Abstract
The human noroviruses (NoVs) are genetically diverse, rapidly evolving RNA viruses and are the major cause of epidemic gastroenteritis of humans. Serum antibodies that block the interaction of NoVs and NoV viruslike particles (VLPs) with host attachment factors are considered surrogate neutralizing antibodies in the absence of cell culture and small-animal replication models for the human NoVs. A serological assay for NoV-blocking antibodies was used to assess the breadth of the heterotypic antibody response in the context of an experimental challenge study with a human NoV. Heterotypic histo-blood group antigen (HBGA)-blocking activity against GI.4, GI.7, and GII.4 NoVs increased significantly in the serum of individuals (n = 18) infected with Norwalk virus (GI.1). Although the fold increases and peak titers of heterotypic antibody were more modest than titers of antibody reactive with the challenge antigen, Norwalk virus infection elicited a serological rise even against the novel Sydney variant of GII.4 NoVs. These observations indicate that the development of a broadly cross-protective NoV vaccine containing a limited number of genotypes may be possible.
INTRODUCTION
Noroviruses (NoVs) are the predominant agents of acute, epidemic gastroenteritis in humans. The NoVs have been described as “perfect pathogens” in large part because they are highly transmissible, highly genetically diverse, and constantly evolving (1). NoVs can be classified into 6 genogroups on the basis of the major capsid protein sequence (2). Of these, genogroup I (GI) and genogroup II (GII) comprise most human NoVs. Although one genotype, GII.4, is responsible for up to 60 to 80% of all NoV disease worldwide since 2002 (47–49), there are at least 29 genetically distinct, cocirculating genotypes of human NoVs (2, 3). Furthermore, the periodic emergence of novel, antigenically distinct GII.4 variants, including the most recent GII.4-2012 Sydney variant, illustrates the need for a better understanding of heterotypic NoV immunity (4–6). Clinical data on the breadth and persistence of human NoV immunity have substantial implications for ongoing vaccine development, which if efficacious would be a cost-effective means to control transmission (7).
NoV infection elicits a robust humoral immune response, and most adults have detectable NoV-specific serum antibody (8). Early experimental challenge studies suggested that the duration of immunity elicited by NoV infection may be short (<6 months) (9, 10). However, the epochal pattern of evolution observed for GII.4 NoVs suggests that NoV immunity may be more complex (11–13). Indeed, a recent mathematical modeling study based on NoV incidence estimates, the prevalence of mutations known to influence resistance to infection, and the natural history of NoV infection suggested that the duration of NoV immunity is closer to 4 to 9 years (14). The impact of NoV diversity on the breadth and duration of protective immunity remains poorly understood. Some previous experimental NoV challenge studies and epidemiologic studies suggested that NoVs elicit antibodies with intragenogroup cross-reactivity (15–22), while other studies reported evidence for intergenogroup cross-reactive serum antibody (23–28).
Not all antibody elicited by NoV infection is protective. Serum antibodies that block NoV binding to the histo-blood group antigens (HBGA), host glycans that are the putative attachment receptors for NoVs, are a correlate of protection from NoVs (29–31). In the absence of cell culture and small-animal replication models for the human NoVs, these “blocking antibodies” are considered to be surrogate neutralizing antibodies. The breadth of cross-reactivity and persistence of this clinically relevant, functional subset of serum antibodies have not been well described. We therefore systematically investigated heterotypic blocking antibody responses to multiple genotypes and variants using archival serum samples from a placebo-controlled challenge study in which healthy adult volunteers were challenged with Norwalk virus (GI.1), the prototypical human NoV (32). The kinetics, breadth of HBGA-blocking antibody reactivity, and persistence of heterotypic blocking antibodies were measured against 4 heterologous NoVs (GI.4, GI.7, GII.4 HOV, GII.4 Sydney) in the context of experimental Norwalk virus infection (n = 18 individuals).
MATERIALS AND METHODS
Serum samples.
An experimental infection study of five dosing cohorts of healthy adults with Norwalk virus was carried out between 2004 and 2011 in Houston, TX. Serum samples from the first three of the five cohorts were utilized for this serological study, and the third cohort was completed in March 2008. All participants provided written informed consent, and the experimental infection study was performed as described previously (32). Briefly, sera were collected prechallenge (designated day 0 [d0]) and over a 6-month follow-up period. Infection was defined based on detection of a 4-fold or greater increase in Norwalk virus-specific total serum antibody (IgG, IgA, and IgM) between d0 and d28 by enzyme-linked immunosorbent assay (ELISA) or direct detection of viral antigen or RNA in the stool (by either ELISA or reverse transcription [RT]-PCR, respectively). Norwalk virus-infected persons experiencing the following signs and symptoms were considered to have viral gastroenteritis, as previously reported: 1 episode of vomiting plus one other symptom (abdominal cramps, nausea, bloating, watery stool, headache, fever of >37.6°C) or moderate diarrhea (watery feces of at least 200 g) for any continuous 24-hour period (32, 33).
VLP production.
NoV viruslike particles (VLPs) were produced as described elsewhere (34) by expressing the NoV subgenomic sequence, which encodes the major and minor capsid proteins, in a baculovirus expression system. The NoV strains (GenBank number; abbreviation, if any) for which VLPs were produced for this study included the following: GI.1 Norwalk virus (NC_001959; GI.1 NV), GI.4 (GQ413970), GI.7 (JN005886), GII.4 Houston virus (EU310927; GII.4 HOV), and GII.4 Sydney virus (JX459908). The GII.4 NoV variants were selected for this study on the basis of their clinical and epidemiological significance. The GII.4 HOV variant was isolated in 2002, approximately 2 years before the start of the study period, from a child with gastroenteritis in Houston, TX. The Sydney variant was first described in 2012, approximately 4 years after sample collection ended for this study, and was responsible for 73% of NoV outbreaks in the United States between 2012 and 2013 (35). The GI.7 NoV variant was selected because this is the first serological study, to our knowledge, to report serum-blocking antibody reactive to a GI.7 NoV in the context of a challenge study. To facilitate comparison of our results with those of others in the field, we also chose to include a GI.4 NoV because one other group (15) has previously reported serum antibody responses to a GI.4 variant in the context of a human challenge study with Norwalk virus.
HBGA-blocking assays.
An assay to measure serum antibody with the ability to block VLP binding to known NoV glycan ligands was developed based on a previously published protocol (30) and optimized for each VLP on the basis of the glycan binding preference of each VLP selected for the study. The term “homotypic” refers to the antibody response measured in a blocking assay using the VLP antigen corresponding to the challenge virus (GI.1 NV). The term “heterotypic” refers to the antibody response measured in a blocking assay using any other norovirus VLP. Briefly, a fixed and optimized concentration of each VLP (ranging from 0.32 μg/ml for GI.1 NV to 20 μg/ml for GI.7) was preincubated with equal volumes of serial 2-fold dilutions of human serum starting at either 1:25 (GI.1 NV, GII.4 HOV, GI.7) or 1:50 (GI.4, GII.4 Sydney). All reagents and test specimens were diluted in 0.1 M sodium phosphate buffer, pH 6.1, with 0.25% fatty-acid-free bovine serum albumin (Sigma-Aldrich, St. Louis, MO). Synthetic polyvalent H-type 3-PAA-biotin, H-type 1-PAA-biotin, or Lewis(x)-PAA-biotin (Glycotech, Gaithersburg, MD) was immobilized on a NeutrAvidin-coated ELISA plate (Thermo Fisher Scientific, Rockford, IL) for 2 h at 22°C. Serum-VLP solutions were incubated on the glycan-coated plates for 2 h at 4°C. Bound VLPs were detected with mouse monoclonal NS14 (blocking assay for GI.4, GII.4 HOV, GII.4 Sydney) or rabbit polyclonal serum raised against GI.1 NV (blocking assays for GI.1 NV and GI.7) followed by either goat anti-mouse horseradish peroxidase (HRP)-conjugated or goat anti-rabbit HRP-conjugated antibody (Southern Biotech, Birmingham, AL). Color development was detected using Ultra 3,3′,5,5′-tetramethylbenzidine (TMB) (Thermo Fisher Scientific, Rockford, IL) by following the manufacturer's instructions, and optical density was measured at 450 nm (OD450) by using the SpectraMax M5 (Molecular Devices, Sunnyvale, CA) plate reader. Positive VLP binding control wells (no test serum added) and blank wells with buffer only were included on each plate. A binding curve was generated for each VLP. The concentration of VLP used for the binding control and for interrogating the cohort of serum samples was optimized by linear-range determination from the binding curve. To limit interplate variability for each VLP screen, plates were rejected if the positive control did not fall within the upper 50% of the linear range. Fifty-percent blocking titers (BT50), defined as the titer at which OD readings (after subtraction of the blank) were 50% of the positive control, were determined for each sample by linear interpolation. Serum samples from placebo recipients were tested only for HBGA-blocking activity reactive to the challenge antigen (GI.1 NV) and to GII.4 Sydney.
Statistical analysis.
For statistical analysis, a BT50 of 12.5 was assigned to samples with a BT50 less than 25 (or 25 for samples where the initial dilution was 1:50 and the BT50 was less than 50). Individual BT50 values were converted to the natural log scale for calculation of summary statistics and 95% confidence intervals (CIs). Geometric mean titers (GMTs) of HBGA-blocking antibody were calculated using the BT50 as the endpoint titer for each individual. A seroresponse was defined as a 4-fold or greater increase in serum antibody level between pre- and postinfection sera at each study time point. Peak GMTs of blocking antibody were calculated using the maximum BT50 detected against each antigen, which may represent the d14 or d28 titer based on the individual. The Wilcoxon signed-rank test was used to compare peak and prechallenge geometric mean titers, as well as mean fold increases in blocking antibody, by study cohort against different antigens. The Mann-Whitney U test was used to compare geometric mean titers and mean fold increases between groups. All reported P values represent two-sided tests.
RESULTS
Norwalk virus infection elicits a robust homotypic blocking antibody response.
Between September 2004 and March 2008, a total of 29 persons were challenged with Norwalk virus (an additional 5 persons received placebo [sterile water]). Of these 29, 18 persons became infected with Norwalk virus. As reported previously, all 18 experienced a blocking antibody seroresponse (defined as a ≥4-fold rise in blocking antibody titer between prechallenge and peak titer) against the homotypic challenge antigen by 14 days postinfection (30). The rise in titer was sustained and remained detectable at 6 months postinfection (Table 1). No seroresponses were observed among placebo recipients.
TABLE 1.
Magnitude and frequency of BT50 seroresponse, by NoV antigen, among NV-infected individuals and placebo recipientsa
| Group | Parameter | Result |
Peak titer | |||
|---|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 28 | Day 180 | |||
| Infected (n = 18 individuals) | GI.1 NV | |||||
| GMT (95% CI) | 47.9 (26.5, 86.4) | 725.6 (433.9, 1,213.4)b | 871.0 (551.7, 1,375.1)b | 527.8 (350.6, 794.6)b | 954.9 (632.1, 1,442.7)b | |
| % seroresponse | NA | 89 | 100 | 72 | 100 | |
| GI.4 | ||||||
| GMT (95% CI) | 42.3 (28.7, 62.2) | 347.9 (181.7, 666.3)b | 422.0 (238.8, 745.6)b | 49.3 (21.1, 115.5)c | 500.8 (279.1, 898.8)b | |
| % seroresponse | NA | 67 | 67 | 0 | 72 | |
| GI.7 | ||||||
| GMT (95% CI) | 12.5 (12.5, 12.5)c | 25.3 (16.5, 38.6)c | 18.5 (11.5, 29.8) | 12.5 (12.5, 12.5)c | 30.0 (17.5, 51.4)b,c | |
| % seroresponse | NA | 13 | 7 | 0 | 19 | |
| GII.4 HOV | ||||||
| GMT (95% CI) | 68.5 (41.4, 113.4)c | 153.7 (113.7, 207.8)b,c | 133.2 (94.8, 187.2)b,c | 99.9 (62.4, 159.7)c | 190.3 (144.6, 250.4)b,c | |
| % seroresponse | NA | 29 | 18 | 7 | 29 | |
| GII.4 Sydney | ||||||
| GMT (95% CI) | 35.5 (25.6, 49.3) | 90.1 (59.3, 136.7)b | 87.5 (54.5, 140.4) b | 35.4 (26.8, 46.9) | 101.3 (64.3, 159.6) b | |
| % seroresponse | NA | 33 | 33 | 0 | 39 | |
| Placebo (n = 5 individuals) | GI.1 NV | |||||
| GMT (95% CI) | 40.6 (13.7, 210.2) | 34.1 (12.4, 163.2) | 35.4 (11.8, 185.8) | NT | 35.8 (7.7, 165.8) | |
| % seroresponse | NA | 0 | 0 | NT | 0 | |
| GII.4 HOV | ||||||
| GMT (95% CI) | 44.0 (33.2, 58.3) | 43.2 (32.0, 58.5) | 41.7 (30.6, 56.9) | 42.4 (34.9, 60.4) | 45.9 (34.9, 60.4) | |
| % seroresponse | NA | 0 | 0 | 0 | 0 | |
| GII.4 Sydney | ||||||
| GMT (95% CI) | 90.3 (34.8, 234.5)c | 73.3 (21.6, 249.1) | 102.4 (38.1, 275.4)c | 92.0 (32.8, 257.9)c | 106.8 (38.4, 297.1)c | |
| % seroresponse | NA | 0 | 0 | 0 | 0 | |
BT50, 50% blocking titer; GMT, geometric mean titer; NA, not applicable; NT, not tested; seroresponse, a 4-fold or greater rise in blocking antibody titer at a given time point relative to prechallenge titer for a particular antigen. In the last column, % seroresponse refers to the percentage of volunteers who demonstrated a 4-fold or greater rise in the day of peak blocking antibody titer relative to the prechallenge titer. d0 data was not available for 1 volunteer each for the GI.4 and GI.7 assays. In these cases, the d7 value was substituted for d0 in the presentation of these summary statistics and the calculation of fold increase between prechallenge and peak titer.
P < 0.05, Wilcoxon ranked-sum test (with respect to BT50 GMT at d0).
Experiment was performed on a subset of samples in certain cases, as follows. Sample statistics represent n = 17 for GII.4 HOV d0, d14, d28, and peak titer, n = 16 for GI.7 d0, d14, d180, and peak titer, n = 15 for GII.4 HOV d180 and GI.7 d28, n = 6 for GI.4 d180, and n = 4 for all time points for placebo serum reactivity against GII.4 Sydney.
Norwalk virus infection elicits heterotypic blocking antibodies reactive to VLPs representing GI and GII NoVs.
Although the peak geometric mean titer (GMT) of blocking antibody was highest in magnitude for the homotypic antigen (954.9 [95% CI of 632.1, 1442.7]), Norwalk virus infection also elicited an increase in blocking titer against heterotypic human NoVs (Table 1). Among the GI NoVs tested in this study, striking differences were observed between the blocking antibody responses to GI.4 VLPs and GI.7 VLPs, as illustrated by median BT50 values on the day of peak blocking titer (412.7 and 18.8 for GI.4 VLPs and GI.7 VLPs, respectively). Consistent with previously reported phylogenetic analysis (36), the proportion of seroresponders and the mean fold increase (MFI) between prechallenge and peak titer of heterotypic blocking antibody to a GI.4 NoV (72% and MFI of 21.9 [95% CI of 10.2, 33.5]) were substantially higher than those against a GI.7 NoV genotype (19% and MFI of 4.9 [95% CI of 1.3, 8.5]) (P = 0.008, Wilcoxon signed-rank test).
Despite the antigenic distance between the GI and GII NoVs tested in this study, seroresponses were also observed against GII NoVs in this cohort of Norwalk virus-infected persons (Table 1). The antigens tested represent two different variants of GII.4 NoVs. Infection with GI.1 NV elicited a blocking antibody seroresponse against each of these GII.4 variants in some infected persons (the median BT50 values on the day of peak blocking titer were 161.0 for GII.4 HOV and 150.7 for GII.4 Sydney). However, the seroresponse frequency and magnitude of the mean fold increase for GII.4 HOV (29% and MFI of 4.5 [95% CI of 2.0, 6.9]) and GII.4 Sydney (39% and MFI of 4.3 [95% CI of 2.5, 6.1]) were both substantially lower than those for the homotypic blocking antibody response (P < 0.001 for each MFI comparison, Wilcoxon signed-rank test).
As reported previously by our group (30, 37), preexisting homotypic blocking antibody is a correlate of protection from viral gastroenteritis following experimental infection with Norwalk virus. However, preexisting heterotypic blocking antibody was not associated with protection from viral gastroenteritis. Furthermore, no differences were detected in the frequency of diarrhea and/or vomiting following challenge with Norwalk virus between “broad seroresponders” (two or more heterotypic antigens; n = 9) and “narrow seroresponders” (zero or one heterotypic antigen; n = 9), whether analyzed as the categorical outcome of viral gastroenteritis (P = 0.119, chi-square test) or as a modified Vesikari score based on individual symptoms of fever, diarrhea, and vomiting (P = 0.087, Mann-Whitney U test) (data not shown).
Heterotypic seroresponse patterns to heterotypic antigens vary by individual.
Individual seroresponse patterns to the panel of heterotypic antigens showed substantial interindividual variation with respect to fold increases between prechallenge titer and peak titer. The ranges in individual fold increases against the homotypic and heterotypic antigens tested, respectively, are as follows: GI.1 NV, 5.8 to 87.5; GI.4, 1.0 to 87.7; GI.7, 1.0 to 30.2; GII.4 HOV, 0.5 to 19.4; GII.4 Sydney, 1.0 to 13.1. There was also interindividual variation with respect to the antigen against which Norwalk virus infection elicited serum reactivity (Fig. 1a). At least one individual developed a heterotypic seroresponse against each of the heterotypic antigens. Of the 18 Norwalk-virus infected persons in our cohort, 9 developed a seroresponse to two or more heterotypic antigens. In fact, only two persons (no. 704 and 734) failed to develop a blocking antibody seroresponse to any antigen other than the challenge virus. Both of these individuals had high (>400) preexisting titers of challenge virus-specific HBGA-blocking antibody (not shown). There was also substantial interindividual variation in preexisting homotypic total serum antibody (measured by ELISA), but titers of this GI.1 NV-specific binding antibody were not associated with heterotypic HBGA-blocking antibody seroresponse (Fig. 1b); furthermore, there was no difference in the GMT of total GI.1 NV-reactive serum antibody between “broad” and “narrow” heterotypic blocking antibody seroresponders at any study time point.
FIG 1.
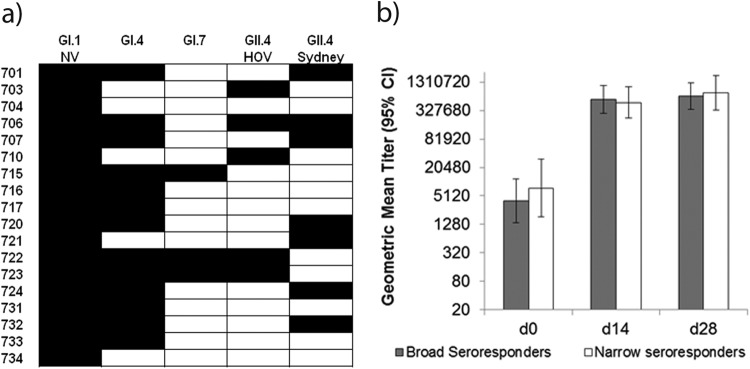
Patterns of heterotypic seroresponse vary among individuals (n = 18) challenged with Norwalk virus (a), independent of total preexisting NoV-reactive antibody in serum (b). Values shown in panel a represent seroresponse outcomes for each Norwalk virus-infected individual (n = 18). A seroresponse (black box) is defined as a 4-fold or greater increase between prechallenge and peak BT50 of serum antibody reactive to a particular NoV antigen. Values shown in panel b represent the geometric mean titers of total preexisting homotypic antibody measured by serum ELISA of heterotypic seroresponders (gray boxes; n = 9) compared to narrow heterotypic responders (white boxes; n = 9) at three time points relative to Norwalk virus challenge. Broad heterotypic seroresponders are defined as individuals who developed a blocking antibody seroresponse to 2 or more heterotypic antigens. Narrow heterotypic seroresponders are defined as individuals who developed a blocking antibody seroresponse to 0 or 1 heterotypic antigen. Error bars represent 95% confidence intervals of geometric mean titers. Geometric mean titers between broad and narrow heterotypic seroresponders were compared using the Mann-Whitney U test at each time point; the differences between the geometric mean titers were not statistically significant at any time point.
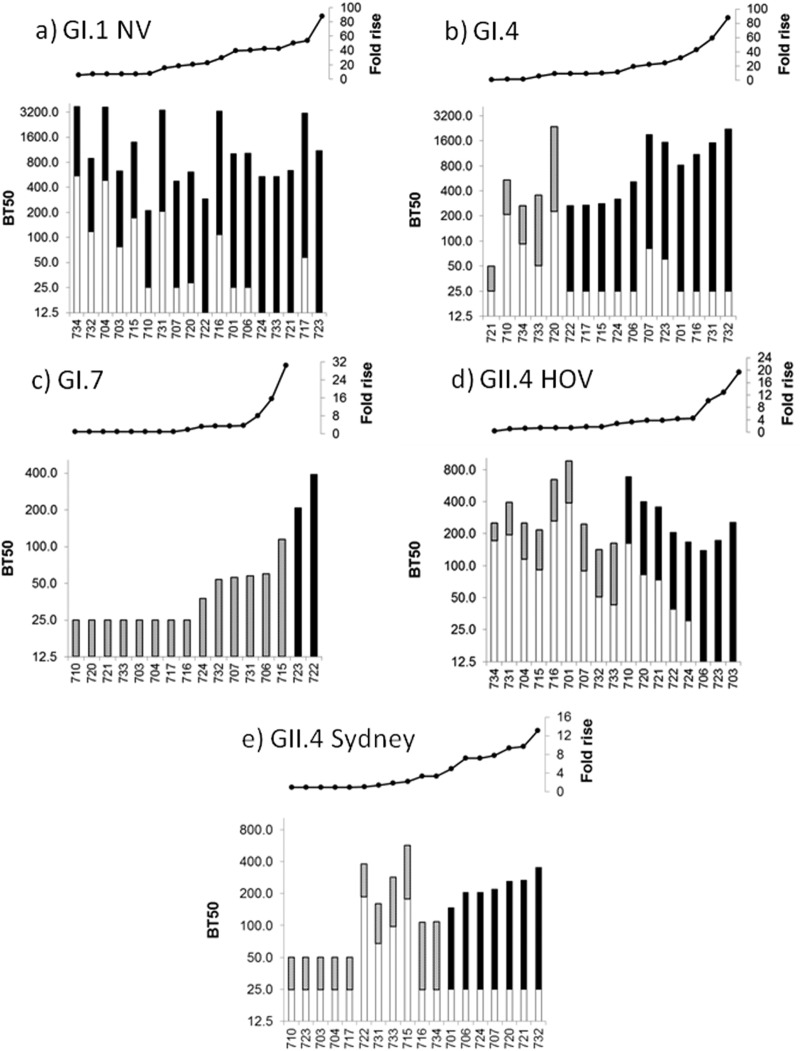
Preexisting heterotypic HBGA-blocking antibody titer correlates with heterotypic seroresponse outcome for GII.4 HOV.
Blocking antibody reactive to each NoV antigen tested, with the exception of GI.7, could be detected in prechallenge sera of this cohort. The antigen with the highest prechallenge GMT of blocking antibody was GII.4 HOV (68.5 [95% CI of 41.4, 113.4]); furthermore, blocking antibodies reactive to this antigen were detectable in all placebo recipients tested (n = 5). (Fig. 2, Table 1). Prechallenge blocking antibody titer against GII.4 HOV was associated with seroresponse outcome to this heterotypic antigen; individuals who developed a seroresponse to the heterotypic GII.4 HOV antigen over the course of experimental Norwalk virus infection had a significantly lower prechallenge titer to GII.4 HOV than those who did not have a seroresponse to GII.4 HOV (P = 0.002, Mann-Whitney U test). In contrast, there were no such differences in the prechallenge blocking antibody GMTs for GI.4 and GII.4 Sydney antigens when comparing seroresponders and nonresponders with respect to each of those antigens (P = 0.2 and P = 0.1, respectively, Mann-Whitney U test).
FIG 2.
Relationship between preexisting HBGA-blocking antibody and seroresponse outcome for homotypic (a) and heterotypic (b to e) human NoVs, by individual. Values shown across the top of each panel represent the arithmetic fold increase between prechallenge and peak antigen-specific blocking antibody titer and correspond to the values shown in the stacked bar graph below. Values shown in the lower portion of each panel represent the BT50, or the endpoint serum dilution at which 50% of NoV VLP binding to HBGA was blocked, at prechallenge (white bars) and peak titer (gray or black bars). Seroresponders to each antigen are defined as individuals who developed a 4-fold or greater increase in BT50 against that antigen (black bars), and nonresponders are those who did not (gray bars).
Prechallenge ELISA antibody titer against GI.1 NV was not associated with the likelihood of a seroresponse against GII.4 HOV antigen (P = 0.7, Mann-Whitney U test; data not shown). Similarly, there was no correlation between the outcome of broad heterotypic seroresponse (GI.1 NV and ≥2 heterotypic antigens) and baseline GI.1 NV-reactive blocking antibody (P = 0.182, Mann-Whitney U test). There was also no correlation between the outcome of broad heterotypic seroresponse and either fold rise (the quotient of peak titer/preexisting titer) of blocking antibody reactive to GI.1 NV (P = 0.659, Mann-Whitney U test) or peak GI.1 NV-reactive blocking antibody titer (P = 0.199, Mann-Whitney U test).
DISCUSSION
NoVs are estimated to cause 19 to 21 million cases of gastroenteritis each year in the United States alone, of which 56,000 to 71,000 cases result in hospitalization and 570 to 800 cases result in death (38). Given the high NoV disease burden and the highly infectious nature of these pathogens, an NoV vaccine has the potential to dramatically reduce disease and economic burden (7). To successfully achieve this objective, an NoV vaccine will need to contend with the high genetic diversity among the human NoVs. The choice of vaccine antigen is complicated by the simultaneous circulation of multiple NoV genotypes as well as documented antigenic drift among the GII.4 NoVs (3–5), underscoring the need for empirical data on which to base such decisions.
We investigated the breadth of seroreactivity of blocking antibodies elicited by human NoV infection. Experimental infection with Norwalk virus (GI.1 NV) elicited surrogate neutralizing antibodies to the challenge antigen and to heterologous NoV antigens; in fact, 16 of 18 Norwalk virus-infected volunteers developed a 4-fold or greater rise in blocking antibody to one or more heterologous antigens. Furthermore, 11 of 18 NV-infected volunteers developed a heterotypic blocking antibody seroresponse to one or more GII NoV antigens, despite the substantial genetic and antigenic distance between these VLPs.
Heterotypic blocking antibody responses were more modest than the homotypic blocking antibody response. This observation is consistent with previous reports (18, 19, 27). In our study, peak geometric mean blocking antibody titers observed among heterotypic seroresponders in our study ranged from 30.0 (95% CI of 17.5, 51.4; GI.7) to 500.8 (95% CI of 279.1, 898.8; GI.4), compared to 954.9 (95% CI of 632.1, 1,442.7) for the Norwalk antigen. Furthermore, our study suggests that experimental infection of healthy adults with Norwalk virus (a GI NoV) can elicit blocking antibody against heterotypic NoV antigens both within the GI genogroup as well as to the more divergent GII NoVs. This observation extends previous descriptions of broadly cross-reactive serum activity (20, 23–26) and is the first description, to our knowledge, of the development of a serum-blocking antibody response with cross-genogroup specificity in the context of experimental human challenge with an NoV.
The complexity of NoV immunity is reflected by differing observations in the literature regarding the breadth of heterotypic antibody elicited by NoV infection; some studies have found that total serum antibody responses or blocking antibody responses are genogroup restricted (18–22, 24). These different observations may be explained, in part, by various assay methodologies. Several of the studies that did not detect genogroup cross-reactive serum antibody were conducted prior to the development of serological assays specific for HBGA-blocking antibody (17, 18, 21). Among the more recent studies that did report HBGA-blocking antibody data, some report higher initial serum dilutions than we used in our study and may therefore have had lower sensitivity than our assay (18, 19). Finally, the choice of challenge virus may also have had an impact on the outcome of our study. To our knowledge, no studies have directly investigated this question, but it has been suggested that infections caused by GI NoVs can elicit more robust heterotypic responses than infections caused by GII NoVs (19).
The mechanisms underlying the development of heterotypic antibody responses to NoV infection remain unknown. It is possible that the broad heterotypic serum-blocking activity observed in some individuals in our study is due to a mixed population of genotype-specific blocking antibodies, reflecting an “archeological record” of past NoV infections. Precise ascertainment of an individual's infection history is precluded by the fact that molecular diagnosis and genotyping are not routinely carried out in the medical care of individuals with NoV infection. Furthermore, serological studies are confounded by the short duration of antibody responses in serum and cross-reactivity of serum antibody. However, our observation that the breadth of the heterotypic blocking antibody response is not associated with preexisting GI.1 NV-specific serum IgG or the homotypic blocking antibody seroresponse at any time point (data not shown) is consistent with the idea that that many blocking antibodies may be at least genogroup specific. The identification of distinct HBGA binding sites in the protruding domain of GI and GII NoVs by structural studies is also consistent with this interpretation (39, 40).
However, the possibility that a subset of HBGA-blocking antibodies may be broadly cross-reactive could explain the observation of epidemiological patterns consistent with herd immunity following NoV outbreaks (11). In fact, a recent study has described evidence for the existence of conserved epitopes that are distant from the primary HBGA-binding domain but mediate broad HBGA-blocking activity among antigenically distinct GII.4 NoV variants (39). Support for the presence of broadly cross-reactive capsid epitopes shared by antigenically distinct NoVs is also provided by observations that multivalent immunization of animal models can elicit broadly heterotypic NoV antibodies with reactivity to antigens not included in the immunogen cocktail (19, 41–44). Our own results are also consistent with the presence of cross-reactive epitopes. The GII.4 Sydney variant is the most recent product of ongoing antigenic drift described among the GII.4 NoVs, to which most human NoV disease is attributable (44, 45). We found that, despite the temporal mismatch between the study period (September 2004 to March 2008) and the emergence of the GII.4 Sydney variant (2012), Norwalk virus infection elicited a seroresponse in some individuals to the GII.4 Sydney variant (44, 45). This observation supports the possibility of the development of a broadly protective human NoV vaccine composed of antigens representing a limited number of NoV genotypes. These broadly reactive blocking antibodies need not bind directly to conserved sites within the glycan binding domain in order to exert broad HBGA-blocking activity; they could induce global conformational changes in the capsid that interrupt glycan binding or could bind conserved secondary sites in the P domain that modulate HBGA binding.
However, challenges remain to our understanding of NoV immunity and the evaluation of NoV vaccine candidates. The contribution of preexisting NoV antibody in primed individuals, in particular, presents a significant hurdle to our understanding of patterns of NoV immunity, given the high seroprevalence of NoV in children and adults (8, 45, 46). In our study, higher prechallenge titers of HOV-reactive blocking antibody were inversely correlated with seroresponse to this GII.4 antigen over the course of infection with a GI NoV (P = 0.002, Mann-Whitney U test). Together with our novel observation that experimental human infection with a GI NoV can elicit blocking antibodies to GII NoVs, this new observation suggests that factors other than antigenic relatedness contribute to the breadth of blocking antibody responses.
Preexisting homotypic HBGA-blocking antibody is a correlate of protection from NoV gastroenteritis (29–31). However, the observed independence between preexisting heterotypic blocking antibody and the clinical outcomes of challenge with Norwalk virus in this study suggests that preexisting HBGA-blocking antibody may offer limited protection upon reexposure to human NoVs. This observation may be explained by several factors, including the lack of statistical power to detect subtle differences in clinical outcomes between “broad” and “narrow” heterotypic seroresponders, the high virus inoculum used in the challenge study, NoV genotype-dependent differences in the affinity of blocking antibodies, and the possibility of poor persistence of heterotypic blocking antibody in serum at titers high enough to protect from viral gastroenteritis; in fact, an efficacy trial of a bivalent, VLP-based NoV vaccine candidate has reported evidence for a threshold effect for homotypic serum-blocking-antibody-associated protection from viral gastroenteritis (29).
Future human infection studies and clinical trials of NoV vaccine candidates should prospectively investigate the effect of preexisting blocking antibody on the breadth and quality of protective immunity following repeated immunization and/or experimental infection. The knowledge about NoV immunity contributed by such studies may be critical for the identification of ideal age groups and target populations for the development of effective vaccination strategies for the human NoVs. Studies to elucidate the molecular mechanism of protection by blocking antibodies and to identify individual human antibodies with broad cross-blocking activity are currently ongoing in our group and will also help to inform antigen selection for vaccine development and contribute to a better understanding of NoV immunity.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (PO1 AI057788 to M.K.E. and R.L.A. and P30DK5638 to M.K.E.). It was also supported by a graduate fellowship to R.C. (Agriculture and Food Research Initiative competitive grant no. 2011-68003-30395 from the USDA National Institute of Food and Agriculture).
We thank Baijun Kou (BCM) for her kind contribution of Sydney VLPs and Jan Vinjé and Everardo Vega (CDC) for their contribution of the now publicly available GI.4 NoV sequence and for many interesting discussions concerning VLP production. We also thank the excellent staff of the Recombinant Protein and Monoclonal Antibody Production Shared Resource at the Baylor College of Medicine, which is supported by P30 CA125123 from the NIH.
REFERENCES
- 1.Hall AJ. 2012. Noroviruses: the perfect human pathogens? J Infect Dis 205:1622–1624. doi: 10.1093/infdis/jis251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramani S, Atmar RL, Estes MK. 2014. Epidemiology of human noroviruses and updates on vaccine development. Curr Opin Gastroenterol 30:25–33. doi: 10.1097/MOG.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroneman A, Vega E, Vennema H, Vinjé J, White PA, Hansman G, Green K, Martella V, Katayama K, Koopmans M. 2013. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol 158:2059–2068. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Beek J, Ambert-Balay K, Botteldoorn N, Eden JS, Fonager J, Hewitt J, Iritani N, Kroneman A, Vennema H, Vinjé J, White PA, Koopmans M. 2013. Indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveill 18:8–9. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20345. [PubMed] [Google Scholar]
- 5.Giammanco GM, De Grazia S, Terio V, Lanave G, Catella C, Bonura F, Saporito L, Medici MC, Tummolo F, Calderaro A, Bányai K, Hansman G, Martella V. 2014. Analysis of early strains of the norovirus pandemic variant GII.4 Sydney 2012 identifies mutations in adaptive sites of the capsid protein. Virology 450–451:355–358. doi: 10.1016/j.virol.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Eden J-S, Hewitt J, Lim KL, Boni MF, Merif J, Greening G, Ratcliff RM, Holmes EC, Tanaka MM, Rawlinson WD, White PA. 2014. The emergence and evolution of the novel epidemic norovirus GII.4 variant Sydney 2012. Virology 450–451:106–113. doi: 10.1016/j.virol.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. 2012. The potential economic value of a human norovirus vaccine for the United States. Vaccine 30:7097–7104. doi: 10.1016/j.vaccine.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker SP, Cubitt WD, Jiang XJ, Estes MK. 1994. Seroprevalence studies using a recombinant Norwalk virus protein enzyme immunoassay. J Med Virol 42:146–150. doi: 10.1002/jmv.1890420209. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. 1990. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis 161:18–21. doi: 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]
- 10.Parrino TA, Schreiber DS, Trier JS, Kapikian AZ, Blacklow NR. 1977. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med 297:86–89. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- 11.Cannon JL, Lindesmith LC, Donaldson EF, Saxe L, Baric RS, Vinjé J. 2009. Herd immunity to GII.4 noroviruses is supported by outbreak patient sera. J Virol 83:5363–5374. doi: 10.1128/JVI.02518-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindesmith LC, Beltramello M, Donaldson EF, Corti D, Swanstrom J, Debbink K, Lanzavecchia A, Baric RS. 2012. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog 8:e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siebenga JJ, Vennema H, Renckens B, de Bruin E, van der Veer B, Siezen RJ, Koopmans M. 2007. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J Virol 81:9932–9941. doi: 10.1128/JVI.00674-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons K, Gambhir M, Leon J, Lopman B. 2013. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis 19:1260–1267. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindesmith LC, Donaldson E, Leon J, Moe CL, Frelinger JA, Johnston RE, Weber DJ, Baric RS. 2010. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol 84:1800–1815. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanstrom J, Lindesmith LC, Donaldson EF, Yount B, Baric RS. 2013. Characterization of blockade antibody responses in GII.2.1976 SMV-infected subjects. J Virol 88:829–837. doi: 10.1128/JVI.02793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyatt RG, Dolin R, Blacklow NR, DuPont HL, Buscho RF, Thornhill TS, Kapikian AZ, Chanock RM. 1974. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis 129:709–714. doi: 10.1093/infdis/129.6.709. [DOI] [PubMed] [Google Scholar]
- 18.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. 2005. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol 79:2900–2909. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LoBue AD, Lindesmith L, Yount B, Harrington PR, Thompson JM, Johnston RE, Moe CL, Baric RS. 2006. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 24:5220–5234. doi: 10.1016/j.vaccine.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 20.Noel JS, Ando T, Leite JP, Green KY, Dingle KE, Estes MK, Seto Y, Monroe SS, Glass RI. 1997. Correlation of patient immune responses with genetically characterized small round-structured viruses involved in outbreaks of nonbacterial acute gastroenteritis in the United States, 1990 to 1995. J Med Virol 53:372–383. doi:. [DOI] [PubMed] [Google Scholar]
- 21.Iritani N, Seto T, Hattori H, Natori K, Takeda N, Kubo H, Yamano T, Ayata M, Ogura H, Seto Y. 2007. Humoral immune responses against norovirus infections of children. J Med Virol 79:1187–1193. doi: 10.1002/jmv.20897. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, Matson DO, Ruiz-Palacios GM, Hu J, Treanor J, Pickering LK. 1995. Expression, self-assembly, and antigenicity of a snow mountain agent-like calicivirus capsid protein. J Clin Microbiol 33:1452–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treanor JJ, Jiang X, Madore HP, Estes MK. 1993. Subclass-specific serum antibody responses to recombinant Norwalk virus capsid antigen (rNV) in adults infected with Norwalk, Snow Mountain, or Hawaii virus. J Clin Microbiol 31:1630–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockx B, Baric RS, de Grijs I, Duizer E, Koopmans MPG. 2005. Characterization of the homo- and heterotypic immune responses after natural norovirus infection. J Med Virol 77:439–446. doi: 10.1002/jmv.20473. [DOI] [PubMed] [Google Scholar]
- 25.Madore HP, Treanor JJ, Buja R, Dolin R. 1990. Antigenic relatedness among the Norwalk-like agents by serum antibody rises. J Med Virol 32:96–101. doi: 10.1002/jmv.1890320206. [DOI] [PubMed] [Google Scholar]
- 26.Higo-Moriguchi K, Shirato H, Someya Y, Kurosawa Y, Takeda N, Taniguchi K. 2014. Isolation of cross-reactive human monoclonal antibodies that prevent binding of human noroviruses to histo-blood group antigens. J Med Virol 86:558–567. doi: 10.1002/jmv.23734. [DOI] [PubMed] [Google Scholar]
- 27.Farkas T, Thornton SA, Wilton N, Zhong W, Altaye M, Jiang X. 2003. Homologous versus heterologous immune responses to Norwalk-like viruses among crew members after acute gastroenteritis outbreaks on 2 US Navy vessels. J Infect Dis 187:187–193. doi: 10.1086/367809. [DOI] [PubMed] [Google Scholar]
- 28.Green KY, Lew JF, Jiang X, Kapikian AZ, Estes MK. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J Clin Microbiol 31:2185–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, Mendelman PM. 2011. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med 365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeck A, Kavanagh O, Estes MK, Opekun AR, Gilger MA, Graham DY, Atmar RL. 2010. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 202:1212–1218. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. 2002. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol 76:12335–12343. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Ramani S, Hill H, Ferreira J, Graham DY. 2014. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 209:1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY. 2008. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 14:1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang X, Wang M, Graham DY, Estes MK. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol 66:6527–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinjé J. 2013. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009-2013. J Clin Microbiol 52:147–155. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng D-P, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. 2006. Norovirus classification and proposed strain nomenclature. Virology 346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Czakó R, Atmar RL, Opekun AR, Gilger MA, Graham DY, Estes MK. 2012. Serum hemagglutination inhibition activity correlates with protection from gastroenteritis in persons infected with Norwalk virus. Clin Vaccine Immunol 19:284–287. doi: 10.1128/CVI.05592-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastañaduy PA, Vinjé J, Parashar UD. 2013. Norovirus disease in the United States. Emerg Infect Dis 19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J-M, Hutson AM, Estes MK, Prasad BVV. 2008. Atomic resolution structural characterization of recognition of histo-blood group antigens by Norwalk virus. Proc Natl Acad Sci U S A 105:9175–9180. doi: 10.1073/pnas.0803275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao S, Lou Z, Tan M, Chen Y, Liu Y, Zhang Z, Zhang XC, Jiang X, Li X, Rao Z. 2007. Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol 81:5949–5957. doi: 10.1128/JVI.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamminen K, Huhti L, Vesikari T, Blazevic V. 2013. Pre-existing immunity to norovirus GII-4 virus-like particles does not impair de novo immune responses to norovirus GII-12 genotype. Viral Immunol 26:167–170. doi: 10.1089/vim.2012.0082. [DOI] [PubMed] [Google Scholar]
- 42.LoBue AD, Thompson JM, Lindesmith L, Johnston RE, Baric RS. 2009. Alphavirus-adjuvanted norovirus-like particle vaccines: heterologous, humoral, and mucosal immune responses protect against murine norovirus challenge. J Virol 83:3212–3227. doi: 10.1128/JVI.01650-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parra GI, Bok K, Taylor R, Haynes JR, Sosnovtsev SV, Richardson C, Green KY. 2012. Immunogenicity and specificity of norovirus consensus GII.4 virus-like particles in monovalent and bivalent vaccine formulations. Vaccine 30:3580–3586. doi: 10.1016/j.vaccine.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debbink K, Lindesmith LC, Donaldson EF, Swanstom J, Baric RS. 2014. Chimeric GII.4 norovirus virus-like particle based vaccines induce broadly blocking immune responses. J Virol 88:7256–7266. doi: 10.1128/JVI.00785-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cubitt WD, Green KY, Payment P. 1998. Prevalence of antibodies to the Hawaii strain of human calicivirus as measured by a recombinant protein based immunoassay. J Med Virol 54:135–139. doi:. [DOI] [PubMed] [Google Scholar]
- 46.Hale A, Mattick K, Lewis D, Estes M, Jiang X, Green J, Eglin R, Brown D. 2000. Distinct epidemiological patterns of Norwalk-like virus infection. J Med Virol 62:99–103. doi:. [DOI] [PubMed] [Google Scholar]
- 47.Noel JS, Fankhauser RL, Ando T, Monroe SS, Glass RI. 1999. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J Infect Dis 179:1334–1344. doi: 10.1086/314783. [DOI] [PubMed] [Google Scholar]
- 48.Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng D-P, Vinje J, Baric RS. 2008. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med 5:e31. doi: 10.1371/journal.pmed.0050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siebenga JJ, Lemey P, Kosakovsky Pond SL, Rambaut A, Vennema H, Koopmans M. 2010. Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathog 6:e1000884. doi: 10.1371/journal.ppat.1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]