Abstract
Activity of matrix metalloproteinase-9 increases following cerebral ischemia/reperfusion, and is associated with cerebral microvascular permeability, blood-brain barrier destruction, inflammatory cell infiltration and brain edema. Matrix metalloproteinase-9 also likely participates in thrombolysis. A rat model of middle cerebral artery infarction was established by injecting autologous blood clots into the internal carotid artery. At 3 hours following model induction, urokinase was injected into the caudal vein. Decreased neurological severity score, reduced infarct volume, and increased expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 were observed in the cerebral cortex 24 hours after urokinase thrombolysis. These results suggest that urokinase can suppress damage in the acute-early stage of cerebral infarction.
Keywords: cerebral infarction, urokinase, thrombolysis, matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, neural regeneration
Abbreviation:
MMP-9, matrix metalloproteinase-9; TIMP-1, tissue inhibitor of metalloproteinase-1; NSS, neurological severity score; TTC, 2,3,5-triphenyltetrazolium chloride
INTRODUCTION
The optimal therapeutic measure for acute cerebral infarction is to recanalize occluded cerebral vessels, and to recover blood supply before irreversible ischemic injury occurs to brain tissues[1,2]. Thrombolytic therapy is an effective method for acute ischemic stroke, but this method can increase the risk of hemorrhagic transformation following ischemia[3,4]. Thrombolysis-associated hemorrhagic transformation is associated with free radical release, increased activities of neutrophils and macrophages, and protease release following ischemia/reperfusion[5,6]. Matrix metalloproteinase-9 (MMP-9) is mainly synthesized and secreted by neutrophils, monocytes, vascular endothelial cells, smooth muscle cells, astrocytes, microglia and macrophages[7,8]. MMP-9 activation is mediated by proteins that activate the proenzyme and is negatively regulated by inhibitors[9]. MMP-9 activity increases following cerebral ischemia/reperfusion, and its expression is strongly linked to cerebral microvascular permeability, blood-brain barrier destruction, inflammatory cell infiltration and brain edema[10,11], suggesting that it plays an important role in cerebral ischemia/reperfusion by degrading collagen, laminin and fibronectin, which are the main components of the perivascular basement membrane. In this study, we investigated changes in MMP-9 and tissue inhibitor of metalloproteinase-1 (TIMP-1) expression, and their effects on thrombolysis after urokinase administration in rats with focal cerebral infarction.
RESULTS
Quantitative analysis of experimental animals
A total of 100 rats were included in this study, of which 10 were included in the sham-surgery group, and the remaining 90 participated in the middle cerebral artery infarction model of ischemic injury. Of these, 80 rats underwent successful middle cerebral artery infarction, and were randomly assigned to model and urokinase groups. At 3 hours following blood clot injection, saline or urokinase was injected into the caudal vein of rats in the model and urokinase groups, respectively. Sham-operated rats were only used for determining infarct volume.
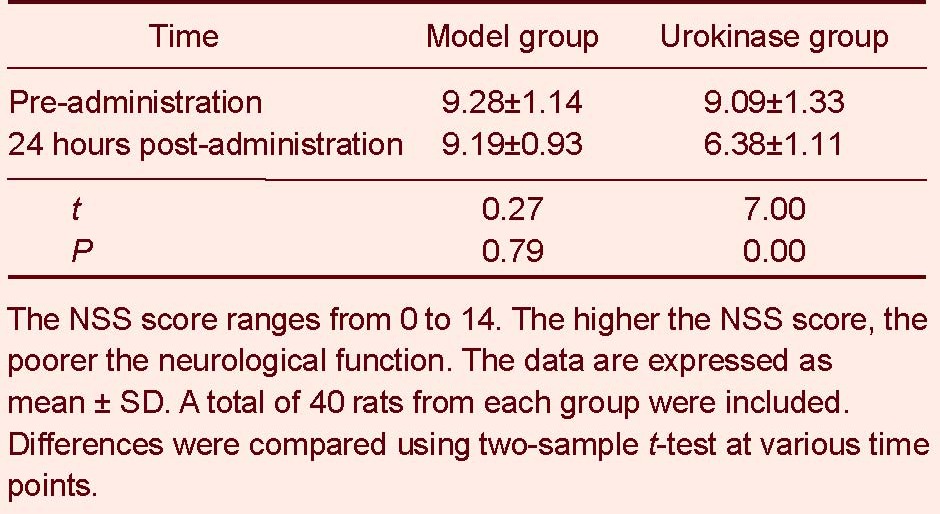
Urokinase thrombolysis lowered the neurological severity score (NSS) in rats with cerebral infarction
NSS scores did not change at 24 hours following saline administration in rats in the model group (P > 0.05). NSS scores were significantly reduced in the urokinase group at 24 hours following urokinase administration (P < 0.01; Table 1).
Table 1.
Neurological severity score (NSS) scores before and after thrombolysis in rats with cerebral infarction
Urokinase thrombolysis reduced infarct volume in rats with cerebral infarction
2,3,5-triphenyltetrazolium chloride (TTC) staining demonstrated that the infarcted region comprised the area supplied by the left middle cerebral artery, primarily the cortex, but also the basal ganglia and hippocampus. At 24 hours following thrombolysis, infarct volume in rats in the urokinase group (59.24 ± 8.25 mm3) was significantly smaller than in the model group (94.90 ± 11.09 mm3, t = 13.494, P = 0.00; Figure 1).
Figure 1.
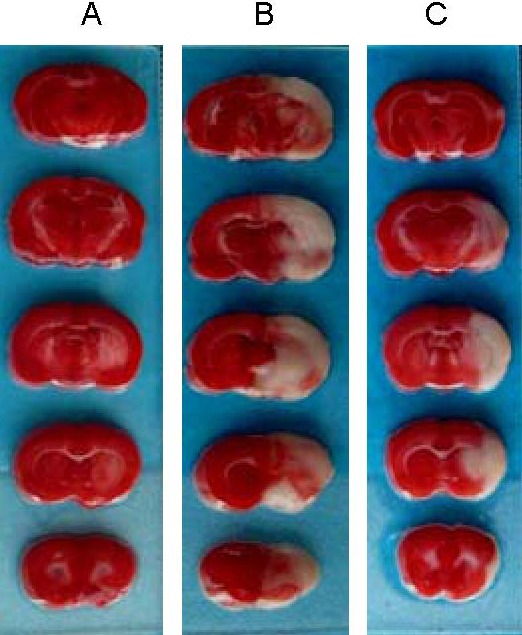
Infarct volume at 24 hours following thrombolysis (2,3,5-triphenyltetrazolium chloride staining).
Unaffected brain regions are stained red, and infarcted regions are unstained. (A) Sham-surgery group; (B) model group; (C) urokinase group.
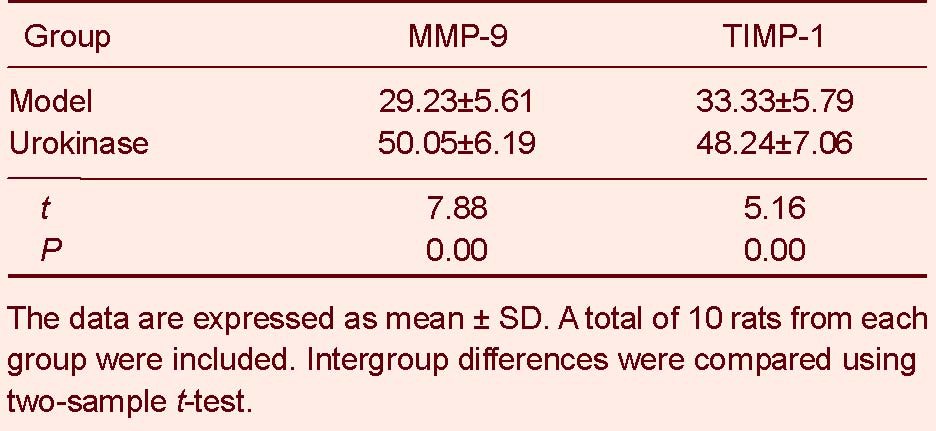
Urokinase thrombolysis increased MMP-9 and TIMP-1 expression in rats with cerebral infarction
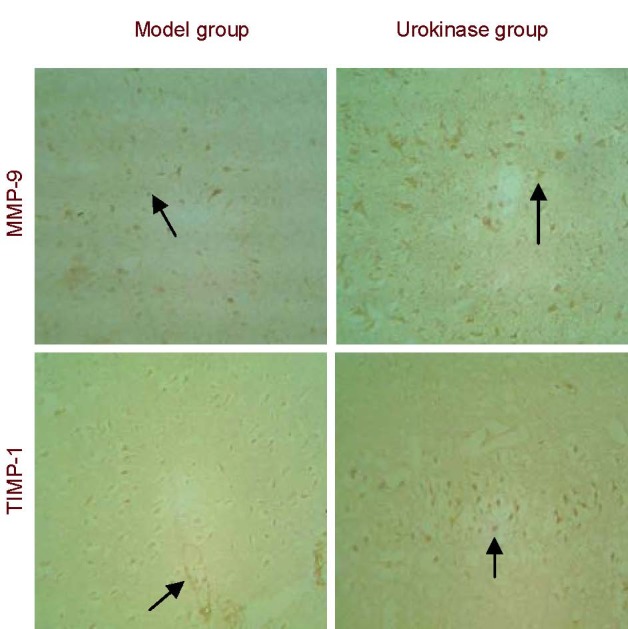
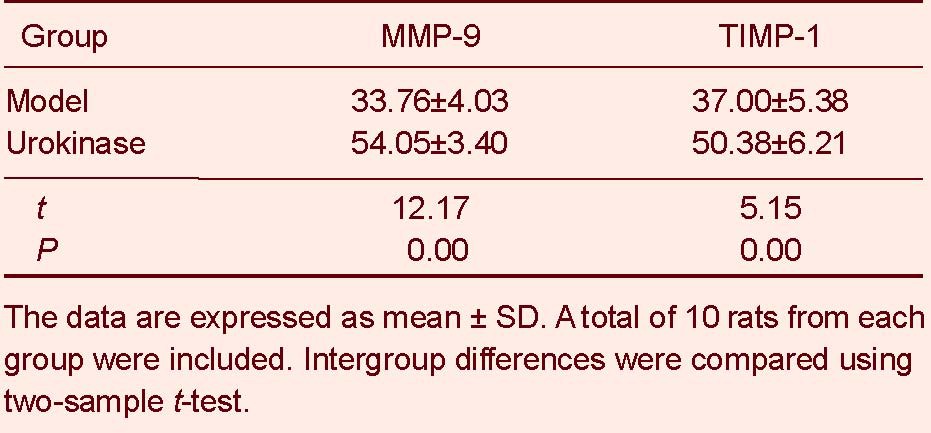
Immunohistochemical staining revealed MMP-9 and TIMP-1 expression in the cytoplasm of cells surrounding the infarct region in the model and urokinase groups. MMP-9 and TIMP-1 expression in the rat cortex was significantly greater in the urokinase group than in the model group (P < 0.01; Figure 2, Table 2).
Figure 2.
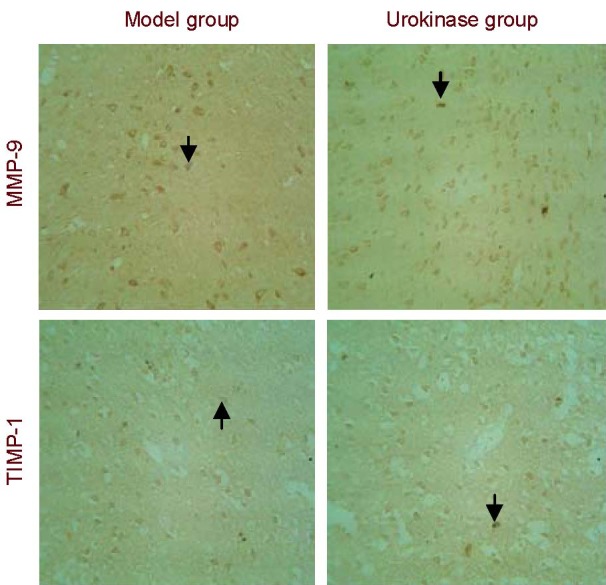
Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) expression in the cortex of rats with cerebral infarction (immunohistochemical staining, × 200).
MMP-9 and TIMP-1 expression in the rat cortex was significantly greater in the urokinase group than in the model group. Arrows indicate positive expression.
Table 2.
Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) expression in brain tissues of rats with cerebral infarction (absorbance; immunohistochemistry staining)
Urokinase thrombolysis increased MMP-9 and TIMP-1 mRNA expression in rats with cerebral infarction
In situ hybridization showed MMP-9 and TIMP-1 mRNA expression in the cytoplasm of cells surrounding the infarct region in the model and urokinase groups. MMP-9 and TIMP-1 mRNA expression in the rat cortex was significantly greater in the urokinase group than in the model group (P < 0.01; Figure 3, Table 3).
Figure 3.
Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) mRNA expression in the cortex of rats with cerebral infarction (in situ hybridization, × 200).
MMP-9 and TIMP-1 mRNA expression in the cortex was significantly greater in the urokinase group than in the model group. Arrows indicate positive expression. MMP-9 and TIMP-1 mRNA-positive cells exhibit a brown cytoplasm.
Table 3.
Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1 (TIMP-1) expression in brain tissues of rats with cerebral infarction (absorbance; in situ hybridization)
DISCUSSION
Blood-brain barrier disruption is the pathophysiological basis for the hemorrhagic transformation of cerebral infarction. MMP-9 has been shown to play a critical role in reperfusion-induced blood vessel destruction. A previous study showed that the MMP inhibitor BB-94 can decrease the risk of secondary hemorrhage in rats with cerebral infarction[12]. A monoclonal antibody to MMP-9 noticeably diminished infarct volume in a rat model of local ischemia[13,14,15]. TIMP therapy decreased vasogenic brain edema and infarct volume[16,17]. In this study, NSS scores did not change 24 hours following saline injection, but were significantly reduced 24 hours following urokinase thrombolysis. Moreover, infarct volume was decreased following urokinase thrombolysis. These results suggest that urokinase thrombolysis has positive effects on cerebral infarction in the acute-early stage, which is consistent with previous results[18,19]. In the present study, immunohistochemistry and in situ hybridization demonstrated that MMP-9 protein and mRNA levels were significantly increased following urokinase thrombolysis in rats with cerebral infarction. This enhanced expression might be induced by ischemia/reperfusion injury, or it may be due to urokinase, as this enzyme can enhance MMP-9 expression in the ischemic region, as shown by a previous study[19]. These observations indicate that MMP-9 plays an important role in secondary brain injury following thrombolysis. Our present study also demonstrated that TIMP-1 protein and mRNA expression was greater in the urokinase group than in the model group, indicating upregulation of endogenous TIMP expression after thrombolysis, which might be a protective reaction secondary to MMP-9 upregulation.
In summary, urokinase thrombolysis for 24 hours increases MMP-9 and TIMP-1 expression in the cerebral cortex of rats with cerebral infarction, improving neurological function. This demonstrates that urokinase thrombolysis has therapeutic effects on cerebral infarction in the acute-early stage.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
Experiments were performed at the Laboratory of the Institute of Cerebrovascular Disease, Medical College, Qingdao University, China, from January 2008 to June 2009.
Materials
A total of 100 clean healthy male Wistar rats, aged 10 weeks old and weighing 280–320 g, were purchased from the Experimental Animal Center, Medical College, Shandong University, China (license No. SCXK (Lu) 20080006). The rats were housed at 21–27°C with a humidity of 45–55%. Protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[20].
Methods
Preparation of autologous thrombus
Autologous thrombus was prepared in accordance with the method of Busch et al[21]. Briefly, rats were intraperitoneally anesthetized with 10% chloral hydrate. 1 mL of blood from the right common iliac vein was placed in a No. 22 indwelling needle for 2 hours at room temperature. A strip of thrombus (0.5 mm diameter) was obtained, washed in saline, stored in a water bath at 37 °C for 24 hours, and then trimmed into 0.5-mm-diameter particles.
Establishment of cerebral infarction model
The rats were intraperitoneally anesthetized with 10% chloral hydrate, 350 mg/kg. A medium incision was made on the neck. The left common carotid, external carotid and the internal carotid arteries were isolated under a microscope (Olympus, Tokyo, Japan). After thermal coagulation, the external carotid artery was cut, and the trunk of the artery was ligated and dissociated. The common carotid and internal carotid arteries were temporarily blocked. The external carotid artery stump was dragged slightly, and a small opening was made in the external carotid artery. The No. 24 indwelling needle was inserted, and the core was pulled out. Six thrombus particles were injected into the internal carotid artery, passing through the common carotid artery bifurcation point and the internal carotid artery, traversing into the anterior cerebral artery. The blood supply of the left middle cerebral artery was blocked. The external carotid artery stump was ligated, and blood flow to the common carotid artery was restored[22,23]. Rectal temperature was maintained at 37 ± 0.5°C. After the rats regained consciousness, NSS testing was performed in accordance with a previous study[24]. NSS scores > 7 represent successful model establishment[25,26]. Rats with successful cerebral infarction were characterized by Horner's syndrome on the left, weak or absent corneal and panic reflexes, right limb paralysis, difficult crawling (not straight), circling in position and balance disturbance. The internal carotid artery of sham-operated rats was injected with 0.4 mL saline.
Thrombolysis
At 3 hours following blood clot injection, 0.4 mL saline and 1.5 × 104 U urokinase were respectively injected into the caudal vein of rats in the model and urokinase groups.
Neurological impairment following thrombolysis detected by NSS
Ten rats each from the model and urokinase groups were tested 24 hours following thrombolysis. NSS[24] was used for assessment of neurological impairment. The NSS score ranges from 0 to 14. The higher the score, the more severe the injury. The mean value was obtained.
Infarct volume as determined by TTC
Ten rats were randomly obtained from each group at 24 hours following thrombolysis, anesthetized and sacrificed. At a low temperature, tissues, 2 mm anterior and posterior to the optic chiasma of the left brain[27,28], were serially sliced into 5 μm-thick coronal sections, placed in 2% TTC saline buffer and incubated at 37°C for 15 minutes. Infarct volume was calculated using MPIAS-500 Multimedia Color Pathologic Image Analysis System (Tongji Medical University, Wuhan, China).
MMP-9 and TIMP-1 expression in the cerebral cortex, as detected by immunohistochemical staining
Ten rats were randomly obtained from each group at 24 hours following thrombolysis. After anesthesia, the thoracic cavity was incised to expose the heart. Following left ventricular puncture, the right auricle was perfused with 250 mL heparinized saline, and then fixed in 250 mL 4% paraformaldehyde. The entire brain was placed in 4% paraformaldehyde for 48 hours, embedded in paraffin at 4°C, and then sliced into 6 μm-thick sections. The specimens were treated with xylene and ethanol, deparaffinized, and then incubated in 30 mL hydrogen peroxide for 5 minutes. After a wash in distilled water, sections were immersed in 0.01 M citrate buffer.
Antigens were retrieved by microwave heating. The sections were blocked with 5% bovine serum albumin for 20 minutes at room temperature, and then incubated with mouse anti-rat MMP-9 and TIMP-1 monoclonal antibodies (1:200; Boster, Wuhan, China) at 4°C overnight. Following three washes in 0.02 M PBS, each 3 minutes, the sections were incubated with biotinylated goat anti-mouse IgG (1:200; Boster) at 37°C for 30 minutes. Following three washes in PBS, each 3 minutes, the sections were incubated with streptavidin biotinylated peroxidase complex (Boster) for 30 minutes at 37°C. Following three washes in PBS, each 3 minutes, the sections were developed using diaminobenzidine. The reaction was terminated by immersion in running water. The specimens were counterstained with hematoxylin, dehydrated with a graded ethanol series, permeabilized in xylene, and mounted in neutral resin. PBS served as the negative control, in place of primary antibody. Absorbance values were quantitatively calculated using image pro plus software (Media Cybernetics, Bethesda, MD, USA).
MMP-9 and TIMP-1 mRNA expression in the cerebral cortex, as determined by in situ hybridization
Ten rats were randomly obtained from each group at 24 hours following thrombolysis. After anesthesia, the brain was dehydrated, embedded in paraffin, and sliced into 6-8-μm-thick sections. The slide was coated with polylysine. Sections were digested with pepsin diluted in 3% citric acid for 15 minutes at 37°C or room temperature. The sections were prehybridized, and then hybridized with digoxin-labeled MMP-9 and TIMP-1 oligonucleotide probes. The rat MMP-9 probe sequences are as follows: (1) 5’-TCC CTG CCC CAG ACT GGT GAG CTG GAC AGC-3’; (2) 5’-CAA CTC GGC AGA GAG ATG TGC GTC TTC CC-3’; (3) 5’-CCA GGT GGA CCA CGT GGC CTA CGT GAC CTA-3’. The rat TIMP-1 probe sequences are as follows: (1) 5’-ACC ACC TTA TAC CAG CGT TAT GAG ATC AAG ATG AC-3’; (2) 5’-CAC AAG TCC CAG AAC CGC AGC GAG GAG TTT CTC AT-3’. The sections were incubated in hybridization solution at 38-42°C in a thermostat-regulated container. After the coverslip was removed, the sections were washed in 2 × sodium citrate buffer for 5 minutes at 37°C, twice, and then immersed in 0.5 × saline sodium citrate at 37°C for 15 minutes. The sections were incubated with blocking buffer at 37°C for 30 minutes, and then with biotinylated mouse anti-digoxin (1:500; Boster) at 37°C for 60 minutes, followed by four washes in PBS, each 5 minutes. The sections were incubated with streptavidin-biotin complex 37°C for 20 minutes, washed in PBS for 5 minutes, three times, incubated with biotinylated peroxidase (Boster) at 37°C for 20 minutes, and then washed in PBS for 5 minutes, four times. The sections were developed with diaminobenzidine, dehydrated in ethanol, permeabilized in xylene, and then mounted. Absorbance values were quantitatively calculated using image pro plus software.
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA), and expressed as mean ± SD. Two-sample t-test was used. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Professor Chen Zhang from the Department of Neurology, the Affiliated Hospital of Medical College, Qingdao University, China for offering access to related works, and for providing guidance during study design.
Footnotes
Funding: This project was funded by the Natural Science Foundation of Shandong Province (Therapeutic effects and mechanisms of low-frequency ultrasound combined with urokinase thrombolysis in treatment of cerebral infarction in rats), No. 2009ZRB14007.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Medical College, Qingdao University, China.
(Edited by Liu B, Feng JC/Qiu Y/Song LP)
REFERENCES
- [1].Yoo DS, Won YD, Huh PW, et al. Therapeutic results of intra-arterial thrombolysis after full-dose intravenous tissue plasminogen activator administration. AJNR Am J Neuroradiol. 2010;31(8):1536–1540. doi: 10.3174/ajnr.A2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Millán M, Dorado L, Dávalos A. Fibrinolytic therapy in acute stroke. Curr Cardiol Rev. 2010;6(3):218–226. doi: 10.2174/157340310791658758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wasay M, Barohi H, Malik A, et al. Utilization and outcome of thrombolytic therapy for acute stroke in Pakistan. Neurol Sci. 2010;31(2):223–225. doi: 10.1007/s10072-009-0159-y. [DOI] [PubMed] [Google Scholar]
- [4].Rabadi MH, Blass JP. Randomized clinical stroke trials in 2007. Open Neurol J. 2008;2:55–65. doi: 10.2174/1874205X00802010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sharma SR, Sharma N. Hyperacute thrombolysis with recombinant tissue plasminogen activator of acute ischemic stroke: feasibility and effectivity from an Indian perspective. Ann Indian Acad Neurol. 2008;11(4):221–224. doi: 10.4103/0972-2327.44556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang SH, Li Q, Deng ZH, et al. Neanthes japonica (Iznka) fibrinolytic enzyme reduced cerebral infarction, cerebral edema and increased antioxidation in rat models of focal cerebral ischemia. Neurosci Lett. 2011;489(1):16–19. doi: 10.1016/j.neulet.2010.11.057. [DOI] [PubMed] [Google Scholar]
- [7].Liu W, Hendren J, Qin XJ, et al. Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem. 2009;108(3):811–820. doi: 10.1111/j.1471-4159.2008.05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tian W, Kyriakides TR. Matrix metalloproteinase-9 deficiency leads to prolonged foreign body response in the brain associated with increased IL-1beta levels and leakage of the blood-brain barrier. Matrix Biol. 2009;28(3):148–159. doi: 10.1016/j.matbio.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee SR, Tsuji K, Lee SR, et al. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J Neurosci. 2004;24(3):671–678. doi: 10.1523/JNEUROSCI.4243-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Asahi M, Asahi K, Jung JC, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20(12):1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- [11].Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25(27):6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34(8):2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- [13].Hashimoto T, Wen G, Lawton MT, et al. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003;34(4):925–931. doi: 10.1161/01.STR.0000061888.71524.DF. [DOI] [PubMed] [Google Scholar]
- [14].Tejima E, Guo S, Murata Y, et al. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J Neurotrauma. 2009;26(11):1935–1941. doi: 10.1089/neu.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guo M, Cox B, Mahale S, et al. Pre-ischemic exercise reduces matrix metalloproteinase-9 expression and ameliorates blood-brain barrier dysfunction in stroke. Neuroscience. 2008;151(2):340–351. doi: 10.1016/j.neuroscience.2007.10.006. [DOI] [PubMed] [Google Scholar]
- [16].Montaner J, Alvarez-Sabín J, Molina C, et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001;32(8):1759–1766. doi: 10.1161/01.str.32.8.1759. [DOI] [PubMed] [Google Scholar]
- [17].Tai SH, Chen HY, Lee EJ, et al. Melatonin inhibits postischemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J Pineal Res. 2010;49(4):332–341. doi: 10.1111/j.1600-079X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- [18].Meng R, Ji X, Li B, et al. Dynamical levels of plasma F(1+2) and D-dimer in patients with acute cerebral infarction during intravenous urokinase thrombolysis. Neurol Res. 2009;31(4):367–370. doi: 10.1179/174313209X443991. [DOI] [PubMed] [Google Scholar]
- [19].Ji X, Li K, Li W, et al. The effects of blood pressure and urokinase on brain injuries after experimental cerebral infarction in rats. Neurol Res. 2009;31(2):204–208. doi: 10.1179/174313209X393924. [DOI] [PubMed] [Google Scholar]
- [20].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [21].Busch E, Krüger K, Hossmann KA. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res. 1997;778(1):16–24. doi: 10.1016/s0006-8993(97)01008-1. [DOI] [PubMed] [Google Scholar]
- [22].Wakabayashi K, Nagai A, Sheikh AM, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88(5):1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- [23].Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem. 2009;9(14):1240–1260. doi: 10.2174/156802609789869619. [DOI] [PubMed] [Google Scholar]
- [24].Seyfried DM, Han Y, Yang D, et al. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 2008;1224:12–19. doi: 10.1016/j.brainres.2008.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Boyko M, Ohayon S, Goldsmith T, et al. Morphological and neuro-behavioral parallels in the rat model of stroke. Behav Brain Res. 2011;223(1):17–23. doi: 10.1016/j.bbr.2011.03.019. [DOI] [PubMed] [Google Scholar]
- [26].Ma M, Ma Y, Yi X, et al. Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci. 2008;9:117. doi: 10.1186/1471-2202-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tu XK, Yang WZ, Liang RS, et al. Effect of baicalin on matrix metalloproteinase-9 expression and blood-brain barrier permeability following focal cerebral ischemia in rats. Neurochem Res. 2011;36(11):2022–2028. doi: 10.1007/s11064-011-0526-y. [DOI] [PubMed] [Google Scholar]
- [28].Foley LM, Hitchens TK, Barbe B, et al. Quantitative temporal profiles of penumbra and infarction during permanent middle cerebral artery occlusion in rats. Transl Stroke Res. 2010;1(3):220–229. doi: 10.1007/s12975-010-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


