Abstract
Pneumonia and acute exacerbation of chronic illness are leading causes of influenza-related hospitalization. Therefore, influenza and pneumococcal vaccinations are strongly recommended for adults with comorbidities. Using a hospital-based influenza surveillance system, we performed a multicenter, prospective cohort study of patients visiting emergency rooms with influenza-like illness (ILI) during the influenza epidemic period in 2013 to 2014. Patients aged ≥19 years were enrolled, and clinical data were collected. Multivariate analyses were performed to estimate the effectiveness of influenza and pneumococcal vaccination in preventing pneumonia development and hospitalization. During study periods, 2,262 patients with ILI were registered. Among 2,217 patients with available vaccination records, 31.9% (707 patients) and 9.7% (216 patients) had received influenza and pneumococcal vaccines, respectively. Among patients who had been administered a pneumococcal vaccine, 94.4% had received the 23-valent polysaccharide vaccine (PPV23). The adjusted rates of effectiveness of the influenza vaccine for preventing pneumonia development and hospitalization were 64.0% (95% confidence interval [CI] = 29% to 81%) and 35.0% (95% CI = 12% to 52%), respectively. Pneumococcal vaccination did not reduce pneumonia development or hospitalization. In conclusion, influenza rather than PPV23 vaccination may reduce pneumonia development and hospitalization in patients with preceding ILI.
INTRODUCTION
Influenza epidemics are responsible for approximately 20,000 to 40,000 deaths and 114,000 hospitalizations annually (1, 2). Community-acquired pneumonia (CAP) is the most common and serious complication after influenza infection. The incidence of influenza-related pneumonia has been reported to range from 0.1% to ≥10%, depending on the level of epidemic and predominant subtypes (3–5). Among diverse bacterial pneumonias, pneumococcal pneumonia is especially common following influenza infections (6). Influenza infections may lead to overexpression of pneumococcal binding receptors, impaired alveolar macrophage phagocytosis, and neutrophil dysfunction, leading to increased host susceptibility to pneumococcal infection (7).
Vaccination has been considered the most effective measure to prevent both influenza and pneumococcal pneumonia. In the Republic of Korea (ROK), the influenza vaccine coverage rate was an estimated 80% in elderly individuals, and pneumococcal vaccination with the 23-valent pneumococcal polysaccharide vaccine (PPV23) was adopted as the national immunization program for elderly individuals (≥65 years of age) in May 2013 (8, 9). This study evaluated the effectiveness of influenza and pneumococcal vaccination alone or in combination to prevent pneumonia and hospitalization following influenza-like illness (ILI).
MATERIALS AND METHODS
Study design.
In the ROK, a multicenter prospective cohort study was performed in 10 hospitals that had participated in a hospital-based influenza surveillance system (Hospital-Based Influenza Morbidity and Mortality [HIMM] system) since 2011 (10). During the 2013-2014 influenza season (1 November 2013 to 30 April 2014), emergency room (ER)-based surveillance was conducted by using ILI criteria; patients aged ≥19 years were enrolled. Clinical data and respiratory specimens were collected prospectively based on a standard protocol (10). A rapid influenza detection test was performed at the bedside, and nasal/throat swab specimens were transported to the central HIMM laboratory for multiplex respiratory viral PCR testing. Patient clinical data were obtained by using a structured case report form, which included demographics, underlying diseases, influenza/pneumococcal vaccination, and 30-day case fatalities. The study design was approved by the ethics committee of each participating hospital, and written informed consent was obtained from all enrolled subjects.
Definition.
ILI was defined as sudden onset of fever (≥38°C) accompanied by one or more respiratory symptoms, including cough, sore throat, or nasal symptoms (10). Clinical, radiological, and microbiological findings of all enrolled cases were evaluated to determine if patients fulfilled the following clinical and radiological CAP criteria: (i) acute pulmonary infiltrate consistent with pneumonia evident on chest radiographs within 48 h after admission, (ii) confirmatory findings on clinical examination, and (iii) acquisition of infection outside the hospital setting (11). Patients with health care-associated pneumonia or hospital-acquired pneumonia were excluded (12).
Statistical analysis.
Statistical analyses were performed by using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Significant differences in variable distributions between groups were estimated by using the chi-square or Fisher exact test for categorical variables and the Student t test for quantitative variables. Quantitative data were expressed as means ± standard deviations. A bilateral P value of <0.05 was considered statistically significant. Logistic regression was used to estimate the odds ratio for pneumonia development and hospitalization in vaccinated versus unvaccinated subjects. Vaccine effectiveness (VE) was calculated as follows: [100 × (1 − the odds ratio for pneumonia development or hospitalization in vaccinated versus nonvaccinated persons)]. Logistic regression models were adjusted for age, body mass index (BMI), and the presence of high-risk medical conditions.
RESULTS
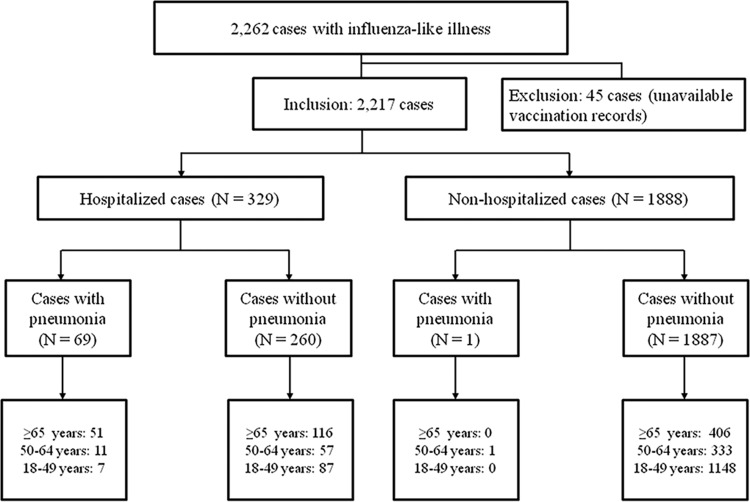
During the 2013-2014 ROK influenza season, 2,262 patients with ILI were enrolled in the HIMM surveillance system, and vaccination records were available for 2,217 (98.0%). Among these 2,217 patients, 988 (44.6%) were laboratory confirmed as having influenza, 70 (3.2%) had ILI accompanied by pneumonia, and 329 (14.8%) were hospitalized (Fig. 1). Twenty-two (1.0%) patients were admitted to the intensive care unit (ICU), and 13 (0.6%) patients died. Overall, 31.9% (707 patients) and 9.7% (216 patients) had received influenza and pneumococcal vaccines, respectively. A total of 193 patients (8.7%) had previously received both the influenza and the pneumococcal vaccine. Among patients who had been administered a pneumococcal vaccine, 94.4% (204 of 216 patients) had received the PPV23. Both influenza and pneumococcal vaccination rates were significantly higher in elderly individuals (aged ≥65 years) than in young adults (Fig. 2).
FIG 1.
Flowchart of cohort-enrolled subjects.
FIG 2.
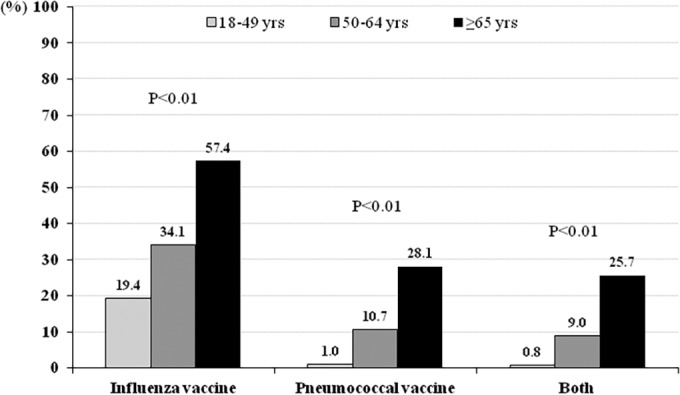
Age-stratified influenza and pneumococcus vaccine coverage rates.
The demographic and clinical characteristics of patients with ILI are compared with respect to accompanying pneumonia in Table 1. Patients with pneumonia were much older than those without pneumonia (P < 0.01); 72.9% of patients with pneumonia were 65 years or older. Underlying medical diseases were more common in patients with pneumonia than in those without diabetes mellitus, chronic heart diseases, chronic lung diseases, chronic renal diseases, and cerebrovascular diseases (P < 0.01). Patients with pneumonia were more likely to be admitted to the ICU; they also died more often. Univariate analysis showed no difference in influenza vaccination rates between both groups. In contrast to what we expected, the pneumococcal vaccination rate was higher in cases with pneumonia.
TABLE 1.
Demographic and clinical characteristics with respect to pneumonia development
| Characteristica | Cases with pneumonia (n = 70) | Cases without pneumonia (n = 2,147) | P value |
|---|---|---|---|
| Age (yr; median ± SD) | 70.1 ± 15.2 | 47.2 ± 19.4 | <0.01 |
| Male sex, no. (%) | 35 (50.0) | 923 (43.0) | 0.24 |
| Body mass index (kg/m2) | 22.4 ± 4.1 | 23.1 ± 3.7 | 0.13 |
| Lab-confirmed influenza, no. (%) | 68 (97.1) | 920 (42.9) | <0.01 |
| Type A | 41 | 631 | |
| Type B | 22 | 258 | |
| Undetermined | 0 | 31 | |
| Current smoking, no. (%) | 9 (12.9) | 342 (15.9) | 0.62 |
| Underlying diseases, no. (%) | |||
| Diabetes mellitus | 16 (22.9) | 238 (11.1) | <0.01 |
| Chronic heart diseases | 11 (15.7) | 122 (5.7) | <0.01 |
| Chronic lung diseases | 12 (17.1) | 50 (2.3) | <0.01 |
| Chronic renal diseases | 7 (10.0) | 56 (2.6) | <0.01 |
| Chronic liver diseases | 2 (2.9) | 39 (1.8) | 0.53 |
| Malignancies | 7 (10.0) | 142 (6.6) | 0.23 |
| HIV infection | 1 (1.4) | 6 (0.3) | 0.09 |
| Immunosuppressant use | 2 (2.9) | 36 (1.7) | 0.34 |
| HIV infection | 1 (1.4) | 6 (0.3) | 0.09 |
| Cerebrovascular diseases | 9 (12.9) | 78 (3.6) | <0.01 |
| Influenza vaccination, no. (%) | 20 (28.6) | 687 (32.0) | 0.55 |
| Pneumococcal vaccination, no. (%) | 14 (20.0) | 202 (9.4) | <0.01 |
| Admission, no. (%) | 69 (98.6) | 260 (12.1) | <0.01 |
| ICU admission, no. (%) | 11 (15.7) | 11 (0.5) | <0.01 |
| Mortality, no. (%) | 9 (12.9) | 4 (0.2) | <0.01 |
HIV, human immunodeficiency virus; ICU, intensive care unit.
After adjustment for age, body mass index (BMI), current smoking status, and the presence of chronic medical conditions, the overall effectiveness of the influenza vaccine against pneumonia development and hospitalization was 64% (95% confidence interval [CI] = 29% to 81%) and 35.0% (95% CI = 12% to 52%), respectively (Tables 2 and 3). Pneumococcal vaccination did not reduce the development of pneumonia (odds ratio, 1.48; 95% CI, 0.68 to 3.20) or hospitalization (odds ratio, 1.01; 95% CI, 0.68 to 1.52). Old age and chronic lung and renal diseases were independent risk factors for pneumonia development and hospitalization. Although insignificant for pneumonia development, multivariate analysis showed chronic heart diseases to be an independent risk factor for hospitalization.
TABLE 2.
Multivariate logistic regression analysis of pneumonia risk factors
| Variable | Odds ratio (95% CI) | P value |
|---|---|---|
| Age (yr) | 1.07 (1.05-−1.09) | <0.01 |
| Body mass index | 0.96 (0.89–1.03) | 0.28 |
| Current smoking | 1.11 (0.52–2.36) | 0.79 |
| Diabetes mellitus | 0.90 (0.45–1.78) | 0.76 |
| Chronic heart diseases | 1.14 (0.54–2.43) | 0.73 |
| Chronic lung diseases | 4.16 (1.98–8.75) | <0.01 |
| Chronic renal diseases | 2.96 (1.12—7.87) | 0.03 |
| Cerebrovascular diseases | 1.61 (0.70–3.69) | 0.27 |
| Influenza vaccination | 0.36 (0.19–0.71) | <0.01 |
| Pneumococcal vaccination | 1.48 (0.68–3.20) | 0.33 |
TABLE 3.
Multivariate logistic regression analysis of hospitalization risk factors
| Variable | Odds ratio (95% CI) | P value |
|---|---|---|
| Age | 1.04 (1.03–1.05) | <0.01 |
| Body mass index | 1.01 (0.97–1.04) | 0.78 |
| Current smoking | 0.83 (0.57–1.22) | 0.34 |
| Diabetes mellitus | 0.72 (0.50–1.04) | 0.08 |
| Chronic heart diseases | 2.16 (1.44–3.24) | <0.01 |
| Chronic lung diseases | 2.84 (1.64–4.94) | <0.01 |
| Chronic renal diseases | 1.96 (1.06—3.60) | 0.03 |
| Cerebrovascular diseases | 1.27 (0.77–2.11) | 0.36 |
| Influenza vaccination | 0.65 (0.48–0.88) | <0.01 |
| Pneumococcal vaccination | 1.01 (0.68–1.52) | 0.95 |
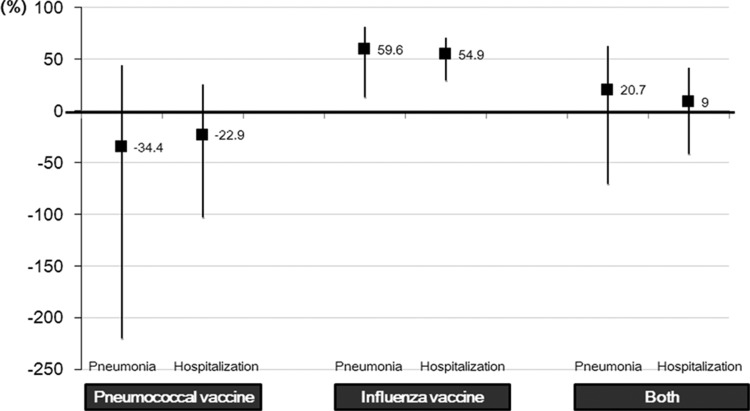
Analysis of age-stratified VE showed the influenza vaccine to be effective in preventing pneumonia (59.6%) and hospitalization (54.9%) in elderly individuals (≥65 years of age) (Table 4; Fig. 3). In comparison, pneumococcal vaccination was not effective. Combined influenza and pneumococcal vaccination did not show a synergistic effect for prevention of pneumonia (VE = 20.7%; 95% CI = −70.8% to 63.2%) or hospitalization (VE = 9.0%; 95% CI = −41.9% to 41.6%) (Fig. 3). In young adults aged 18 to 64 years, low vaccine coverage rates as well as low pneumonia and hospitalization case numbers impeded VE estimation.
TABLE 4.
Age-stratified vaccine effectiveness for prevention of pneumonia and hospitalization
| Category | Vaccination | Age group (yr) (no. of subjects) | No. (%) of cases with pneumonia | No. (%) of cases hospitalized | No. (%) of cases without pneumonia | No. (%) of cases hospitalized | % adjusted VEa (95% CI) |
|---|---|---|---|---|---|---|---|
| Prevention of pneumonia | Influenza vaccine | ≥65 (573) | 17/51 (33.3) | 312/522 (59.8) | 59.6 (13.1 to 81.2) | ||
| 19–64 (1,644) | 3/19 (15.8) | 375/1,625 (23.1) | 78.1 (−0.3 to 95.3) | ||||
| Pneumococcal vaccine | ≥65 (573) | 12/51 (23.5) | 149/522 (28.5) | −34.4 (−223.0 to 44.1) | |||
| 19–64 (1,644) | 2/19 (10.5) | 53/1,625 (3.3) | −142.9 (−1,500.8 to 63.3) | ||||
| Prevention of hospitalization | Influenza vaccine | ≥65 (573) | 73/167 (43.7) | 256/406 (63.1) | 54.9 (29.4 to 71.2) | ||
| 19–64 (1,644) | 44/162 (27.2) | 334/1,482 (22.5) | 10.7 (−36.5 to 41.6) | ||||
| Pneumococcal vaccine | ≥65 (573) | 43/167 (25.7) | 118/406 (29.1) | −22.9 (−103.4 to 25.8) | |||
| 19–64 (1,644) | 10/162 (6.2) | 45/1,482 (3.0) | −10.7 (−148.3 to 50.6) |
VE, vaccine effectiveness.
FIG 3.
Additive effect of influenza and pneumococcal vaccination against pneumonia development and hospitalization.
DISCUSSION
In this study, we evaluated the effectiveness of influenza and pneumococcal vaccines for prevention of pneumonia and hospitalization based on a large prospective cohort of patients with ILI. Influenza vaccination reduced the risk of pneumonia development and hospitalization in patients with ILI who visited the ER. In comparison, pneumococcal vaccination did not show significant preventive effectiveness.
Preceding influenza infection has been suggested to enhance susceptibility to pneumococcal pneumonia as well as transmission between close contacts (6, 7). Increased susceptibility is related to the paradoxical suppression of the host immune system and enhanced bacterial adherence to the respiratory epithelium. As in previous reports, elderly people and patients with chronic lung diseases were at the greatest risk for postinfluenza pneumonia in this study (6, 13). In the ROK, immunization of elderly individuals (aged ≥65 years) with the PPV23 has been included in the national immunization program (NIP) since May 2013, and overall vaccine coverage rates were estimated to reach about 40% by the end of 2013 (9). It is important to evaluate the effectiveness of the pneumococcal NIP for elderly individuals in order to reduce influenza- and pneumonia-related morbidity and mortality.
Recently, the Spanish influenza surveillance system reported low VE in seasons with predominant circulation of influenza A/H3N2 (14). Similarly, in the ROK, circulation of influenza A/H3N2 was dominant in the past 3 years, with reportedly low influenza VE (15, 16). Of note, this study showed that influenza vaccination would be beneficial for elderly individuals and those with comorbidities despite statistically insignificant influenza VE. A previous retrospective study found influenza vaccination to be associated with a 56%-reduced risk of hospitalization due to acute exacerbation of cardiopulmonary disease despite suboptimal VE (17). Although the influenza vaccine showed limited ability to prevent influenza infection, it might have affected illness severity. Influenza virus hemagglutinin (HA) is active early in respiratory tract infections; therefore, it has been the main target of current influenza vaccines. Influenza VE might decrease in proportion to the degree that circulating viral HA differs from the HA of the vaccine strain. We can hypothesize three mechanisms for the unexpected protective effectiveness against pneumonia and hospitalization. First, even low inhibitory activity against variant HA might prevent severe influenza infection and its complications. Second, the effective antineuraminidase (anti-NA) activity of the influenza vaccine might prevent pneumonia development and hospitalization irrespective of infection itself. Although NA does not have a significant role in initial infection, it might play an important role in viral spread within the host and contribute to development of pneumonia and other influenza-related complications. However, there is no serological data to support the present explanation. Finally, vaccine-induced T-cell immunity against influenza virus might be a factor in preventing influenza-related complications also.
Unlike the influenza vaccine, the pneumococcal vaccine was not effective in preventing pneumonia and hospitalization after influenza infection. Pneumococcus is well known as the most common causative pathogen of secondary bacterial pneumonia after influenza infection (6). Theoretically, the pneumococcal vaccine reduces influenza-related pneumonia and hospitalization. In this study, pneumococcal vaccine coverage was quite low (9.7%), and more than 90% of recipients received the PPV23. Unlike protein-conjugated vaccines, polysaccharide vaccines may not induce mucosal immunity and cannot suppress nasopharyngeal colonization of pneumococci (18). A meta-analysis showed that polysaccharide vaccines were consistently ineffective in preventing pneumococcal pneumonia, all-cause pneumonia, and related deaths (19). Nevertheless, previous studies have shown additive or synergistic effects between influenza and pneumococcal vaccines in preventing pneumonia and influenza-related admission (20–22). In the ROK, with increasing PPV23 vaccination rates in elderly individuals (aged ≥65 years), the effectiveness of PPV23 must be reassessed; in this study, 26 (37.1%) among 70 ILI patients with pneumonia were actually infected by Pneumococcus, but only 4 of them received pneumococcal vaccination. In patients aged 19 to 64 years, pneumonia, though statistically insignificant, was rather common among pneumococcal vaccine recipients. This unexpected finding was related to underlying medical conditions; diabetes (9.1% versus 4.3%, P = 0.10), malignancy (9.1% versus 4.3%, P = 0.10), chronic heart diseases (10.9% versus 1.8%, P < 0.01), and chronic lung diseases (10.9% versus 0.8%, P < 0.01) were more common among pneumococcal vaccine recipients than among unvaccinated patients. Considering the high disease burden of nonbacteremic pneumococcal pneumonia exclusively in elderly individuals and adults with chronic medical conditions (6), the 13-valent protein-conjugated vaccine (PCV13) may be beneficial. In addition to vaccine effectiveness, the cost-effectiveness of each vaccine (PPV23 versus PCV13) is also important in establishing a national immunization strategy.
In summary, rather than the PPV23, the influenza vaccine may reduce postinfluenza pneumonia and hospitalization. Besides the protective effect against influenza itself, the influenza vaccine may prevent complications and reduce severity in elderly adults and those with comorbidities.
ACKNOWLEDGMENTS
We appreciate all the colleagues participating in the hospital-based influenza surveillance system (HIMM system) for their assistance with the data collection as well as their clinical support.
This study was supported by a grant from the Korea Healthcare Technology R&D Project of the Ministry of Health & Welfare of the Republic of Korea (A103001).
J.Y.S. and H.J.C. received unrestricted grant funding from Pfizer and Novartis for unrelated studies. All other authors report no potential conflicts.
REFERENCES
- 1.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. 2000. The impact of influenza epidemics on hospitalizations. J Infect Dis 181:831–837. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Peltola VT, Murti KG, McCullers JA. 2005. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis 192:249–257. doi: 10.1086/430954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Haber M, Ray S, Farley MM, Panozzo CA, Klugman KP. 2012. Invasive pneumococcal pneumonia and respiratory virus co-infections. Emerg Infect Dis 18:294–297. doi: 10.3201/eid1802.102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholson KG. 1998. Human influenza, p 219–264. In Nicholson KG, Webster RG, Hay AJ (ed), Textbook of influenza. Blackwell Science Ltd, London, United Kingdom. [Google Scholar]
- 6.Song JY, Nahm MH, Cheong HJ, Kim WJ. 2014. Impact of preceding flu-like illness on the serotype distribution of pneumococcal pneumonia. PLoS One 9:e93477. doi: 10.1371/journal.pone.0093477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballinger MN, Standiford TJ. 2010. Postinfluenza bacterial pneumonia: host defenses gone awry. J Interferon Cytokine Res 30:643–652. doi: 10.1089/jir.2010.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kee SY, Lee JS, Cheong HJ, Chun BC, Song JY, Choi WS, Jo YM, Seo YB, Kim WJ. 2007. Influenza vaccine coverage rates and perceptions on vaccination in South Korea. J Infect 55:273–281. doi: 10.1016/j.jinf.2007.04.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim ES, Shin JK, Oh HK. 2014. Elderly immunization program against invasive pneumococcal disease in Korea 2013. Public Health Wkly Rep KCDC 7:182–186. [Google Scholar]
- 10.Song JY, Cheong HJ, Choi SH, Baek JH, Han SB, Wie SH, So BH, Kim HY, Kim YK, Choi WS, Moon SW, Lee J, Kang GH, Jeong HW, Park JS, Kim WJ. 2013. Hospital-based influenza surveillance in Korea: hospital-based influenza morbidity and mortality study group. J Med Virol 85:910–917. doi: 10.1002/jmv.23548. [DOI] [PubMed] [Google Scholar]
- 11.Bodí M, Rodríguez A, Solé-Violán J, Gilavert MC, Garnacho J, Blanquer J, Jimenez J, de la Torre MV, Sirvent JM, Almirall J, Doblas A, Badía JR, García F, Mendia A, Jordá R, Bobillo F, Vallés J, Broch MJ, Carrasco N, Herranz MA, Rello J. 2005. Antibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis 41:1709–1716. doi: 10.1086/498119. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society Infectious Diseases Society of America. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 13.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. 2012. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis 206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savulescu C, Jiménez-Jorge S, Delgado-Sanz C, de Mateo S, Pozo F, Casas I, Larrauri A, Spanish Influenza Surveillance System . 2014. Higher vaccine effectiveness in seasons with predominant circulation of seasonal influenza A(H1N1) than in A(H3N2) seasons: test-negative case-control studies using surveillance data, Spain, 2003–2011. Vaccine 32:4404–4411. doi: 10.1016/j.vaccine.2014.06.063. [DOI] [PubMed] [Google Scholar]
- 15.Choi WS, Noh JY, Seo YB, Baek JH, Lee J, Song JY, Park DW, Lee JS, Cheong HJ, Kim WJ. 2013. Case-control study of the effectiveness of the 2010-2011 seasonal influenza vaccine for prevention of laboratory-confirmed influenza virus infection in the Korean adult population. Clin Vaccine Immunol 20:877–881. doi: 10.1128/CVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi WS, Noh JY, Baek JH, Seo YB, Lee J, Song JY, Park DW, Lee JS, Cheong HJ, Kim WJ. Suboptimal effectiveness of the 2011-2012 seasonal influenza vaccine in adult Korean populations. PLoS One, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo YB, Choi WS, Baek JH, Lee J, Song JY, Lee JS, Cheong HJ, Kim WJ. 2014. Effectiveness of the influenza vaccine at preventing hospitalization due to acute exacerbation of cardiopulmonary disease in Korea from 2011 to 2012. Hum Vaccin Immunother 10:423–427. doi: 10.4161/hv.26858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanford M. 2012. Pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed): in older adults. Drugs 72:1243–1255. doi: 10.2165/11209330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Huss A, Scott P, Stuck AE, Trotter C, Egger M. 2009. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christenson B, Hedlund J, Lundbergh P, Ortqvist A. 2004. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J 23:363–368. doi: 10.1183/09031936.04.00063504. [DOI] [PubMed] [Google Scholar]
- 21.Domínguez A, Castilla J, Godoy P, Delgado-Rodríguez M, Saez M, Soldevila N, Astray J, Mayoral JM, Martín V, Quintana JM, González-Candelas F, Galán JC, Tamames S, Castro A, Baricot M, Garín O, Pumarola T. 2013. Effectiveness of vaccination with 23-valent pneumococcal polysaccharide vaccine in preventing hospitalization with laboratory confirmed influenza during the 2009-2010 and 2010-2011 seasons. Hum Vaccin Immunother 9:865–873. doi: 10.4161/hv.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichol KL. 1999. The additive benefits of influenza and pneumococcal vaccinations during influenza seasons among elderly persons with chronic lung disease. Vaccine 17(Suppl 1):S91–S93. doi: 10.1016/S0264-410X(99)00114-0. [DOI] [PubMed] [Google Scholar]