Abstract
Isolated hypoglossal nerve palsy (HNP), or neurapraxia, a rare postoperative complication after airway management, causes ipsilateral tongue deviation, dysarthria, and dysphagia. We reviewed the pathophysiological causes of hypoglossal nerve injury and discuss the associated clinical and procedural characteristics of affected patients. Furthermore, we identified procedural factors potentially affecting HNP recovery duration and propose several measures that may reduce the risk of HNP. While HNP can occur after a variety of surgeries, most cases in the literature were reported after orthopedic and otolaryngology operations, typically in males. The diagnosis is frequently missed by the anesthesia care team in the recovery room due to the delayed symptomatic onset and often requires neurology and otolaryngology evaluations to exclude serious etiologies. Signs and symptoms are self-limited, with resolution occurring within 2 months in 50% of patients, and 80% resolving within 4 months. Currently, there are no specific preventive or therapeutic recommendations. We found 69 cases of HNP after procedural airway management reported in the literature from 1926–2013.
Introduction
Solitary hypoglossal nerve palsy (HNP) after airway management during general anesthesia is a rare complication that may occur after a variety of surgeries. By the end of the first postoperative day, patients typically present with ipsilateral tongue deviation and may exhibit speech and swallowing difficulties. HNP is often diagnosed postoperatively after a thorough workup to exclude stroke, hematoma, impending airway obstruction, and endotracheal trauma. Early consultation with otolaryngology and neurology can guide the diagnostic workup and help promptly identify these serious conditions. We believe that HNP is frequently missed by the anesthesia care team due to rapid hospital turnover of outpatients (i.e., same-day surgery) and delayed onset of symptoms characteristic of nerve palsy. Furthermore, residual anesthesia can hinder accurate neurological examinations and the characteristic delayed onset of symptoms. This review identifies the clinical signs and symptoms associated with HNP that help define its differential diagnosis. The current literature was reviewed for factors associated with the HNP diagnosis, including demographics, predisposing anatomical findings and procedural and airway-related characteristics. Management options, expected clinical course and factors affecting recovery duration as well as recommendations on preventive measures conclude the review.
Methods
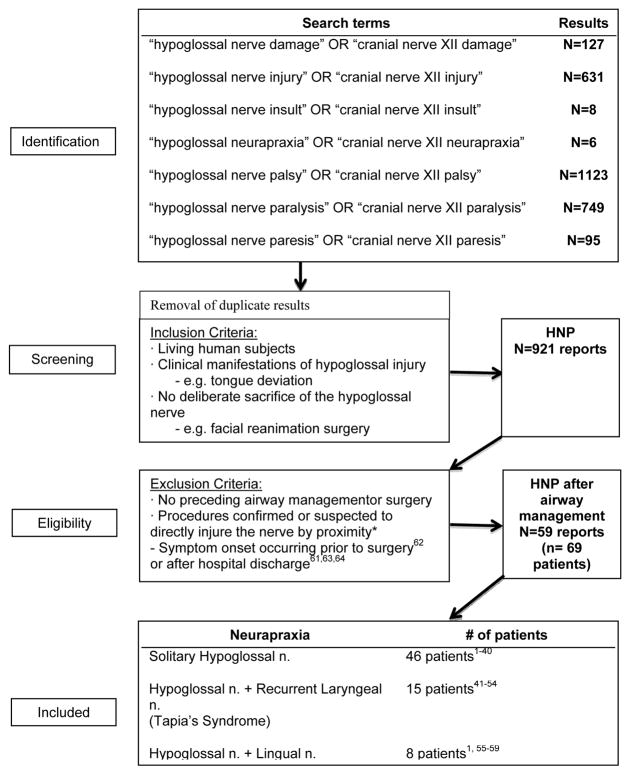
We searched the National Library of Medicine (PubMed) and MEDLINE databases for publications reporting on patients manifesting symptoms of hypoglossal nerve injury after procedural airway management from 1926 through 2013. (Appendix Figure 1) Reports of airway management techniques containing endotracheal tube (ETT), supraglottic devices such as the laryngeal mask airway (LMA), and the Combitube were included1–59 (Table 1). Cases in which hypoglossal nerve injury was likely due to the surgery itself, noted preoperatively or weeks after surgery, were excluded.60–63 We obtained demographic (age, gender), surgical (type of surgery and specific procedure, positioning, anesthetic duration), airway and anesthetic management details (laryngoscope blade type and size, ETT or LMA size, cuff pressure or volume, side of tube securement, use of nitrous oxide (N2O)), as well as neurapraxia course and time of onset. Recovery status was ascertained from each case report based on clinical observations of complete resolution (i.e., no further tongue deviation or symptoms), partial improvement (resolved tongue deviation with persistent symptoms), or no recovery (persistent tongue deviation and symptoms). The time until recovery or final clinic encounter (for patients without recovery) was recorded as the number of days after the procedure was completed. Information regarding neuromuscular blockade monitoring and recovery were inconsistently reported and thus excluded. Cases were included regardless of whether the above-stated characteristics were mentioned in each individual article. We retrieved HNP patient payment data from the American Society of Anesthesiologists Closed Claims database (1980-present), for which the data analysis methods have been previously reported.64
Appendix Figure 1.
Literature search results for hypoglossal nerve palsy (HNP) after procedural airway management. The subgroups of combined neurapraxias, in addition to solitary HNP, and their respective patient counts are listed. * schwannoma resections, parapharyngeal/carotid body tumor resections, neck dissections, carotid endarterectomies and reconstructive procedures, neck dissections and parathyroid excisions.s33–s77
Table 1.
Characteristics of hypoglossal nerve palsy (HNP) patients after procedural airway management
| Demographics | |
|---|---|
|
| |
| Age (N=66) | Mean: 40.9 +/− 17.3 years (Range: 9 months – 74 years) Unspecified: 3 patients |
| Gender (N=66) | Male: 47 patients1–44 Female: 19 patients1,45–57 Unspecified: 3 patients |
| Laterality (N=64) | Left: 28 patients2,5,9,12,13,16,18,19,21,23,25,26,30,32,34,36,40,43,46,48,49,53,55,56 Right: 29 patients1, 3, 4, 8, 10, 11, 14, 15, 17, 20, 22,24,31,37–39,41,42,44,45,47, 50, 52, 54 Bilateral: 7 patients6,7,27–29,33,35 Unspecified: 5 patients |
|
| |
| Airway Type (N=69) | ETT: 57 patients1–18,20,22,23,25,27,30,33,35,36,38–49,51–53,55–59 LMA: 11 patients19, 21,24,26,28,29,31,32,34,37,50 Combitube: 1 patient54 |
|
| |
| Laryngoscope Blade Type (N=12) | Macintosh: 12 patients2,7,8,15,27,30,45,47,48,52,54,57 Unspecified: 57 patients |
|
| |
| Airway Size (N=33) | |
| ETT size (N=21) | Median: 8mm (Range 7–9mm)2,5,7,10–12,15,16,27,30,36,39,44–48,52,57 |
| LMA size (N=11) | Median: 4 (Range 1.5–5) 19,21,24,26,28,29,32,50 |
| Combitube (N=1) | 37 French54 Unspecified: 36 patients |
|
| |
| Operative Duration (N=37) | Mean: 131.0 +/− 77.2 minutes (range: 25–330 minutes) Unspecified: 32 patients |
|
| |
| Symptom Onset (N=33) | |
| POD0 | 15 patients7,11,16,19,23,25,26,28,31,34,36,44,46–48 |
| POD1 or thereafter | 18 patients1,2,10–12,15,22,24,32,33,39,40,42,45,50,52,54,57 Unspecified: 36 patients |
|
| |
| Treatment (N=14) | |
| Corticosteroids | 8 patients7,24,26,28,33,42,43,46 |
| Vitamin B Complex | 1 patient8 |
| Combined treatment | 5 patients2,5,15,50,53 Unspecified: 55 patients |
|
| |
| Recovery Status (N=65) | |
| Complete | 45 patients (69.2%) 1,2,5–8,10–12,14,16,17,19–32,34–36,38–40,42,44–48,50,52,54,57 Median follow-up*: 7 weeks; Range: 6 days – 6 months |
| Partial | 11 patients (16.9%) 1,9,15,18,33,37,41,43,49,53 Median follow-up: 7 weeks; Range: 2 weeks – 30 months |
| None | 9 patients (15%) 1,3,4,13,55,56 Median follow-up**: 11 months; Range: 4 weeks – 12 months Unspecified: 4 patients |
“N” represents the number of patient reports with the available information relevant to each field. No reported follow-up interval on 1 patient with complete recovery (*) and 4 patients with no recovery (**) POD = postoperative day; ETT = endotreacheal tube; LMA = laryngeal mask airway.
Statistical Analysis
Descriptive data are presented as mean with standard deviation (SD) and as percentage where appropriate. Patients were grouped based on the recovery status of their tongue deviation at time of follow-up (complete or partial recovery vs. no recovery), and analyzed using a Kaplan-Meier survival technique. Using the statistical software R version 3.0.0 (R Foundation for Statistical Computing, Vienna, Austria), we conducted a log-rank test to compare the time-to-recovery curves between airway type, gender, diagnosis (isolated vs multiple cranial nerves) and treatment subgroups. Kaplan-Meier summary statistics provide the mean and median times to recovery, and the times to recovery for the 25th, 50th and 75th patient quartiles. For patients with complete or partial recovery, a Pearson’s correlation coefficient was calculated to examine the relationship between age or operative duration and the reported follow-up interval.
Results and Discussion
HYPOGLOSSAL NERVE PALSY: AIRWAY-RELATED MECHANISMS AND CLINICAL MANIFESTATIONS
Diagnosis
We identified 59 publications reporting 69 patients with HNP after procedural airway management in the literature through 2013 (Table 1). Diagnoses include isolated unilateral or bilateral HNP (n=46), as well as combined hypoglossal-lingual nerve neurapraxia (n=8) or hypoglossal-recurrent laryngeal neurapraxia (Tapia’s syndrome) (n=15).
Clinical symptoms of HNP are nonspecific and include dysarthria (difficulty with articulation), dysphagia, and even dyspnea. On examination, unilateral deviation and elevation of the tongue ipsilateral to the injured side are pathognomonic for hypoglossal nerve injury and can be attributed to paralysis of the superior and inferior longitudinal muscles.65 Later physical examination findings revealed unilateral atrophy and genioglossus muscle fasciculation, signifying denervation-reinnervation injury.66,67
Radiographic imaging, including computed tomography and magnetic resonance imaging, can help exclude ischemic stroke and hemorrhage, and provides confirmation of both supraglottic airway trauma and tongue atrophy68,69(Figure 1). Extracranial Doppler and ultrasound studies can aid in the diagnosis of vascular dissection as a cause of HNP.11,16 In persistent cases of HNP, electromyographic and nerve conduction studies demonstrated damage to the neural elements, a pathology that is not typical of transient neurapraxia.6
Figure 1.
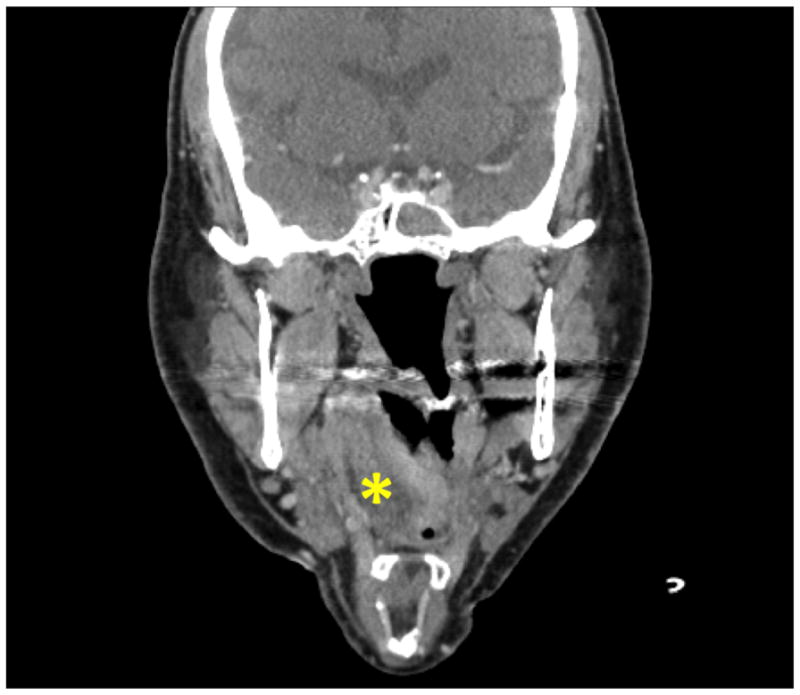
Non-contrast computed tomography (CT) scan of the neck in a patient with hypoglossal nerve palsy (HNP). Asymmetric heterogeneous soft tissue swelling in the right anterolateral oropharynx, marked with an asterix (*), extending from base of the tongue to vallecula, is seen on the coronal section.
Proposed HNP Mechanisms
Most reported cases of HNP after airway management suggest involvement of the extracranial section of the hypoglossal nerve, which exits the skull through the hypoglossal canal and descends caudally, along with the internal carotid artery and jugular vein. At the mandibular angle, it passes anteriorly, deep to the posterior belly of the digastric muscle and reaches the submandibular region to enter the tongue.67 At the undersurface of the tongue, numerous branches pass upward to supply its intrinsic muscles.11,29,67 The 4 mechanisms of injury leading to HNP proposed in the literature are described in Figure 2. Tapia’s syndrome (unilateral recurrent laryngeal nerve and hypoglossal nerve paralysis), a subset of hypoglossal nerve injury, is attributed to compression injury to intersecting extracranial fibers of both the hypoglossal and vagus nerves at the base of the tongue.13,18,33,37,53
Figure 2.
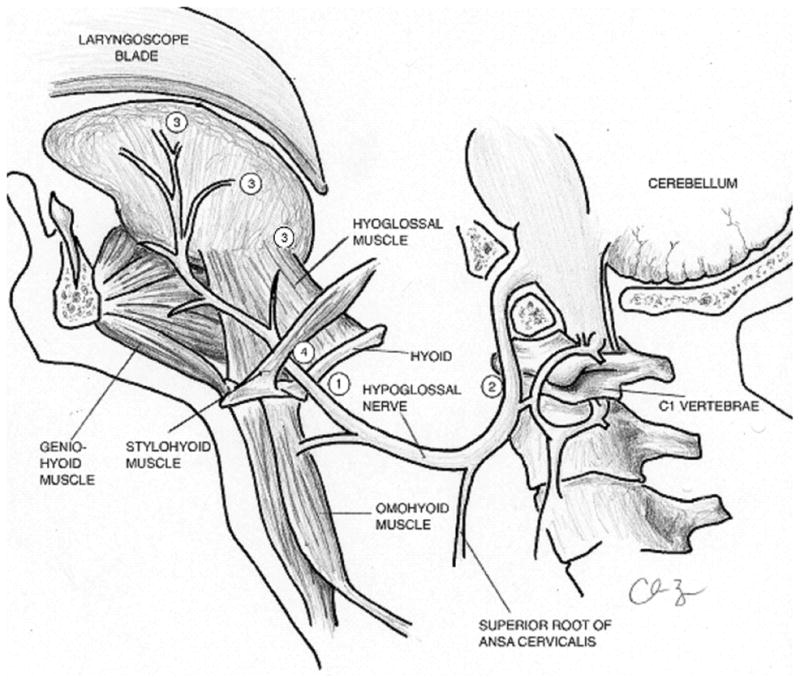
Anatomic locations for hypoglossal nerve injury during airway management. (1) Nerve compression or impingement can occur at the hyoid bone where the nerve is relatively superficial in its course. 11, 23, 47, 48, 50, 79 (2) Nerve stretching can occur at the lateral aspect of the transverse process of the first cervical vertebrae (C1). (3) Pressure exerted by the laryngoscope blade can lead to lateral retraction and shearing of the distal nerve fibers that supply motor input to the tongue. (4) A calcified stylohyoid ligament has also been reported in association with hypoglossal nerve impingement. Drawing courtesy of Dr. C. Barnes.
PATIENT CHARACTERISTICS
Demographics
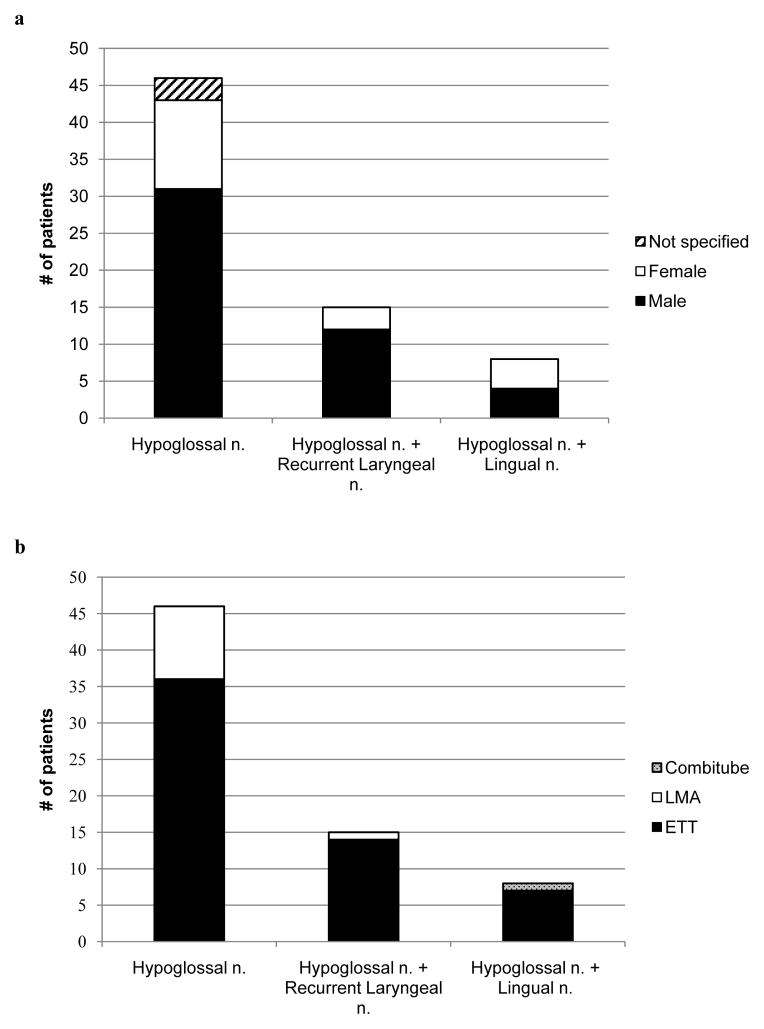
Table 1 and Figure 3a present the demographics of patients with HNP after procedural airway management. The majority of patients with isolated hypoglossal neurapraxia and Tapia’s syndrome are male. No differences in demographics are seen between cases of solitary hypoglossal injury and combined cranial nerve neurapraxia. Although reporting bias must be considered, morphometric and forensic studies of the hyoid bone demonstrate greater absolute dimensions in males.70,71 Ito et al. show that males have a longer length of the greater cornu (33.8 mm vs 29.8 mm), larger hyoid volume (4.31 cm3 vs 2.95 cm3) and exhibit earlier ossification of the connection between the hyoid body and greater cornu. Given these anatomical differences, male patients are more likely to experience hypoglossal nerve compression at the hyoid cornu level.
Figure 3.
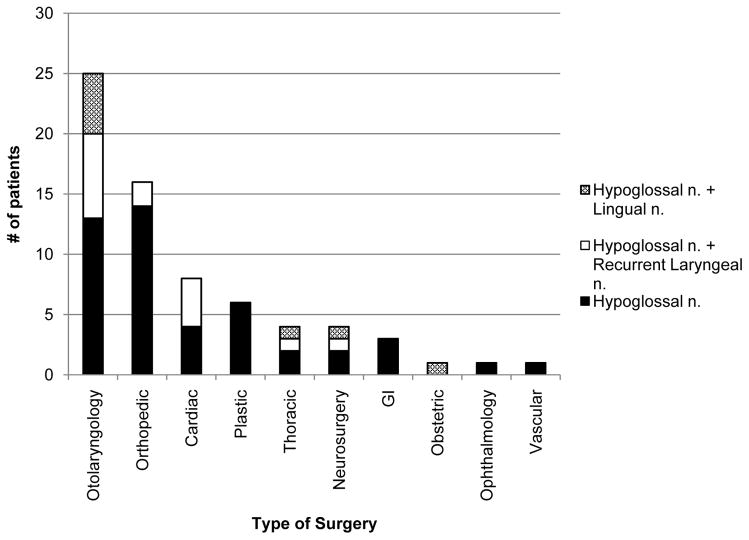
Figures 3a–c. Distribution of hypoglossal nerve palsy (HNP) diagnoses. Gender (a), airway type (b), and surgery type (c) subgroups are separately delineated within each stacked column. Patients of male gender or those receiving an endotracheal tube composed the majority of reported hypoglossal neurapraxia cases. Twelve of 25 (48%) otolaryngology operations and 4 of 8 (50%) cardiac surgeries were associated with multiple cranial nerve palsies. LMA = laryngeal mask airway; ETT = endotracheal tube.
There does not appear to be any specific age range associated with anecdotal reports of HNP. Harnett et al. found a higher incidence of minor airway complications in infants receiving a LMA,72 and multiple authors report postoperative HNP after LMA placement in adolescents.26,28,31 On the other hand, several authors report findings of a calcified stylohyoid ligament on radiographic imaging in elderly patients.17,30,38,50,59 In addition, Nagai et al. report a patient with rheumatoid arthritis, which is known to cause cervical (C1-C2) joint instability and bony ligamentous abnormalities.50 It is conceivable that these anatomical abnormalities could stretch the nerve at the angle of the mandible and cause HNP. Additionally, a short neck may predispose to nerve stretching during laryngoscopy.1,34
Laterality of HNP
An earlier report of HNP suggests that right-sided neurapraxias aremore common, a finding that was originally attributed to the fact that most anesthesiologists are right-handed.8 Nevertheless, most operators would use their right hand to introduce the ETT into the trachea regardless of their handedness. Theoretically, pressure exerted from the laryngoscope blade could predispose to unilateral hypoglossal injury when sweeping the tongue from the right to left before intubation. However, multiple subsequent case reports have demonstrated the bilateral incidence of this neurapraxia. Moreover, in those reports that mention the tube being taped to the right side, there is an equal prevalence of neurapraxic symptoms on either side.7,15,16,30,52
AIRWAY MANAGEMENT CHARACTERISTICS
Intubation
Various case reports address airway management strategies including tube size, method of laryngoscopy, and blade type/size. All studies that provide laryngoscope information for orotracheal intubation report the use of a Macintosh blade, either size 3 or 4.2,7,8,15,27,30,45,47,48,52, 54,57 Figure 3b displays the reported airway management techniques that were used in patients who subsequently developed isolated or multiple cranial nerve neurapraxia. In their review, Dziewas and Ludemann show that HNP occurs after direct laryngoscopy and endotracheal intubation, LMA placement, and even after bronchoscopy.11 Zamora and Saha discuss HNP after Combitube placement.54 We found a greater number of patients with HNP after ETT placement. During orotracheal intubation, neck hyperextension stretches the hypoglossal nerve on the anterior aspect of the C1 transverse process by as much as 1.3 cm. In addition, direct pressure exerted by the Macintosh blade at the base of the tongue causes soft tissue compression against the hyoid bone, possibly exacerbating the neurapraxia.1,3,12,15,26,30,46,49,73,74
Cuff Insufflation
Some authors suggest that ETT cuff pressure and LMA cuff insufflation may be associated with HNP, suggesting injury at the hyoid bone.21,29,31,32,37,45,53,54 Seven patients were reported to have received at least 30 minutes of N2O as part of their anesthetic management, which would predispose to diffusion of N2O into the cuff resulting in increased cuff pressures.12,19,29,32,36,45,50
ETT cuff pressure
In 9 patients, the ETT cuff pressure was maintained < 20 cmH2O, 2,7,10,12,16,22,27,52 while in 1 patient, the intracuff pressure was maintained at 30 cmH2O before surgical draping.10 Al-Benna described hypoglossal nerve injury in a patient with a maximum measured ETT cuff pressure of 34 cmH20.45 While there is an anatomical disparity between the location of the inflated ETT cuff and the hypoglossal nerve, it is possible that ETT cuff-related damage may be explained by anatomical variants, such as a low-looping hypoglossal nerve or tongue innervation from the superior root of the ansa cervicalis.
LMA cuff volume
Eight cases with LMA use mention cuff insufflation volume in the range of 15–40 ml. of air,19,21,28,29,31,32,37,50 but did not mention goals for cuff pressure titration or intraoperative monitoring. Lumb and Wrigley demonstrated that LMA cuff pressures can increase by as much as 50% during brief periods of N2O anesthesia.75 Similarly, Trumpelmann and Cook reported on an overdistended LMA cuff after removal in a patient who had received N2O during anesthesia.32 Although the cuff insufflation volume varies considerably by LMA size and type, these unanticipated increases in cuff volume during longer cases can compress the hypoglossal nerve against the hyoid bone and cause HNP symptoms.
SURGICAL CHARACTERISTICS
Operative Duration and Reintubation
Anesthetic and procedural duration in the reported cases vary (Table 1). Aside from the complications associated with prolonged intubation, no studies have evaluated its relative contribution specifically to HNP. On the other hand, repeated airway management attempts,5,29 intra- and postoperative reintubations,6,27,28,40,57 and prolonged ventilatory support27,35,40 increase the risk of iatrogenic trauma to the airway mucosa and underlying nerve structures. Three patients required reintubation due to respiratory failure.6,27,40 Two additional patients required LMA replacement28 or conversion to ETT34 for preoperative supraglottic airway device dislocation. All 5patients with bilateral isolated HNP included1 of these factors of complex airway management.6,27–29,35
Surgical Considerations
Surgical subspecialties associated with subsequent isolated HNP or combined neurapraxias are displayed in Figure 3c and listed in Appendix Table 1. However, HNP is frequently reported after otolaryngologic surgery. Dysarthria and ipsilateral tongue deviation are mentioned after rhinoplasty3,7,22,38,52 and sinus surgery,30,34 as well as after tonsillectomy1,4,49,51,58,59 and periglottic excisions.1,8,14 Throat pack placement during these surgeries can create pressure at the greater cornu of the hyoid,2 and their frequent use is linked to combined hypoglossal-recurrent (Tapia’s syndrome) and lingual nerve palsies.12,22,52 Similarly, hematoma and other postsurgical upper airway swelling can result in delayed symptoms and dysarthria due to nerve compression.9,10,19,76 The otolaryngology team can detect tongue deviation in patients with subclinical HNP (i.e., without symptomatic dysarthria) through frequent routine neurologic examinations that arenot consistently used in other specialties, contributing to an increased diagnostic rate and reporting bias.
Appendix Table 1.
HNP after Procedural Airway Management, 1926–2014
| Author | Age | Sex | Primary diagnosis |
Surgery | Position | Surgery length (min.) |
Blade | Airway | Size (ETT or LMA) |
Cuff pressure (cmH20) or volume (ml) |
Side taped (ETT) |
Side | N20? | Associated lingual/ recurrent injuries |
Recovery | Symptom onset |
Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Benna 201345 | 24 | F | breast ptosis | breast augmentation | 90 | Mac 3 | ETT | 7.5 | R | Yes | No | 2 weeks, Complete | POD1 | ||||
| Haslam 201347 | 56 | F | osteoarthritis | TSA | beach chair | Mac 3 | ETT | 7 | R | R | No | No | 6 days, Complete | POD0 | |||
| Pariente 201339 | 62 | M | OA | shoulder arthroplasty | beach chair | 200 | ETT | 7.5 | R | No | No | 4 weeks, Complete | POD3 | ||||
| Varedi 20132 | 27 | M | zygomatic complex fracture | ORIF | Mac 3 | ETT | 7 | 20 cmH2O | L | No | Recurrent laryngeal (Tapias) | 9 months, Complete | POD1 | Vitamin B, cortico-steroids | |||
| Weissman 201340 | 34 | M | Burn, 4-% TBSA (20% full thickness) | multiple debridements | supine | ETT | L | No | 4 weeks, Complete | POD1 | |||||||
| Lykoudis 201222 | 32 | M | open rhinoplasty | ETT | <20 cmH20 | R. | No | Recurrent laryngeal (Tapias) | 4 months, Complete | POD1 | |||||||
| Nalladaru 201242 | 49 | M | CAD | CABG | ETT | R | Recurrent laryngeal (Tapias) | 10 weeks, Complete | POD1 | cortico-steroids | |||||||
| Turan 201233 | 15 | M | ALL | tracheostomy | ETT | R+L | No | Recurrent laryngeal (Tapias) | 14 days, Partial | POD21 | cortico-steroids | ||||||
| Wadelek 201237 | 57 | M | impingement syndrome | arthroscopic acromioplasty | semi-supine | 70 | LMA | 4 | 30 ml. air | R | Recurrent laryngeal (Tapias) | 3 months, Partial | |||||
| Trujillo 201131 | 0.8 | M | b/lretino-blastoma | EUA, laser tx OU cryotherapy OS | supine, neutral | 45 | N/A | LMA | 1.5 | minimum volume | R | No | No | 3 weeks, Complete | POD0 | ||
| Park 201141 | 42 | M | cerval spine herniated disk | C3-4 discectomy | ETT | R | Yes | 2 months, Partial | |||||||||
| Rotondo 200943 | 72 | M | aortic valvular disease | AVR, MVR | supine | ETT | L | Recurrent laryngeal (Tapias) | 3 months, Partial | cortico-steroids | |||||||
| Hung 200916 | 57 | M | rotator cuff tear | RCR, arthroscopy | beach-chair | 108 | ETT | 7.5 | <20 cmH2O | R | L. | No | No | 3 weeks, Complete | POD0 | ||
| Lopes 200948 | 36 | F | N/A | breast reduction, abdominoplasty | semi-sitting (60)(120m) --> dorsal decubitus (150m) | 270 | ETT | 7 | L | No | No | 6 months, Complete | POD0 | ||||
| Lopes 200948 | 64 | F | s/p mastectomy for breast CA | breast reconstruction | lateral decubitus (160m) --> sitting position (120m) supine, 20 degrees | 330 | Mac 3 | ETT | 7.5 | L | No | No | 6 months, Complete | ||||
| Hong 200915 | 37 | M | cholelithiasis | laparoscopic CCY | semi- upright | 85 | Mac 4 | ETT | 7.5 | 22cm, R | R. | No | No | 8 weeks, Partial | POD1 | cortico- steroids, vitamin B | |
| Kashyap 200918 | 41 | M | Fractures of R. parasymphis and L. condyle of mandible | ORIF facial fractures | ETT | L | Recurrent laryngeal (Tapias) | 16 months, No | |||||||||
| Rhee 200825 | 41 | M | R. traumatic shoulder dislocation | Bankart repair | beach chair, 70 --> 30 for Bankart repair | 130 | ETT | L | No | 6 weeks, Complete | POD0 | ||||||
| Rhee 200825 | 71 | M | wear and tear | mini-open rotator cuff repair, arthroscopy | beach chair, 70 --> 30 for Bankart repair | 120 | ETT | L | No | 12 weeks, complete | |||||||
| Rodriguez Ogando 200826 | 15 | M | SVT | electro-physiologic study, RFA | supine | N/A | LMA | 4 | N/A | L | No | No | 15 days, Complete | POD0 | cortico-steroids | ||
| Zamora 200854 | 24 | F | pregnancy- induced hypertension | caesarean delivery | supine/LUD | 180 | Mac 3, 4 | Combi-tube | 37 Fr | 85 ml. air (pharyngeal), 12 ml. air (distal) | N/A | R | No | Lingual | 3 months, Complete | POD1 | |
| Nam 200724 | 51 | M | ulnar nerve palsy | ORIF supracondylar fx | supine | 175 | N/A | LMA | 4 | 60–70 cmH2O | N/A | R | No | No | 12 days, Complete | POD1 | cortico-steroids |
| Yelken 200738 | 22 | M | difficulty breathing | septoplasty | supine, routine neck flexion | 120 | ETT | R | No | 2 months, Complete | |||||||
| Sotiriou 200757 | 52 | F | CAD | CABG | supine | Mac 3 | ETT | 8 | Recurrent laryngeal (Tapias) | 4 weeks, Complete | POD1 | ||||||
| Lo 200621 | 48 | M | humerusfx | humerusfx repair | 120 | N/A | LMA | 3 | 10–15 cmH2O | N/A | L | No | 2 weeks, Complete | ||||
| Batjom 200646 | 33 | F | N/A | b/l breast enhancement | semi-sitting | 90 | ETT | 7 | L | No | 2 months, Complete | POD0 | cortico-steroids | ||||
| Soyal 200644 | 32 | M | rheumatic heart disease | AVR, MVR | supine | 300 | ETT | 8 | R | No | 3 months, Complete | POD0 | |||||
| Tesei 200652 | 30 | F | N/A | rhinoplasty | semirecum bent | 100 | Mac 3 | ETT | 7 | <20 cmH2O | R | R | No | Recurrent laryngeal (Tapias) | 4 weeks, Complete | POD1 | |
| Una 200635 | 28 | M | yolk sac tumor | diagnostic mediatin- oscopy | supine | N/A | ETT | R+L | No | 4 months, Complete | |||||||
| Bramer 20066 | 63 | M | sigmoid colon CA | hemicolectomy | supine | ETT | R+L | No | No | 7 months, Complete | |||||||
| Cinar 20057 | 20 | M | N/A | rhinoplasty | semirecum bent | 180 | Mac 4 | ETT | 8.5 | <20 cmH2O | middle | R+L | No | Recurrent laryngeal (Tapias) | 4 weeks, Complete | POD0 | cortico-steroids |
| Trumpelman 200532 | 28 | M | comminuted tibia/fibul a fracture | ORIF | supine | 210 | N/A | LMA | 5 | 40 ml. air | L | Yes | No | 4 months, Complete | POD1 | ||
| Sommer 200428 | 15 | M | scar tissue behind ears | excision | extreme side-rotation of head | 180 | N/A | LMA | 4 | air | R+L | No | No | 4 weeks, Complete | POD0 | cortico-steroids | |
| Yavuzer 200453 | 42 | F | septal deviation, dorsal nasal hump | septo-rhinoplasty | 65 | ETT | midline | L | Recurrent laryngeal (Tapias) | cortico-steroids, vitamin B6-B12 | |||||||
| Dogan 20039 | 56 | M | CAD, MI | CABG | supine | ETT | L | No | 3 months, Partial | ||||||||
| Boisseau 20025 | 42 | M | recurrent dislocation of Glenohumeral joint | arthroscopy | upright sitting | 130 | ETT | 8 | L | Recurrent laryngeal (Tapias) | 3 months, Complete | cortico- steroids, vitamin B1-B6 | |||||
| Dziewas 200211 | 32 | M | b/l shoulder dislocation | open repair of L. greater tuberucle of humerus | 75 | ETT | 8 | R. | No | No | 1 week, Complete | POD0 | |||||
| Dziewas 200211 | 74 | M | esophageal perforation | esophageal resection, esophago-gastrostomy | 285 | ETT | 8 | R. | No | No | 2 week, Complete | POD1 | |||||
| Rubio- Nazabal 200227 | 63 | M | AAA rupture | aneurysm excision, graft | supine | 240 | Mac | ETT | 8 | <20 cmH2O | R | R+L | No | No | 3 months, Complete | ||
| Stewart 200229 | 54 | M | OA | knee arthroscopy | 45 | N/A | LMA | 5 | 40 ml. air | N/A | R+L | Yes | No | 6 weeks, Complete | |||
| Umapathy 200134 | 46 | M | sinus issues | sinus surgery | LMA | 4 | L | No | 6 weeks, Complete | POD0 | |||||||
| Drouet 199910 | 20 | M | Recurrent L. shoulder dislocation | shoulder surgery (thrust) | hyper- extension- inflexion, rotate 30 degrees to right | 78 | ETT | 8 | 30 cmH2O | R. | No | 2 months, Complete | POD2 | ||||
| Evers 199912 | 56 | M | acromegaly | hypo-physectomy | supine | 180 | ETT | 9 | <20 cmH2O | L. | Yes | lingualis | 4 months, Complete | POD3 | |||
| Sengupta 199956 | 35 | F | tuberculosis/Pott’s disease | C3-4 corpectomy w/graft, C2-5 plating | ETT | L | No | 18 months, No | |||||||||
| Streppel 199730 | 35 | M | sinus issues | paranasal sinus surgery | supine | 85 | Mac 4 | ETT | 9 | R | L. | No | 4 weeks, Complete | ||||
| Venkatesh 199736 | 65 | M | CDH | b/l CDH drainage | supine | ETT | 8 | side | L. | Yes | No | 6 days, Complete | POD0 | ||||
| Baum-garten 19973 | 45 | M | septum deviation | nasal septoplasty | bronchoscope | ETT | R. | No | Unknown, No | ||||||||
| Condado 19948 | 44 | M | vocal cord hyperplasia | DL, excision | 70 | Mac | ETT | R. | lingualis | 1 month, Complete | B1-B6-B12 | ||||||
| King 199419 | 55 | M | humerusfx | orthopedic removal of Rush pins | 25 | N/A | LMA | 4 | 25 ml. air | L | Yes | No | 8 days, Complete | POD0 | |||
| Nagai 199450 | 62 | F | rheumatoid arthritis | L. TSA | Supine -- > R. lateral (donut- pillow and soft cushions) | 180 | N/A | LMA | 3 | 20 ml. air | R. | Yes | No | 1 week, Complete | POD1 | cortico- steroids, vitamin B12 | |
| Smoker 199351 | 17 | F | unknown | tonsillectomy | ETT | No | |||||||||||
| Mullins 199223 | 40 | M | L. rotator cuff tear | RCR, arthroscopy | beach chair (70 degrees), down to 30 degrees for Bankart repair | 70 | ETT | L. | No | 8 weeks, Complete | POD0 | ||||||
| Donati 199155 | 3 | F | recurrent tonsillitis | tonsillectomy | ETT | L | No | 6 months, No | |||||||||
| Donati 199155 | 12 | F | recurrent tonsillitis | tonsillectomy | ETT | L | No | 11 months, No | |||||||||
| Michel 199049 | 42 | F | tonsillitis | tonsillectomy | ETT | L. | No | 30 months, Partial | |||||||||
| Gelmers 198313 | 41 | M | CAD | CABG | 180 | ETT | L | Recurrent laryngeal (Tapias) | 12 months, No | ||||||||
| Gelmers 198313 | 36 | M | bronchiectasis | thoracotomy | 120 | ETT | L | Recurrent laryngeal (Tapias) | 12 months, No | ||||||||
| Boenninghaus 19824 | 36 | M | tonsillitis | tonsillectomy | ETT | R. | No | Unknown, No | |||||||||
| Hinze 197614 | 27 | M | vocal cord polyp | DL, excision | ETT | R. | lingualis | 3 months, Complete | |||||||||
| Bumm 197458 | tonsillectomy | ETT | No | ||||||||||||||
| Bumm 197458 | bronchoscopy | ETT | No | ||||||||||||||
| Agnoli 19701 | 48 | F | vocal cord hyperplasia | DL, excision | 40 | ETT | R. | lingualis | 4 weeks, Complete | POD1 | |||||||
| Agnoli 19701 | 24 | F | tonsillectomy | ETT | R. | lingualis | Unknown, No | ||||||||||
| Agnoli 19701 | 57 | F | DL, excision | ETT | R. | lingualis | Unknown, No | ||||||||||
| Agnoli 19701 | 71 | F | vocal cord hyperplasia | DL, excision | 65 | ETT | R. | No | 13 weeks, Partial | ||||||||
| Agnoli 19701 | 53 | M | vocal cord polyposis | DL, excision | 50 | ETT | R. | No | 7 weeks, Partial | ||||||||
| Konrad 196020 | 32 | M | aortic arch abnormality, unspecified | cardiac aortic arch surgery | ETT | R. | No | 12 months, Complete | |||||||||
| Kaess 195517 | 58 | M | lung disease, unspecified | diagnostic bronchoscopy | ETT | R. | lingualis | 6 weeks, Complete | |||||||||
| Guthrie 192659 | unknown | tonsillectomy | bronch-oscope | ETT | No |
ALL = acute lymphoblastic leukemia, AAA = abdominal aneurysm repair, b/l = bilateral, CA = cancer, CAD = coronary artery disease, CABG = coronary artery bypass graft, CCY = cholecystectomy, CDH = congenital diaphragmatic hernia, DL = direct laryngoscopy, ETT = endotracheal tube, fx = fracture, LMA = laryngeal mask airway, Mac= Macintosh (blade), MI = myocardial infarction, MVR = mitral valve repair, N2O = nitrous oxide, OA = osteoarthritis, OD = post-operative day, ORIF = open reduction and internal fixation, OSA = obstructive sleep apnea, RCR = rotator cuff repair, RFA = radiofrequency ablation, SVT = supraventricular tachycardia, TBSA = total body surface area
Several authors report HNP after shoulder surgeries.10,11,16,19,21,23,25,50 Neck rotation and undetected head movement underneath the surgical drapes can lead to prolonged traction of the hypoglossal nerve throughout the case.5,16,37,46 During cardiac surgery, neck hyperextension and lateral flexion during sternotomy can compress the ETT cuff against the hypoglossal-recurrent laryngeal nerve, resulting in Tapia’s syndrome.13,42,43,57 Similarly, unanticipated position changes resulting in accidental extubation,36 LMA malposition,28 or change in airway management34(e.g., switching from LMA to ETT) are associated with HNP.
Routine position changes after intubation, such as from semisupine (30 degrees) to the Fowler’s position (70 degrees), can cause pressure injury to the nerve throughout its superficial course anterior to the mandible.7,16,23,25,38,48,52 It is possible that even small position changes after airway securement, including during surgical preparation and draping, could predispose to hypoglossal nerve trauma. Conrardy et al. demonstrated that the ETT cuff can migrate from 3.8 – 6.4 cm with neck flexion or extension during intubation,77 potentially injuring the subglottis. This pattern of trauma would more likely result in recurrent laryngeal nerve injury and dysphonia as in Tapia’s syndrome.
CLINICAL COURSE AND MANAGEMENT
Symptom Onset
HNP is typically diagnosed in a delayed fashion, with more than half of the reported cases diagnosed the day after surgery. Nevertheless, all but 3 patients exhibited tongue deviation by the end of the first postoperative day.10,12,33 Residual anesthesia may interfere with an early diagnosis of neurapraxia. Some patients after ear-nose and throat or general surgery do not exhibit signs or symptoms until their first postoperative day or later.11,15,22,24,32,52 Due to the delayed onset of symptoms, neurapraxia can potentially develop after discharge and remain undiagnosed.
Recovery
HNP appears to be largely self-limited; of 60 patients with a reported recovery status and follow-up interval, 26 patients (43.3%) achieved resolution within 6 weeks after surgery, and an additional 24 patients (40.0%) were symptom-free within 6 months of their operative date. The 25th percentile, median, and 75th percentile are 28, 60 and 120 days, respectively. Several authors reported complete resolution 1 year after diagnosis,6,9,12,14,20,22 while others found only partial recovery at variable follow-up periods.1,15,33,49 Five patients (8.3%) had persistent tongue deviation and dysarthria at follow-up intervals. The follow-up interval was not reported for four additional patients with persistent symptoms.1,3,4,13,55,56 Patients with partial recovery demonstrated similar demographics and operative durations when compared to fully recovered patients. More than half of the patients with partial recovery are associated with Tapia’s syndrome, and remaining neurologic deficits include persistent tongue deviation15 or vocal fold immobility. Patients with isolated or combined cranial nerve neurapraxias (recurrent laryngeal, lingual, or glossopharyngeal nerves) are reported to recover at similar follow-up intervals (p=0.34). However, patients receiving an ETT exhibitedlater recovery postoperatively than patients in whom an LMA was used (p=0.003) (Figure 4). Indeed, the invasive technique (direct laryngoscopy), neck positioning and cuff pressures associated with ETT placement can be more traumatic to the airway mucosa and require longer healing times. In addition, the reported follow-up period for patients exhibiting complete recovery is similar between genders (p=0.09), and age is poorly correlated with the recovery follow-up interval (r=−0.090). There is a moderate positive correlation between operative duration and follow-up interval for patients with reported recovery status (r=0.49).
Figure 4.
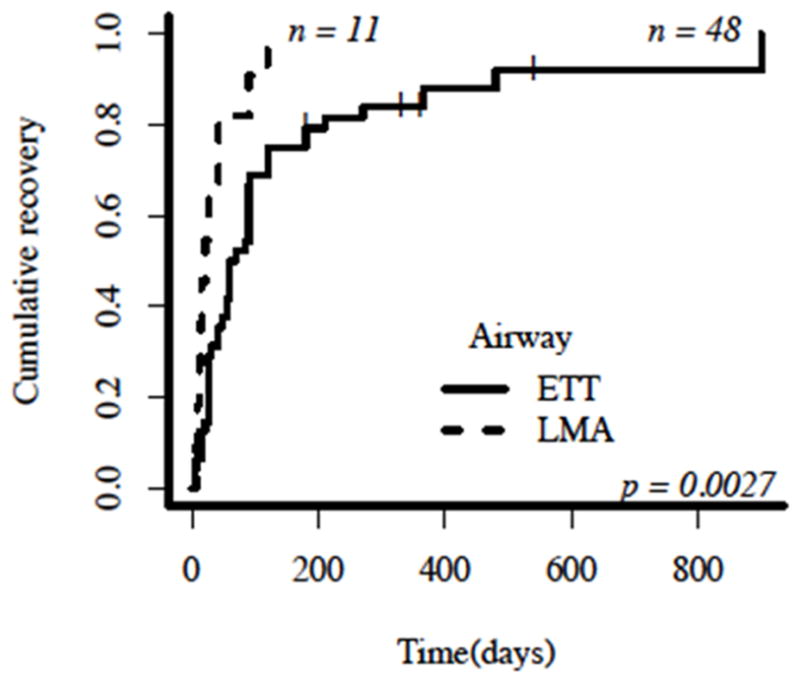
Time-to-event curve demonstrating hypoglossal nerve palsy (HNP) recovery status based on airway management device. A vertical dash (|) represents patients with persistent tongue deviation (no recovery) at the time of follow-up reported in each individual case report; these patients are right-censored as their recovery status after the reported follow-up period is unknown. The x-axis describes the number of days after airway removal until the reported follow-up in each individual case study. The y-axis describes the cumulative recovery represented as the number of patients with a positive recovery status at any follow-up interval divided by the total number of patients in the subgroup. One patient who received Combitube was excluded from the airway subgroup analysis. LMA = laryngeal mask airway; ETT = endotracheal tube.
A few shortcomings must be considered when interpreting these recovery estimates and analyses. The reported data are extracted retrospectively from individual publications and several authors instead of 1 study. In addition, patients may have recovered earlier than the reported follow-up period in each publication, and patients with longer recovery times may be disproportionately represented in this sample of case reports. On the other hand, the correlations between age or operative duration and time to recovery exclude patients with persistent HNP who may have needed longer recovery times past the last recorded encounter.
Possible Preventive Measures
Potentially preventive measures are deduced by the postulated mechanisms of injury, with an emphasis on the use of less invasive methods of airway management (LMA instead of ETT) (Appendix Table 2). Indeed, some authors postulate that routine cuff pressure monitoring could decrease the incidence of HNP after surgery.31,32 Although no neurapraxias were noted in their study of 200 patients receiving an LMA for ambulatory surgery, Seet et al. demonstrated a decrease in dysphagia and dysphonia at 1 hour and 1 day after surgery in patients whose LMA cuff pressures were limited to <60 cmH2O.78 Similarly, Ratnaraj et al. showed that maintaining ETT cuff pressure < 20 cmH2O in patients undergoing cervical spine surgery significantly decreased the incidence of sore throat 24 hours after extubation.79 Intermittent pressure cuff lowering during long operations or pressure-relief valves can decrease the risk of nerve compression,29,75,80 and it follows that LMA or ETT cuff deflation during surgical positioning could also prevent iatrogenic injury to the hypoglossal and recurrent laryngeal nerves, respectively. Bohner et al. described the first use of a nerve stimulator for the successful identification and continuous monitoring of the hypoglossal nerve during an anatomically challenging carotid endarterectomy under general anesthesia.81
Appendix Table 2.
Proposed Measures to Reduce the Risk of HNP Associated with General Anesthesia
| - Use a supraglottic airway device (e.g., LMA) rather than ETT for short procedures (≤2 hours), if deemed safe after individual patient evaluation |
| - Avoid neck hyperextension, traumatic or multiple laryngoscopies by using a fiberoptic intubation technique when these situations are anticipated |
| - Check patient positioning intermittently, with special attention to the patient’s head and airway securement |
| - Implement intermittent cuff pressure monitoring +/− cuff desufflation, especially during longer operations and when nitrous oxide (N2O) is administered. |
| - Initiate early specialty consultation and diagnostic workup of patients with multiple neurologic abnormalities to evaluate for neurovascular abnormalities (e.g., stroke, carotid dissection) |
| - Identify promptly patients with impending airway compromise and triage appropriately (e.g., does the patient need a longer duration of close monitoring, medical treatment, or retinubation?) |
| - Follow-up with outpatients with questionable symptoms or complaints, especially within the first few days after orthopedic and otolaryngology procedures |
Treatment
Supportive measures for HNP during initial evaluation in the immediate postoperative period may include supplemental oxygen and respiratory monitoring. Ear-nose and throat-guided rehabilitation measures include dietary modifications, logopedic treatment and electrical stimulation therapy.82 Corticosteroid therapy has been shown to accelerate spontaneous recovery after Bell’s palsy,83 and multiple authors advocate a short course of high-dose steroids such as prednisone if airway edema is suspected.5,7,15,24,26,28,33,46,50,53 However, there are no controlled studies of the benefits of these treatments on neurapraxic patients after surgery. In our review, patients receiving corticosteroid treatment demonstrated complete or partial recovery at similar follow-up periods compared to nontreated patients.
Closed Claims Data
There are only 4 nonsurgical hypoglossal nerve injury claims in the Anesthesia Closed Claims database (1980-present:10,093 claims). A difficult intubation with pharyngeal injury occurred in 1 claim, and an LMA was used in 2 claims. Three of the nonsurgical injuries were permanent, and 1 temporary. Only 1 of these 4 claims resulted in payment ($30,500 in 2012 inflation-adjusted dollars), a significantly smaller proportion when compared to other surgical anesthesia claims (58%, p=0.012). (Domino KB: Personal communication, 17th March 2014)
Conclusion
Hypoglossal neurapraxia after airway extubation is repeatedly reported after various surgeries. Nerve compression and overstretching can occur during both unexpected and routine position changes, including neck hyperextension for laryngoscopy and surgical positioning. Male patients may be more vulnerable given their larger hyoid bone dimensions. Excessive pressure in the ETT or LMA cuff, perhaps exacerbated by the use of N2O, may produce injurious malposition of the airway devices. Early postoperative detection of tongue deviation and dysarthria, as well as consultation with neurology and otolaryngology consultants, can help exclude other serious etiologies including stroke and carotid dissection. Minimizing airway instrumentation during endotracheal intubation, along with consideration for intermittent pressure monitoring of the ETT cuff and position during long surgical procedures, may decrease the incidence of cranial nerve neurapraxias. While a short course of steroids may decrease swelling after airway removal, further studies need to be performed to ascertain their effect on the incidence of postoperative HNP and the recovery period for neurapraxic patients.
Acknowledgments
Funding: The Institute for Translational Health Sciences (ITHS) at University of Washington, Seattle, WA, provided statistical support for this project. ITHS is funded by Grant UL1TR000423 from the NIH National Center for Advancing Translational Sciences through the Clinical and Translational Sciences Awards Program (CTSA). For more information, please visit: http://www.iths.org/
The authors thank Dr. Karen Domino for providing data from the ASA Closed Claims Database. We also thank Dr. Allan Goldman and Paul Constanthin for their critical review of our manuscript.
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
This report was previously presented, in part, at the Academic Evening, May 6, 2014, University of Washington Department of Anesthesiology & Pain Medicine.
DISCLOSURES:
Name: Aalap C. Shah, MD
Contribution: This author helped prepare the manuscript, conduct the literature review, choose and execute statistical tests
Attestations: I, Aalap C. Shah, approve the final manuscript and attest to the integrity of the analysis reported in the manuscript. I am the archival author.
Name: Christopher Barnes, MD
Contribution: This author helped prepare original artwork, the figure layout, manuscript preparation
Attestations: I, Christopher Barnes, approve the final manuscript and attest to the integrity of the analysis reported in the manuscript.
Name: Charles F. Spiekerman, PhD
Contribution: This author helped prepare the manuscript and choose and execute statistical tests
Attestation: I, Charles Spiekerman, approve the final manuscript and attest to the integrity of the analysis reported in the manuscript.
Name: Laurent A. Bollag, MD
Contribution: This author helped prepare the manuscript and to choose and execute the statistical tests
Attestation: I, Laurent Bollag, approve the final manuscript and attest to the integrity of the analysis reported in the manuscript.
Contributor Information
Aalap C. Shah, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, Washington.
Christopher Barnes, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, Washington.
Charles F. Spiekerman, Institute for Translational Health Sciences (ITHS), University of Washington, Seattle, Washington.
Laurent A. Bollag, Department of Anesthesiology and Pain Medicine, University of Washington, Seattle, Washington.
References
- 1.Agnoli A, Strauss P. Isolated paresis of hypoglossal nerve and combined paresis of hypoglossal nerve and lingual nerve following intubation and direct laryngoscopy. HNO. 1970;18:237–239. [PubMed] [Google Scholar]
- 2.Varedi P, Shirani G, Karimi A, Khiabani K, Bohluli B. Tapia syndrome after repairing a fractured zygomatic complex: A case report and review of the literature. J Oral Maxillofac Surg. 2013;71:1665–1669. doi: 10.1016/j.joms.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Baumgarten V, Jalinski W, Bohm S, Galle E. Hypoglossal paralysis after septum correction with intubation anesthesia. Anaesthesist. 1997;46:34–37. doi: 10.1007/s001010050368. [DOI] [PubMed] [Google Scholar]
- 4.Boenninghaus HG, Denecke U. Paralysis of the hypoglossal nerve after tonsillectomy? (author’s transl) Laryngol Rhinol Otol (Stuttg) 1982;61:189–192. [PubMed] [Google Scholar]
- 5.Boisseau N, Rabarijaona H, Grimaud D, Raucoules-Aime M. Tapia’s syndrome following shoulder surgery. Br J Anaesth. 2002;88:869–870. doi: 10.1093/bja/88.6.869. [DOI] [PubMed] [Google Scholar]
- 6.Bramer S, Koscielny S, Witte OW, Terborg C. Bilateral hypoglossal nerve palsy following intubation. Nervenarzt. 2006;77:204–207. doi: 10.1007/s00115-005-1985-7. [DOI] [PubMed] [Google Scholar]
- 7.Cinar SO, Seven H, Cinar U, Turgut S. Isolated bilateral paralysis of the hypoglossal and recurrent laryngeal nerves (bilateral Tapia’s syndrome) after transoral intubation for general anesthesia. Acta Anaesthesiol Scand. 2005;49:98–99. doi: 10.1111/j.1399-6576.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 8.Condado MA, Morais D, Santos J, Alonso-Vielba J, Miyar V. Hypoglossal nerve paralysis after intubation and direct laryngoscopy. Acta Otorrinolaringol Esp. 1994;45:477–479. [PubMed] [Google Scholar]
- 9.Dogan M, Erdal O. Isolated unilateral hypoglossal nerve paralysis: A report of two cases. Kulak Burun Bogaz Ihtis Derg. 2003;11:125–128. [PubMed] [Google Scholar]
- 10.Drouet A, Straboni JP, Gunepin FX. Paralysis of the hypoglossal nerve after orotracheal intubation for general anesthesia. Ann Fr Anesth Reanim. 1999;18:811–812. doi: 10.1016/s0750-7658(00)88462-2. [DOI] [PubMed] [Google Scholar]
- 11.Dziewas R, Ludemann P. Hypoglossal nerve palsy as complication of oral intubation, bronchoscopy and use of the laryngeal mask airway. Eur Neurol. 2002;47:239–243. doi: 10.1159/000057906. [DOI] [PubMed] [Google Scholar]
- 12.Evers KA, Eindhoven GB, Wierda JM. Transient nerve damage following intubation for trans-sphenoidalhypophysectomy. Can J Anaesth. 1999;46:1143–1145. doi: 10.1007/BF03015523. [DOI] [PubMed] [Google Scholar]
- 13.Gelmers HJ. Tapia’s syndrome after thoracotomy. Arch Otolaryngol. 1983;109:622–623. doi: 10.1001/archotol.1983.00800230058014. [DOI] [PubMed] [Google Scholar]
- 14.Hinze FLH. Kombiniertehypoglossus/lingualis-schädigungnachdirekterlaryngoskopie. Akt Neurol. 1976:233–235. [Google Scholar]
- 15.Hong SJ, Lee JY. Isolated unilateral paralysis of the hypoglossal nerve after transoral intubation for general anesthesia. Dysphagia. 2009;24:354–356. doi: 10.1007/s00455-008-9197-5. [DOI] [PubMed] [Google Scholar]
- 16.Hung NK, Lee CH, Chan SM, Yeh CC, Cherng CH, Wong CS, Wu CT. Transient unilateral hypoglossal nerve palsy after orotracheal intubation for general anesthesia. Acta Anaesthesiol Taiwan. 2009;47:48–50. doi: 10.1016/S1875-4597(09)60022-9. [DOI] [PubMed] [Google Scholar]
- 17.Kaess H. Transitory hypoglossal paralysis following bronchoscopy. HNO. 1955;5:115–116. [PubMed] [Google Scholar]
- 18.Kashyap SA, Patterson AR, Loukota RA, Kelly G. Tapia’s syndrome after repair of a fractured mandible. Br J Oral Maxillofac Surg. 2010;48:53–54. doi: 10.1016/j.bjoms.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 19.King C, Street MK. Twelfth cranial nerve paralysis following use of a laryngeal mask airway. Anaesthesia. 1994;49:786–787. doi: 10.1111/j.1365-2044.1994.tb04452.x. [DOI] [PubMed] [Google Scholar]
- 20.Konrad RM, Lakomy J. Combined peripheral hypoglossal paralysis after intubation anesthesia. Anaesthesist. 1960;9:206–208. [PubMed] [Google Scholar]
- 21.Lo TS. Unilateral hypoglossal nerve palsy following the use of the laryngeal mask airway. Can J Neurol Sci. 2006;33:320–321. doi: 10.1017/s0317167100005217. [DOI] [PubMed] [Google Scholar]
- 22.Lykoudis EG, Seretis K. Tapia’s syndrome: An unexpected but real complication of rhinoplasty: Case report and literature review. Aesthetic Plast Surg. 2012;36:557–559. doi: 10.1007/s00266-011-9849-y. [DOI] [PubMed] [Google Scholar]
- 23.Mullins RC, Drez D, Jr, Cooper J. Hypoglossal nerve palsy after arthroscopy of the shoulder and open operation with the patient in the beach-chair position. A case report. J Bone Joint Surg Am. 1992;74:137–139. [PubMed] [Google Scholar]
- 24.Nam SB, Chang CH, Lee YW, Lee JS, Yang HG, Jang DJ. Hypoglossal nerve injury following the use of the Cobra PLA. Eur J Anaesthesiol. 2007;24:556–557. doi: 10.1017/S026502150600216X. [DOI] [PubMed] [Google Scholar]
- 25.Rhee YG, Cho NS. Isolated unilateral hypoglossal nerve palsy after shoulder surgery in beach-chair position. J Shoulder Elbow Surg. 2008;17:e28–30. doi: 10.1016/j.jse.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez Ogando A, Miranda Herrero MC, Avellón Liaño H, Castro de Castro P, Vázquez López M. Hypoglossal nerve palsy as a complication of the use of laryngeal mask airway. An Pediatr. 2008;70(3):312. doi: 10.1016/j.anpedi.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Rubio-Nazabal E, Marey-Lopez J, Lopez-Facal S, Alvarez-Perez P, Martinez-Figueroa A, Rey del Corral P. Isolated bilateral paralysis of the hypoglossal nerve after transoral intubation for general anesthesia. Anesthesiology. 2002;96:245–247. doi: 10.1097/00000542-200201000-00040. [DOI] [PubMed] [Google Scholar]
- 28.Sommer M, Schuldt M, Runge U, Gielen-Wijffels S, Marcus MA. Bilateral hypoglossal nerve injury following the use of the laryngeal mask without the use of nitrous oxide. Acta Anaesthesiol Scand. 2004;48:377–378. doi: 10.1111/j.0001-5172.2004.0332.x. [DOI] [PubMed] [Google Scholar]
- 29.Stewart A, Lindsay WA. Bilateral hypoglossal nerve injury following the use of the laryngeal mask airway. Anaesthesia. 2002;57:264–265. doi: 10.1046/j.1365-2044.2002.02231.x. [DOI] [PubMed] [Google Scholar]
- 30.Streppel M, Bachmann G, Stennert E. Hypoglossal nerve palsy as a complication of transoral intubation for general anesthesia. Anesthesiology. 1997;86:1007. doi: 10.1097/00000542-199704000-00036. [DOI] [PubMed] [Google Scholar]
- 31.Trujillo L, Anghelescu D, Bikhazi G. Unilateral hypoglossal nerve injury caused by a laryngeal mask airway in an infant. Paediatr Anaesth. 2011;21:708–709. doi: 10.1111/j.1460-9592.2011.03572.x. [DOI] [PubMed] [Google Scholar]
- 32.Trumpelmann P, Cook T. Unilateral hypoglossal nerve injury following the use of a proseal laryngeal mask. Anaesthesia. 2005;60:101–102. doi: 10.1111/j.1365-2044.2004.04056.x. [DOI] [PubMed] [Google Scholar]
- 33.Turan I, Yildirim ZK, Tan H. Bilateral Tapia syndrome secondary to oropharyngeal intubation. J Neurosurg Anesthesiol. 2012;24:78. doi: 10.1097/ANA.0b013e31823769ef. [DOI] [PubMed] [Google Scholar]
- 34.Umapathy N, Eliathamby TG, Timms MS. Paralysis of the hypoglossal and pharyngeal branches of the vagus nerve after use of a LMA and ETT. Br J Anaesth. 2001;87:322. [PubMed] [Google Scholar]
- 35.Una E, Gandia F, Duque JL. Tongue paralysis after orotracheal intubation in a patient with primary mediastinal tumor: A case report. Cases J. 2009;2:9301. doi: 10.1186/1757-1626-2-9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkatesh B, Walker D. Hypoglossal neuropraxia following endotracheal intubation. Anaesth Intensive Care. 1997;25:699–700. doi: 10.1177/0310057X9702500635. [DOI] [PubMed] [Google Scholar]
- 37.Wadelek J, Kolbusz J, Orlicz P, Staniaszek A. Tapia’s syndrome after arthroscopic shoulder stabilisation under general anaesthesia and lma. Anaesthesiol Intensive Ther. 2012;44:31–34. [PubMed] [Google Scholar]
- 38.Yelken K, Guven M, Kablan Y, Sarikaya B. Isolated unilateral hypoglossal nerve paralysis following open septoplasty. Br J Oral Maxillofac Surg. 2008;46:308–309. doi: 10.1016/j.bjoms.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Pariente L, Camarena P, Koo M, Sabate A, Armengol J. Hypoglossal nerve neuropraxia after shoulder hemiarthroplasty. Rev Esp Anestesiol Reanim. 2013 doi: 10.1016/j.redar.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Weissman O, Farber N, Berger E, GrabovNardini G, Zilinsky I, Winkler E, Haik J. Hypoglossal nerve paralysis in a burn patient following mechanical ventilation. Ann Burns Fire Disasters. 2013;26:86–89. [PMC free article] [PubMed] [Google Scholar]
- 41.Park J, Ahn R, Weon Y, Yang D. Diagnosing Tapia syndrome using a videofluoroscopic swallowing study and electromyography after anterior cervical spine surgery. Am J Phys Med Rehabil. 2011;90:948–953. doi: 10.1097/PHM.0b013e31823286e0. [DOI] [PubMed] [Google Scholar]
- 42.Nalladaru Z, Wessels A, DuPreez L. Tapia’s syndrome--a rare complication following cardiac surgery. Interact Cardiovasc Thorac Surg. 2011;14:131–132. doi: 10.1093/icvts/ivr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotondo F, De Paulis S, Modoni A, Schiavello R. Peripheral Tapia’s syndrome after cardiac surgery. Eur J Anaesthesiol. 2009;27:575–576. doi: 10.1097/EJA.0b013e3283340ac3. [DOI] [PubMed] [Google Scholar]
- 44.Soyal OB, Turan S, Durak P, Erdemli O. Transient palsy of peripheral cranial nerves following open heart surgery. Singapore Med J. 2006;47:422–424. [PubMed] [Google Scholar]
- 45.Al-Benna S. Right hypoglossal nerve paralysis after tracheal intubation for aesthetic breast surgery. Saudi Journal of Anesthesia. 2013;7:341–343. doi: 10.4103/1658-354X.115331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batjom E, Coron T, Mercier F, Benhamou D. Hypoglossal nerve palsy, a rare complication of orotracheal intubation. Ann Fr Anesth Reanim. 2006;25:541–542. doi: 10.1016/j.annfar.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Haslam B, Collins S. Unilateral hypoglossal neurapraxia following endotracheal intubation for total shoulder arthroplasty. AANA. 2013;81:233–236. [PubMed] [Google Scholar]
- 48.Lopes G, Denoel C, Desuter G, Docquier MA. Two cases of isolated unilateral paralysis of hypoglossal nerve after uncomplicated orotracheal intubation. Acta Anaesthesiol Belg. 2009;60:191–193. [PubMed] [Google Scholar]
- 49.Michel O, Brusis T. Hypoglossal nerve paralysis following tonsillectomy. Laryngorhinootologie. 1990;69:267–270. doi: 10.1055/s-2007-998187. [DOI] [PubMed] [Google Scholar]
- 50.Nagai K, Sakuramoto C, Goto F. Unilateral hypoglossal nerve paralysis following the use of the laryngeal mask airway. Anaesthesia. 1994;49:603–604. doi: 10.1111/j.1365-2044.1994.tb14230.x. [DOI] [PubMed] [Google Scholar]
- 51.Smoker The hypoglossal nerve. Neuroimaging Clin N Am. 1993:193–206. [Google Scholar]
- 52.Tesei F, Poveda LM, Strali W, Tosi L, Magnani G, Farneti G. Unilateral laryngeal and hypoglossal paralysis (Tapia’s syndrome) following rhinoplasty in general anaesthesia: Case report and review of the literature. Acta Otorhinolaryngol Ital. 2006;26:219–221. [PMC free article] [PubMed] [Google Scholar]
- 53.Yavuzer R, Basterzi Y, Ozkose Z, YucelDemir H, Yilmaz M, Ceylan A. Tapia’s syndrome following septorhinoplasty. Aesthetic Plast Surg. 2004;28:208–211. doi: 10.1007/s00266-003-3037-7. [DOI] [PubMed] [Google Scholar]
- 54.Zamora JE, Saha TK. Combitube rescue for cesarean delivery followed by ninth and twelfth cranial nerve dysfunction. Can J Anaesth. 2008;55:779–784. doi: 10.1007/BF03016352. [DOI] [PubMed] [Google Scholar]
- 55.Donati F, Pfammatter JP, Mauderli M, Vassella F. Neurological complications following tonsillectomy. Schweiz Med Wochenschr. 1991;121:1612–1617. [PubMed] [Google Scholar]
- 56.Sengupta DK, Grevitt MP, Mehdian SM. Hypoglossal nerve injury as a complication of anterior surgery to the upper cervical spine. Eur Spine J. 1999;8:78–80. doi: 10.1007/s005860050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sotiriou K, Balanika M, Anagnostopoulou S, Gomatos C, Karakitsos D, Saranteas T. Postoperative airway obstruction due to Tapia’s syndrome after coronary bypass grafting surgery. Eur J Anaesthesiol. 2007;24:378–379. doi: 10.1017/S0265021506001542. [DOI] [PubMed] [Google Scholar]
- 58.Bumm P. Peripheral hypoglossal paralysis. Laryngol Rhinol Otol (Stuttg) 1974;53:274–283. [PubMed] [Google Scholar]
- 59.Guthrie D. Hypoglossal paralysis following tonsillectomy. J Laryng Otol. 1926;41:662–663. [Google Scholar]
- 60.Gevorgyan A, Nedzelski JM. A late recognition of Tapia syndrome: A case report and literature review. Laryngoscope. 2013;123:2423–2427. doi: 10.1002/lary.24070. [DOI] [PubMed] [Google Scholar]
- 61.Johnson TM, Moore HJ. Cranial nerve x and xii paralysis (tapia’s syndrome) after an interscalene brachial plexus block for a left shoulder mumford procedure. Anesthesiology. 1999;90:311–312. doi: 10.1097/00000542-199901000-00040. [DOI] [PubMed] [Google Scholar]
- 62.Sharp CM, Borg HK, Kishore A, MacKenzie K. Hypoglossal nerve paralysis following tonsillectomy. J Laryngol Otol. 2002;116:389–391. doi: 10.1258/0022215021910870. [DOI] [PubMed] [Google Scholar]
- 63.Zollner B, Herrmann IF. Horner’s syndrome hypoglossal and laryngeal nerve paralyses as inflammatory late complications following tonsillectomy. Monatsschr Ohrenheilkd Laryngorhinol. 1971;105:228–232. [PubMed] [Google Scholar]
- 64.Cheney FW, Posner K, Caplan RA, Ward RJ. Standard of care and anesthesia liability. JAMA. 1989;261:1599–603. [PubMed] [Google Scholar]
- 65.Ropper AH, Adams RD, Victor M, Samuels MA. Adams and Victor’s Principles of Neurology. New York: McGraw-Hill Medical; 2009. [Google Scholar]
- 66.Gowers WR. A manual of diseases of the nervous system. Darien, Conn: Hafner Pub. Co; 1970. [Google Scholar]
- 67.Lin HC, Barkhaus PE. Cranial nerve XII: The hypoglossal nerve. Semin Neurol. 2009;29:45–52. doi: 10.1055/s-0028-1124022. [DOI] [PubMed] [Google Scholar]
- 68.Khoo SG, Ullah I, Wallis F, Fenton JE. Isolated hypoglossal nerve palsy: A harbinger of malignancy. J Laryngol Otol. 2007;121:803–805. doi: 10.1017/S0022215107006275. [DOI] [PubMed] [Google Scholar]
- 69.Lindsay FW, Mullin D, Keefe MA. Subacute hypoglossal nerve paresis with internal carotid artery dissection. Laryngoscope. 2003;113:1530–1533. doi: 10.1097/00005537-200309000-00022. [DOI] [PubMed] [Google Scholar]
- 70.Ito K, Ando S, Akiba N, Watanabe Y, Okuyama Y, Moriguchi H, Yoshikawa K, Takahashi T, Shimada M. Morphological study of the human hyoid bone with three-dimensional ct images -gender difference and age-related changes. Okajimas Folia AnatJpn. 2012;89:83–92. doi: 10.2535/ofaj.89.83. [DOI] [PubMed] [Google Scholar]
- 71.Kindschuh SC, Dupras TL, Cowgill LW. Determination of sex from the hyoid bone. Am J PhysAnthropol. 2010;143:279–284. doi: 10.1002/ajpa.21315. [DOI] [PubMed] [Google Scholar]
- 72.Harnett M, Kinirons B, Heffernan A, Motherway C, Casey W. Airway complications in infants: Comparison of laryngeal mask airway and the facemask-oral airway. Can J Anaesth. 2000;47:315–318. doi: 10.1007/BF03020944. [DOI] [PubMed] [Google Scholar]
- 73.Stone M. Toward a model of three-dimensional tongue movement. J Phon. 1991;19:309–320. [Google Scholar]
- 74.Rodrigues MA, Gillies D, Charters P. A biomechanical model of the upper airways for simulating laryngoscopy. Comput Methods Biomech Biomed Engin. 2001;4:127–148. doi: 10.1080/10255840008908001. [DOI] [PubMed] [Google Scholar]
- 75.Lumb AB, Wrigley MW. The effect of nitrous oxide on laryngeal mask cuff pressure. In vitro and in vivo studies. Anaesthesia. 1992;47:320–323. doi: 10.1111/j.1365-2044.1992.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 76.Complications in arthroscopy: The knee and other joints. Committee on complications of the arthroscopy association of north america. Arthroscopy. 1986;2:253–258. [PubMed] [Google Scholar]
- 77.Conrardy PA, Goodman LR, Lainge F, Singer MM. Alteration of endotracheal tube position. Flexion and extension of the neck. Crit Care Med. 1976;4:7–12. doi: 10.1097/00003246-197601000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Seet E, Yousaf F, Gupta S, Subramanyam R, Wong DT, Chung F. Use of manometry for laryngeal mask airway reduces postoperative pharyngolaryngeal adverse events: A prospective, randomized trial. Anesthesiology. 2010;112:652–657. doi: 10.1097/ALN.0b013e3181cf4346. [DOI] [PubMed] [Google Scholar]
- 79.Ratnaraj J, Todorov A, McHugh T, Cheng MA, Lauryssen C. Effects of decreasing endotracheal tube cuff pressures during neck retraction for anterior cervical spine surgery. J Neurosurg. 2002;97:176–179. doi: 10.3171/spi.2002.97.2.0176. [DOI] [PubMed] [Google Scholar]
- 80.Brimacombe J, Clarke G, Keller C. Lingual nerve injury associated with the proseal laryngeal mask airway: A case report and review of the literature. Br J Anaesth. 2005;95:420–423. doi: 10.1093/bja/aei187. [DOI] [PubMed] [Google Scholar]
- 81.Bohner H, Terorde N, Goretzki PE. Monitoring of the hypoglossal nerve during general anesthesia. J Vasc Surg. 2005;41:734. doi: 10.1016/j.jvs.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 82.Laskawi R, Rohrbach S. Impaired motor functions. Surgical and conservative procedures for restoring motor functions of the facial nerve, accessory nerve, hypoglossal nerve. Laryngorhinootologie. 2005;84 (Suppl 1):S142–155. doi: 10.1055/s-2005-861153. [DOI] [PubMed] [Google Scholar]
- 83.Lagalla G, Logullo F, Di Bella P, Provinciali L, Ceravolo MG. Influence of early high-dose steroid treatment on bell’s palsy evolution. Neurol Sci. 2002;23:107–112. doi: 10.1007/s100720200035. [DOI] [PubMed] [Google Scholar]