein RZ, Alia-Klein N, Tomasi D, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164(1):43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Franken IH, de Haan HA, van der Meer CW, et al. Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. J Subst Abuse Treat. 1999;16(1):81–85. doi: 10.1016/s0740-5472(98)00004-x. [DOI] [PubMed] [Google Scholar]
- [20].Lu L, Xu NJ, Ge X, et al. Reactivation of morphine conditioned place preference by drug priming: role of environmental cues and sensitization. Psychopharmacology (Berl) 2002;159(2):125–132. doi: 10.1007/s002130100885. [DOI] [PubMed] [Google Scholar]
- [21].Laufs H, Hamandi K, Salek-Haddadi A, et al. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp. 2007;28(10):1023–1032. doi: 10.1002/hbm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mazoyer B, Zago L, Mellet E, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- [23].Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- [24].Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- [25].McKeown MJ, Makeig S, Brown GG, et al. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Arbabshirani MR, Calhoun VD. Functional network connectivity during rest and task: comparison of healthy controls and schizophrenic patients. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:4418–4421. doi: 10.1109/IEMBS.2011.6091096. [DOI] [PubMed] [Google Scholar]
- [27].Lee K, Tak S, Ye JC. A data-driven sparse GLM for fMRI analysis using sparse dictionary learning with MDL criterion. IEEE Trans Med Imaging. 2011;30(5):1076–1089. doi: 10.1109/TMI.2010.2097275. [DOI] [PubMed] [Google Scholar]
- [28].Lulham A, Bogacz R, Vogt S, et al. An Infomax algorithm can perform both familiarity discrimination and feature extraction in a single network. Neural Comput. 2011;23(4):909–926. doi: 10.1162/NECO_a_00097. [DOI] [PubMed] [Google Scholar]
- [29].Schwartzman A, Dougherty RF, Lee J, et al. Empirical null and false discovery rate analysis in neuroimaging. Neuroimage. 2009;44(1):71–82. doi: 10.1016/j.neuroimage.2008.04.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]


