Abstract
Chronic cerebral ischemia is a pathological process in many cerebrovascular diseases and it is induced by long-term hyperlipidemia, hypertension and diabetes mellitus. After being fed a high-fat diet for 4 weeks, rats were subjected to permanent occlusion of bilateral common carotid arteries to establish rat models of chronic cerebral ischemia with hyperlipidemia. Intercellular adhesion molecule-1 expression in rat hippocampal CA1 region was determined to better understand the mechanism underlying the effects of hyperlipidemia on chronic cerebral ischemia. Water maze test results showed that the cognitive function of rats with hyperlipidemia or chronic cerebral ischemia, particularly in rats with hyperlipidemia combined with chronic cerebral ischemia, gradually decreased between 1 and 4 months after occlusion of the bilateral common carotid arteries. This correlated with pathological changes in the hippocampal CA1 region as detected by hematoxylin-eosin staining. Immunohistochemical staining showed that intercellular adhesion molecule-1 expression in the hippocampal CA1 region was noticeably increased in rats with hyperlipidemia or chronic cerebral ischemia, in particular in rats with hyperlipidemia combined with chronic cerebral ischemia. These findings suggest that hyperlipidemia aggravates chronic cerebral ischemia-induced neurological damage and cognitive impairment in the rat hippocampal CA1 region, which may be mediated, at least in part, by up-regulated expression of intercellular adhesion molecule-1.
Keywords: hyperlipidemia, chronic cerebral ischemia, intercellular adhesion molecule-1, hippocampus, CA1, water maze test, cognitive function, neural regeneration
Abbreviation:
2VO, occlusion of bilateral common carotid arteries; ICAM-1, intercellular adhesion molecule-1; HL, hyperlipidemia
INTRODUCTION
Chronic disruption of cerebral blood flow resulting from carotid insufficiency can induce neurological deficits and dementia, such as vascular dementia, Alzheimer's disease and Binswanger's disease. In 1992, permanent occlusion of bilateral common carotid arteries (2VO) was used to induce chronic cerebral ischemia, which led to morphological abnormalities in hippocampal cells and quantifiable cell loss within 7 months of blood flow reduction[1]. Hippocampal atrophy correlates with impairment on visuospatial memory tasks[1,2]. While the 2VO model reduces cerebral blood flow, there are numerous additional factors that influence cerebral morphology in humans, such as hyperlipidemia (HL), hypertension and hyperglycemia. To investigate the pathological changes associated with chronic cerebral ischemia, we established a HL rat model using 2VO. It has been proposed that HL disrupts the function and integrity of endothelial cells[3]. This appears to be due to a series of chain reactions. First, hypercholesterolemia, especially high plasma levels of low-density lipoprotein cholesterol, can stimulate the expression of intracellular adhesion molecule 1 (ICAM-1), which promotes the rolling of a large number of leukocytes, strong adhesion, and ultimately, transmigration[4,5]. The final result is the production of thrombosis and atherosclerosis, which are the pathological basis of chronic cerebral ischemia.
ICAM-1 interaction with cognate receptors on leukocytes, lymphocyte function-associated antigen-1 and macrophage antigen-1 is crucial for leukocyte adhesion and transendothelial migration[6,7,8,9]. Thus, we chose ICAM-1 as a marker of HL in this study.
A previous study showed that at 2 months after the 2VO procedure, a large number of leukocytes and T cells infiltrated into the ischemic region, especially around the regions of vascular infarction[10]. The concentration of these cells in the ischemic penumbra is tightly linked to brain damage[11]. However, little is known about the effect of ICAM-1 on the inflammatory response in chronic cerebral ischemia, or even in HL with chronic cerebral ischemia. We established a rat model of HL with chronic cerebral ischemia and examined the expression of ICAM-1 in the rat hippocampal CA1 to investigate the relationship between ICAM-1 expression and HL with chronic cerebral ischemia.
RESULTS
Quantitative analysis of experimental animals
A total of 105 Wistar rats were used in the study. Rats were randomly divided into four groups: control, 2VO, HL and HL + 2VO groups. Each group was divided into three subgroups: 1st, 2nd and 4th months. During experimentation, 15 rats died due to anesthesia or inability to tolerate the operation in the latter three groups. In total, 15 rats from the control group, 20 from the 2VO group, 25 from the HL group and 30 from the HL + 2VO group were included in the final analysis.
Blood lipid results
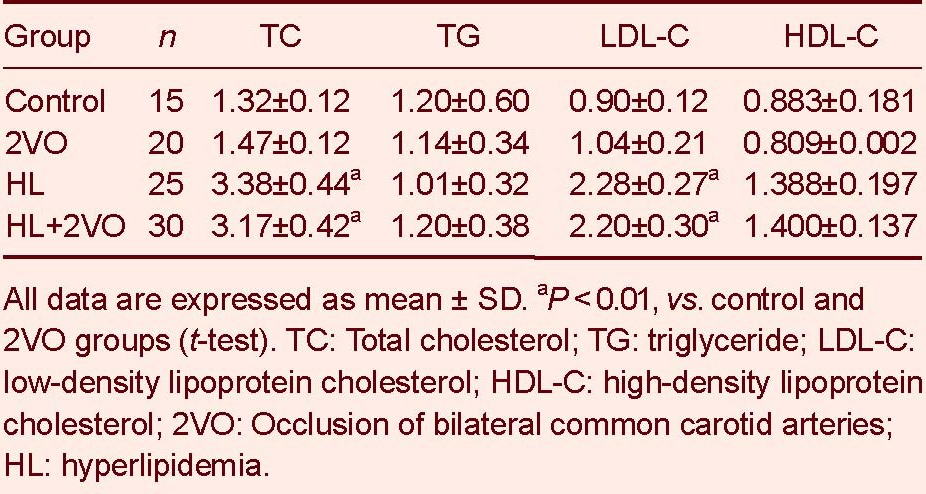
Blood lipid results demonstrated that total cholesterol (TC) and low density lipoprotein-cholesterol (LDL-C) were significantly higher in the HL and HL + 2VO groups compared with the control and 2VO groups (P < 0.01, Table 1).
Table 1.
The levels of blood lipids (mM) and apolipoprotein in rats
Cognitive function
Before the 2VO operation, all rats were subjected to the Morris water maze test to eliminate sub-standard rats (unintelligent rats) to reduce variability between them.
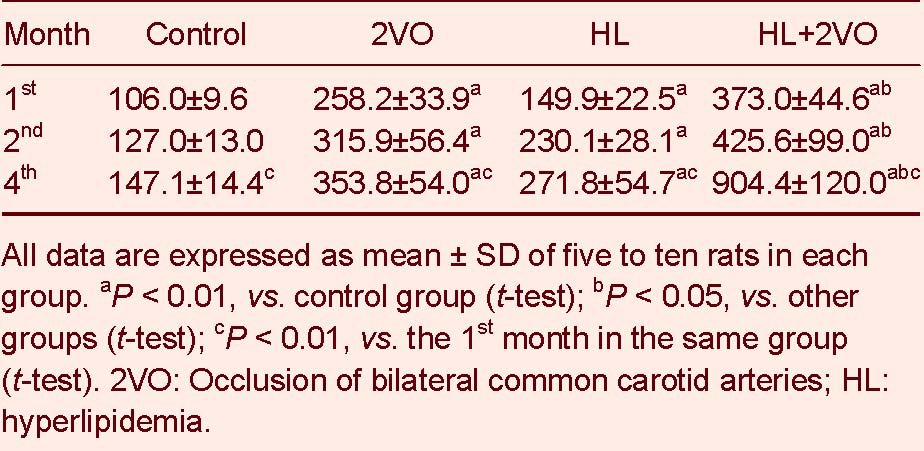
Compared to the control group, rat cognitive function significantly declined between the 1st and 4th months in the 2VO, HL and HL + 2VO groups. The worst cognitive function was displayed in the HL + 2VO group (P < 0.05, Tables 2-4).
Table 2.
The escape latency (second) of rats after the 2VO operation
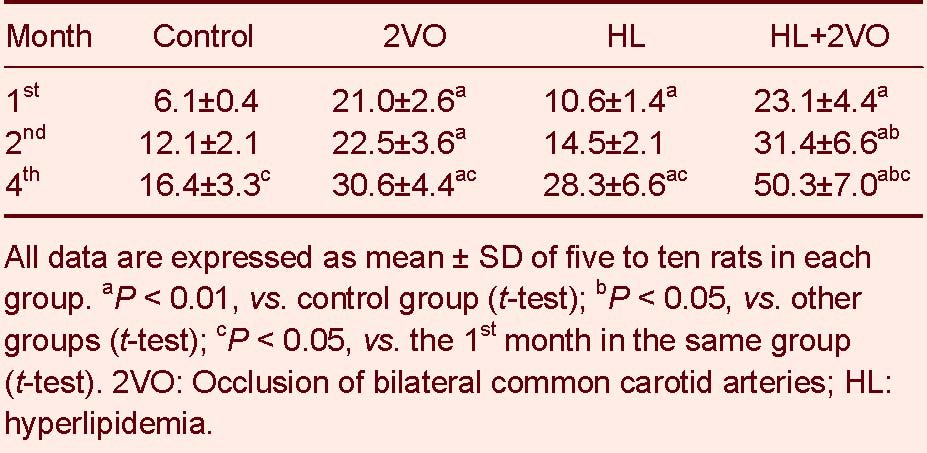
Table 4.
Spatial probing (times of crossing the platform per 3 minutes) of rats after the 2VO operation
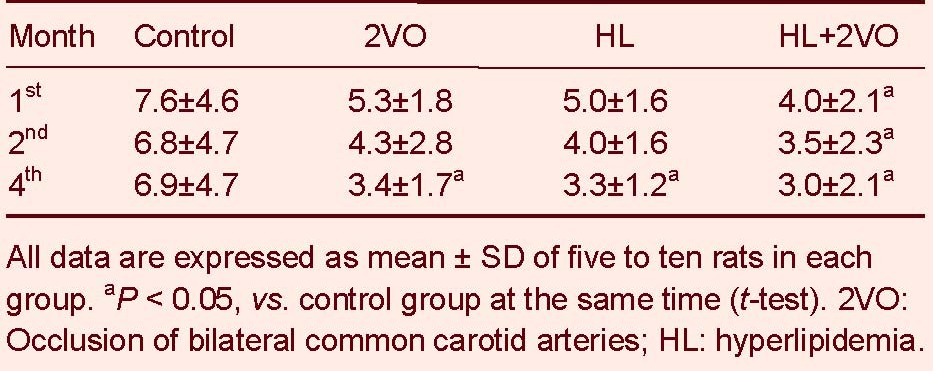
Table 3.
The search length (cm) of rats after the 2VO operation
Pathological change
Hematoxylin-eosin staining showed that the pathological lesions in the hippocampal CA1 region increased between the 1st and 4th months in the 2VO, HL and HL+2VO groups. The most severe pathological lesions were present in the HL + 2VO group (Figure 1).
Figure 1.
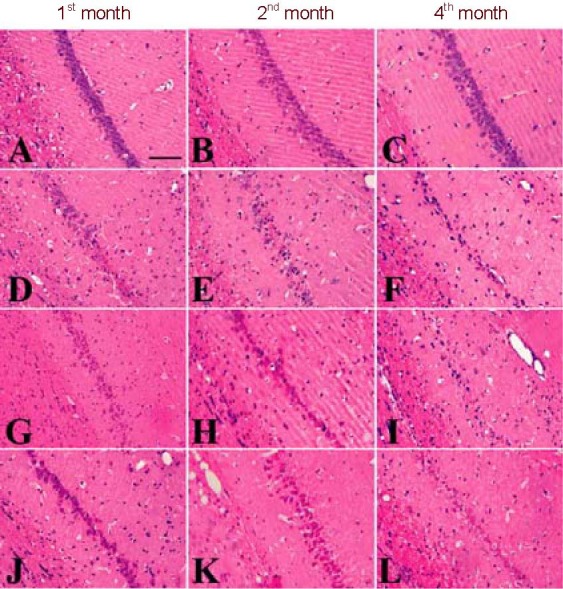
Hematoxylin-eosin staining of hippocampal CA1 area.
(A–C) There was no obvious pathological change in the hippocampal CA1 subregion in the 1st, 2nd or 4th months after 2VO in the control group.
(D) In the 1st month after 2VO, the pyramidal layer is distorted, the pyramidal cells are degenerated, necrotic and even lost, and inflammatory cells surround the vessels.
(E) In the 2nd month after 2VO, many swollen cells with microcavities and a shrunken appearance are observed. Glial cells have proliferated and a large number of inflammatory cells surround the vessels.
(F) In the 4th month after 2VO, many pyramidal cells are lost and the cell layer is incomplete or disappears. Glial cells proliferate. Vascular endothelial cells shrink and many inflammatory cells surround them.
The pathological changes in the HL group (G–I) were similar to those in the 2VO group. The pathological changes in the HL + 2VO group (J-L) were more severe than in any other group. Scale bar: 50 µm. 2VO: Occlusion of bilateral common carotid arteries; HL: hyperlipidemia.
ICAM-1 expression in the hippocampal CA1
There was no ICAM-1-positive vessel found in the control group at any time point. ICAM-1 expression markedly increased in the microvascular endothelial cells in the 2VO, HL and HL + 2VO groups between the 1st and 2nd months. However, in the 4th month, there were significantly fewer ICAM-1-positive vessels in the hippocampal CA1 than in the 2nd month. At each time point, the HL + 2VO group had the largest number of ICAM-1-positive vessels (P < 0.05; Figure 2).
Figure 2.
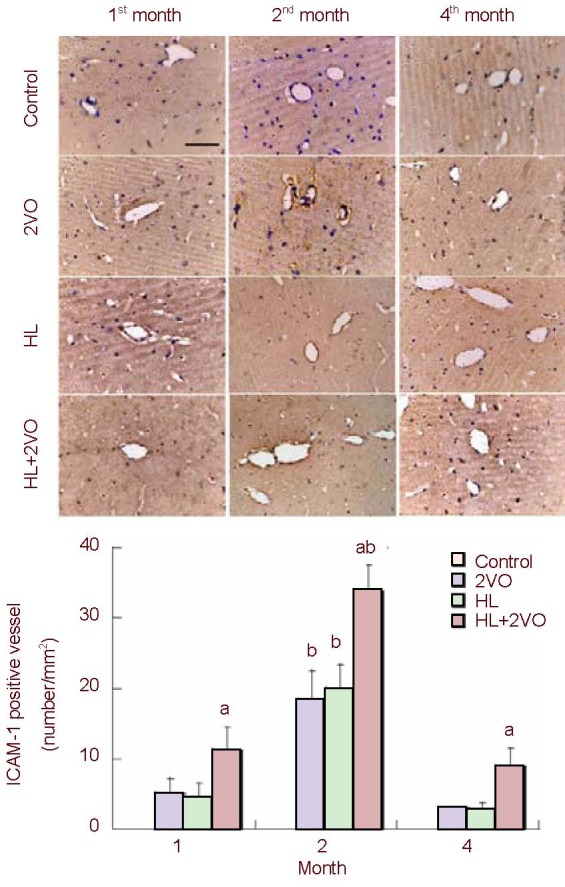
The ICAM-1-positive vessels in the hippocampal CA1 in the control, 2VO, HL and HL + 2VO groups at 1, 2 and 4 months post-surgery. Scale bar: 20 µm.
Values are expressed as mean ± SD of five rats in each group. aP < 0.05, vs. other groups (Student's t-test); bP < 0.05, vs. other time points (Student's t-test). ICAM-1: Intracellular adhesion molecule 1; 2VO: Occlusion of bilateral common carotid arteries; HL: hyperlipidemia.
In the control group, there were no ICAM-1-positive cells in the 1st month, and only a few in the 2nd and 4th months. In the 2VO, HL and HL + 2VO groups, a large number of positive cells were observed in the 1st month. The positive cells were considered to be pyramidal cells according to their shape and position, and most of them were stained weakly. Cell numbers increased between the 1st and 4th months. In the 2nd month, some positive cells were glial cells, and by the 4th month, most positive cells were glial cells, which stained strongly, and there were only a few pyramidal cells. At each time point, the HL + 2VO group had the largest number of ICAM-1-positive cells (P < 0.05; Figure 3).
Figure 3.
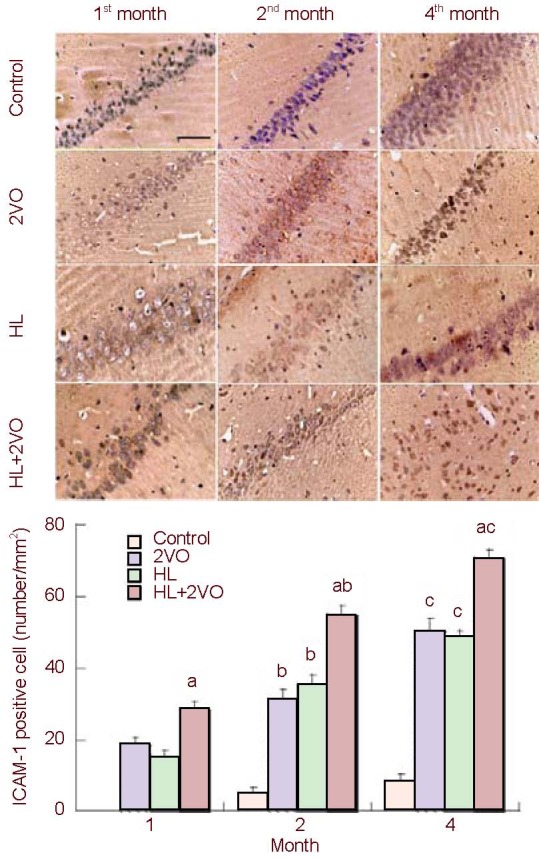
The ICAM-1-positive cells in the hippocampal CA1 in the control, 2VO, HL and HL + 2VO groups at 1, 2 and 4 months post-surgery. Scale bar: 20 µm.
Values are expressed as mean ± SD of five rats from each group. aP < 0.05, vs. other groups (Student's t-test); bP < 0.05, vs. the 1st month in the same group (Student's t-test); cP < 0.05, vs. other time points (Student's t-test). ICAM-1: Intracellular adhesion molecule 1; 2VO: Occlusion of bilateral common carotid arteries; HL: hyperlipidemia.
DISCUSSION
The pathological basis of HL is impaired endothelial cell function and integrity[7]. Vascular endothelium expresses low levels of ICAM-1, and inflammatory stimuli can markedly increase ICAM-1 surface expression[12,13]. Hackman et al[14] reported that soluble ICAM-1 levels were elevated in patients with HL in both the hypercholesterolemic and hypertriglyceridemic groups compared with the control group. Scalia et al[15] found that the surface of intestinal microvascular endothelial cells in rabbits fed a high cholesterol diet expressed high levels of ICAM-1.
Accumulating evidence suggests that the immune inflammatory response plays a vital role in chronic cerebral ischemia, similar to its previously identified role in acute ischemia[16,17]. Leukocyte migration and glial cell activation are two major components of immune inflammatory response in cerebral ischemia. Hypoxic-ischemic brain damage can lead to the expression of cytokines, such as interleukin (IL)-1, tumor necrosis factor-alpha (TNF-α), nuclear factor kappa B (NF-κB), IL-6, IL-8 and IL-17, that up-regulate expression of ICAM-1 on the surface of vascular endothelial cells, which, in turn, induces blood leukocytes to undergo transendothelial migration[18,19,20,21,22]. Numerous animal models of in vivo inflammation have been reported previously[23,24,25,26]. We consider these leukocytes to be inflammatory cells, which release various toxic substances and cause proliferating glial cells to damage the brain in humans and in the various animal models. In hypoxic-ischemic brain injury, the infiltration of inflammatory cells, caused by elevated endothelial ICAM-1 expression, likely mediates tissue damage. Clinical trials have shown that increased soluble ICAM-1(sICAM-1) and angiotensin-converting enzyme levels in cerebrospinal fluid are closely linked to reduced cerebral blood flow[27]. Cechetti et al[28] reported that at 4 months after 2VO in rats, neurons had degenerated and glial cells were activated, and at 7 months, neuronal degeneration and cortical atrophy were observed. Farr et al[29] studied 2VO models and concluded that at 2 months after chronic cerebral ischemia, microglia were activated widely and in different forms, and a large number of T cells had infiltrated the ischemic brain tissue, especially around the vascular infarction. The cortex was the most affected region, followed by the hippocampus and the white matter. The activated microglial cells produce a variety of cytokines and toxins, exacerbating the immune response and inflicting damage to the brain, particularly to the white matter[30].
A previous study showed that the damage to the white matter in rats with chronic cerebral ischemia was related to the proliferation and activation of astrocytes[31]. Farkas et al[32] found that at 13 weeks after 2VO in rats, there was a large number of proliferating and activated microglial cells in the corpus callosum, internal capsule and optic tract, which produce inflammatory cytokines to damage neurons and the white matter.
In this study, we showed that at 1 and 2 months after 2VO operation in rats, ICAM-1 was expressed on the surface of endothelial cells. After 4 months, it was expressed on the surface of glial cells, but not on the surface of endothelial cells. As mentioned above, ICAM-1 could induce leukocytes to gather and adhere to the endothelial cells to damage the blood-brain barrier, and infiltrate into the brain to release neurotoxic substances that injure neurons. In the 4th month, we speculate that a large number of endothelial cells were damaged and that glial cells continued to proliferate, leading to ICAM-1 expression by glial cells which helps mediate leukocyte infiltration and neuronal destruction. Our results also showed that the temporal pattern of expression of ICAM-1 in chronic cerebral ischemia with HL was similar to that in chronic cerebral ischemia or HL. However, ICAM-1 expression and the injury to the brain in chronic cerebral ischemia with HL was much greater than in the latter two. These findings suggest that HL can aggravate neurologic damage and cognitive impairment in rats with induced chronic cerebral ischemia, which may be mediated, at least in part, by up-regulated expression of ICAM-1.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
Experiments were performed at the Experiment Center of Bethune Medical Collage of Jilin University in China from May 2007 to March 2008.
Materials
Male Wistar rats, weighing 250-300 g, supplied by the Laboratory Animal Center, Jilin University (license No. SCXK (Ji) 2008-0005), were used in this study. All rats received humane care in compliance with the guidelines of the First Hospital of Jilin University in China and the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[33].
Methods
Establishment of HL/2VO rat models
From the first week, rats from the HL and HL + 2VO groups were fed a high-fat diet, which contained 70% basic food and 30% high-fat food. The percentage of high-fat food was gradually increased each week until rats were fed with high-fat food only. After 4 weeks, tail blood was collected to measure the level of blood lipids. HL was considered to have been successfully induced when the levels of total cholesterol and low-density lipoprotein cholesterol in these rats were significantly higher than in rats given normal food (P < 0.01). At this stage, rats were subjected to permanent 2VO, a model of chronic ischemia. Rats from the HL and HL + 2VO groups were fed high-fat food until they were killed. The rats in the 2VO and control groups were fed basic food. The high-fat diet contained 10% lard, 2% cholesterol, 0.2% sodium cholate, 0.2% propylthiouracil and 87.6% basic food. The basic and high-fat foods were supplied by the Laboratory Animal Center, Jilin University, China.
Morris water maze for behavioral tests
Each rat was subjected to the Morris water maze test, which included place navigation and spatial probing before and after 2VO induction[34]. Place navigation measures comprised escape latency and searching length, which reflect learning ability (lower measures indicate better performance). The platform was removed after the place navigation test, and spatial probing was performed, in which the time spent by the rats in the platform area was recorded to assess memory (longer duration indicates better function). All data were collected automatically by a computer.
Hematoxylin-eosin staining for pathological changes in the hippocampal CA1 region
Paraffin coronal sections of the rat brain, 5-μm thick, were stained with hematoxylin-eosin. The images were captured by a microscopic digital camera system (Olympus, Tokyo, Japan). An independent observer who was blinded to the study performed subsequent assessments, with a gross survey and a detailed evaluation of the hippocampal CA1 regions.
Immunohistochemical staining for detection of ICAM-1 expression
The hippocampal sections were immunostained on slides. The primary antibody was mouse anti-rat-ICAM-1 (1:500 dilution; Boster, Wuhan, China). The secondary antibody was goat anti-mouse (1:100 dilution; Boster). Five slices from each sample were selected for the same region. The ICAM-1-positive vessels and cells in the hippocampal CA1 region were counted using a light microscope with an eyepiece grid under 400 × magnification (Olympus). After immunohistochemical staining, ICAM-1-expressing cells and vessels appeared brown. The positive cells or vessels were calculated as the total number in one field. Results were expressed as the average positive ratio of three visual fields.
Statistical analysis
Data were expressed as mean ± SD and differences between two groups were analyzed by t-tests. Data were analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA). A probability value P < 0.05 was regarded as statistically significant.
Footnotes
Conflicts of interest: None declared.
Ethical approval: All protocols for these animal studies were approved by the Animal Care and Ethics Committee of Jilin Medical University, China.
(Edited by Su ZQ, Ma R/Song LP)
REFERENCES
- [1].de la Torre JC, Fortin T, Park GA, et al. Chronic cerebrovascular insufficiency induces dementia-like deficits in aged rats. Brain Res. 1992;582(2):186–195. doi: 10.1016/0006-8993(92)90132-s. [DOI] [PubMed] [Google Scholar]
- [2].Pappas BA, de la Torre JC, Davidson CM, et al. Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res. 1996;708(1-2):50–58. doi: 10.1016/0006-8993(95)01267-2. [DOI] [PubMed] [Google Scholar]
- [3].Cooke JP. Therapeutic interventions in endothelial dysfunction: endothelium as a target organ. Clin Cardiol. 1997;20(11 Suppl 2) II:45–51. [PubMed] [Google Scholar]
- [4].Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- [5].Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2(6):515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- [6].Ding ZM, Babensee JE, Simon SI, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163(9):5029–5038. [PubMed] [Google Scholar]
- [7].Siasos G, Tousoulis D, Oikonomou E, et al. Inflammatory markers in hyperlipidemia: from experimental models to clinical practice. Curr Pharm Des. 2011;17(37):4132–4146. doi: 10.2174/138161211798764780. [DOI] [PubMed] [Google Scholar]
- [8].Henderson RB, Lim LH, Tessier PA, et al. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J Exp Med. 2001;194(2):219–226. doi: 10.1084/jem.194.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lyck R, Reiss Y, Gerwin N, Greenwood J, et al. T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: the cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood. 2003;102(10):3675–3683. doi: 10.1182/blood-2003-02-0358. [DOI] [PubMed] [Google Scholar]
- [10].Liu ZR, Bian XH, Li LS, et al. Effect of cyclosporin A on actication of immune cells in brain after chronic cerebral hypoperfusion in aged rats. Xiandai Kangfu. 2001;5:46–47. [Google Scholar]
- [11].Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66(3):232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- [12].Dustin ML, Rothlein R, Bhan AK, et al. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137(1):245–254. [PubMed] [Google Scholar]
- [13].Dustin ML, Springer TA. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hackman A, Abe Y, Insull W, Jr, et al. Levels of soluble cell adhesion molecules in patients with dyslipidemia. Circulation. 1996;93(7):1334–1338. doi: 10.1161/01.cir.93.7.1334. [DOI] [PubMed] [Google Scholar]
- [15].Scalia R, Appel JZ, 3rd, Lefer AM. Leukocyte-endothelium interaction during the early stages of hypercholesterolemia in the rabbit: role of P-selectin, ICAM-1, and VCAM-1. Arterioscler Thromb Vasc Biol. 1998;18(7):1093–1100. doi: 10.1161/01.atv.18.7.1093. [DOI] [PubMed] [Google Scholar]
- [16].Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem. 2009;9(14):1240–1260. doi: 10.2174/156802609789869619. [DOI] [PubMed] [Google Scholar]
- [17].Melani A, Cipriani S, Corti F, et al. Effect of intravenous administration of dipyridamole in a rat model of chronic cerebral ischemia. Ann N Y Acad Sci. 2010;1207:89–96. doi: 10.1111/j.1749-6632.2010.05732.x. [DOI] [PubMed] [Google Scholar]
- [18].Gerritsen ME, Bloor CM. Endothelial cell gene expression in response to injury. FASEB J. 1993;7(6):523–532. doi: 10.1096/fasebj.7.6.8472891. [DOI] [PubMed] [Google Scholar]
- [19].Iigo Y, Suematsu M, Higashida T, et al. Constitutive expression of ICAM-1 in rat microvascular systems analyzed by laser confocal microscopy. Am J Physiol. 1997;273(1 Pt 2):H138–147. doi: 10.1152/ajpheart.1997.273.1.H138. [DOI] [PubMed] [Google Scholar]
- [20].Klein CL, Bittinger F, Kohler H, et al. Comparative studies on vascular endothelium in vitro. 3. Effects of cytokines on the expression of E-selectin, ICAM-1 and VCAM-1 by cultured human endothelial cells obtained from different passages. Pathobiology. 1995;63(2):83–92. doi: 10.1159/000163938. [DOI] [PubMed] [Google Scholar]
- [21].Scholz D, Devaux B, Hirche A, et al. Expression of adhesion molecules is specific and time-dependent in cytokine-stimulated endothelial cells in culture. Cell Tissue Res. 1996;284(3):415–423. doi: 10.1007/s004410050602. [DOI] [PubMed] [Google Scholar]
- [22].Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52(3):349–374. [PubMed] [Google Scholar]
- [23].Feng D, Nagy JA, Pyne K, et al. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187(6):903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hoshi O, Ushiki T. Scanning electron microscopic studies on the route of neutrophil extravasation in the mouse after exposure to the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMLP) Arch Histol Cytol. 1999;62(3):253–260. doi: 10.1679/aohc.62.253. [DOI] [PubMed] [Google Scholar]
- [25].Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83(2):309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- [26].Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109(2):181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- [27].Janciauskiene SM, Erikson C, Warkentin S. A link between sICAM-1, ACE and parietal blood flow in the aging brain. Neurobiol Aging. 2009;30(9):1504–1511. doi: 10.1016/j.neurobiolaging.2007.08.025. [DOI] [PubMed] [Google Scholar]
- [28].Cechetti F, Pagnussat AS, Worm PV, et al. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res Bull. 2012;87(1):109–116. doi: 10.1016/j.brainresbull.2011.10.006. [DOI] [PubMed] [Google Scholar]
- [29].Farr TD, Seehafer JU, Nelles M, et al. Challenges towards MR imaging of the peripheral inflammatory response in the subacute and chronic stages of transient focal ischemia. NMR Biomed. 2011;24(1):35–45. doi: 10.1002/nbm.1553. [DOI] [PubMed] [Google Scholar]
- [30].Sharkey J, Butcher SP. Immunophilins mediate the neuroprotective effects of FK506 in focal cerebral ischaemia. Nature. 1994;371(6495):336–339. doi: 10.1038/371336a0. [DOI] [PubMed] [Google Scholar]
- [31].Shibata M, Ohtani R, Ihara M, et al. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35(11):2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- [32].Farkas E, Donka G, de Vos RA, et al. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol. 2004;108(1):57–64. doi: 10.1007/s00401-004-0864-9. [DOI] [PubMed] [Google Scholar]
- [33].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [34].Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]


