Abstract
Atherosclerosis plays an important role in ischemic stroke, and oxidative stress participates in the entire process of atherosclerosis. Glutathione S-transferase (GST) acting with other antioxidant enzymes can eliminate reactive oxygen species and protect cells against oxidative damage. To assess the association of glutathione S-transferase (GSTT1 and GSTM1) gene polymorphisms with ischemic stroke in the Chinese Han population, the present study selected 315 patients with ischemic stroke and 210 healthy controls for comparison. GSTT1 and GSTM1 genotypes were determined using polymerase chain reactions, electrophoresis and imaging analysis. No obvious evidence of GSTT1-null, GSTM1-null and GSTT1/GSTM1-double null genotype distribution differences was found between case and control groups or between genders. Subgroup analysis showed that the risk of stroke was increased when hypertension was accompanied by GSTT1-null (odds ratio (OR) = 2.996, P < 0.001) and GSTM1-null (OR = 3.680, P < 0.001) genotypes; diabetes mellitus was accompanied by GSTT1-null (OR = 1.860, P = 0.031) and GSTM1-null (OR = 2.444, P = 0.002) genotypes, and smokers showed a GSTT1-null genotype (OR = 2.276, P = 0.003). GSTT1- and GSTM1-null genotypes may interact synergistically with hypertension, diabetes mellitus and smoking to increase the incidence risk of ischemic stroke.
Keywords: glutathione S-transferase, GSTT1, GSTM1, gene polymorphism, ischemic stroke, risk factors, stroke, neural regeneration
Abbreviations
AS, atherosclerosis; OS, oxidative stress; GST, glutathione S-transferase
INTRODUCTION
Atherosclerosis (AS) is the pathological foundation of ischemic stroke and its promotion is due to structural and functional injury of endothelial cells. Under a normal condition, oxidative and anti-oxidative status is maintained as a balance in the human body. Oxidative stress (OS) appears in situations of excess reactive oxygen species and functional reduction of the anti-oxidative defense system[1]. Free radicals and harmful substances, which are produced during this process, can lead to lipid peroxidation of the cell membrane, damage to proteins and DNA, and acceleration of AS advancement.
OS is involved in the development of many diseases including diabetes mellitus, some cancer types and AS[2]. The correlation between glutathione S-transferases (GSTs) and a variety of diseases has been investigated[3,4,5,6,7]. Evidence[8,9,10] indicates that GSTT1 (–) increases the risk of Meningioma and bladder cancer among smokers, and GSTM1 (–) is associated with susceptibility to inflammation and smoking-related cancers. Several studies[11,12,13] regarding the association of GSTT1- and GSTM1-null genotypes with coronary artery disease have been conducted. Only one study[14] did not discover any apparent differences in GSTT1 and GSTM1 gene polymorphisms between ischemic stroke and control groups of Caucasians. The correlation between GSTT1 and GSTM1 gene polymorphisms, and ischemic stroke remains poorly understood in the Chinese population. In the present study, we investigated GSTT1 and GSTM1 gene polymorphisms to assess their association with ischemic stroke in the Chinese Han population.
RESULTS
General clinical data of subjects
Due to incomplete information and population matching, 10 ischemic stroke patients and 17 controls were excluded. A total of 525 individuals were recruited in the final analysis consisting of 315 ischemic stroke patients and 210 controls. The general clinical features and some stroke risk factors of case and control group subjects are listed in Table 1.
Table 1.
General clinical information of patients and controls
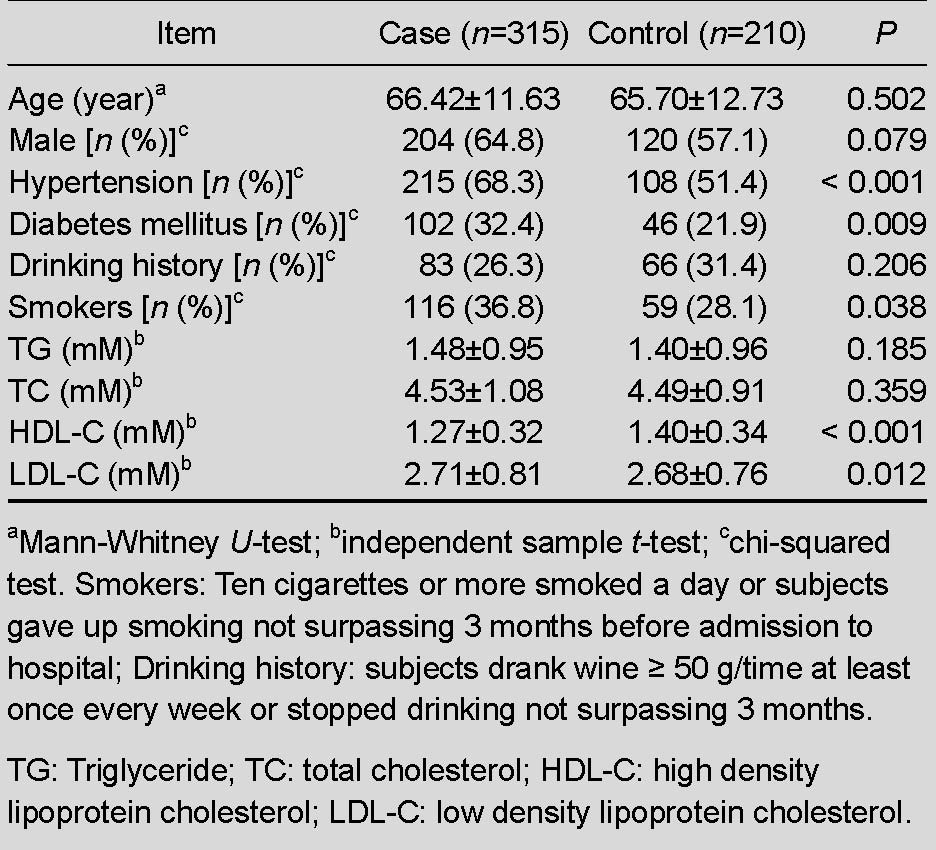
Differences of age and gender between groups were not significant, which indicated that the included subjects were matched. There were no significant differences in triglycerides and total cholesterol between groups. The case group exhibited a higher level of low-density lipoprotein, and lower levels of high-density lipoprotein than the control group (P < 0.05). As expected, the case group exhibited a greater frequency of hypertension, diabetes mellitus and smokers than the control group (OR > 1, P < 0.05).
GSTT1 and GSTM1 genotype detection
PCR and electrophoresis were used for gene amplification and genotyping. The results are shown in Figure 1.
Figure 1.
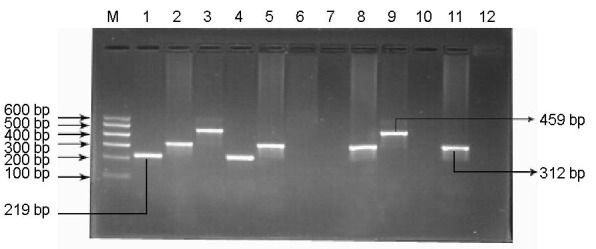
Electrophoretogram of GSTT1 and GSTM1 gene amplification products.
M: Marker (100–600 bp); lanes 1–3 represent sample “a” (GSTM1+/GSTT1+), lanes 4–6 represent sample “b” (GSTM1+/GSTT1–), lanes 7–9 represent sample “c” (GSTM1–/GSTT1+), lanes 10–12 represent sample “d” (GSTM1–/GSTT1–). GSTM1(+) and GSTM1(+/–) were detected as a fragment at 219 bp, CYP1A1 exon7 was the fragment at 312 bp, GSTT1 (+) and GSTT1(+/-) was the fragment at 459 bp; GSTM1 (–) and GSTT1 (–) were detected as no corresponding fragment. GST: Glutathione S-transferase.
Heterozygous deletion and the present genotype could not be distinguished in the electrophoretogram.
GSTT1 and GSTM1 genotype frequency distribution of participants
No significant differences were found for each genotype distribution between the two groups (Table 2).
Table 2.
Genotype frequencies [n (%)] of GSTT1 and GSTM1 in case and control groups
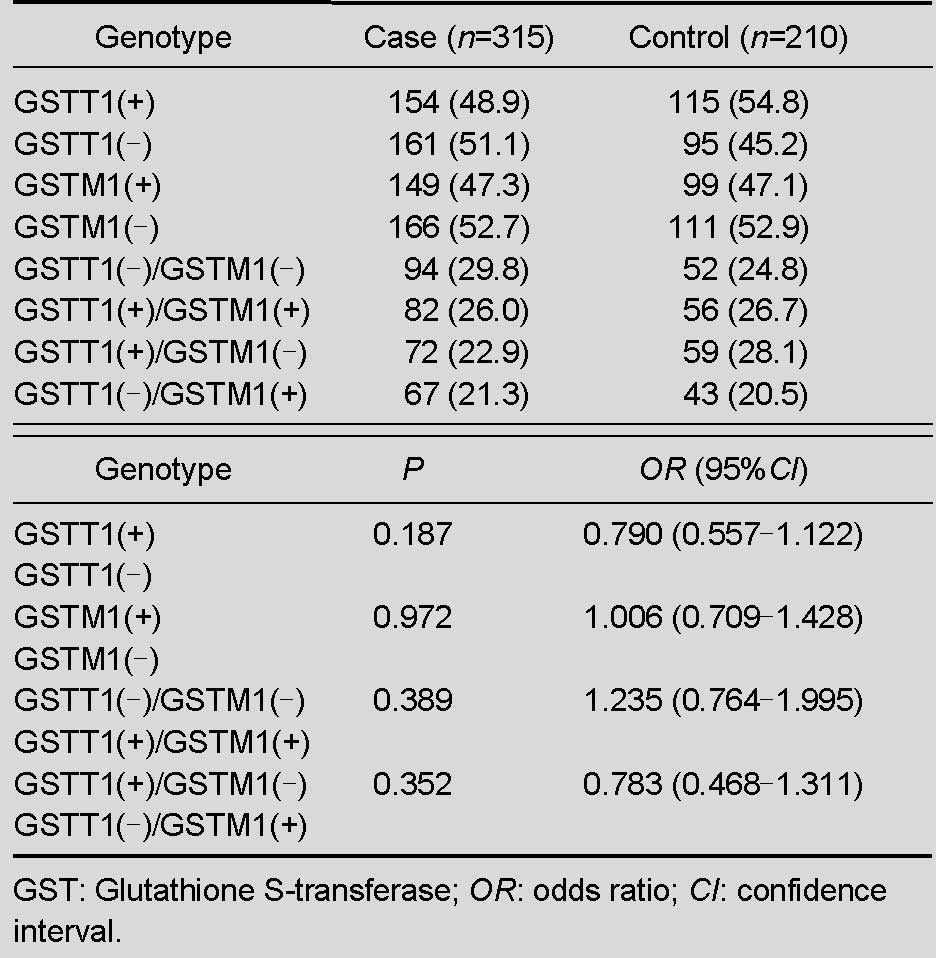
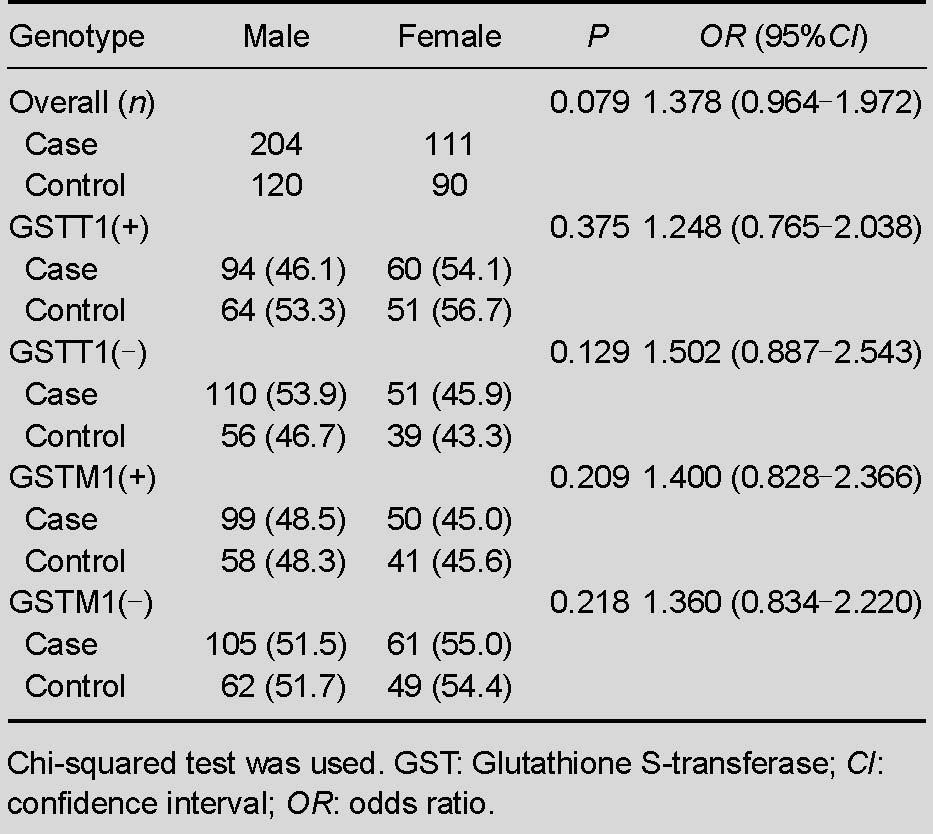
Because heterozygous genotypes could not be distinguished from the present genotype in electrophoreograms, we were unable to determine whether these genotypes were in Hardy-Weinberg equilibrium. Randomization and a large number of subjects were involved in the present research to maintain the equilibrium. Results showed that the frequency of GSTT1 (–) was 51.1% in the case group and 45.2% in the control group, and 52.7% and 52.9% for GSTM1 in case and control groups, respectively. Compared with the control group (24.8%), both null genotypes of GSTT1 and GSTM1 were slightly higher in the case group (29.8%). In addition, each genotype distribution of different genders between the two groups is shown in Table 3 and no differences were significant (P > 0.05).
Table 3.
Genotype frequencies [n (%)] of GSTT1 and GSTM1 among genders between case and control groups
Correlation analysis of GSTT1 and GSTM1 genotypes
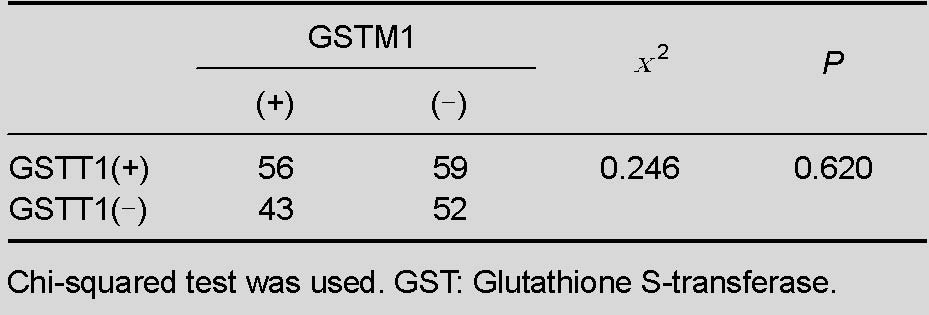
Combined expression of GSTT1 and GSTM1 genes was used to confirm if there was a correlation between GSTT1 and GSTM1 genotypes among the control group. As shown in Table 4, the correlation analysis result was (χ2 = 0.246, P = 0.620), indicating no association between the two genes.
Table 4.
Interaction analysis of GSTT1 and GSTM1 genotypes
Genotype frequency distribution in subgroups
Subgroups were established according to the risk factors of ischemic stroke for genotype analysis among subgroups (Table 5).
Table 5.
Genotype analysis [n (%)] of GSTT1 and GSTM1 in subgroups according to stroke risk factors
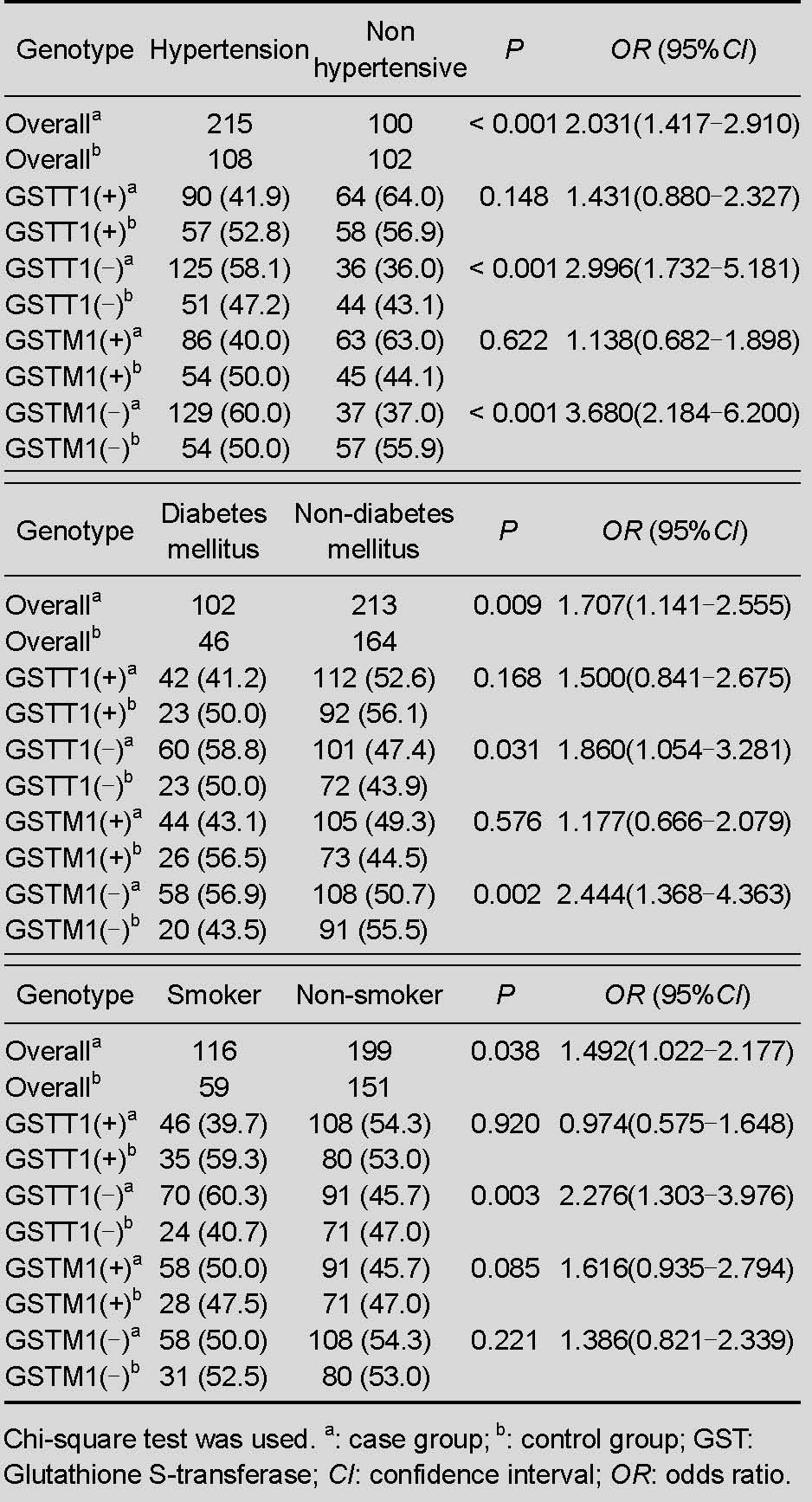
The proportion of hypertensive stroke patients to hypertensive controls compared with the proportion of normotensive stroke patients to normotensive controls was 2.996 (P < 0.001) when subjects had a GSTT1-null genotype. For the GSTM1-null genotype, the proportion was 3.68 (P < 0.001) when hypertensive (stroke/control) subjects were compared with normotensive (stroke/control) subjects.
Similarly, in the diabetes mellitus subgroup, the proportion of diabetic stroke patients to diabetic controls compared with the proportion of non-diabetic stroke patients to non-diabetic controls was 1.86 (P = 0.031) when subjects had a GSTT1-null genotype, and 2.444 (P = 0.002) for diabetes mellitus with a GSTM-null genotype. The proportion was 2.276 (P = 0.003) for smokers with the GSTT1-null genotype. The remaining genotype frequencies were not significantly different in each subgroup.
Logistic regression results of high risk factors for ischemic stroke
Logistic regression analysis was used to assess the association between risk factors and ischemic stroke. Age, lipid parameters, smoking history, hypertension and diabetes mellitus were attributed to the variables. Results revealed that together with increased age every 10 years, the odds ratio of stroke risk was 1.34. Smoking history, hypertension, diabetes mellitus and increased low density lipoprotein-cholesterol were also confirmed as susceptibility factors for ischemic stroke and the risk of stroke increased by 1.566-, 1.707-, 1.621- and 2.313-fold, respectively.
DISCUSSION
As major factors in cardiovascular and cerebrovascular diseases, OS and inflammation are emerging as a unifying mechanism of cardiovascular diseases[15]. OS selectively induces the expression of inflammation-related genes, which is a common mechanism of traditional risk factors leading to AS[16], implying a close relationship between OS and AS. Under the action of damaging factors, cells release a massive amount of reactive oxygen species, leading to OS. OS promotes the formation and breakage of atherosclerosis plaque and triggers ischemic stroke.
Various cellular detoxification systems and defense mechanisms against OS exist in the human body. The intracellular and extracellular redox equilibrium status is adjusted by small molecular antioxidants and antioxidases[17]. GSTs are a super family of polymorphic isozymes that operate in Phase II detoxification and antioxidant function by promoting the combination of glutathione and many kinds of electrophiles and OS products. Glutathione eliminates reactive oxygen species and is transformed into the oxidized form (GSSG). The ratio of glutathione and GSSG has been considered as an index of lipid peroxidation injury. At least eight classes of GSTs exist in the human body (Alpha, Mu, Kappa, Omega, Pi, Sigma, Theta and Zeta)[18], and each class includes several isoenzymes. Members of the GSTs use various products of OS as substrates to protect cells against oxidative damage[19]. As a result of gene polymorphisms in GSTs, the function of detoxification and the susceptibility to OS damage are different[20]. GSTT1 belongs to the Theta type, and GSTM1 belongs to Mu. GSTT1 and GSTM1 genes are mapped to chromosomes 22q11.2 and 1p13.3, respectively. GSTT1 uses oxidized lipids, and GSTM1 has an affinity for epoxide. Both gene polymorphisms consist of entire gene deletions. Subjects with at least one functional allele for GSTM1 or GSTT1 are grouped into the positive genotypes. The homozygous deletion genotype loses the function of the encoded isozymes and is grouped into the null genotype that increases susceptibility to OS.
Previous research shows that the frequencies of GSTT1- and GSTM1-null genotypes vary from 13% to 26% and 42% to 60% in Caucasians, respectively[21]. Another study[22] showed that frequencies of the GSTT1-null (48.6%) and GSTM1-null (59%) genotypes in the normal Chinese population that is not completely similar to the Caucasian population due to regional and racial differences of the included participants. According to a Turkish study[14], the percentage of GSTT1- and GSTM1-null genotypes were 19.8% and 50.6% in their ischemic stroke group, and 21% and 56.2% in the control group, respectively. Results from the present study revealed that the frequency of the GSTT1-null genotype was 51.1% in the ischemic stroke group and 45.2% in the control group. For the GSTM1-null genotype, the case group (52.7%) had a similar percentage to that of the control group (52.9%). No significant differences were found in the frequency of GSTT1- and GSTM1-null genotypes between the two groups, which was consistent with a previous study[14]. The present study possibly revealed that GSTT1- and GSTM1-null genotypes are not associated with ischemic stroke risk in the Chinese Han population. The differences of genotype distribution between genders were not significant between the two groups and indicated that the ischemic stroke risk between genders was not different between the two groups in the same genotype, because the two genes are mapped to common chromosomes and are not associated with sex-linked inheritance, which is consistent with a previous study[22]. The combined expression of the two genes was detected (Table 4) and interaction analysis results among the various genotypes was determined by a chi-squared test, which indicates that GSTT1 and GSTM1 are mutually independent. This result is consistent with previous research of the normal Chinese population[22].
Subgroup analyses were subsequently conducted according to traditional risk factors. Capoluongo et al[23] observed that GSTM1 (–) increases the risk of hypertension, but no connection was found between GSTT1 (–) and hypertension. According to another study[24], both GSTT1- and GSTM1-null genotypes might be a genetic factor to predict the development of essential hypertension and permit early therapeutic intervention in the Egyptian population. In the present study, the risk of ischemic stroke was 2.996 times in hypertension subjects with GSTT1 (–), compared with that in non-hypertension subjects with the same genotype. Similarly, hypertension subjects with GSTM1 (–) had a 3.68 times higher risk of stroke than that of non-hypertension subjects with the same genotype. Gene deletion reduces GST activity, and the fact that these patients present lower GST activity might be a susceptibility factor for OS injury. Furthermore, OS might be associated with the occurrence of hypertension[25], a traditional risk factor for ischemic stroke. This observation indicates that the null genotype possibly leads to hypertension and indirectly increases the risk of ischemic stroke or hypertension together with GSTT1 (–). Moreover, GSTM1 (–) plays a synergistic role in the incidence of ischemic stroke, which is consistent with a related study[14].
Bid et al[26] observed that GSTM1 (–) is a genetic marker to predict the incidence of type 2 diabetes mellitus. Some researches[20,27] reported that the vGSTT1 genotype reduces the incidence rate of type 2 diabetes mellitus and might be a protective factor. According to another study[28], GSTT1 (–) and GSTT1 (–)/GSTM1 (–) are independent risk factors of type 2 diabetes mellitus for Japanese people. Results from the present study showed that diabetes mellitus together with GSTT1- and GSTM1-null genotypes showed a 1.86-fold and 2.444-fold increased risk of ischemic stroke, compared with that of non-diabetes mellitus subjects with the same genotype. β-cells are very sensitive to OS damage because they express very few antioxidant enzymes[29]. The null genotype has a decreased capability to detoxify some carcinogens and oxygen metabolites. The accumulation of free radicals raises the OS status and plays a key role in the formation and progression of diabetes mellitus, a risk factor for ischemic stroke, while increasing reactive oxygen species are produced in diabetic patients. This observation indicates that diabetes mellitus with gene deletion potentially increases the risk of ischemic stroke, which is inconsistent with previous research[14]. The reasons might be related to the differences of selected criterion, race and subjects sampled.
Endothelial cell dysfunction, which is caused by the accumulation of reactive oxygen species and vascular inflammation, is the cause of cardiovascular diseases[30,31]. Subjects lacking GSTT1/GSTM1 genotypes have a higher incidence of inflammation and OS compared with those with the genotype present. The association of GSTT1 and GSTM1 gene polymorphisms with cardiovascular diseases has been investigated by some studies[11,12,13]. One study[32] demonstrated that smokers with GSTM1 (–) show a 1.5-fold higher risk of cardiovascular diseases, relative to that of smokers with GSTM (+), and a 2-fold higher risk relative to that of non-smokers with GSTM1 (–). Smokers with GSTT1 (+) show a 4-fold increased risk of cardiovascular diseases, compared with that of non-smokers with GSTT1 (+). Masetti et al[33] confirmed that smokers with GSTT1/GSTM1-null genotypes have a 4-fold increased risk of cardiovascular diseases, compared with that of non-smokers with GSTT1/GSTM1 genotypes. In contrast, GSTT1 (–) reduces the incidence rate of atherosclerotic disease[34]. A common pathological foundation (AS) exists in coronary diseases and ischemic stroke. Therefore, in the present study, we compared genotype distribution among subgroups according to smoking history. Compared with non-smokers with a GSTT1-null genotype, the risk of ischemic stroke was 2.276-fold in smokers with the corresponding genotype. This result is not completely consistent with related research[14]. The reasons might due to the differences of race, kinds of cigarette and severity of smoking. The combination of the null genotype and increased OS caused by smoking would result in a high risk of inflammation and injury of endothelial cells, furthermore leading to atherosclerotic diseases.
The present study analyzed the correlation between gene polymorphisms of GSTT1 and GSTM1, and ischemic stroke risk in the Chinese Han population for the first time. Results were not consistent with previous research[14], because of differences in the selection criteria of subjects, region, race, habits and customs of the participants. However, there were also some limitations. We did not assess the genotype frequency of different types of ischemic stroke, which are based on the classification standard of the Trial of Org 10172 in Acute Stroke Treatment, and we failed to analyze the genotype frequency difference between anterior and posterior circulation ischemic stroke.
In conclusion, there are no significant differences in the genotype distributions of GSTT1 and GSTM1 between ischemic stroke and control groups in the Chinese Han population. GSTT1- and GSTM1-null genotypes may interact synergistically with hypertension and/or diabetes mellitus to increase the risk of ischemic stroke, and smoking together with the GSTT1-null genotype plays a possible synergistic role in the incidence of ischemic stroke. However, a larger sample investigation is required to confirm these results.
SUBJECTS AND METHODS
Design
A case control study of gene polymorphism.
Time and setting
This study was performed in Qingdao, Shandong province, China, from October 2010 to November 2011.
Subjects
Case group
A total of 315 ischemic stroke patients were selected as the case group, who were admitted to the Department of Neurology, Affiliated Hospital of Qingdao University Medical College from March 2010 to June 2011. Subjects consisted of 204 males, 111 females, aged 66.4 ± 11.6 years. Patients were diagnosed according to the diagnostic standards for cerebrovascular diseases in the Fourth Academic Conference of Cerebrovascular Diseases, issued by the China Medical Association in 1995. Lesions were confirmed by cranial CT or MRI scans. Individuals were excluded based on tumors, autoimmune disease, serious infection, heart valve disease, atrial fibrillation, hematological disease, liver and kidney dysfunction, heart dysfunction, surgery or trauma history in the previous 4 weeks, the type of cerebral infarction resulting from arteritis, aneurism or malformation of the cerebral artery and youth stroke. Cerebral embolism of the heart source was eliminated by cardiac ultrasound and dynamic electrocardiogram.
Control group
The control group (210 subjects) selected from the population of the same region, underwent daily physical examination and consisted of 120 males and 90 females, aged 65.70 ± 12.73 years. Subjects did not have previous transient ischemic attack or an ischemic stroke history and common information was recorded in detail.
Methods
Definition of index
Systolic pressure ≥ 140 mm Hg (18.7 kPa) and/or diastolic pressure ≥ 90 mm Hg (12.0 kPa) or taking drugs were considered as hypertension. The criterion of diabetes mellitus was consistent with the diagnostic standard formulated by the World Health Organization in 1999[35]. Hyperlipemia agreed with the Cardiovascular Branch of the Chinese Medical Association on the standard for high blood fats[36]. The following criteria were considered for the smoking group: 10 or more cigarettes smoked a day or subjects gave up smoking not surpassing 3 months before admission to hospital. Drinking history: subjects drank wine ≥ 50 g/time at least once a week or stopped drinking not surpassing 3 months.
DNA extraction
Genomic DNA was isolated from elbow vein blood leukocytes by a genomic DNA extraction kit (DP319-01; Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer's instructions. The absorbance value was measured by a spectrophotometer to normalize the DNA concentration and calculate the amount of DNA for PCR.
PCR amplification, electrophoresis and genotyping
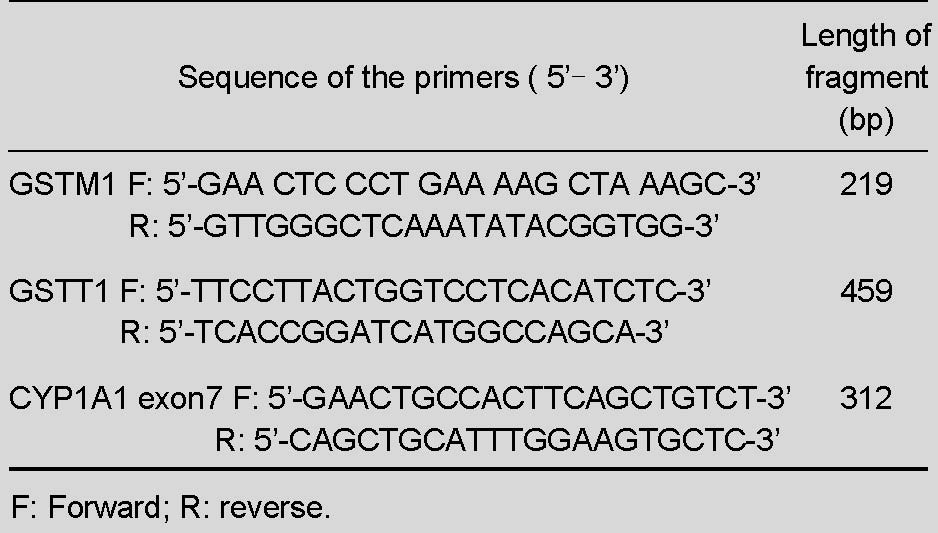
The region of DNA containing GSTT1 and GSTM1 polymorphisms was amplified by PCR using conditions similar to those of previous reports[14], except for the annealing temperature (63°C for 45 seconds). According to a previously published report[14], three pairs of primers were synthesized by Sangon Biotech (Shanghai, China) and are listed in Table 6.
Table 6.
Nucleotide sequences of the three primer pairs
Amplified fragments were detected on 2% agarose gels. The genotype or heterozygous deletion genotype of GSTT1 was detected as a fragment of 459 bp, and for GSTM1, as a fragment of 219 bp. As an internal control, CYP1A1 exon7 was detected as a fragment of 312 bp. When GSTT1 or GSTM1 was the homozygous deletion genotype, no corresponding electrophoretic bands appeared. As a result of no PCR products, routine sequencing technology could not test the sequences of homozygous deletion genotypes. Samples with the genotypes present were selected to test (performed by Sangon Biotech of Shanghai, China) and the amplified DNA fragment was consistent with the sequence found in the National Center of Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/pubmed/).
The sequences are as follows:
GSTT1 (459 bp):
TTC CTT AAC TGT CCT CAC ATC TCC TTA GCT GAC CTC GTA GCC ATC ACG GAG CTG ATG CAT GTG AGT GCT GTG GGC AGG TGA ACC CAC TAG GCA GGG GGC CCT GGC TAG TTG CTG AAG TCC TGC TTA TGC TGC CAC ACC GGG CTA TGG CAC TGT GCT TAA GTG TGT GTG CAA ACA CCT CCT GGA GAT CTG TGG TCC CCA AAT CAG ATG CTG CCC ATC CCT GCC CTC ACA ACC ATC CAT CCC CAG TCT GTA CCC TTT TCC CCA CAG CCC GTG GGT GCT GGC TGC CAA GTC TTC GAA GGC CGA CCC AAG CTG GCC ACA TGG CGG CAG CGC GTG GAG GCA GCA GTG GGG GAG GAC CTC TTC CAG GAG GCC CAT GAG GTC ATT CTG AAG GCC AAG GAC TTC CCA CCT GCA GAC CCC ACC ATA AAG CAG AAG CTG ATG CCC TGG GTG CTG GCC ATG ATC CGG TGA
GSTM1 (219 bp):
GAA CTC CCT GAA AAG CTA AAG CTC TAC TCA GAG TTT CTG GGG AAG CGG CCA TGG TTT GCA GGA AAC AAG GTA AAG GAG GAG TGA TAT GGG GAA TGA GAT CTG TTT TGC TTC ACG TGT TAT GGA GGT TCC AGC CCA CAT ATT CTT GGC CTT CTG CAG ATC ACT TTT GTA GAT TTT CTC GTC TAT GAT GTC CTT GAC CTC CAC CGT ATA TTT GAG CCC AAC
Statistical analysis
Statistical analysis was performed by SPSS 17.0 software (SPSS Inc, Chicago, IL, USA). Data were expressed as the mean ± SD (continuous variables) or by the concrete value and the percentage (categorical variables). The independent sample t-test was applied to compare the continuous variable in normal distribution data and the non-parameter test (Mann-Whitney U-test) for skewed data. A chi-squared test was used to assess genotype frequencies between case and control groups. An odds ratio was used to evaluate the affect of genotype on ischemic stroke risk and a 95% confidence interval was calculated. Statistical significance was considered at P < 0.05. Logistic regression analysis with the backward stepping method was performed to analyze the association of high risk factors and ischemic stroke.
Acknowledgments
We thank all the included subjects and their families for cooperating in this investigation. We also thank the reagent providers for assistance with DNA extraction, PCR and sequencing.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Institutional Review Board of Qingdao Medical College, Shandong Province, China. Informed consent was obtained from all subjects.
(Edited by Liu SG, Li JS/Su LL/Song LP)
REFERENCES
- [1].Rodrigo R, Prat H, Passalacqua W, et al. Relationship between oxidative stress and essential hypertension. Hypertens Res. 2007;30(12):1159–1167. doi: 10.1291/hypres.30.1159. [DOI] [PubMed] [Google Scholar]
- [2].Lyons TJ. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes? Diabet Med. 1991;8(5):411–419. doi: 10.1111/j.1464-5491.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- [3].Yalin S, Hatungil R, Tamer L, et al. Glutathione S-transferase gene polymorphisms in Turkish patients with diabetes mellitus. Cell Biochem Funct. 2007;25(5):509–513. doi: 10.1002/cbf.1339. [DOI] [PubMed] [Google Scholar]
- [4].Singh M, Shah PP, Singh AP, et al. Association of genetic polymorphisms in glutathione S-transferases and susceptibility to head and neck cancer. Mutat Res. 2008;638(1-2):184–194. doi: 10.1016/j.mrfmmm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- [5].Oniki K, Hori M, Takata K, et al. Association between glutathione S-transferase A1, M1 and T1 polymorphisms and hypertension. Pharmacogenet Genomics. 2008;18(3):275–277. doi: 10.1097/FPC.0b013e3282f56176. [DOI] [PubMed] [Google Scholar]
- [6].Kiyohara C, Miyake Y, Koyanagi M, et al. GST polymorphisms, interaction with smoking and pesticide use, and risk for Parkinson's disease in a Japanese population. Parkinsonism Relat Disord. 2010;16(7):447–452. doi: 10.1016/j.parkreldis.2010.04.009. [DOI] [PubMed] [Google Scholar]
- [7].Keenan BT, Chibnik LB, Cui J, et al. Effect of interactions of glutathione S-transferase T1, M1, and P1 and HMOX1 gene promoter polymorphisms with heavy smoking on the risk of rheumatoid arthritis. Arthritis Rheum. 2010;62(11):3196–3210. doi: 10.1002/art.27639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rebbeck TR. Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev. 1997;6(9):733–743. [PubMed] [Google Scholar]
- [9].Brockmoller J, Cascorbi I, Kerb R. Combined analysis of inherited polymorphisms in arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1, microsomal epoxide hydrolase, and cytochrome P450 enzymes as modulators of bladder cancer risk. Cancer Res. 1996;56(17):3915–3925. [PubMed] [Google Scholar]
- [10].Elexpuru-Camiruaga J, Buxton N, Kandula V, et al. Susceptibility to astrocytoma and meningioma: influence of allelism at glutathione S-transferase (GSTT1 and GSTM1) and cytochrome P-450 (CYP2D6) loci. Cancer Res. 1995;55(19):4237–4239. [PubMed] [Google Scholar]
- [11].Taspinar M, Aydos S, Sakiragaoglu O, et al. Impact of genetic variations of the CYP1A1, GSTT1, and GSTM1 genes on the risk of coronary artery disease. DNA Cell Biol. 2012;31(2):211–218. doi: 10.1089/dna.2011.1252. [DOI] [PubMed] [Google Scholar]
- [12].Nomani H, Mozafari H, Ghobadloo SM, et al. The association between GSTT1, M1, and P1 polymorphisms with coronary artery disease in Western Iran. Mol Cell Biochem. 2011;354(1-2):181–187. doi: 10.1007/s11010-011-0817-2. [DOI] [PubMed] [Google Scholar]
- [13].Wang J, Zou L, Huang S, et al. Genetic polymorphisms of glutathione S-transferase genes GSTM1, GSTT1 and risk of coronary heart disease. Mutagenesis. 2010;25(4):365–369. doi: 10.1093/mutage/geq014. [DOI] [PubMed] [Google Scholar]
- [14].Turkanoglu A, Can Demirdogen B, Orhan A, et al. Association analysis of GSTT1, GSTM1 genotype polymorphisms and serum total GST activity with ischemic stroke risk. Neurol Sci. 2010;31(6):727–734. doi: 10.1007/s10072-010-0330-5. [DOI] [PubMed] [Google Scholar]
- [15].Ridker PM, Brown NJ, Vaughan DE, et al. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109(25)(Suppl 1):IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- [16].Alexander RW. Hypertension and the pathogenesis of atherosclerosis oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25(2):155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- [17].Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic Biol Med. 2009;47(12):1673–1706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- [19].Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation. 1997;96(9):3243–3247. doi: 10.1161/01.cir.96.9.3243. [DOI] [PubMed] [Google Scholar]
- [20].Wang GY, Zhang L, Li QF. Genetic polymorphisms of GSTT1, GSTM1, and NQO1 genes and diabetes mellitus risk in Chinese population. Biochem Biophys Res Commun. 2006;341(2):310–313. doi: 10.1016/j.bbrc.2005.12.195. [DOI] [PubMed] [Google Scholar]
- [21].Garte S, Gaspari L, Alexandrie AK, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1239–1248. [PubMed] [Google Scholar]
- [22].Ren WB, Fan ZL, Guo YS, et al. Study on the genetic polymorphisms of human GSTT1 and GSTM1 in health population of He Bei province. Nao yu Shenjing Jibing Zazhi. 2009;17(2):88–92. [Google Scholar]
- [23].Capoluongo E, Onder G, Concolino P, et al. GSTM1-null polymorphism as possible risk marker for hypertension: results from the aging and longevity study in the Sirente Geographic Area (ilSIRENTE study) Clin Chim Acta. 2009;399(1-2):92–96. doi: 10.1016/j.cca.2008.09.017. [DOI] [PubMed] [Google Scholar]
- [24].Bessa SS, Ali EM, Hamdy SM. The role of glutathione S- transferase M1 and T1 gene polymorphisms and oxidative stress-related parameters in Egyptian patients with essential hypertension. Eur J Intern Med. 2009;20(6):625–630. doi: 10.1016/j.ejim.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [25].de Champlain J, Wu R, Girouard H, et al. Oxidative stress in hypertension. Clin Exp Hypertens. 2004;26(7-8):593–601. doi: 10.1081/ceh-200031904. [DOI] [PubMed] [Google Scholar]
- [26].Bid HK, Konwar R, Saxena M, et al. Association of glutathione S-transferase (GSTM1, T1 and P1) gene polymorphisms with type 2 diabetes mellitus in north Indian population. J Postgrad Med. 2010;56(3):176–181. doi: 10.4103/0022-3859.68633. [DOI] [PubMed] [Google Scholar]
- [27].Nowier SR, Kashmiry NK, Rasool HA, et al. Association of type 2 diabetes mellitus and glutathione S-transferase (GSTM1 and GSTT1) genetic polymorphism. Res J Med Med Sci. 2009;4:181–188. [Google Scholar]
- [28].Hori M, Oniki K. Combined glutathione S-transferase T1 and M1 positive genotypes afford protection against Type 2 diabetes in Japanese. Pharmacogenomics. 2007;8(10):1307–1314. doi: 10.2217/14622416.8.10.1307. [DOI] [PubMed] [Google Scholar]
- [29].Tiedge M, Lortz S, Drinkgern J. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46(11):1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- [30].Dominguez LJ, Galioto A, Pineo A, et al. Age, homocysteine, and oxidative stress: Relation to hypertension and type 2 diabetes mellitus. J Am Coll Nutr. 2010;29(1):1–6. doi: 10.1080/07315724.2010.10719810. [DOI] [PubMed] [Google Scholar]
- [31].Higashi Y, Noma K, Yoshizumi M, et al. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73(3):411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- [32].Li R, Boerwinkle E. Glutathione S-transferase genotype as a susceptibility factor in smoking-related coronary heart disease. Atherosclerosis. 2000;149(2):451–462. doi: 10.1016/s0021-9150(99)00483-9. [DOI] [PubMed] [Google Scholar]
- [33].Masetti S, Botto N, Manfredi S, et al. Interactive effect of the glutathione S-transferase genes and cigarette smoking on occurrence and severity of coronary artery risk. J Mol Med. 2003;81(8):488–489. doi: 10.1007/s00109-003-0448-5. [DOI] [PubMed] [Google Scholar]
- [34].Li R, Folsom AR, Sharrett AR, et al. Interaction of the glutathione S-transferase genes and cigarette smoking on risk of lower extremity arterial disease: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2001;154(3):729–738. doi: 10.1016/s0021-9150(00)00582-7. [DOI] [PubMed] [Google Scholar]
- [35].Geneva: World Health Organization; 1999. World Health Organization. Definition, diagnosis and classification of diabetes mellitus. Report of a WHO consulation. [Google Scholar]
- [36].Cardiovascular Branch of the Chinese Medical Association. Prevention suggestions of dyslipidemia. Zhonghua Xinxueguan Bing Zazhi. 1997;25(3):169–173. [Google Scholar]


