Abstract
Nearly all Streptococcus agalactiae (group B streptococcus [GBS]) strains express a protein which belongs to the so-called alpha-like proteins (Alps), of which Cα, Alp1, Alp2, Alp3, Rib, and Alp4 are known to occur in GBS. The Alps are chimeras which form mosaic structures on the GBS surface. Both N- and C-terminal stretches of the Alps possess immunogenic sites of dissimilar immunological specificity. In this review, we have compiled data dealing with the specificity of the N- and C-terminal immunogenic sites of the Alps. The majority of N-terminal sites show protein specificity while the C-terminal sites show broader cross-reactivity. Molecular serotyping has revealed that antibody-based serotyping has often resulted in erroneous Alp identification, due to persistence of cross-reacting antibodies in antisera for serotyping. Retrospectively, this could be expected on the basis of sequence analysis results. Some of the historical R proteins are in fact Alps. The data included in the review may provide a basis for decisions regarding techniques for the preparation of specific antisera for serotyping of GBS, for use in other approaches in GBS research, and for decision making in the context of GBS vaccine developments.
INTRODUCTION
Immunologic classification of hemolytic streptococci began in 1933 when Rebecca Lancefield defined serogroups based on serogroup-specific polysaccharides (1). Streptococcus agalactiae (serogroup B; group B streptococcus [GBS]), an important cause of infections in humans, notably in neonates, and an important cause of mastitis in cattle, was divided into serotypes I, II, and III on the basis of capsular polysaccharide (CPS) antigens (2). Currently, CPS types Ia, Ib, and II to IX have been recognized among group B streptococci (GBS).
Protein antigens of GBS were originally named Ii antigen (3), later changed to Ic antigens (4), and were changed again to Ibc proteins by Lancefield in 1975 (5). The history of the alpha-like proteins (Alps) of GBS appears to have begun in 1971 with the demonstration of two precipitinogens in an HCl extract of GBS. One of the antigens was sensitive to trypsin, and the other was trypsin resistant (4). The trypsin-sensitive antigen (Cβ) was a non-Alp, but the trypsin-resistant antigen might have been the alpha antigen (Cα). In 1979, Bevanger and Maeland (6) described two precipitinogens in HCl extracts from the CPS type Ia strain A909 and the type Ib strain H36B and one precipitinogen in the type Ia strain 335 (6). The strain 335 antigen cross-reacted immunologically with one of the two A909 and H36B antigens and was trypsin resistant but sensitive to pepsin digestion. This antigen was named alpha (Cα). The second A909 and H36B antigen which was sensitive to trypsin was called Cβ. Today, we know that the trypsin-resistant 335 antigen was Alp1, which at that time was erroneously considered to be Cα due to immunological cross-reactivity (see below), while the trypsin-resistant A909 and H36B antigens were true Cα (6). Strain A909 has since been a prototype strain for Cα. However, trypsin-resistant protein antigens were first described in group A streptococci (GAS) as R antigens, i.e., the R28 protein of GAS (7) and the R proteins R1, R2, R3, and R4 (8).
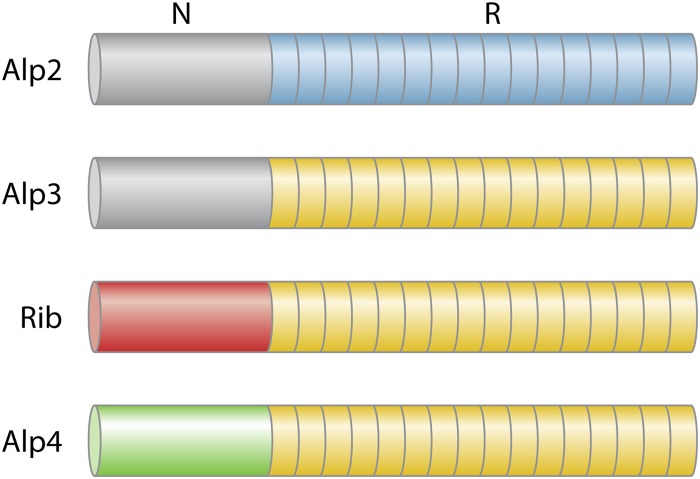
All Alps belong to the same protein antigen family of GBS based on a variety of shared characteristics revealed by analysis both of the Alp genes and of the gene products (Fig. 1). When expressed in clinical isolates, Alps are high-molecular-mass proteins with a signal peptide of ∼50 amino acids (aa), an N terminus of 170 to 180 aa, and a C-terminal area of 40 to 50 aa with a cell wall-anchoring motif, which probably ensures binding to the cell wall (9); some Alps also contain an intermediate region before a repeat region with tandemly arranged repeats of ∼80 aa, mostly 8 to 10 repeats in clinical isolates. For extended review and references, see reference 10. On SDS-PAGE and Western blotting, Alps form ladder-like banding patterns with the number of bands in the ladder corresponding to the number of repeats and a distance between neighboring bands corresponding to ∼8 kDa, i.e., the molecular mass of a single repeat. Although clinical isolates usually contain 9 or 10 repeats, the number may vary and the size of the protein varies correspondingly. The Alp genes have been sequenced, resulting in a great increase in knowledge about these genes and gene products. This has led to improved understanding of the relationship between different Alps and development of methods for identification of the proteins by molecular methods, which has facilitated serotyping (11, 12, 13). Alps are chimeras with a mosaic structural arrangement, probably as a result of lateral gene or gene fragment transfer and recombinational events (14, 15). GenBank accession numbers of the Alp genes are as follows: for bca (Cα), M97256; for alp1 (Alp1), U33554; for alp2 (Alp2), AF208158; for alp3 (Alp3), AF245663; for rib (Rib), U58333; and for alp4 (Alp4), AJ488912. Alp genes are allelic, meaning that any of the genes may occupy one and the same genomic location (15). Nearly all GBS possess at least one of the Alp genes, but approximately 95% of GBS strains possess only one of the genes (16, 17). The genes show considerable, but variable, homology. Some GBS domains may show up to 100% similarity, which has been interpreted as an indication of a common ancestor. Specific Alps show a preference for certain CPS types, exemplified by frequent Rib association with CPS type III and frequent CPS type V-Alp3 and type Ia-Alp1 associations (16, 17, 18). Alps are immunogenic, give rise to protective antibodies, and are potential vaccine candidates (19, 20, 21, 22). Antibodies raised against particular Alps show variable cross-reactivities with other Alps, as would be expected on the basis of their considerable structural similarities. At least one of the Alps, Cα, the prototype Alp, functions as an important invasin in cervical epithelial cells and mediates adherence, transmembrane passage, and translocation of GBS. This process is facilitated by Cα N-terminal sites which attach to α1β1-integrin or to glycosaminoglycans associated with eukaryotic cells (23, 24, 25). The potential invasive properties of other Alps have not been studied as extensively as they have for Cα but may be comparable to those of Cα (23, 26). Thus, Alps play important roles in the immunology of GBS and as virulence factors.
FIG 1.

Schematic presentation of the major domains of Alps, the signal peptide (S), the N-terminal region (N), the repeat region (R), and the C-terminal region (C). ID indicates the immunodominant regions. The arrow indicates the N-terminal end of the intermediate area in Alp2 and Alp3. For more details of structural features, see the text and reference 10.
Over the years, antisera to native Alps have been used by many researchers in a variety of experimental approaches, such as infection models in animals and in serotyping, often without assurance regarding the immunological specificity of the antibodies, including which Alp domain was targeted by the antibodies. This has certainly resulted in some misleading conclusions, including failure to discriminate between different but cross-reacting Alps in antibody-based serotyping, for example, failure to discriminate between Cα and Alp1 or between Alp3 and Rib. The introduction of GBS serotyping by molecular methods has disclosed the pitfalls related to the cross-reacting antibodies which cause inadequate specificity of antisera. On the other hand, since it has been possible to develop protein-specific molecular methods such as PCRs for Alp identification in spite of considerable homology between Alps, it seems tempting to speculate that antibody-based techniques with specificity comparable to that of molecular methods can be established. In this survey, we have compiled published data in order to elucidate the immunological specificity of major immunogenic domains of the Alps.
Cα.
Cα was defined in 1979 (6). The Cα-encoding gene (bca) of strain A909, serotype Ia/Cα,Cβ, was sequenced in 1992 (27). The major segments of the gene and its product were found to be the signal sequence, which consisted of 41 aa; the N terminus, initially calculated to contain 185 aa; the repeat region, composed of 749 aa with 82 aa within each repeat unit; and the C-terminal anchoring region, with 45 aa (27). Cα contained protective epitopes located both in the N terminus and in the C-terminal repeat region, also demonstrated by using a monoclonal anti-Cα antibody (20). Studies using animal models showed that when GBS was grown in vivo in the presence of Cα antibodies, the isolate could generate mutants with reduced numbers of Cα repeats compared to the parent strain (28). The mutations produced profound effects on the binding affinity of Cα C-terminal antibodies; antibodies elicited against Cα with many repeats recognized the protein with many repeats but barely recognized Cα with only one or two repeats (19, 29). In contrast, antibodies elicited against Cα with few repeats recognized both few-repeat and many-repeat Cα (29). This was explained by assuming that antibodies against conformational epitopes predominated in the many-repeat antisera while antibodies against sequential epitopes predominated in the few-repeat antisera (29). The mutational change to fewer repeats combined with variability of the affinity of antibodies generated against Cα with a variable number of repeats probably represents a mechanism by which GBS escape host immune responses (19, 29). The variability in binding affinity of anti-Cα antibodies was mirrored by the protective capacity of antibodies elicited with antigen with a high or low number of repeats (19, 29). Mutations to fewer repeats have also been observed in GBS infection in humans (30).
A puzzling phenomenon observed with Cα is that the number of repeats altered the immunogenicity of the protein. Specifically, immunogenicity weakened with increasing numbers of repeats, with the most notable reductions occurring in antibody production to the N-terminal region. For example, immunization with a Cα with as many as 16 repeats failed to induce anti-N-terminal-region antibodies and also affected the protective activity of the antibodies elicited (19, 22, 29). Similar observations have been made in our laboratory (31). Immunization with purified recombinant Cα N terminus or the N terminus as part of a fusion protein resulted in a strong antibody response when the immunogenicity of the Cα N terminus was not suppressed by multiple C-terminal repeats (22, 29). Available information supports the inference that repeat-number-dependent downregulation of the immunogenicity of the N terminus is a mechanism by which GBS prevents generation of anti-N-terminal-region antibodies, known to be highly efficient in immunoprotection (19, 22, 29). Thus, it seems that the number of Cα repeats is a factor which modulates the overall Cα antibody response, the binding affinity of the Cα antibodies generated, and most notably the production of N-terminal-region antibodies, by mechanisms which remain unexplained.
Although antibodies to the Cα N terminus have been used experimentally, including in protection studies in animals (20, 22), it does not appear that the immunological specificity of these antibodies has been thoroughly tested, which explains the question mark in Table 1. The N-terminal region of the product of bca, the gene encoding Cα, showed 70% homology with the N termini of the products of alp2 and alp3 and 60% homology with the Rib N terminus, but antibodies against the corresponding proteins showed no immunological cross-reactivity with Cα (22, 31, 32, 33). This should imply that the Cα N terminus induces Cα-specific antibodies when used for immunization without being bound to the Cα C terminus with multiple repeats. The bca strech encoding the Cα N terminus has at least one site which is the target for a 24-mer reverse primer in a Cα-specific PCR (11). It is a possibility that this site could correspond to either the whole or part of a Cα-specific epitope, but this is largely speculation. In conclusion, available data support the notion that immunization with the Cα N terminus under certain conditions generates a Cα-specific antibody response.
TABLE 1.
Putative targets of cross-reacting and protein-specific antibodies in anti-Alp sera
| Antiserum target | Cross-reacting domain(s) | Protein-specific domain |
|---|---|---|
| Cα | Alp1 C term.d | Cα N term.? |
| Alp1a | Cα C term. | Alp1 N term. |
| Alp2b | Alp3 N term. | Alp2 C term. |
| Alp3c | Alp2 N term.; Rib C term.; Alp4 C term. | None |
| Rib | Alp3 C term.; Alp4 C term. | Rib N term. |
| Alp4 | Alp3 C. term.; Rib C term. | Alp4 N term. |
Alp1 also possessed a site of low immunogenicity which cross-reacted with Alp2 and Alp3, of uncertain location in these proteins (28).
A Streptococcus dysgalactiae subsp. equisimilis strain contained a hybrid gene (dys-alp; GenBank accession numbers DQ380235 and DQ380236) with an N terminus nearly identical to that of rib and a tandem B repeat area (15) nearly identical to that of alp2 (14).
R28, a protein expressed by many Streptococcus pyogenes strains, is virtually identical to the Alp3 protein of GBS (10, 15, 26).
term., terminal.
On the other hand, the Cα repeat region cross-reacted strongly with the Alp1 (Epsilon) repeat region as expected, since Cα and Alp1 repeats showed nearly 100% identity at the nucleotide level (31) (Fig. 2; Table 1). This explains why serotyping GBS with anti-Cα polyclonal or monoclonal antibodies has erroneously identified Alp1 as Cα, with the result that CPS Ia strains have been closely associated with Cα. In contrast, molecular typing has shown that Ia strains usually possess alp1, which encodes Alp1, and, more rarely, possess bca, which encodes Cα (16, 18, 34).
FIG 2.
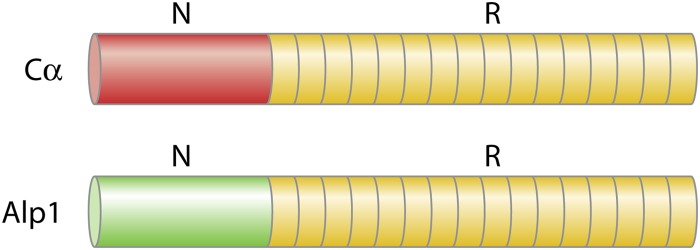
Schematic presentation of the immunodominant domains of Cα and Alp1, with the protein-specific, N-terminal domains indicated by red and green, respectively, and cross-reacting repeat area domains indicated by yellow.
Alp1.
Alp1 was formerly called Epsilon and considered a mosaic variant of Cα (GenBank accession numbers AY345596 and U33554). Actually, it appears that Alp1 and Cα are more closely related to each other than to the other Alps (Fig. 2; Table 1).When alp1 sequences from the CPS type Ia/Alp1 strain 515 (GenBank accession no. AAJP00000000) and sequences from the strain A909 bca (GenBank accession no. M97256) were aligned, it was found that the Alp1 and Cα repeats were virtually identical (31). This explains why the C-terminal regions from Alp1 and Cα behaved immunologically as two identical antigens, including the ability of both repeat regions to bind a monoclonal antibody raised against the Cα from A909 (31). The Alp1 and Cα repeat regions are thus indistinguishable immunologically due to their structural similarity but appear to be unique to these two Alps, as the repeat regions from Alp1 and Cα showed no cross-reactivity with other Alps (31). When antiserum against Alp1 had been cross-absorbed by a Cα-expressing strain in order to remove antibodies against the shared Alp1 and Cα C-terminal domain, antibodies remained which recognized only Alp1-expressing strains, consistent with the understanding that the Alp1 N terminus harbored an Alp1-specific epitope (31). The N termini of the products of alp1 and bca possess divergent stretches which have enabled construction of both alp1- and bca-specific primers for Alp1- and Cα-specific PCRs (11). It remains to be elucidated if the Alp1-specific primer site and the Alp1-specific epitope(s) are overlapping stretches. Consequently, Alp1 harbors one C-terminal antigenic domain which probably is identical immunologically to the C-terminal antigenic domain of Cα and one antigenic N-terminal domain which is Alp1 specific (Fig. 2; Table 1).
Alp2 and Alp3.
Immunological testing of the CPS type V strain CJB-110 was described in 1996, and it was shown that this isolate expressed a ladder-forming protein which was called Alp2 (35). The gene (alp2) encoding Alp2 and the gene (alp3) encoding the ladder-forming protein Alp3 expressed by the CPS type VIII strain JM9-130013 were sequenced and described in 2000 (15). Both proteins had features characteristic of Alp family proteins; the two proteins shared structural similarities, but structural differences were also noted. The N termini containing 172 aa shared identical sequences (15). The intermediate region between the N terminus and the repeat areas consisted of 239 aa in Alp2 and 188 aa in Alp3, with stretches which were nearly identical in the two Alps. In Alp2 and Alp3, the repeats consisted of 76 and 79 aa, respectively, with 67% similarity between the repeats (15). The Alp3 repeats were virtually identical to the Rib repeats, and Alp3 as a whole was virtually identical to the GAS protein R28 (see below). Further, it was shown by Western blotting that antiserum against an Alp3-expressing strain targeted both Alp2 and Alp3, in accordance with the common Alp2/Alp3 epitope(s) (15). This was later confirmed by enzyme-linked immunosorbent assay (ELISA)-based testing (32). In view of the findings of sequence identity of the Alp2 and Alp3 N termini, it seems obvious that these domains should cross-react immunologically. When the Alp2/Alp3 cross-reacting antibodies were removed by cross-absorption, the anti-Alp2 serum retained anti-Alp2 antibody activity but demonstrated no binding to Alp3 or other Alps. Thus, the Alp2 C-terminal polypeptide contained at least one Alp2-specific antigenic site, which can be considered an Alp2-specific antigenic marker (32). This marker may be located in the Alp2 repeats despite the finding that these repeats showed as much as 67% homology with the non-cross-reacting Alp3 repeats. Alternatively, the Alp2-specific marker may be on an Alp2 site outside the Alp2 repeats. Thus, sequence data have provided a rational basis for interpreting Alp2/Alp3 immunological cross-reactivity and for the observation that PCR with primers targeting sequences within the Alp2/Alp3 N termini failed to discriminate between the genes alp2 and alp3 (11). However, primers which provide alp2- and alp3-specific PCRs have been designed and used in molecular serotyping (12).
After removing the Alp2/Alp3 cross-reacting antibodies from an Alp3 antiserum, it was shown that Alp3 antibody activity remained and that these antibodies combined equally well with the homologous Alp3 and the heterologous Rib protein (32, 33). Since the Alp3 and Rib repeats are nearly identical (15), these structures were likely to be the targets of the Alp3/Rib cross-reacting antibodies. Thus, Alp3 which shares its N-terminal antigenic domain with Alp2 appears to share the C-terminal antigenic domain with Rib. Thus, the major immunogenic domains of Alp3 appear to cross-react with other Alps, making Alp3 the only known Alp that lacks a protein-specific immunogenic marker. As a consequence, it may not be possible to identify Alp3 by antibody-based methods without using combinations of specific antibodies. It remains to be seen whether more refined immunological techniques will result in detection of an Alp3-specific antigenic marker. Due to its cross-reactivity with other Alps, Alp3 may be of considerable interest as a vaccine candidate in a CPS/Alp3 conjugate vaccine, as a free protein, or as a recombinant version of antigenic Alp3 domains (36). A summary of immunological characteristics of Alp2 and Alp3 is included in Fig. 3 and Table 1.
FIG 3.
Schematic presentation of the immunodominant domains of Alp2, Alp3, Rib, and Alp4. Cross-reacting N-terminal domains in gray; protein-specific N- or C-terminal domains in blue, red, and green, respectively; and cross-reacting repeat area domains in yellow.
Immunological cross-reactivity between Cα and Alp2 has been reported (35), as has cross-reactivity of Alp1 with Alp2 and Alp3 (31). The basis for this cross-reactivity has not been fully clarified, for instance, with respect to the binding site of the cross-reacting antibodies, a site which seems to be weakly immunogenic and perhaps is of minor importance (31). In conclusion, Alp2 and Alp3 share an immunogenic marker located in the N termini, and Alp2 possesses a protein-specific marker in its C-terminal region, but Alp3 does not appear to have a protein-specific antigenic marker.
Rib.
The initial description of the Alp Rib appeared in 1993, including immunochemical characterization; differential expression of Rib by GBS strains, most notably by CPS type III strains; and the protective ability of anti-Rib antibodies tested in a mouse model (21). The gene rib, encoding Rib, was sequenced in 1996 and showed a molecular structure representative of Alp genes (37). The N terminus of the product of rib contained 174 aa, and without an intermediate area, the C terminus of the product of rib had multiple identical repeats composed of 79 aa. Anti-Rib antibodies showed protective activity in experimental infections with GBS strains expressing Rib and with infection that was caused by a Streptococcus strain which was Rib negative but expressed a cross-reacting surface protein (26, 38). Immunological testing and sequence analysis have shown that Rib is identical to the historical R4 protein (39, 40). When antibodies were elicited by immunization with intact Rib with multiple C-terminal repeats, the repeat region was immunodominant and the N terminus was nonimmunodominant. In contrast, when immunization with fusion proteins was made with N-terminal or repeat peptides, the N-terminal component was immunodominant (22), resembling the results obtained with Cα regions (see above). Both Rib anti-N-terminal-region and antirepeat antibodies were protective in animal models (22). Based on antiserum against a serotype III/Rib GBS strain and antiserum against purified Rib, immunological testing using appropriately cross-absorbed antisera provided evidence that antibodies against the Rib N terminus recognized only rib PCR-positive GBS, i.e., evidence that these antibodies were Rib specific and thus targeted a unique antigenic marker of Rib (33). Experiments designed to test the immunological specificity of antigenic sites of the Rib repeat region produced results that were consistent with results expected based on sequence analysis. GBS which harbored either the alp3 or the rib gene were recognized by anti-Rib C-terminal-region antibodies, whereas strains negative for these genes failed to bind the antibodies (33). Antibodies which targeted Rib and Alp3 repeat areas, which are virtually identical, also targeted strain 9828, which possessed Alp4, and the R28 protein of GAS, presumably binding to repeat sites of these proteins (A. I. Kvam, personal communication). Over the years, the cross-reactivity of the Rib repeat area with Alp3 has resulted in false typing of Alp3-expressing GBS as Rib (R4)-expressing strains when anti-Rib sera have been used for serotyping. In conclusion, the N terminus of Rib, which has at least one site for a primer that allows a rib-specific PCR (11), also has one or more sites which induce generation of Rib-specific antibodies. In contrast, the C-terminal region of Rib elicits highly cross-reactive antibodies in accordance with sequence analysis results (Fig. 3; Table 1).
Alp4.
Strain 9828 (Compton; Prague 25/60; NCTC 9828) has for many years been considered a prototype for the GBS proteins R3 and R4 (Rib) and also a prototype strain for another R-like antigen called the BPS protein (group B protective surface protein [41]). The isolate is not CPS typeable by means of anti-CPS sera, but analysis of the 9828 cps gene cluster indicated CPS type II (42). Testing of strain 9828 by PCR with primer sets designed for rib resulted in amplicon production with one primer set but not with other sets designed for rib (12). For this reason, it was considered that 9828 did not contain an ordinary R4 (Rib) but contained another Alp, which was called Alp4 (12). Based on sequence data (GenBank accession no. AJ488912), a PCR which amplified a 110-bp segment of alp4 that corresponded to the N-terminal region of the alp4 product was designed and provided an Alp4-specific PCR (11). Hundreds of human GBS strains from various geographical areas have been tested for alp4 possession, but 9828 seems to be the only isolate that possesses alp4, the Alp4-encoding gene (12, 16, 17).
In experiments performed in our laboratory (not yet reported), using rabbit antisera against strain 9828, we demonstrated that antibodies which recognized Rib, Alp3, and the R28 protein of GAS (see above), presumably by binding to the repeats of these Alps, recognized Alp4 equally well. This suggests that the C-terminal region of Alp4 is similar, if not identical, to those of Rib, Alp3, and R28. This conclusion was supported by the finding that our Rib monoclonal antibody which recognized the repeat area of Rib, Alp3, and R28 (31) also recognized Alp4. This cross-reactivity is certainly the reason why strain 9828 for many years was erroneously considered a prototype strain for R4 (Rib). The antiserum used in our experiments also contained another antibody which recognized only 9828 among a number of GBS strains tested, presumably by targeting an Alp4-specific antigenic site(s) in the Alp4 N terminus, in accordance with N-terminal primer sites for an alp4-specific PCR (11). Thus, it seems that Alp4 is constructed similarly to other Alps with respect to immunogenic domains, one protein-specific domain located in the N terminus and one cross-reacting domain located in the repeat-containing C-terminal region (Fig. 3; Table 1).
R proteins and Alps.
The term R protein was introduced in 1952 for streptococcal proteins which were resistant to trypsin digestion (7). Later, the R proteins R1, R2, R3, and R4 were described (8). R2 probably never occurs in GBS, and R4 is identical to Rib (see above). The identity of R1 has been confusing. Anti-R1 serum has often been prepared from antiserum raised against strain D136C (NEM316; ATCC 12403), previously serotyped as a type III/R1 strain and considered an R1 prototype strain (43, 44). However, D136C is currently classified as a type III/Alp2 strain by PCR and by detection of alp2 in the D136C genome (45). Putative R1-specific antibodies in strain D136C antiserum generated banding patterns in Western blotting when used as a probe against purified Alp2 from the strain CJB-110 (35). R1 antibodies prepared in a classical manner (46) recognized all of 26 GBS strains which possessed either alp2 or alp3 but not strains which were PCR negative for these genes (47). Taken together, these data support the interpretation that the Alp2 and Alp3 N termini, which are identical (15), were the targets of the putative R1 antibodies in traditionally prepared anti-R1 sera. R1 most likely is nonexistent as a distinct antigen different from the Alps, also meaning that the results of former experiments attributed to R1 actually may represent results which can be attributed to Alp2 and/or Alp3. However, theoretically, R1 might be one of the 30 putative surface proteins with a cell wall-sorting signal motif identified by D136C/NEM316 genomic sequencing (45).
R3 is a high-molecular-mass protein among others expressed by the prototype strains 10/84 (ATCC 49447) and 9828 (NCTC 9828). This protein generated patterns with multiple bands on Western blotting whether probed with polyclonal antibodies or probed with monoclonal antibodies (48, 49). R3 antibodies showed no cross-reactivity with any of the well-defined Alps, which would have been unusual if R3 had belonged to the Alp family (49). This protein may belong to another family of streptococcal proteins, but R3 awaits full characterization and sequencing of the encoding gene.
Alps in non-group B streptococci.
A variety of Gram-positive cocci and an occasional Gram-negative bacterium harbor genes which encode high-molecular-weight proteins with a structural organization which mimics that of the Alps of GBS (10). These proteins have been termed Alp related and may have repeats with some sequence similarity to the Alps of GBS. In most instances, however, the similarity is below 50%, which probably means that it is below levels that could provide immunological cross-reactivity with GBS Alps.
Within the genus Streptococcus, the two species Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis include strains which possess true Alps. The protein R28 was detected in 1952 and occurred in both GBS and GAS strains (7). In a later study, it was found that R28 was probably identical to the T28 antigen of GAS (50). Sequence analysis showed that the R28 gene was virtually identical to the GBS gene alp3, which encodes the GBS protein Alp3 (15, 26). Lateral transfer from GBS to GAS probably had taken place in the past. The R28 and Rib repeats showed 95% residue identity (10, 26), and in accordance with this, immunological cross-reactivity between the repeats would be expected. This cross-reactivity most likely mediated the cross-protection between R28-expressing GAS and Rib-expressing GBS, demonstrated in animal models (26, 38). The N terminus of R28 is not expected to mediate protective immunity against Rib-expressing GBS but may have the potential to provide cross-protection against GBS which express either Alp2 or Alp3 (32), due to nearly identical N termini (15, 32). To the best of our knowledge, however, these relationships have not been clarified. Immunogenicity and immunological specificity of the intermediate region present in R28, Alp2, and Alp3 also remain unknown. In 2006, an additional Alp was described, detected in a Streptococcus dysgalactiae subsp. equisimilis strain (14). This Alp, which was named Dys-Alp, and the encoding gene dys-alp (GenBank accession numbers DQ380235 and DQ380236) possessed a signal sequence, and the Alp possessed an N terminus with 95% amino acid identity to the Rib N terminus and a C-terminal region with repeats (called B repeats) that were virtually identical to the repeats of Alp2 of GBS (14). Immunological testing of Dys-Alp did not conclusively determine the immunological relatedness of this protein to other Alps, possibly due to testing by antisera which were not quite appropriate for the situation. We are not aware of detection of similar N-terminal/C-terminal combinations in GBS Alps. The dys-alp structural arrangement may serve as an illustration of the manner by which the mosaicism of the Alps has arisen.
Alps in bovine GBS.
It has been claimed that human and bovine GBS have evolved along different phylogenetic lines (51, 52, 53), and a bovine GBS genome contained numerous genes which encoded putative surface-localized proteins that were not found in human GBS (54). In one study, 173 human and 52 bovine GBS strains were tested, mostly by PCR, for 18 different surface-localized markers, including the Alps; all of these markers were detected in isolates of both categories, although there were some differences in the frequency of occurrence of the markers (55). The Alps showed essentially similar CPS-Alp associations in the two strain categories and behaved similarly in immunological tests with specific antisera, without displaying results that could point to immunological differences between the Alps of human and bovine GBS, or differences between the non-Alp proteins Z1, Z2, and R3 (49, 55). More detailed comparative testing, including comparative sequence analysis, may be required to determine whether surface-exposed protein markers of human and bovine GBS differ significantly. Sequence differences between human and bovine cps genes involved in CPS synthesis have been reported (56). These differences may represent the underlying basis for the frequent failure of antibodies raised against CPSs of human GBS strains to recognize CPSs of bovine GBS strains (56, 57).
Comments.
The data compiled in this review and summarized in Table 1 support the notion that the Alps identified to date possess two major antigenic domains. One domain is located in the N-terminal region, and the other domain is located in the repeat-containing C-terminal region. Alp3 appears to be relatively unique among the Alps as it does not appear to have an immunologically specific domain. For the other Alps, one of these domains, usually the N-terminal domain, is protein specific. Alp2 varies from this trend, as the repeat region appears to harbor the protein-specific antigenic domain. All non-protein-specific domains show immunological cross-reactivity with a corresponding domain in one or more of the other Alps. These inferences, which are based on immunological testing, are consistent with results of genetic sequence analysis, since cross-reacting domains were located in regions with identical or virtually identical nucleotide sequences. Non-cross-reacting domains, in contrast, usually showed less than 70% sequence identity, further emphasizing the genetic basis for immunological specificity. The mosaicism and constructs of antigenic Alp domains have complicated the task of preparing protein-specific antisera for serotyping. Cross-reacting antibodies are common in these antisera, and this has significant potential to produce confusion due to erroneous results. In order to avoid these problems, antiserum raised against intact Alp has to be cross-absorbed by appropriate bacteria or Alp in order to make it protein specific. Although molecular biology-based serotyping methods have largely replaced antibody-based typing methods over the last few years, presenting a significant technological advance, domain-specific antibodies will still be required for future research, including vaccine development studies, or for studies of GBS virulence factors. In these and other contexts, detailed knowledge of Alp epitope structures would be desirable. Experiments designed for detailed epitope mapping have not been successful to date (20), and we have encountered similar difficulties in our laboratory (unpublished data). Success in research along these lines may eventually result in specifically tailored peptides for a variety of experimental purposes, including vaccine developments.
Antigenic specificity is of utmost importance in the choice of vaccine components. The data reviewed here point to the possibility that a two-component vaccine, one immunogenic peptide corresponding to a repeat area stretch of Cα or Alp1, either of which cross-reacts strongly (Fig. 2; Table 1), and one peptide corresponding to a repeat area stretch of Alp3 or Rib, either of which also cross-reacts strongly (Fig. 3; Table 1), may provide broad protective activity. Theoretically, protection might be provided against 91 and 97%, respectively, of isolates of an African (16) and a European (18) strain collection, provided that antibodies were generated against both vaccine components, which could be recombinant peptides. Cα/Alp1 repeat area antibodies may provide protection against the majority of CPS type Ia and Ib isolates, and Alp3/Rib repeat area antibodies may protect against the majority of CPS type III, type V, and type VIII isolates (16, 18) and against R28-expressing Streptococcus pyogenes strains (26). The repeat area vaccine may also protect against the highly virulent GBS clone of the sequence type ST-17, often of the serotype III/Rib (58, 59). This may be possible since the great majority of isolates of the two strain collections (16, 18) expressed one, occasionally two, of the Alps with cross-reacting repeat regions, either the Cα/Alp1 region or the Alp3/Rib region. It is important to note that cross-reacting antibodies provide protective immunity (36, 38) and that anti-repeat area antibodies induce immunoprotection in experimental models (20, 22). A vaccine based on N-terminal epitopes would require more vaccine components in order to achieve comparable broadness of protection. On the other hand, anti-N-terminal-region antibodies may be more protective than anti-repeat area antibodies (22). Of single Alps, Alp3 seems to be of particular interest as a vaccine candidate (36). Further research is required to clarify among a variety of problems related to GBS vaccine development.
REFERENCES
- 1.Lancefield RC. 1933. A serological differentiation of human and other groups of haemolytic streptococci. J Exp Med 57:571–595. doi: 10.1084/jem.57.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lancefield RC. 1934. A serological differentiation of specific types of bovine haemolytic streptococci (group B). J Exp Med 59:441–458. doi: 10.1084/jem.59.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson HW, Moody MD. 1969. Serological relationships of type I antigens of group B streptococci. J Bacteriol 97:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson HW, Eagon RG. 1971. Type-specific antigens of group B type Ic streptococci. Infect Immun 4:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lancefield RC, McCarty M, Everly WN. 1975. Multiple mouse-protective antibodies directed against group B streptococci. J Exp Med 142:165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bevanger L, Maeland JA. 1979. Complete and incomplete Ibc protein fraction in group B streptococci. Acta Pathol Microbiol Immunol Scand B 87:51–54. [DOI] [PubMed] [Google Scholar]
- 7.Lancefield RC, Perlmann GE. 1952. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28, and in certain streptococci of other serological groups. J Exp Med 96:83–97. doi: 10.1084/jem.96.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson HW. 1972. Comparison of streptococcal R antigens. Appl Microbiol 24:669–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarre WW, Schneewind O. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63:174–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindahl G, Stålhammar-Carlemalm M, Areschoug T. 2005. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin Microbiol Rev 18:102–127. doi: 10.1128/CMR.18.1.102-127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creti R, Fabretti F, Orefici G, von Hunolstein C. 2004. Multiplex PCR assay for direct identification of group B streptococcal alpha-protein-like protein genes. J Clin Microbiol 42:1326–1329. doi: 10.1128/JCM.42.3.1326-1329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong F, Gowan S, Martin D, James G, Gilbert GL. 2002. Molecular profiles of group B streptococcal surface protein antigen genes: relationship to molecular serotypes. J Clin Microbiol 40:620–626. doi: 10.1128/JCM.40.2.620-626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Z, Kong F, Gilbert GL. 2006. Reverse line blot assay for direct identification of seven Streptococcus agalactiae protein antigen genes. Clin Vaccine Immunol 13:145–149. doi: 10.1128/CVI.13.1.145-149.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creti R, Imperi M, Baldassarri L, Pataracchia M, Alfarone G, Orefici G. 2007. Lateral transfer of alpha-like protein gene cassettes among streptococci: identification of a new family member in Streptococcus agalactiae subsp. equisimilis. Lett Appl Microbiol 44:224–227. doi: 10.1111/j.1472-765X.2006.02045.x. [DOI] [PubMed] [Google Scholar]
- 15.Lachenauer CS, Creti R, Michel JL, Madoff LC. 2000. Mosaicism in the alpha-like protein genes of group B streptococci. Proc Natl Acad Sci U S A 97:9630–9635. doi: 10.1073/pnas.97.17.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mavenyengwa RT, Maeland JA, Moyo SR. 2008. Distinctive features of surface-anchored proteins of Streptococcus agalactiae strains from Zimbabwe revealed by PCR and dot blotting. Clin Vaccine Immunol 15:1420–1424. doi: 10.1128/CVI.00112-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyo SR, Maeland JA, Berg K. 2002. Typing of human isolates of Streptococcus agalactiae (group B streptococci) strains from Zimbabwe. J Med Microbiol 51:595–600. [DOI] [PubMed] [Google Scholar]
- 18.Persson E, Berg S, Bevanger L, Bergh K, Valsø-Lyng R, Trollfors B. 2008. Characterisation of invasive group B streptococci based on investigation on surface proteins. Clin Microbiol Infect 14:66–73. doi: 10.1111/j.1469-0691.2007.01877.x. [DOI] [PubMed] [Google Scholar]
- 19.Gravekamp C, Kasper DL, Michel JL, Kling DE, Carey V, Madoff LC. 1997. Immunogenicity and protective efficacy of the alpha C protein of group B streptococci are inversely related to the number of repeats. Infect Immun 65:5216–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kling DE, Gravekamp C, Madoff LC, Michel JL. 1997. Characterization of two distinct opsonic and protective epitopes within the alpha C protein of group B Streptococcus. Infect Immun 65:1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stålhammar-Carlemalm M, Stenberg L, Lindahl G. 1993. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med 177:1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stålhammar-Carlemalm M, Waldemarsson J, Johnsson E, Areschoug T. 2007. Nonimmunodominant regions are effective as building blocks in a streptococcal fusion protein vaccine. Cell Host Microbe 2:427–434. doi: 10.1016/j.chom.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Auperin TC, Bolduc GR, Baron MJ, Heroux A, Filman DJ, Madoff LC, Hogle JM. 2005. Crystal structure of the N-terminal domain of the group B Streptococcus alpha C protein. J Biol Chem 280:18245–18252. doi: 10.1074/jbc.M412391200. [DOI] [PubMed] [Google Scholar]
- 24.Baron MJ, Filman DJ, Prophete GA, Hogle JM, Madoff LC. 2007. Identification of a glycosaminoglycan binding region of the alpha C protein that mediates entry of group B streptococci into host cells. J Biol Chem 282:10526–10536. doi: 10.1074/jbc.M608279200. [DOI] [PubMed] [Google Scholar]
- 25.Bolduc GR, Madoff LC. 2007. The group B streptococcal alpha C protein binds α1β1-integrin through a novel KTD motif that promotes internalization of GBS within epithelial cells. Microbiology 153:4039–4049. doi: 10.1099/mic.0.2007/009134-0. [DOI] [PubMed] [Google Scholar]
- 26.Stålhammar-Carlemalm M, Areschoug T, Larsson C, Lindahl G. 1999. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confer protective immunity and promotes binding to human epithelial cells. Mol Microbiol 33:208–219. doi: 10.1046/j.1365-2958.1999.01470.x. [DOI] [PubMed] [Google Scholar]
- 27.Michel JL, Madoff LC, Olson K, Kling DE, Kasper DL, Ausubel FM. 1992. Large, identical, tandem repeating units in the C protein alpha antigen gene, bca, of group B streptococci. Proc Natl Acad Sci U S A 89:10060–10064. doi: 10.1073/pnas.89.21.10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madoff LC, Michel JL, Gong EW, Kling DE, Kasper DL. 1996. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc Natl Acad Sci U S A 93:4131–4136. doi: 10.1073/pnas.93.9.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravekamp C, Horensky DS, Michel JL, Madoff LC. 1996. Variation in repeat number within the alpha C protein of group B streptococci alters antigenicity and protective epitopes. Infect Immun 64:3576–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hervas JA, Gonzales L, Gil J, Paoletti LC, Madoff LC, Benedi VJ. 1993. Neonatal group B streptococcal infection in Mallorca, Spain. J Infect Dis 16:714–718. [DOI] [PubMed] [Google Scholar]
- 31.Kvam AI, Mavenyengwa RT, Radtke A, Maeland JA. 2011. Streptococcus agalactiae alpha-like protein 1 possesses both cross-reacting and Alp1-specific epitopes. Clin Vaccine Immunol 18:1365–1370. doi: 10.1128/CVI.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeland JA, Bevanger L, Lyng RV. 2004. Antigenic determinants of alpha-like proteins of Streptococcus agalactiae. Clin Vaccine Immunol 11:1035–1039. doi: 10.1128/CDLI.11.6.1035-1039.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeland JA, Bevanger L, Lyng RV. 2005. Immunological markers of the R4 protein of Streptococcus agalactiae. Clin Vaccine Immunol 12:1305–1310. doi: 10.1128/CDLI.12.11.1305-1310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brimil N, Barthell E, Heindrichs U, Kuhn M, Lütticken R, Spellerberg B. 2006. Epidemiology of Streptococcus agalactiae colonization in Germany. Int J Med Microbiol 296:39–44. doi: 10.1016/j.ijmm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Lachenauer CS, Madoff LC. 1996. A protective surface protein from type V group B streptococci shares N-terminal sequence homology with the alpha C protein. Infect Immun 64:4255–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H-H, Mascuch SJ, Madoff LC, Paoletti LC. 2008. Recombinant group B Streptococcus alpha-like protein 3 is an effective immunogen and carrier protein. Clin Vaccine Immunol 15:1035–1041. doi: 10.1128/CVI.00030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wästfelt M, Stålhammar-Carlemalm M, Delisse A-M, Cabezon T, Lindahl G. 1996. Identification of a family of streptococcus surface proteins with extremely repetitive structure. J Biol Chem 271:18892–18897. doi: 10.1074/jbc.271.31.18892. [DOI] [PubMed] [Google Scholar]
- 38.Stålhammar-Carlemalm M, Areschoug T, Larsson C, Lindahl G. 2000. Cross-protection between group A and group B streptococci due to cross-reacting surface proteins. J Infect Dis 182:142–149. doi: 10.1086/315693. [DOI] [PubMed] [Google Scholar]
- 39.Bevanger L, Kvam AI, Maeland JA. 1995. A Streptococcus agalactiae R protein analysed by polyclonal and monoclonal antibodies. APMIS 103:731–736. doi: 10.1111/j.1699-0463.1995.tb01430.x. [DOI] [PubMed] [Google Scholar]
- 40.Smith BL, Flores A, Dechaine J, Krepela J, Bergdall A, Ferrieri P. 2004. Gene encoding the group B streptococcal protein R4, its presence in clinical reference laboratory isolates & R4 protein pepsin sensitivity. Indian J Med Res 119(Suppl):213–220. [PubMed] [Google Scholar]
- 41.Erdogan S, Fagan PK, Talay SR, Rohde M, Ferrieri P, Flores AE, Guzman CA, Walker MJ, Chhatwal GS. 2002. Molecular analysis of group B protective surface protein, a new cell surface protective antigen of group B streptococci. Infect Immun 70:803–811. doi: 10.1128/IAI.70.2.803-811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong F, Gowan S, Martin D, James G, Gilbert GL. 2002. Serotype identification of group B streptococci by PCR and sequencing. J Clin Microbiol 40:216–226. doi: 10.1128/JCM.40.1.216-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flores AE, Ferrieri P. 1989. Molecular species of R-protein antigens produced by clinical isolates of group B streptococci. J Clin Microbiol 27:1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flores AE, Ferrieri P. 1996. Molecular diversity among the trypsin resistant surface proteins of group B streptococci. Zentralbl Bakteriol 285:44–51. [DOI] [PubMed] [Google Scholar]
- 45.Glaser P, Rusniok C, Buchrieser C, Chevalier F, Frangeul L, Msadek T, Zouine M, Couvé E, Lalioui L, Poyart C, Trieu-Cuot P, Kunst F. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol Microbiol 45:1499–1513. doi: 10.1046/j.1365-2958.2002.03126.x. [DOI] [PubMed] [Google Scholar]
- 46.Moyo SR, Maeland JA, Lyng RV. 2001. The putative R1 protein of Streptococcus agalactiae as serotype marker and target of protective antibodies. APMIS 109:842–848. doi: 10.1034/j.1600-0463.2001.091206.x. [DOI] [PubMed] [Google Scholar]
- 47.Maeland JA, Lyng RV. 2003. Variants of the R1 protein of group B streptococci, poster P611 13th Eur Congr Clin Microbiol Infect Dis, Glasgow, United Kingdom, 2003. [Google Scholar]
- 48.Mavenyengwa RT, Maeland JA, Moyo SR. 2009. Putative novel surface-exposed Streptococcus agalactiae protein frequently expressed by the group B streptococcus from Zimbabwe. Clin Vaccine Immunol 16:1302–1308. doi: 10.1128/CVI.00133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maeland JA, Radtke A, Lyng RV, Mavenyengwa RT. 2013. Novel aspects of the Z and R3 antigens of Streptococcus agalactiae revealed by immunological testing. Clin Vaccine Immunol 20:607–612. doi: 10.1128/CVI.00581-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson RH. 1975. Characterization of group A streptococcal R28 antigen purified by hydroxy-apatite column chromatography. Infect Immun 12:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bohnsack JF, Whiting AA, Martinez G, Jones N, Adderson EE, Detrick S, Blaschke-Bonkowsky AJ, Bisharat N, Gottschalk M. 2004. Serotype III Streptococcus agalactiae from bovine milk and human neonatal infections. Emerg Infect Dis 10:1412–1419. doi: 10.3201/eid1008.030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dogan B, Schukken YH, Santisteban C, Boor KJ. 2005. Distribution of serotypes and antimicrobial resistance genes among Streptococcus agalactiae isolates from bovine and human hosts. J Clin Microbiol 43:5899–5906. doi: 10.1128/JCM.43.12.5899-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sukhnanand S, Dogan B, Ayodele MO, Zadoks RN, Craver MP, Dumas NB, Schukken YH, Boor KJ, Wiedmann M. 2005. Molecular subtyping and characterization of bovine and human Streptococcus agalactiae isolates. J Clin Microbiol 43:1177–1186. doi: 10.1128/JCM.43.3.1177-1186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards VP, Lang P, Bitar PD, Lefebure T, Schukken YH, Zadoks RN, Stanhope MJ. 2011. Comparative genomics and the role of lateral gene transfer in the evolution of bovine adapted Streptococcus agalactiae. Infect Genet Evol 11:1263–1275. doi: 10.1016/j.meegid.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maeland JA, Radtke A. 2013. Comparison of Z and R3 antigen expression and of genes encoding other antigenic markers in invasive human and bovine Streptococcus agalactiae strains from Norway. Vet Microbiol 167:729–733. doi: 10.1016/j.vetmic.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Z, Kong F, Martinez G, Zeng X, Gottschalk M, Gilbert GL. 2006. Molecular serotype identification of Streptococcus agalactiae of bovine origin by multiplex PCR-based reverse line blot (mPCR/RLB) hybridization assay. FEMS Microbiol Lett 263:236–239. doi: 10.1111/j.1574-6968.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 57.Martinez G, Harel J, Higgins R, Lacouture S, Daignault D, Gottschalk M. 2000. Characterization of Streptococcus agalactiae isolates of bovine and human origin by randomly amplified polymorphic DNA analysis. J Clin Microbiol 38:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones NJ, Bohnsack JF, Takahashi S, Oliver KA, Chan M-S, Kunst F, Glaser P, Rusniok C, Crook DWM, Harding RM, Bisharat N, Spratt BG. 2003. Multilocus sequence typing system for group B streptococcus. J Clin Microbiol 41:2530–2536. doi: 10.1128/JCM.41.6.2530-2536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamy M-C, Dramsi S, Billoét A, Réglier-Poupet H, Tazi A, Raymond J, Guérin F, Couvé E, Kunst F, Glaser P, Trieu-Cuot P, Poyart C. 2006. Rapid detection of the “highly virulent” group B streptococcus ST-17 clone. Microbes Infect 8:1714–1722. doi: 10.1016/j.micinf.2006.02.008. [DOI] [PubMed] [Google Scholar]