Abstract
The tuberculin skin test (TST) and interferon gamma (IFN-γ) release assays (IGRAs) are used as adjunctive tests for the evaluation of suspected cases of active tuberculosis (TB). However, a positive test does not differentiate latent from active TB. We investigated whether flow cytometric measurement of novel combinations of intracellular cytokines and surface makers on CD4 T cells could differentiate between active and latent TB after stimulation with Mycobacterium tuberculosis-specific proteins. Blood samples from 60 patients referred to the Singapore Tuberculosis Control Unit for evaluation for active TB or as TB contacts were stimulated with purified protein derivative (PPD), ESAT-6 and CFP-10, or heparin-binding hemagglutinin (HBHA). The CD4 T cell cytokine response (IFN-γ, interleukin-2 [IL-2], interleukin-17A [IL-17A], interleukin-22 [IL-22], granulocyte-macrophage colony-stimulating factor [GM-CSF], and tumor necrosis factor alpha [TNF-α]) and surface marker expression (CD27, CXCR3, and CD154) were then measured. We found that the proportion of PPD-specific CD4 T cells, defined as CD154+ TNF-α+ cells that were negative for CD27 and positive for GM-CSF, gave the strongest discrimination between subjects with latent and those with active TB (area under the receiver operator characteristic [ROC] curve of 0.9277; P < 0.0001). Also, the proportions and absolute numbers of HBHA-specific CD4 T cells were significantly higher in those with latent TB infection, particularly CD154+ TNF-α+ IFN-γ+ IL-2+ and CD154+ TNF-α+ CXCR3+. Finally, we found that the ratio of ESAT-6- and CFP-10-responding to HBHA-responding CD4 T cells was significantly different between the two study populations. In conclusion, we found novel markers of M. tuberculosis-specific CD4 cells which differentiate between active and latent TB.
INTRODUCTION
The diagnosis of active tuberculosis (TB) is based on clinical, radiological, and epidemiological factors and confirmed by the growth of Mycobacterium tuberculosis or the detection of its DNA in patient samples. Culture of M. tuberculosis is very sensitive, but results take several weeks to become available, and patients with paucibacillary disease may not demonstrate growth of M. tuberculosis in their clinical samples. Nucleic acid tests are quick but expensive and still fall short of culture in terms of sensitivity. The tuberculin skin test (TST) and interferon gamma (IFN-γ) release assays (IGRAs) are often used as adjunctive tests to provide supportive evidence for active TB in cases where the diagnosis is challenging or where initial microbiological testing does not indicate the presence of M. tuberculosis. Although these tests can help to distinguish M. tuberculosis-infected from uninfected patients, they still do not distinguish between active and latent TB cases. A test that can rapidly differentiate active from latent TB will be a welcome addition to the limited tools currently available to clinicians.
The CD4 T cell response is central to the immune control of M. tuberculosis (1), and measurement of this response is the basis of the TST and IGRAs. Recent research into techniques that can more accurately characterize and enumerate the CD4 T cell response against M. tuberculosis antigens has raised hope that the diagnostic capability of these tests may be improved. First among these methods is the flow cytometry technique of intracellular cytokine staining (ICS) (2). Previously, our research (3) found that the ICS technique could measure other cytokines and activation markers besides IFN-γ, which enhanced the ability to discriminate patients with pulmonary TB from those with non-TB pneumonia and healthy controls. More recently, researchers have used ICS to try and discriminate active from latent TB by differences in the combinations of cytokines produced by M. tuberculosis antigen-specific CD4 T cells (4, 5). Another approach has been to measure cell surface proteins associated with particular states of the memory response on M. tuberculosis antigen-specific CD4 T cells. Markers that have been suggested to differentiate the two groups include CD27 (6, 7), CD45RA and CCR7 (8), and CD127 (9). Finally, another possible avenue of discrimination has been the identification of particular M. tuberculosis antigens that appear to have stronger T cell responses in the latent population (10). One of these antigens is heparin-binding hemagglutinin (HBHA) (11), which has been studied in patients with latent and active TB by using IGRAs (12) as well as by ICS (13).
We hypothesized that it may be possible to distinguish active from latent TB by using a combination of all these parameters. To test this, we obtained blood samples from patients evaluated at the Singapore Tuberculosis Control Unit (TBCU) and measured novel combinations of intracellular cytokines and surface markers on CD4 T cells after stimulation with the antigens tuberculin purified protein derivative (PPD), 6-kDa early secretory antigenic target (ESAT-6) and 10-kDa culture filtrate protein (CFP-10), and HBHA by using ICS. We quantified the responding cells as both a proportion of CD4 cells and the absolute number of CD4 cells circulating in the blood, to determine if there were particular combinations of surface markers and cytokine staining that could discriminate subjects with active from those with latent TB.
MATERIALS AND METHODS
Study subjects.
Subjects were recruited from patients evaluated at the TBCU for suspected TB or as close contacts of TB cases. This took place from December 2011 to March 2014 under Ethics Approval DSRB 2011/01775. All subjects were adults and gave written informed consent for study participation. The group definitions were as follows: active (findings radiologically compatible with pulmonary TB plus M. tuberculosis-positive sputum/nucleic acid amplification test) and latent (patients with findings clinically not compatible with pulmonary TB plus negative TB cultures or healthy contacts with normal chest radiographs and positive by either TST or IGRA or with a detectable PPD response by the flow cytometric assay).
Patient clinical tests.
For each subject, full blood counts (Tan Tock Seng Hospital [TTSH] Department of Laboratory Medicine) and CD4 counts were performed. TSTs were performed at the TBCU. Quantiferon assays (Quantiferon Gold In-Tube test), T-Spot TB, and HIV antibody determination tests were performed at the TTSH Department of Laboratory Medicine. For the CD4 count, 100 μl of blood was incubated with a combination of anti-CD45-phycoerythrin (PE), anti-CD3-allophycocyanin (APC), anti-CD4-fluorescein isothiocyanate (FITC), and anti-CD8-Pacific Blue antibodies (Beckman Coulter, USA). After incubation, red blood cells were lysed by using BDFACSLyse solution (BD Biosciences, USA), and samples were analyzed on a BD LSRFortessa flow cytometer. The percentage of CD3+ CD4+ leukocytes was multiplied against the total leukocyte count to give the CD4 count.
Antigen stimulation assay.
We used an adapted version of the whole-blood antigen stimulation method (14). Briefly, 10 ml of Li Heparin blood was collected from each patient and transferred to the laboratory at the National University of Singapore. After storage overnight at room temperature, 1 μg/ml of anti-CD28 and -CD49d (eBioscience, USA) and either 10 μg/ml tuberculin PPD (Statens Serum Institute, Denmark), 10 μg/ml the recombinant M. tuberculosis proteins ESAT-6 and CFP-10 (ImmunoDiagnostics Inc., USA), or, for some patients, 10 μg/ml methylated native heparin-binding hemagglutinin (HBHA) (kindly provided by Camille Locht, Institut Pasteur de Lille, France) plus a no-antigen control were added to 2-ml aliquots of blood. After 1 h of incubation (37°C in 5% CO2), brefeldin A was added, and the sample was incubated for another 5 h. Next, an EDTA–phosphate-buffered saline (PBS) solution was added (2 mM final concentration), and each aliquot was briefly vortexed and left at room temperature for 15 min. The red blood cells were then lysed in a 1× NH4Cl solution (10× solution containing 80.2 g NH4Cl, 8.4 g NaHCO3, and 3.7 g EDTA in H2O), the samples were spun down, and cells were stained with the surface markers anti-CD3-ECD, anti-CD16-APC/Alexa 750 (Beckman Coulter, USA), anti-CD14-APC/e780, anti-CD19-APC/e780 (eBioscience, USA), anti-CD27-Horizon V500, and anti-CXCR3-PcP-Cy5.5 (BD Biosciences, USA), along with Live/Dead Fixable near-infrared (IR) fluorescent dye (Life Technologies, USA). After this step, the cells were fixed and permeabilized (eBioscience fixation and permeabilization kit) and stained with anti-CD4-BV605 (BD Biosciences, USA), anti-CD154-APC, anti-tumor necrosis factor alpha (TNF-α)–PE/Cy7, anti-granulocyte-macrophage colony-stimulating factor (GM-CSF)–PE or -interleukin-22 (IL-22)–PE (BioLegend, USA), anti-IFN-γ–e450, and anti-IL-2–FITC or IL-17A–FITC (eBioscience). The cells were then measured on a Becton Dickinson LSRFortessa flow cytometer.
Flow cytometry data analysis.
The flow cytometric data were analyzed by using FlowJo software (v7.6.4; Treestar, USA) and performed in a blind manner regarding patient clinical status. The gating strategy used to identify the antigen-reactive cells is outlined in Fig. 1. Determination of fluorescent channel spillover values was done by using single-stained anti-mouse Ig beads (Life Technologies, USA). The negative cutoffs for individual cytokine/CD154 staining were determined by comparing PPD-induced cytokine staining to that of the background no-antigen control and were then applied to all the different antigen-stimulated samples. CD27 and CXCR3 cutoffs were determined by visualization of the distributions for the no-antigen control and then applied to different stimulated samples. Total antigen-responsive cells were defined as being CD4+ CD154+ TNF-α+. Cell populations that were negative for this combination but positive for the other cytokines in the antigen-stimulated samples were occasionally seen, but gating on them invariably revealed these results to be due to nonspecific staining and/or an overlap of non-T cell populations in the CD4 gate. For this population, expression of CD27, CXCR3, combinations of the other cytokines, and CD27 on particular cytokine-positive cells was determined.
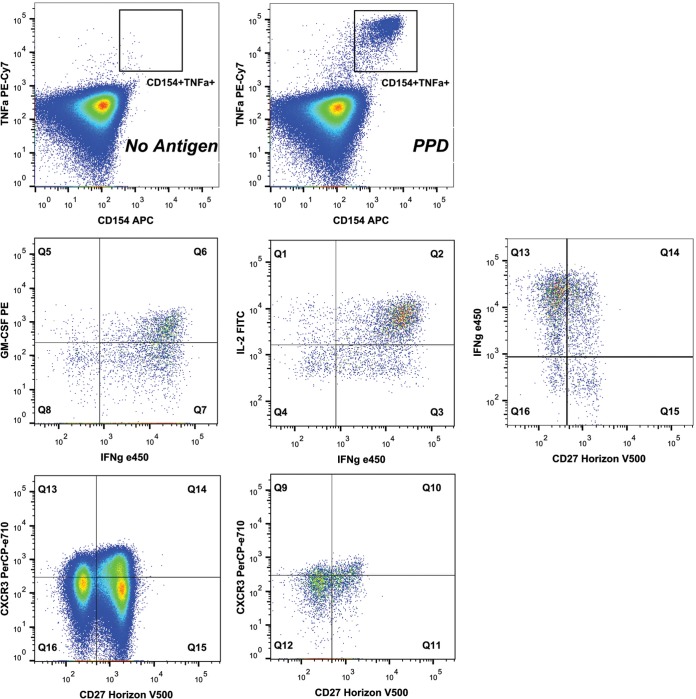
FIG 1.
Flow cytometric gating strategy. Representative flow cytometric plots for an individual subject are shown to detail the gating strategy used. The top row details the CD154+ TNF-α+ region for cells gated positive for CD3 and CD4 and negative for the dump channel (Live/Dead fixable dye positive and CD14+ CD16+ CD19+), stimulated with PPD or with no stimulus. The middle row details cytokine and CD27 staining on the PPD-stimulated cells positive for CD154 and TNF-α. The bottom row shows CD27 and CXCR3 staining on the total CD4 T cells (left) and the PPD-stimulated CD154+ TNF-α+ cells (right).
Statistical analysis.
The two-tailed Mann-Whitney U test was used to determine differences in subset distributions of the two groups, and receiver operator characteristic (ROC) curves were used to indicate the sensitivity and specificity of the parameters (Graph Pad software V5.01). The level of statistical significance was set at a value of <0.05. LASSO regression of the measured variables was analyzed by using the glmnet package in Statistics R, where a 4-fold cross-validation was performed to determine the optimal parameter values.
RESULTS
Study groups.
The demographics of the tested subjects are detailed in Table 1. There were no major differences between the two groups studied.
TABLE 1.
Patient demographics and clinical details
| Parameter | Value for group |
|
|---|---|---|
| Active TB (n = 26) | Latent TB (n = 34) | |
| Median age of patients (yr) (range) | 31 (19–64) | 39 (25–71) |
| No. of males/no. of females | 16/10 | 15/9 |
| No. (%) of patients of ethnicity | ||
| Chinese | 12 (46) | 19 (58) |
| Malay | 2 (8) | 2 (6) |
| Filipino | 4 (15) | 4 (12) |
| Other | 8 (31) | 9 (27) |
| No. of vaccinated patients | 17 | 22 |
| No. of nonvaccinated patients | 6 | 8 |
| No. of patients with unknown vaccination status | 3 | 4 |
| No. of patients with positive TST/total no. of patients (median diam of indurationb [mm] [range]) | 18/19 (16 [5–22]) | 17/24a (14 [0–22]) |
| No. of patients with positive IGRA/total no. of patients | 1/1 | 8/9a |
| No. of patients treated at testing (treatment duration [days]) | 6 (1–13) | 5 (2–28) |
| No. of patients with HIV antibody result(s) | 26/26 negative | 13 negative, 21 unknown |
| Mean CD4 count (cells/ml) ± SD | 647,600 ± 291,900 | 718,800 ± 314,300 |
All subjects in the “latent” group that were negative by a TST and/or IGRA were found to have a detectable response with PPD stimulation by the flow cytometry assay.
The positive cutoff was 10 mm.
Differences in phenotype and frequency of PPD-specific CD4 T cells in active versus latent TB patients.
The frequency and phenotype of the PPD-responding CD4 T cells were measured for each subject. The percentages of total CD4 cells (defined as CD154+ TNF-α+) that responded to PPD activation were not significantly different between the two groups (Table 2).
TABLE 2.
PPD-specific T cell responsesa
| Parameter | Value for group |
P value | |
|---|---|---|---|
| Active TB (n = 26) | Latent TB (n = 33) | ||
| Median % of CD4 cells (25th–75th percentile) | |||
| CD154+ TNF-α+ | 0.237 (0.098–0.430) | 0.377 (0.156–0.470) | 0.239 |
| CD154+ TNF-α+ IFN-γ+ | 0.137 (0.058–0.278) | 0.255 (0.102–0.335) | 0.099 |
| CD154+ TNF-α+ GM-CSF+ | 0.081 (0.037–0.160) | 0.113 (0.065–0.205) | 0.225 |
| CD154+ TNF-α+ IL-2+ | 0.134 (0.056–0.229) | 0.234 (0.105–0.308) | 0.064 |
| CD154+ TNF-α+ IFN-γ+ IL-2+ | 0.102 (0.042–0.179) | 0.182 (0.084–0.258) | 0.043 |
| CD154+ TNF-α+ IFN-γ− IL-2− | 0.040 (0.028–0.086) | 0.042 (0.024–0.076) | 0.737 |
| CD154+ TNF-α+ CD27− | 0.138 (0.066–0.297) | 0.145 (0.055–0.207) | 0.633 |
| CD154+ TNF-α+ IFN-γ+ CD27− | 0.100 (0.029–0.187) | 0.109 (0.044–0.165) | 0.964 |
| CD154+ TNF-α+ GM-CSF+ CD27− | 0.056 (0.023–0.112) | 0.054 (0.025–0.075) | 0.440 |
| CD154+ TNF-α+ IL-2+ CD27− | 0.064 (0.033–0.158) | 0.087 (0.039–0.126) | 0.870 |
| CD4+ TNF-α+ CD154+ CXCR3+ | 0.017 (0.007–0.026) | 0.038 (0.014–0.083) | 0.017 |
| Median % of CD4+ TNF-α+ CD154+ cells (25th–75th percentile) | |||
| IFN-γ+ | 60.5 (54.0–68.8) | 68.5 (61.7–79.3) | 0.005 |
| GM-CSF+ | 32.5 (28.2–40.0) | 34.0 (28.0–41.0) | 0.647 |
| IL-2+ | 57.0 (49.0–70.0) | 72.0 (56.7–77.3) | 0.022 |
| IL-17A+ | 3.0 (1.0–5.3) | 1.0 (0.0–1.3) | 0.003 |
| IL-22+ | 3.0 (1.0–6.0) | 1.0 (1.0–3.0) | 0.126 |
| IL-2− IFN-γ− | 22.5 (15.2–29.3) | 15.5 (11.0–22.0) | 0.033 |
| IL-2+ IFN-γ+ | 42.5 (34.2–50.0) | 54.0 (39.7–66.0) | 0.006 |
| CXCR3+ | 6.0 (3.0–15.3) | 13.0 (7.0–20.0) | 0.055 |
| Median absolute no. of cells/ml (25th–75th percentile) | |||
| CD4+ TNF-α+ CD154+ | 1,808 (550–2,727) | 2,527 (927–3,464) | 0.145 |
| CD4+ TNF-α+ CD154+ IFN-γ+ IL-2+ | 649 (214–1,303) | 1,323 (436–1,968) | 0.024 |
| CD4+ TNF-α+ CD154+ IFN-γ− IL-2− | 309 (125–505) | 342 (132–508) | 0.982 |
| CD4+ TNF-α+ CD154+ CD27− | 1,086 (396–1,659) | 938 (364–1,501) | 0.754 |
| CD4+ TNF-α+ CD154+ IFN-γ+ CD27− | 778 (249–1,122) | 755 (273–1,188) | 0.849 |
| CD4+ TNF-α+ CD154+ GM-CSF+ CD27− | 456 (133–677) | 309 (169–629) | 0.761 |
| CD4+ TNF-α+ CD154+ IL-2+ CD27− | 506 (201–1,003) | 512 (244–1,026) | 0.837 |
| CD4+ TNF-α+ CD154+ CXCR3+ | 105 (33–186) | 226 (75–499) | 0.035 |
Results for Mann-Whitney U test comparisons between the active and latent groups are displayed.
By comparing the different combinations of cytokine staining as a proportion of the PPD-responding cells, it was found that total IFN-γ or IL-2 production had a small but significant increase in the latently infected subjects, while there was no difference for GM-CSF (Table 2). There were different combinations of GM-CSF, IFN-γ, and IL-2 production that had significant differences between the subject groups (Fig. 2), but none on their own gave a clear separation of the two populations. The measured level of IL-17A production in the PPD-responsive CD4 cells was low but was found to be significantly higher in actively infected subjects, while the measured level of the IL-22 response was also low, and no differences between the groups were detected (Table 2).
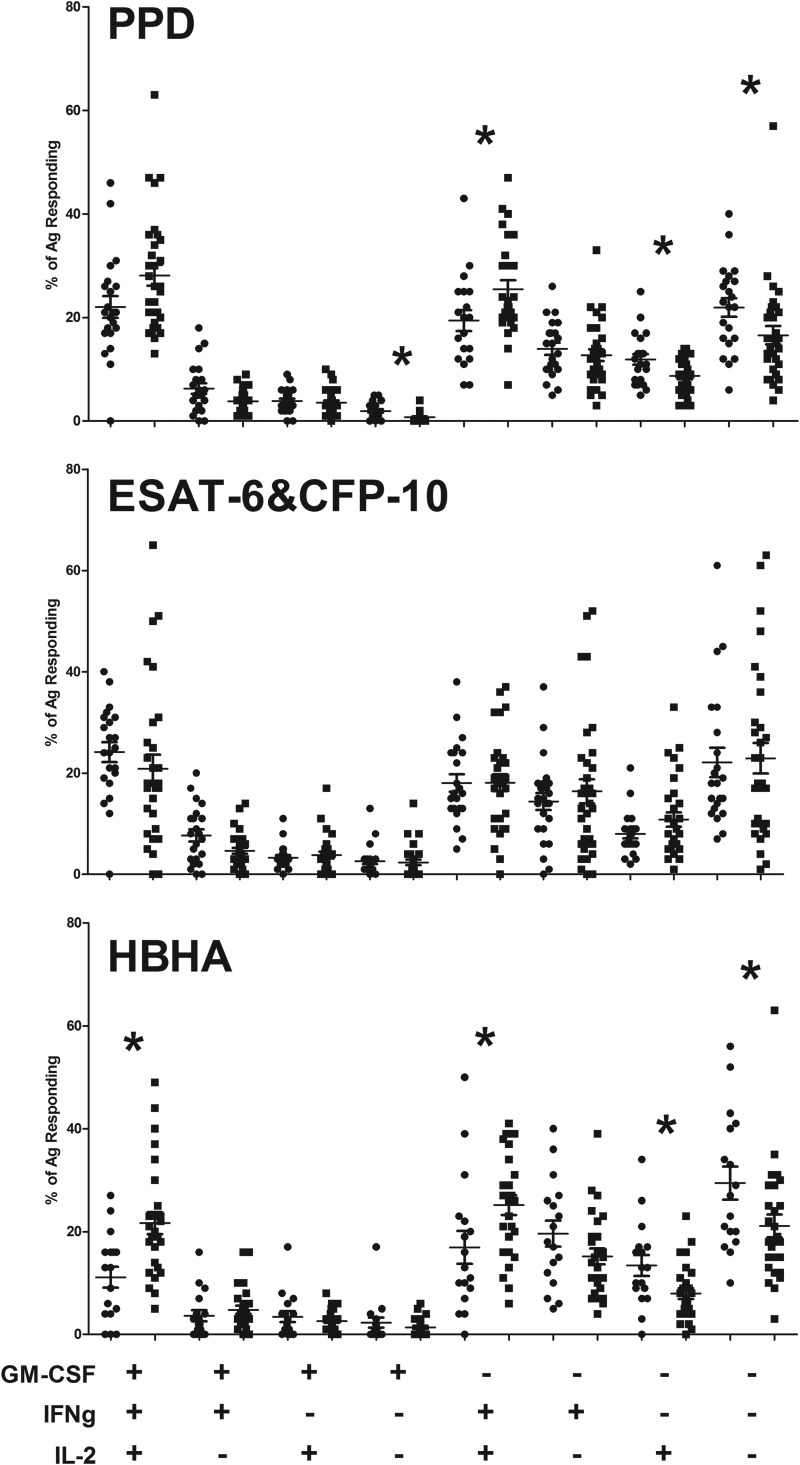
FIG 2.
GM-CSF, IFN-γ, and IL-2 staining in M. tuberculosis antigen-specific cells. The measured percentages of responding cells for each subject (means and standard errors are shown) (circles, actively infected subjects; squares, latently infected subjects) for different combinations of GM-CSF, IFN-γ, and IL-2 staining are shown for the different M. tuberculosis antigens tested. An asterisk indicates significance at a P value of <0.05, as measured by the Mann-Whitney U test for comparisons between the actively and latently infected groups.
Others (5) have reported that the proportion of PPD-specific TNF-α+ IFN-γ− IL-2− CD4 cells was significantly higher in patients with active TB than in those with latent TB. By ignoring the GM-CSF results and looking only at the IFN-γ and IL-2 combinations in PPD-specific CD4 cells, we also found a significant increase in the proportion of IFN-γ− IL-2− cells in the actively infected group. A clearer difference between the two groups was found for the IFN-γ+ IL-2+ population, with latently infected patients having a higher proportion (Table 2).
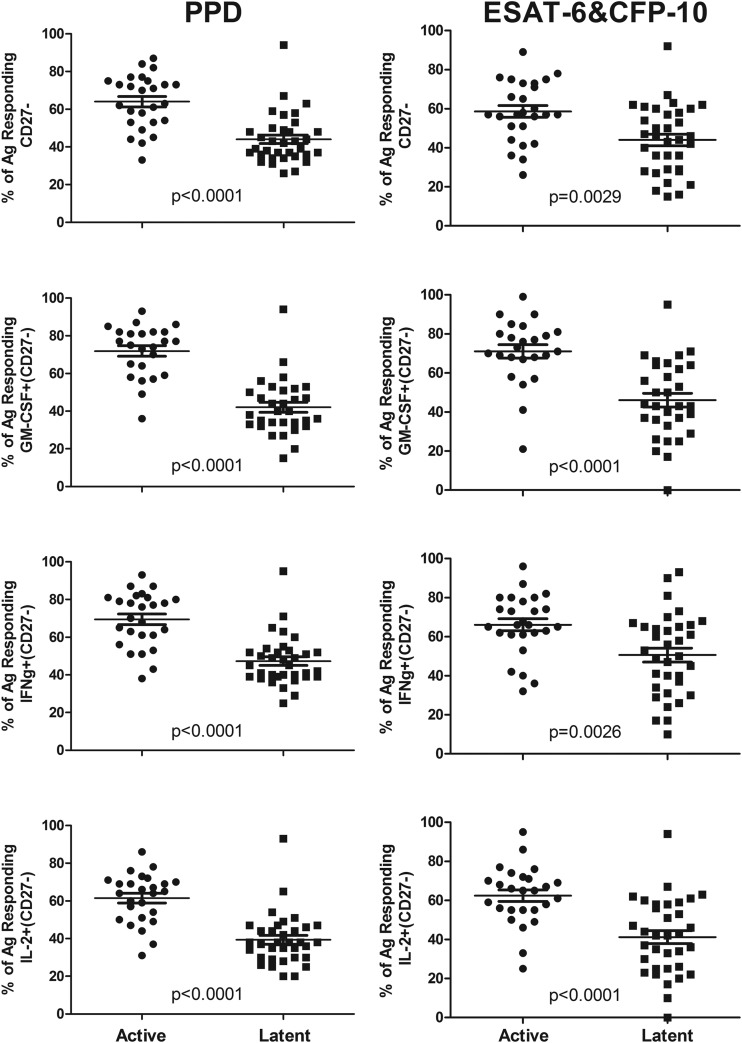
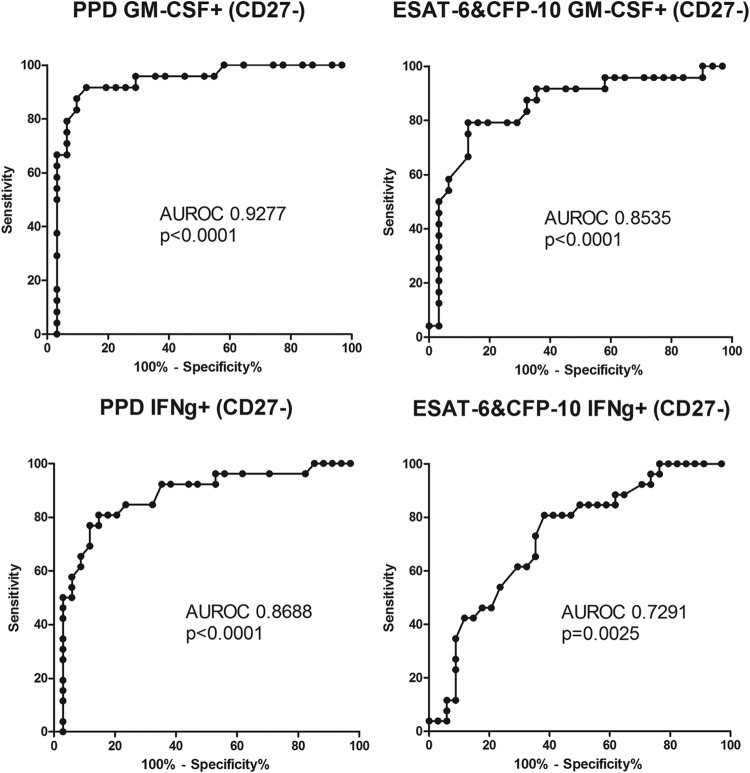
We found significantly reduced CD27 expression levels on total PPD-specific CD4 T cells in the actively versus the latently infected population (Fig. 3). Combined with individual cytokine staining, we found that the proportion of PPD-specific cells negative for CD27 and positive for IFN-γ, IL-2, or GM-CSF was significantly lower in the latent group, with the GM-CSF+ CD27− combination giving the strongest and clearest separation between the two groups (by ROC analysis) (Fig. 4). There was no significant difference in the proportions of PPD-specific cells that were CXCR3+ between the two groups (Table 2).
FIG 3.
Proportions of CD27-negative PPD-responding and ESAT-6- and CFP-10-responding cells significantly differ between subjects with active and those with latent TB. The measured percentages of responding cells for each subject (means and standard errors are shown) that were CD27− are shown for total responding, GM-CSF+, IFN-γ+, or IL-2+ cells. (Left) PPD stimulation; (right) ESAT-6 and CFP-10 stimulation. Results for Mann-Whitney U test comparisons between the actively and latently infected groups are displayed.
FIG 4.
ROC curve of the proportions of antigen-responding GM-CSF+ CD27− cells and antigen-responding IFN-γ+ CD27− cells. ROC analysis of the percentages of PPD-responding and ESAT-6- and CFP-10-responding cells was performed, and the respective areas under the ROC curve (AUROC) and P values are shown.
Unlike the clear differences found for the PPD-specific cell phenotypes, we found no significant differences in CD27 and cytokine expression combinations as a proportion of the total CD4 cells. For the combinations of cytokine staining, independent of CD27 expression, the proportion of CD4 cells that were CD154+ TNF-α+ IFN-γ+ IL-2+ was significantly higher in the latently than in the actively infected population. Also, the percentage of CD4 cells that were CD154+ TNF-α+ CXCR3+ was significantly higher in the latent than in the active TB group (Table 2).
The absolute numbers of the different subsets of PPD-specific CD4 cells were calculated by multiplying the percentages of CD4 cells positive for the staining combinations by the measured CD4 cell count. For the PPD-responding CD4 cells, we found no significant differences in their total absolute numbers or in the CD27 and cytokine combinations identified as being significantly different as a proportion of the PPD-specific cells. However, we measured a significant increase in the numbers of CD154+ TNF-α+ CXCR3+ and CD154+ TNF-α+ IFN-γ+ IL-2+ CD4 cells in the latent TB group (Table 2).
Differences in phenotype and frequency of ESAT-6- and CFP-10-specific CD4 T cells in patients with active versus latent TB.
By comparing the ESAT-6 and CFP-10 responses between the groups, we found the following. First, the total response defined by the percentage of CD154+ TNF-α+ CD4 cells was not significantly different between the latently and actively infected populations. Unlike the PPD response, we found no significant difference in IFN-γ or IL-2 positivity between the two groups as a proportion of the total ESAT-6- and CFP-10-specific response. However, there was a significant decrease in GM-CSF positivity in the latent TB population compared to the active TB population (Table 3). There were no significant differences in combinations of IFN-γ, IL-2, and GM-CSF staining (Fig. 2) or in combinations of IFN-γ and IL-2 staining independent of GM-CSF. The measured IL-17A and IL-22 responses after ESAT-6 and CFP-10 stimulation were greater for both groups than those after PPD stimulation, but there were no significant differences between groups (Table 3).
TABLE 3.
ESAT-6- and CFP-10-specific T cell responsesa
| Parameter | Value for group |
P value | |
|---|---|---|---|
| Active TB (n = 26) | Latent TB (n = 33) | ||
| Median % of CD4 cells (25th–75th percentile) | |||
| CD154+ TNF-α+ | 0.085 (0.034–0.197) | 0.065 (0.022–0.152) | 0.182 |
| CD154+ TNF-α+ IFN-γ+ | 0.065 (0.019–0.155) | 0.038 (0.009–0.107) | 0.152 |
| CD154+ TNF-α+ GM-CSF+ | 0.041 (0.014–0.075) | 0.022 (0.005–0.049) | 0.086 |
| CD154+ TNF-α+ IL-2+ | 0.053 (0.018–0.106) | 0.045 (0.010–0.076) | 0.148 |
| CD154+ TNF-α+ IFN-γ+ IL-2+ | 0.042 (0.014–0.088) | 0.029 (0.006–0.060) | 0.119 |
| CD154+ TNF-α+ IFN-γ− IL-2− | 0.015 (0.010–0.030) | 0.010 (0.006–0.019) | 0.064 |
| CD154+ TNF-α+ CD27− | 0.053 (0.019–0.144) | 0.022 (0.008–0.069) | 0.035 |
| CD154+ TNF-α+ IFN-γ+ CD27− | 0.039 (0.012–0.120) | 0.016 (0.004–0.051) | 0.043 |
| CD154+ TNF-α+ GM-CSF+ CD27− | 0.029 (0.011–0.064) | 0.009 (0.003–0.022) | 0.003 |
| CD154+ TNF-α+ IL-2+ CD27− | 0.035 (0.009–0.067) | 0.012 (0.003–0.028) | 0.011 |
| CD4+ TNF-α+ CD154+ CXCR3+ | 0.007 (0.003–0.015) | 0.005 (0.002–0.014) | 0.526 |
| Median % of CD4+ TNF-α+ CD154+ cells (25th–75th percentile) | |||
| IFN-γ+ | 71.5 (57.5–77.5) | 65.5 (39.7–74.3) | 0.248 |
| GM-CSF+ | 39.0 (34.0–58.0) | 28.0 (24.0–41.0) | 0.026 |
| IL-2+ | 55.0 (45.5–67.0) | 54.0 (37.7–71.5) | 0.748 |
| IL-17A+ | 8.0 (4.0–15.0) | 12.0 (4.0–27.8) | 0.174 |
| IL-22+ | 9.0 (4.0–15.5) | 8.0 (4.5–14.5) | 0.982 |
| IL-2− IFN-γ− | 18.5 (14.0–27.3) | 24.0 (10.7–33.3) | 0.682 |
| IL-2+ IFN-γ+ | 46.5 (35.2–51.3) | 38.0 (23.2–54.3) | 0.163 |
| CXCR3+ | 10.0 (2.7–17.0) | 8.0 (4.0–14.0) | 0.977 |
| Median absolute no. of cells/ml (25th–75th percentile) | |||
| CD4+ TNF-α+ CD154+ | 541 (269–1,097) | 334 (155–1,008) | 0.216 |
| CD4+ TNF-α+ CD154+ IFN-γ+ IL-2+ | 262 (94–484) | 118 (35–468) | 0.172 |
| CD4+ TNF-α+ CD154+ IFN-γ− IL-2− | 106 (57–171) | 71 (41–115) | 0.082 |
| CD4+ TNF-α+ CD154+ CD27− | 335 (123–666) | 144 (39–446) | 0.032 |
| CD4+ TNF-α+ CD154+ IFN-γ+ CD27− | 249 (83–520) | 107 (19–354) | 0.038 |
| CD4+ TNF-α+ CD154+ GM-CSF+ CD27− | 197 (52–274) | 43 (19–137) | 0.012 |
| CD4+ TNF-α+ CD154+ IL-2+ CD27− | 182 (72–395) | 69 (20–182) | 0.017 |
| CD4+ TNF-α+ CD154+ CXCR3+ | 32 (17–90) | 28 (10–72) | 0.541 |
Results for Mann-Whitney U test comparisons between the active and latent groups are displayed.
Similar to the results for PPD, we found a highly significant decrease in the proportions of ESAT-6- and CFP-10-specific cells negative for CD27 in the latent TB group compared to those in subjects with active TB (Fig. 3). By combining CD27 expression with staining for other cytokines, we found that the proportion of IFN-γ-, IL-2-, or GM-CSF-positive and CD27-negative ESAT-6- and CFP-10-specific cells was significantly lower in the latent TB group, with the GM-CSF+ CD27− combination again giving the strongest and clearest separation between the two groups (by ROC analysis) (Fig. 4). Also, when calculated as a percentage of the total CD4 cells, significantly smaller proportions of TNF-α+ CD154+ IFN-γ+ CD27− and TNF-α+ CD154+ GM-CSF+ CD27− cells were found in the latent TB group. There was no difference in CXCR3-positive percentages on the ESAT-6- and CFP-10-specific cells between the active and latent TB groups (Table 3).
There was no significant difference in the absolute numbers of total ESAT-6- and CFP-10-reactive CD4 cells between the two groups. However, we saw a significant reduction in the numbers of TNF-α+ CD154+ IFN-γ+ CD27− and TNF-α+ CD154+ GM-CSF+ CD27− CD4 cells in the latent TB group (Table 3).
Differences in the phenotype and frequency of HBHA-specific CD4 T cells in patients with active versus latent TB.
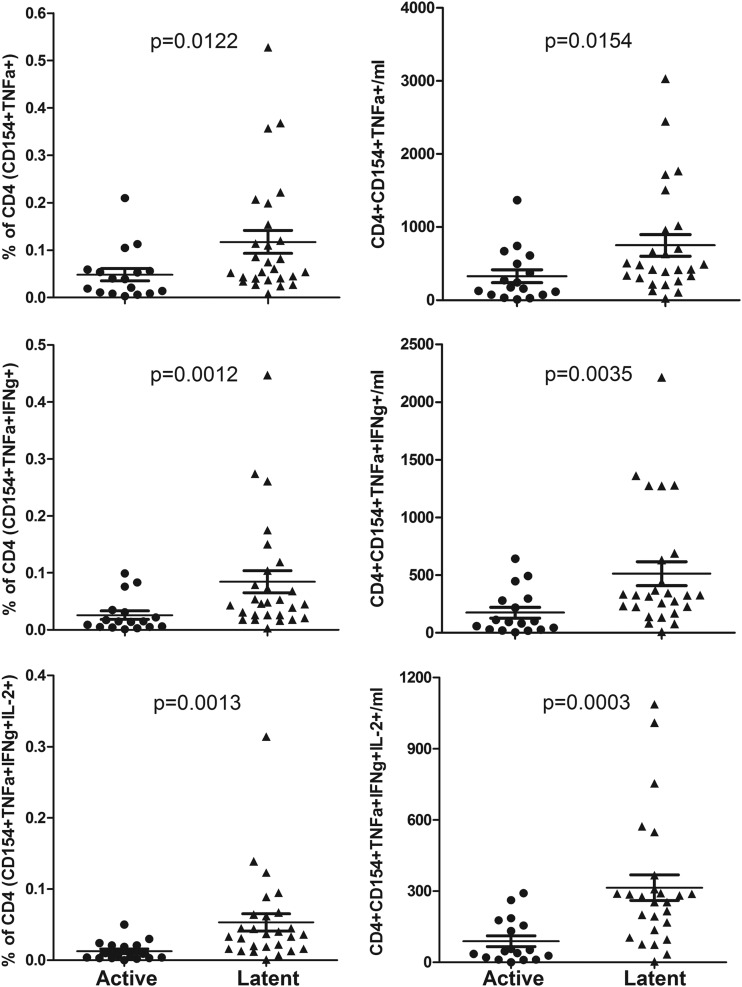
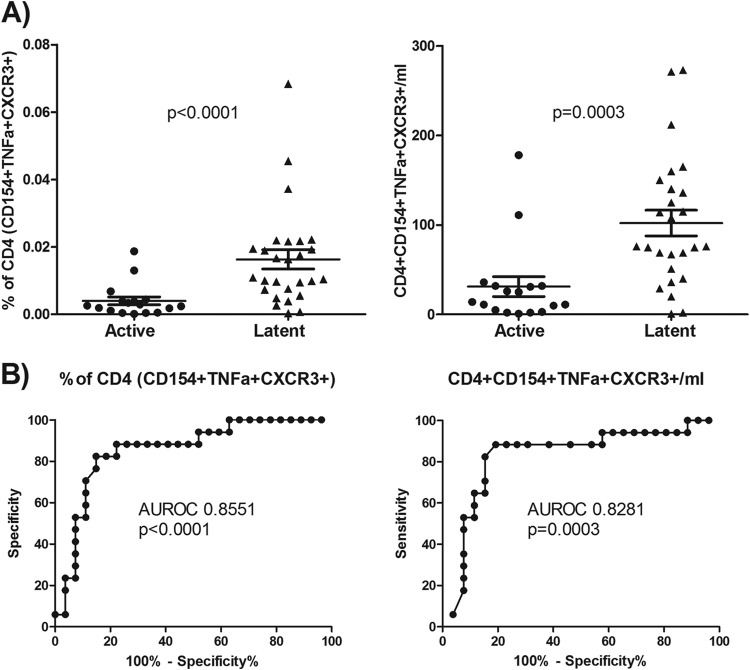
For HBHA stimulation, we studied a subgroup of the total subjects: 26 from the latent TB group and 17 from the active TB group. We found a significantly greater response to HBHA in the latent TB group than in the active TB subjects when measured as a proportion of CD154+ TNF-α+, CD154+ TNF-α+ IFN-γ+, and CD154+ TNF-α+ IFN-γ+ IL-2+ CD4 cells, with the strongest difference being found for the latter combination (Fig. 5). Also, the percentage of CD154+ TNF-α+ CXCR3+ CD4 cells was significantly higher in the latent TB group than in the active TB group (Fig. 6).
FIG 5.
HBHA-responding cells as a percentage of CD4 cells and their absolute numbers. HBHA-responding cells as a percentage of CD4 cells (left) and absolute numbers (right) are shown for CD154+ TNF-α+ (total responding), CD154+ TNF-α+ IFN-γ+, and CD154+ TNF-α+ IFN-γ+ IL-2+ cells. Results for Mann-Whitney U test comparisons between the actively and latently infected groups are displayed.
FIG 6.
CXCR3 expression on HBHA-responding cells. (A) CXCR3 expression on HBHA-responding cells as a percentage of CD4 cells (left) and their absolute numbers (right) for each subject. Results for Mann-Whitney U test comparisons between the actively and latently infected groups are displayed. (B) ROC analysis for HBHA-responding cells as a percentage of CD4 cells (left) and their absolute numbers (right), along with their respective areas under the curve and P values.
As a proportion of the HBHA-specific cells, we found that, similar to the results for PPD-stimulated cells, total IFN-γ or IL-2 staining was significantly increased in the latent compared to the active TB group (Table 4), but there was no significant difference with GM-CSF. For different combinations of GM-CSF, IFN-γ, and IL-2 production, we found some significant differences between the subject groups (Fig. 2), but as with the PPD results, none of these combinations gave a clear separation of the two populations. For the combination of IFN-γ and IL-2 staining alone, we found that the proportion IFN-γ− IL-2− cells was higher in the active TB group and that the proportion of IFN-γ+ IL-2+ cells was higher in the latent TB subjects. The measured IL-17A response was low in both groups but was significantly lower in the latent than in the active TB group. Again, the IL-22 response was small, and no differences between groups were detected (Table 4).
TABLE 4.
HBHA-specific T cell responsesa
| Parameter | Value for group |
P value | |
|---|---|---|---|
| Active TB (n = 17) | Latent TB (n = 26) | ||
| Median % of CD4 cells (25th–75th percentile) | |||
| CD154+ TNF-α+ GM-CSF+ | 0.004 (0.002–0.015) | 0.017 (0.013–0.045) | <0.001 |
| CD154+ TNF-α+ IL-2+ | 0.013 (0.005–0.035) | 0.042 (0.024–0.077) | <0.001 |
| CD154+ TNF-α+ IFN-γ− IL-2− | 0.013 (0.002–0.021) | 0.014 (0.007–0.026) | 0.148 |
| CD154+ TNF-α+ CD27− | 0.014 (0.006–0.035) | 0.029 (0.016–0.063) | 0.101 |
| CD154+ TNF-α+ IFN-γ+ CD27− | 0.011 (0.003–0.023) | 0.020 (0.012–0.042) | 0.030 |
| CD154+ TNF-α+ GM-CSF+ CD27− | 0.003 (0.001–0.011) | 0.010 (0.003–0.017) | 0.041 |
| CD154+ TNF-α+ IL-2+ CD27− | 0.007 (0.003–0.017) | 0.015 (0.007–0.033) | 0.031 |
| Median % of CD4+ TNF-α+ CD154+ cells (25th–75th percentile) | |||
| IFN-γ+ | 56.0 (37.0–64.0) | 67.0 (62.0–76.0) | 0.001 |
| GM-CSF+ | 24.0 (11.2–32.5) | 28.0 (22.0–39.0) | 0.065 |
| IL-2+ | 47.0 (32.0–59.0) | 61.0 (45.0–73.0) | 0.021 |
| IL-17A+ | 5.0 (2.0–10.0) | 2.0 (1.0–3.0) | 0.024 |
| IL-22+ | 2.5 (0.7–8.3) | 1.5 (0.0–2.3) | 0.132 |
| IL-2− IFN-γ− | 29.0 (20.0–47.0) | 21.0 (15.0–30.0) | 0.044 |
| IL-2+ IFN-γ+ | 30.0 (16.0–38.5) | 50.0 (30.0–60.0) | 0.001 |
| CXCR3+ | 12.7 (2.5–18.0) | 17.7 (8.0–26.0) | 0.072 |
| Median % of CD4+ TNF-α+ CD154+ CD27− cells (25th–75th percentile) | |||
| Total | 61.0 (47.0–68.0) | 42.0 (36.0–53.0) | 0.009 |
| IFN-γ+ | 70.0 (49.0–76.5) | 44.0 (33.0–55.0) | 0.016 |
| GM-CSF+ | 66.5 (48.5–78.8) | 40.0 (29.0–51.0) | 0.002 |
| IL-2+ | 53.0 (44.0–66.5) | 38.0 (30.0–44.0) | 0.003 |
| Median absolute no. of cells/ml (25th–75th percentile) | |||
| CD4+ TNF-α+ CD154+ IFN-γ− IL-2− | 71 (12–146) | 82 (48–236) | 0.130 |
| CD4+ TNF-α+ CD154+ CD27− | 101 (36–293) | 190 (115–438) | 0.109 |
| CD4+ TNF-α+ CD154+ IFN-γ+ CD27− | 58 (14–221) | 135 (84–332) | 0.055 |
| CD4+ TNF-α+ CD154+ GM-CSF+ CD27− | 15 (6–71) | 59 (25–106) | 0.025 |
| CD4+ TNF-α+ CD154+ IL-2+ CD27− | 45 (15–128) | 110 (59–174) | 0.043 |
Results for Mann-Whitney U test comparisons between the active and latent TB groups are displayed.
Similar to the results described above, we found a significant decrease in the proportion of HBHA-specific cells negative for CD27 in the latent TB group compared to the subjects with active TB. By combining CD27 expression with staining for other cytokines, we also found that the proportion of IFN-γ-, IL-2-, or GM-CSF-positive and CD27-negative PPD-specific cells was significantly lower in the latent TB group, with the GM-CSF+ CD27− combination giving the clearest separation between the two groups (Table 4).
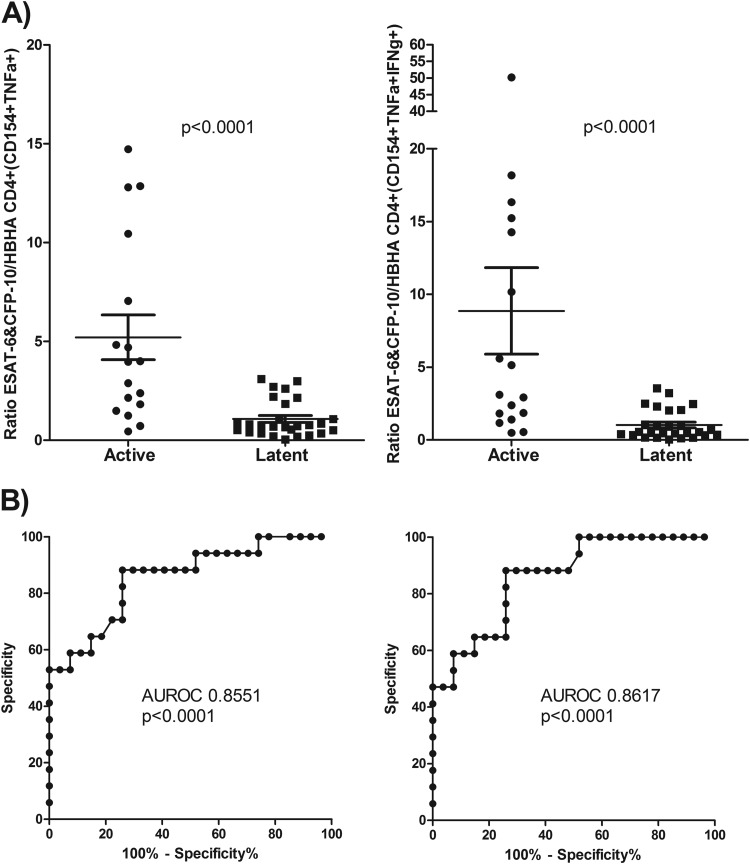
Others have found that comparing an individual's IGRA responses to ESAT-6 and HBHA can increase the discrimination between active and latent TB subjects (15). We thus compared the magnitudes of each individual subject's HBHA as well as ESAT-6 and CFP-10 responses by determining the ratio of the proportion of total responding cells to the proportion of cells that were IFN-γ positive. It was found that there was a striking difference between the two groups for both comparisons (Fig. 7).
FIG 7.
Comparison of HBHA responses and ESAT-6 and CFP-10 responses. (A) Ratio of the percentage of ESAT-6- and CFP-10-responding to HBHA-responding CD154+ TNF-α+ (left) and CD154+ TNF-α+ IFN-γ+ (right) CD4 cells for each subject. Results for Mann-Whitney U test comparisons between the actively and latently infected groups are displayed. (B) ROC analysis of the ratio of ESAT-6 and CFP-10 to HBHA for CD154+ TNF-α+ (left) and CD154+ TNF-α+ IFN-γ+ (right) cells, along with their respective areas under the curve and P values.
Unlike the PPD results and the ESAT-6 and CFP-10 results, we found significant differences in the absolute numbers of total HBHA-specific CD4 T cells between the two groups, with an increase in the latent TB compared to the active TB population (Fig. 5). We also found stronger differences when HBHA-specific cells were classified according to IFN-γ+ and IFN-γ+ IL-2+ staining. Independent of IFN-γ and IL-2 staining, we also found a clear and significant increase in the number of HBHA-specific CD4 cells that expressed CXCR3 for the latent TB group (Fig. 6).
LASSO regression analysis of combined parameter measurements.
To determine if combinations of the measured parameters gave stronger separation between the two groups of subjects than the parameters on their own, we performed a LASSO regressional analysis on the results. However, we found that there was no combination that gave a statistically valid increase in separation above that of the individual parameters.
DISCUSSION
We measured cytokine production and determined the phenotype of M. tuberculosis antigen-specific CD4 T cells to determine if it was possible to describe particular markers that can distinguish active from latent TB infection. We found that a decrease in CD27 expression on M. tuberculosis antigen-specific cells, as well as an increased response to HBHA, significantly corresponded to the latently infected subjects. In particular, the decreased CD27 expression on GM-CSF-producing cells was a clear and strong discriminator of latently infected patients, while comparison of a subject's response to HBHA to their response to ESAT-6 and CFP-10 also gave a strong distinction between the two groups. We also determined that increased CXCR3 expression on PPD- and HBHA-stimulated CD4 T cells was clearly associated with latent TB infection.
We found combinations of M. tuberculosis antigen-induced CD4 T cell cytokine production that produced significant differences between the two patient groups studied. Similar to data reported previously by Sester et al. (4), we found significantly higher frequencies of PPD-induced CD4 IFN-γ+ IL-2+ cells in latently infected individuals than in those with active TB. Also, similar to data reported previously by Harari et al. (5), there was a significantly higher frequency of TNF-α+ IFN-γ− IL-2− PPD-specific CD4 T cells in the active TB patient group. However, our results, while statistically significant, did not have the clear difference seen by Harari et al., and unlike that study, we found no difference with ESAT-6 and CFP-10 stimulation. This inconsistency in cytokine combination results concurs with what others have reported. For example, Petruccioli et al. found no difference in the frequencies of TNF-α+ IFN-γ− IL-2− M. tuberculosis antigen-specific CD4 T cells between active and latent TB populations but saw a significant increase in the TNF-α+ IFN-γ+ IL-2− frequency in active TB patents (8). Another study that measured a range of cytokines found no differences at all (16). The reason for the variability of the studies is probably due to the many variations of the stimulation assays used, including the use of whole blood versus density gradient-separated mononuclear cells, the duration of antigen stimulation, the use of costimulatory antibodies, and the antibody-fluorophore combinations used.
Another approach used for discriminating actively from latently infected individuals is the measurement of CD4 T cell memory markers on M. tuberculosis antigen-specific cells (9). One such marker is CD27, a receptor for costimulation expressed on CD4 T cells but downregulated when T cells progress from a naive to a terminal memory stage (17). Previously, others found that subjects with active TB had a much higher proportion of IFN-γ-producing M. tuberculosis-specific CD4 T cells negative for or weakly expressing CD27 than did those with latent TB infection, for both HIV-negative (6) and HIV-positive (18) subjects. Our results confirmed this, but we also found for the first time that measurement of CD27 expression on GM-CSF-producing M. tuberculosis antigen-specific CD4 T cells was an even better discriminator of the two populations for all the M. tuberculosis antigens measured. ICS measurement of M. tuberculosis antigen-induced GM-CSF production by CD4 T cells in children has been described (19, 20), but as far as we know, ours is the first study to do so with adults. The role of GM-CSF in tuberculosis is not totally clear, but it has been shown that its deletion by gene knockout in mice results in uncontrolled M. tuberculosis growth and increased mortality after pulmonary infection (21), while others have shown in an in vitro model that GM-CSF produced by invariant natural killer T cells can control M. tuberculosis growth (22). Our results also indicate an important role for GM-CSF, as the increase in the frequency of effector memory M. tuberculosis-specific CD4 T cells making GM-CSF in active TB patients supports it being involved in the attempted control of infection by the immune system.
Particular M. tuberculosis proteins that elicit a differential response from T cells in actively and latently infected patients have been identified (23). One of these proteins is HBHA (11), which has been found by IGRAs to induce significantly stronger T cell responses in latently infected individuals than in individuals with active disease (12). Our ICS results confirm and extend these findings. Similar to the findings reported previously by Loxton et al. (24), we found that measurement of the frequency of HBHA-specific IFN-γ+ IL-2+ CD4 cells gave the strongest separation between the two patient populations, but unlike those authors, we did not see the same results with IL-17. We also found that comparison of the ESAT-6- and CFP-10-induced IFN-γ+ IL-2+ responses to those induced by HBHA enabled strong discrimination between the two patents groups. A similar finding by Corbiere et al. using IGRAs (15) gives support to this method of using the HBHA response and the ESAT-6 and CFP-10 response to discriminate the two groups.
Our study is the first to our knowledge to report significant differences in the absolute numbers of circulating M. tuberculosis antigen-specific CD4 T cells between active and latent TB populations. In particular, we found that the changes in HBHA-specific CD4 T cell frequencies were mirrored by changes in absolute numbers, which was not the case for the PPD-specific and the ESAT-6- and CFP-10-specific cells. Others have reported no differences in absolute numbers of Mycobacterium bovis BCG-specific CD4 T cells in patients with TB infection versus healthy, skin test-positive controls (7) or of sonicated M. tuberculosis-specific CD4 T cells from actively infected individuals and M. tuberculosis-exposed healthy controls (25). Why there is a difference in absolute numbers for HBHA stimulation but not with the other antigens is an interesting question, with the higher concentration of HBHA-specific CD4 T cells perhaps indicating that they are part of an ongoing systemic response in the latently infected population. On the other hand, the PPD-responding and the ESAT-6- and CFP-10-responding CD4 T cells may be the ones responsible for the localized response at the site of infection, and as such, sampling of the blood does not give a true reflection of their number. The discordance between the HBHA response and the PPD or the ESAT-6 and CFP-10 response can also be demonstrated by the fact that combining the two results did not enhance the differences measured, indicating that they are independent of each other.
We also report the novel finding that there were significantly higher proportions and higher numbers of CXCR3-expressing PPD- and HBHA-specific CD4 T cells in the latently infected population than in actively infected TB subjects. CXCR3 is a chemokine receptor that has been found to be specifically expressed on Th1 cells (26) and is the receptor for the chemokines IP-10 and Mig3 (27). Both CXCR3 and its ligand IP-10 have been found to have important roles in tuberculosis: CXCR3 knockout mice have been shown to have heightened resistance to TB infection (28), while IP-10 has been suggested as a replacement for IFN-γ in IGRAs, as it appears to be a more sensitive and specific indicator of M. tuberculosis exposure (29). Another study found that the majority of human M. tuberculosis-specific Th1 cells are within the CXCR3+ CCR6+ cell subset (30), which may be supported by our finding of increased CXCR3 expression in latently infected TB subjects, as it could be expected that there would be more protective cells present at this stage.
Our study may have benefited from having the samples set up on the same day as the blood was taken, but due to logistical issues and the need to treat all samples equally, this was not possible. We performed preliminary testing to compare blood stored overnight to fresh blood and found little or no difference in the phenotype of the PPD CD4 T cell response, no decrease in cell viability, and comparable intensities and patterns of staining. Whatever the effects that storage overnight may have had, they would have been consistent across all samples, and so we believe that the differences measured are accurate. The LASSO analysis would have been more precise with increased numbers of patients.
In conclusion, our study found novel markers of M. tuberculosis-specific CD4 T cells that can differentiate actively from latently infected tuberculosis subjects. These results could potentially be of clinical benefit and provide new insights into the type of immune responses mounted during M. tuberculosis infection.
ACKNOWLEDGMENTS
This work was supported by funding from a National Healthcare Group small innovative grant and the Tan Tock Seng Hospital Community Charity Fund.
We acknowledge the assistance of Teo Guo Hui in performing the flow cytometric work and Ong Twee Hee and Xu Wenting for help in performing the LASSO regression analysis.
REFERENCES
- 1.Flynn JL, Chan J. 2001. Immunology of tuberculosis. Annu Rev Immunol 19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest 99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes AJ, Hutchinson P, Gooding T, Freezer NJ, Holdsworth SR, Johnson PD. 2005. Diagnosis of Mycobacterium tuberculosis infection using ESAT-6 and intracellular cytokine cytometry. Clin Exp Immunol 142:132–139. doi: 10.1111/j.1365-2249.2005.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sester U, Fousse M, Dirks J, Mack U, Prasse A, Singh M, Lalvani A, Sester M. 2011. Whole-blood flow-cytometric analysis of antigen-specific CD4 T-cell cytokine profiles distinguishes active tuberculosis from non-active states. PLoS One 6:e17813. doi: 10.1371/journal.pone.0017813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, Faouzi M, Day CL, Hanekom WA, Bart PA, Pantaleo G. 2011. Dominant TNF-alpha+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med 17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Streitz M, Tesfa L, Yildirim V, Yahyazadeh A, Ulrichs T, Lenkei R, Quassem A, Liebetrau G, Nomura L, Maecker H, Volk HD, Kern F. 2007. Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PLoS One 2:e735. doi: 10.1371/journal.pone.0000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang J, Wang X, Wang X, Cao Z, Liu Y, Dong M, Tong A, Cheng X. 2010. Reduced CD27 expression on antigen-specific CD4+ T cells correlates with persistent active tuberculosis. J Clin Immunol 30:566–573. doi: 10.1007/s10875-010-9418-1. [DOI] [PubMed] [Google Scholar]
- 8.Petruccioli E, Petrone L, Vanini V, Sampaolesi A, Gualano A, Girardi E, Palmieri F, Goletti D. 2013. IFNgamma/TNFalpha specific-cells and effector memory phenotype associate with active tuberculosis. J Infect 66:475–486. doi: 10.1016/j.jinf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Pollock KM, Whitworth HS, Montamat-Sicotte DJ, Grass L, Cooke GS, Kapembwa MS, Kon OM, Sampson RD, Taylor GP, Lalvani A. 2013. T-cell immunophenotyping distinguishes active from latent tuberculosis. J Infect Dis 208:952–968. doi: 10.1093/infdis/jit265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demissie A, Leyten EM, Abebe M, Wassie L, Aseffa A, Abate G, Fletcher H, Owiafe P, Hill PC, Brookes R, Rook G, Zumla A, Arend SM, Klein M, Ottenhoff TH, Andersen P, Doherty TM, VACSEL Study Group . 2006. Recognition of stage-specific mycobacterial antigens differentiates between acute and latent infections with Mycobacterium tuberculosis. Clin Vaccine Immunol 13:179–186. doi: 10.1128/CVI.13.2.179-186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locht C, Hougardy JM, Rouanet C, Place S, Mascart F. 2006. Heparin-binding hemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis. Tuberculosis 86:303–309. doi: 10.1016/j.tube.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Hougardy JM, Schepers K, Place S, Drowart A, Lechevin V, Verscheure V, Debrie AS, Doherty TM, Van Vooren JP, Locht C, Mascart F. 2007. Heparin-binding-hemagglutinin-induced IFN-gamma release as a diagnostic tool for latent tuberculosis. PLoS One 2:e926. doi: 10.1371/journal.pone.0000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delogu G, Chiacchio T, Vanini V, Butera O, Cuzzi G, Bua A, Molicotti P, Zanetti S, Lauria FN, Grisetti S, Magnavita N, Fadda G, Girardi E, Goletti D. 2011. Methylated HBHA produced in M. smegmatis discriminates between active and non-active tuberculosis disease among RD1-responders. PLoS One 6:e18315. doi: 10.1371/journal.pone.0018315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suni MA, Picker LJ, Maino VC. 1998. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J Immunol Methods 212:89–98. doi: 10.1016/S0022-1759(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 15.Corbiere V, Pottier G, Bonkain F, Schepers K, Verscheure V, Lecher S, Doherty TM, Locht C, Mascart F. 2012. Risk stratification of latent tuberculosis defined by combined interferon gamma release assays. PLoS One 7:e43285. doi: 10.1371/journal.pone.0043285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemeth J, Winkler HM, Zwick RH, Muller C, Rumetshofer R, Boeck L, Burghuber OC, Winkler S. 2012. Peripheral T cell cytokine responses for diagnosis of active tuberculosis. PLoS One 7:e35290. doi: 10.1371/journal.pone.0035290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. 2013. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol 43:2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 18.Schuetz A, Haule A, Reither K, Ngwenyama N, Rachow A, Meyerhans A, Maboko L, Koup RA, Hoelscher M, Geldmacher C. 2011. Monitoring CD27 expression to evaluate Mycobacterium tuberculosis activity in HIV-1 infected individuals in vivo. PLoS One 6:e27284. doi: 10.1371/journal.pone.0027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Mauff K, Hughes EJ, Moyo S, Brittain N, Lawrie A, Mulenga H, de Kock M, Gelderbloem S, Veldsman A, Hatherill M, Geldenhuys H, Hill AV, Hussey GD, Mahomed H, Hanekom WA, McShane H. 2011. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis 203:1832–1843. doi: 10.1093/infdis/jir195. [DOI] [PubMed] [Google Scholar]
- 20.Mueller H, Detjen AK, Schuck SD, Gutschmidt A, Wahn U, Magdorf K, Kaufmann SH, Jacobsen M. 2008. Mycobacterium tuberculosis-specific CD4+, IFNgamma+, and TNFalpha+ multifunctional memory T cells coexpress GM-CSF. Cytokine 43:143–148. doi: 10.1016/j.cyto.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Juarrero M, Hattle JM, Izzo A, Junqueira-Kipnis AP, Shim TS, Trapnell BC, Cooper AM, Orme IM. 2005. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol 77:914–922. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- 22.Rothchild AC, Jayaraman P, Nunes-Alves C, Behar SM. 2014. iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathog 10:e1003805. doi: 10.1371/journal.ppat.1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govender L, Abel B, Hughes EJ, Scriba TJ, Kagina BM, de Kock M, Walzl G, Black G, Rosenkrands I, Hussey GD, Mahomed H, Andersen P, Hanekom WA. 2010. Higher human CD4 T cell response to novel Mycobacterium tuberculosis latency associated antigens Rv2660 and Rv2659 in latent infection compared with tuberculosis disease. Vaccine 29:51–57. doi: 10.1016/j.vaccine.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loxton AG, Black GF, Stanley K, Walzl G. 2012. Heparin-binding hemagglutinin induces IFN-gamma+ IL-2+ IL-17+ multifunctional CD4+ T cells during latent but not active tuberculosis disease. Clin Vaccine Immunol 19:746–751. doi: 10.1128/CVI.00047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikitina IY, Kondratuk NA, Kosmiadi GA, Amansahedov RB, Vasilyeva IA, Ganusov VV, Lyadova IV. 2012. Mtb-specific CD27low CD4 T cells as markers of lung tissue destruction during pulmonary tuberculosis in humans. PLoS One 7:e43733. doi: 10.1371/journal.pone.0043733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 27.Farber JM. 1997. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 61:246–257. [PubMed] [Google Scholar]
- 28.Chakravarty SD, Xu J, Lu B, Gerard C, Flynn J, Chan J. 2007. The chemokine receptor CXCR3 attenuates the control of chronic Mycobacterium tuberculosis infection in BALB/c mice. J Immunol 178:1723–1735. doi: 10.4049/jimmunol.178.3.1723. [DOI] [PubMed] [Google Scholar]
- 29.Ruhwald M, Dominguez J, Latorre I, Losi M, Richeldi L, Pasticci MB, Mazzolla R, Goletti D, Butera O, Bruchfeld J, Gaines H, Gerogianni I, Tuuminen T, Ferrara G, Eugen-Olsen J, Ravn P, TBNET . 2011. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis 91:260–267. doi: 10.1016/j.tube.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Lindestam Arlehamn CS, Gerasimova A, Mele F, Henderson R, Swann J, Greenbaum JA, Kim Y, Sidney J, James EA, Taplitz R, McKinney DM, Kwok WW, Grey H, Sallusto F, Peters B, Sette A. 2013. Memory T cells in latent Mycobacterium tuberculosis infection are directed against three antigenic islands and largely contained in a CXCR3+CCR6+ Th1 subset. PLoS Pathog 9:e1003130. doi: 10.1371/journal.ppat.1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]