Abstract
Background
Natural antioxidants can reduce oxidative damage caused by high-intensity resistance training (RT). We investigated the in vitro antioxidant potential of hydroethanolic extract (HEE) from Bowdichia virgilioides on muscular damage and oxidative stress in rats subjected to high-intensity RT.
Methods
Thirty-two male Wistar rats were divided into four experimental groups: 1) control group (CG), oral administration (P.O.) of vehicle; 2) trained group (TG), vehicle-treated with RT; 3) B. virgilioides untrained group (BVG), treated with B. virgilioides HEE (200 mg/kg P.O.); and 4) trained B. virgilioides group (TBVG), treated with B. virgiliodes HEE (200 mg/kg P.O.). All animals were habituated to the training apparatus for 1 week. CT and TBVG animals were subjected to the training protocol, which consisted of three sets of 10 repetitions with 75% of the load established using the one-repetition maximum, for four weeks. CG and BVG animals were manipulated and fixed to the apparatus three times a week with no load. Treatment with B. virgilioides HEE or vehicle treatment was initiated after 25 days of RT (5 days; one dose per day). At the end of the experiments, plasmatic and gastrocnemius samples from all groups were obtained for the assessment of lipid peroxidation and creatine kinase activity.
Results
Compared to TG rats, TBVG rats showed decreases in plasma and gastrocnemius tissue lipid peroxidation by 55.68% (p <0.0001) and 66.61% (p <0.0012), respectively. Further, compared to TG rats TBVG rats showed decreases in plasma and gastrocnemius tissue oxidative stress by 62.83% (p <0.0005) and 54.97% (p <0.0197), respectively.
Conclusions
B. virgilioides HEE treatment reduced markers of oxidative stress caused by high-intensity RT. Further, HEE treatment during training significantly reduced the markers of tissue damage.
Keywords: Oxidative stress, Physical exercise, Hydroethanolic extract
Background
Physical exercise is characterized as any form of activity that induces a series of physiological responses in the body and maintains physical fitness [1-3]. Resistance training (RT) is defined as physical activity involving voluntary contractions of skeletal muscles against an external resistance or force that opposes motion, such as one’s own body mass, free weights and other equipment, or manual resistance machines [2,3].
RT is considered a safe form of exercise and is practiced by individuals of various age groups. Further its health benefits are well recognized [4,5], including increased muscular resistance and force [6] and reduced blood sugar levels in diabetic individuals [7]. However, during high-intensity RT, muscles undergo periods of ischemia-reperfusion [8], resulting in increased reactive oxygen species (ROS) levels, including hydroxyl (HO•), alkoxy (RO•), peroxyl (ROO•), and superoxide (O2•-) radicals, and non-radicals such as hydrogen peroxide (H2O2), singlet oxygen (1O2), and ozone (O3) [9,10]. These molecules can react with proteins, lipids, carbohydrates, and nucleic acids, leading to changes in cell function and cell death. Moreover, ROS are associated with post-exercise inflammatory responses, which can propagate muscular damage [11].
Oxidative stress is a detrimental condition characterized by an imbalance of oxidants and antioxidants [12,13]. It can be caused by overtraining, xenobiotics, exposure to pollutants, use of antibiotics, and UV radiation [14]. Further, high-intensity RT can cause microtears in muscular tissue. Leukocytes and other immune cells migrate to the site of tears, thereby triggering increased ROS production and activating inflammatory mediators [15]. Moreover, Hawke [16] and Saxton et al. [17]. stated that muscular damage and inflammation are proportional to exercise intensity. These injuries may be related to both the contractile and non-contractile muscle components, such as the extracellular matrix, sarcolemma, and basal membrane [18-21]. However, other studies suggest that chronic exercise may cause depletion of antioxidants, which may increase exercise-induced oxidative stress and tissue damage if combined with diminished ingestion of antioxidants [22].
Numerous approaches have been developed to prevent or minimize the deleterious effects of oxidative stress, including the use of natural and synthetic antioxidants, such as vitamin C (ascorbic acid), E (α-tocopherol), A (β-carotene), and polyphenols from medicinal plants [9,23,24]. Moreover, recent studies suggest that foods rich in polyphenols can reduce oxidative damage in response to physical exertion caused by high-intensity RT [25-27]. Diminished lipid peroxidation and DNA damage was observed in rodents that received supplementation with grape seed oil extract, which contains a high concentration of polyphenols [28-30]. These data suggest that supplementation with antioxidants might reduce oxidative stress and thereby attenuate muscular damage after high-intensity exercise.
In this study, we investigated the in vitro antioxidant potential and protective effects of the hydroethanolic extract (HEE) of Bowdichia virgilioides on muscular damage in rats subjected to high-intensity RT. We hypothesized that supplementation with B. virgilioides can reduce lipid peroxidation and prevent muscle injury in rats undergoing high-intensity RT.
Methods
Animals and treatment period
Thirty-two male Wistar rats (3 months old, weight: 200–250 g) were obtained from the bioterium at Federal University of Sergipe. The rats were randomly housed (four rats per cages) and maintained in temperature-controlled conditions (22 ± 3°C) with a light–dark cycle of 12 h (lights on between 0600 h and 1800 h), free access to food (Labina®), and water ad libitum. All procedures described in this study were approved by the Animal Research Ethics Committee at Federal University of Sergipe (protocol 10/12).
The animals were divided into four groups: 1) control group (CG, n = 8), composed of healthy, vehicle-treated animals (Tween 80, 3% P.O., Vetec, LTDA, Rio de Janeiro, Brazil) receiving electrostimulation; 2) trained group (TG, n = 8), composed of healthy vehicle-treated animals (Tween 80, 3% P.O.) subjected to the RT protocol; 3) B. virgilioides group (BVG, n = 8), composed of healthy animals treated with B. virgilioides HEE (200 mg/kg, P.O); and 4) trained and B. virgilioides treated group (TBVG, n = 8), composed of animals subjected to RT and treated with B. virgilioides HEE (200 mg/kg, P.O). All animals were either vehicle-treated or received B. virgilioides HEE on day 25 of the RT protocol (5 days total treatment, as described in the training protocol), which is shown in the organogram (Figure 1).
Figure 1.
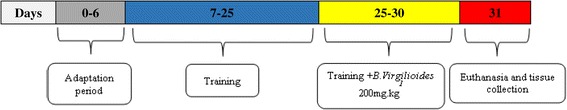
Organogram of the experimental protocol. The experiment was performed over 30 days with all animal groups: Day 0 – 6 were the adaptation period without charge (white bars); day 7–25 of training without the use of B. virgilioides, day 26 – 30 (5 days with intake of the extract B. virgilioides) after initiating RT. At day 31 the animals were euthanized.
The inner bark of B. virgilioides was collected in March 2011 from the village of Fazenda Riachão, in the municipality of Japaratuba, Sergipe, Brazil (10°32′04.49 S, 36°53′57″ W). A reference sample of this species was stored in the herbarium at the Federal University of Sergipe under the reference ASE 23.107.
High-performance liquid chromatography
The high-performance liquid chromatography (HPLC) system used includes a Shimadzu Prominence chromatograph composed of two LC-6 AD pumps, an autoinjector, DGU 20 A5 degasser, a solvent selector valve, and a photodiode detector (DAD SPD M20A). For chromatographic analysis, two C18 columns were used, as well as an analytical column (25.0 × 0.46 cm, 5 mm particles) and a preparatory column (25.0 × 2 cm, 5 mm particles), both manufactured by Shimadzu. To obtain and process the data, we used the chromatographic software LC Solution.
Antioxidant potential and redox properties of hydroethanolic extract of B. virgilioides
HEE samples were dissolved in methanol to obtain a stock solution of 0.5 mg/mL, from which aliquots were removed and added to a solution of 2,2-difenil-1-picrilhidrazina (DPPH•, 40 μg/mL, Sigma-Aldrich, Steinheim, Germany) to obtain a final concentrations of 5, 15, and 25 μg/mL in a reaction volume of 3 mL. The blank was composed of a mixture of the analyzed sample and methanol (Vetec, LTDA, Rio de Janeiro, Brazil). Gallic acid (Abiquim, São Paulo, Brazil) was used as the positive control.
The absorbance value of each sample was obtained using an spectrophotometer at a wavelength of 515 nm, and the readings were taken at 1, 5, and 10 min, and at 10 min intervals thereafter, up to 60 min [31]. The percentage of DPPH remaining (DPPHREM%) was calculated according to previous methods [32] using the following equation:
where [DPPH] T is the concentration of radicals in the reaction medium after reaction with the sample; and [DPPH] T0 is the initial concentration of DPPH. From the DPPHREM% values, the percent inhibition at 60 min was calculated.
Measuring lipid peroxidation in vitro
The capacity of HEE to inhibit lipid peroxidation was determined by monitoring the production of thiobarbituric acid reactive substances (TBARS) in the lipid medium, according to previous methods [32]. For the quantification of TBARS, we used the protocol from Lapenna et al. [33]. Briefly, 1.0 mL of egg yolk homogenate (1% w/v) was completely dissolved in 20 mM phosphate buffer solution (pH 7.4), and then homogenized with 0.1 mL of HEE at varying concentrations (50, 100, and 200 μg/mL) suspended in methanol.
Lipid peroxidation was induced upon the addition of 0.1 mL of 0.17 M 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH, Sigma-Aldrich, Steinheim, Germany) and 0.17 M solution of iron sulfate (FeSO4, Sigma-Aldrich, Steinheim, Germany) at different time points. Trolox (Sigma-Aldrich, Steinheim-Germany) was used as the positive control, and the extract and solvent (water or methanol) were used as the negative control. The reactions were incubated 30 min at 37°C. After cooling, the samples (0.5 mL) were centrifuged in the presence of 0.5 mL of 15% trichloroacetic acid (TCA, Vetec, LTDA, Rio de Janeiro, Brazil) at 1,200 g for 10 min. A 0.5-mL aliquot of the supernatant was mixed with 0.5 mL of 0.67% thiobarbituric acid (TBA, Sigma-Aldrich, Steinheim, Germany) and heated at 95°C for 60 min. After cooling, the absorbance was measured using a spectrophotometer at a wavelength of 532 nm. The results were expressed as percent inhibition.
Training protocol
RT was carried out using a squat machine. The animals wore a leather jacket, connected to a mobile 35 cm long wooden bar, and the loads were allocated. The rats wearing jackets remained sitting down with their back legs bent and supported, according to the model by Tamaki et al. [34]. All animals underwent habituation to the apparatus for one week, where they received electrostimulation. After this period, the CT and TBVG animals were subjected to the training protocol in three sets of 10 repetitions, with rest intervals of 60 s, at an intensity of 75% of the load established using the one-repetition maximum (1RM) test. The RT was performed three times a week on alternate days, for four weeks [35]. The training load and intensity were adjusted every two weeks following a new 1RM test. The CG and BVG animals were manipulated and fixed to the apparatus three times a week on alternate days with electrostimulation, by using three sets of 10 repetitions and a rest interval of 60 s. These animals experienced no load, 0% intensity (Table 1).
Table 1.
Resistance training protocol
| Week | Intensity (%) | Days of the week* | Sets | Repetitions | Interval (s) |
|---|---|---|---|---|---|
| 1st | 75 | 3 | 3 | 10 | 60 |
| 2nd | 75 | 3 | 3 | 10 | 60 |
| 3rd | 75 | 3 | 3 | 10 | 60 |
| 4th | 75 | 3 | 3 | 10 | 60 |
*Alternate days. The training was conducted for 4 weeks on alternate days at 75% intensity defined by MRI with 3 sets and 10 repetitions with 60-s intervals between a series and another.
Electrical stimulation was applied to animals during each set (20 V/0.3 s in duration, 3 s interval) using electrodes (ValuTrode, Model CF3200, Axelgaard, Fallbrook, CA, USA) fixed to the tail and connected to an electrostimulator (BIOSET, Physiotonus Four, Model 3050, Rio Claro, SP, Brazil).
Collection of biological material
Twenty-four hours after the last session, the animals were fasted overnight, anesthetized using sodium thiopental (40 mg/kg, i.p., Cristália, Itapira São Paulo, Brazil) and sacrificed. Blood was collected by cardiac puncture, and the rats were decapitated. After the blood was collected, it was immediately centrifuged at 800 g for 15 min at 4°C. The supernatant was then stored at −80°C. The organs were removed, and the gastrocnemius muscle was washed three times in a solution of 1.15% KCl (Vetec, LTDA, Rio de Janeiro, Brazil), dried, and weighed. The muscle was then homogenized, and each gram of tissue was mixed with 5 mL of KCl, 10 μL of phenylmethylsulfonyl fluoride (PMSF, 100 mmol, Sigma-Aldrich, Steinheim, Germany), and 15 μL of 10% Triton. The homogenate was then centrifuged at 3,000 g for 10 min at 4°C. The supernatant was stored at −70°C until further analyses of oxidative stress and tissue damage markers.
Biochemical analysis
The biological materials (plasmatic fraction) were analyzed for markers of tissue damage and oxidative stress according to the methodology described by Branco et al. [36]. The quantification of tissue damage caused by high-intensity RT was assessed by measuring enzyme markers of tissue damage, such as creatine kinase (CK), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). For quantification, a commercial kit (Labtest®, Santa Lagoa, Minas Gerais, Brazil) was used. Plasma (20 μL) from each animal was homogenized in specific reagents at 37 ± 0.2°C, and readings were taken using a spectrophotometer (Bioespectro Model SP-22 UV/Visible, Minas Gerais, Brazil) at a wavelength of 340 nm.
To determine lipid peroxidation, TBARS was measured according to Lapenna et al. [33]. For the assessment of carbonyl proteins, the oxidation of proteins was assessed by determining carbonyl residues (CR) according to the methodology of Faure and Lafond [37].
Statistical analyses
The results are presented as the mean ± standard deviation (SD). Differences between samples were considered statistically significant when p <0.05. All the analyses were carried out in triplicate. After assessing the normality of the data using the Shapiro Wilk test, the data were statistically analyzed using one-way analysis of variance (ANOVA), followed by the Bonferroni or Dunnett multiple comparison tests, when appropriate. The statistical software Graph Pad Prism version 5.0 was used.
Results
Antioxidant potential and redox properties of B. virgilioides HEE
To verify the antioxidant potential of B. virgilioides, the reduction of the free radical DPPH was evaluated. HEE displayed a dose-dependent enhancement in antioxidant activity (Figure 2), with significant variations between concentrations (5–30 mg/mL), and an IC50 of 33.45 ± 5.97 μg/mL for 60 min.
Figure 2.
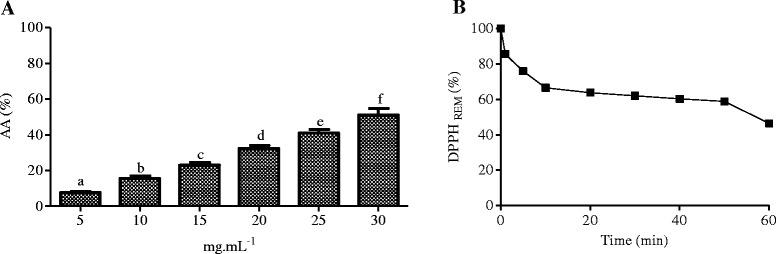
Percentage of antioxidant activity at different concentrations of B. virgilioides HEE. (A). Kinetic behavior of HEE at a concentration of 25 μg/mL to reduce DPPH free radical (B). Results are expressed as the mean ± SD. The statistical difference between the concentrations was determined using one-way ANOVA, followed by Bonferroni post-hoc test. Different letters on the graph stand for a statistical difference between the concentrations of HEE (p <0.05). All experiments herein were performed in triplicate.
From this data, we verified that HEE from B. virgilioides had an antioxidant activity index (AAI) of 0.89 ± 0.05, which classifies it as a moderate antioxidant [38]. We also observed that HEE had moderate reaction kinetics, requiring 60 min to reduce the DPPH radical level by more than half, as shown in Figure 2 via the dose–response curve, showing the percent decrease of remaining DPPH (% DPPHREM) over time (min).
Redox property of B. virgilioides HEE
The hydroethanolic extract of B. virgilioides inhibited AAPH- and iron sulfate-induced lipid peroxidation. HEE also showed potential as a chelating agent of transition metals and neutralized Fenton reactions. HEE inhibited AAPH- and iron sulfate-induced lipid peroxidation to a similar extent (p >0.05) as the positive control, Trolox (Figure 3).
Figure 3.
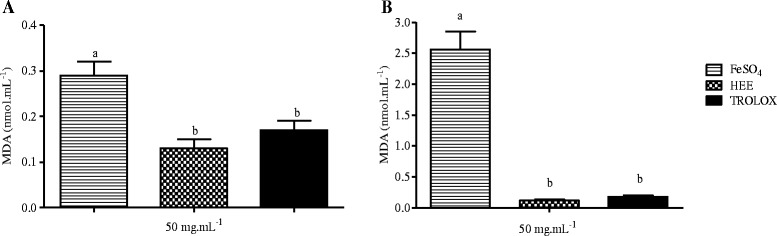
Effect of HEE (50 μg/mL) on lipid peroxidation induced by AAPH (A) and FeSO 4 (B). The results are shown as the concentration of malondialdehyde formed (nmol/mL). Values are expressed as the mean ± SD. Different letters on the graph stand for statistical difference between the groups. The statistical analysis was carried out using one-way ANOVA, followed by Bonferroni post-hoc test (p <0.05). All experiments were performed in triplicate.
Phytochemical profile and the total phenolic content of B. virgilioides HEE
The chromatographic profile of HEE was obtained using HPLC-DAD. The spectra showed a characteristic fingerprint of medium to high polarity substances, similar to phenolic compounds, as shown in Figure 4. These results were similar to those obtained by Im and colleagues [39].
Figure 4.
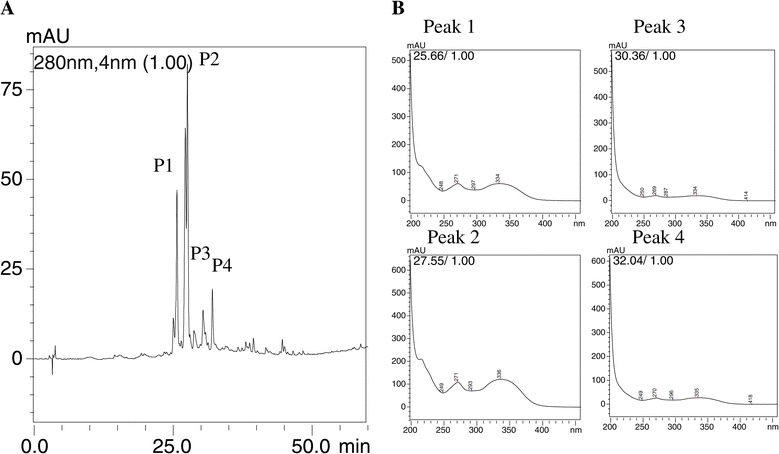
Chromatographic profile of HEE and the respective spectra of the prominent peaks. The experimental 5:100% water/methanol condition gradient, measured at a wavelength of 250 nm - 350 nm with the absorption spectra of UV/Vis prominent peaks (A) of spectra, and segmented (B) for each peak: Peak 1 - band “A” 271 nm, and band “B” = 334 nm; Peak 2 - band “A” 271 nm, and band “B” 336 nm; Peak 3 - band “A” 269 nm, and band “B” 334 nm; Peak 4 - band “A” 270 nm, and band “B” 335 nm.
Quantification of the total phenol content
Total phenol content was quantified using the spectrophotometer, and determined to be 128.05 ± 26.10 mg eq AG/g B. virgilioides extract.
Effect of B. virgilioides HEE on the reduction of oxidative stress induced by high-intensity RT
To assess the effects of HEE in the body, we studied the effects of ingesting HEE in animals undergoing high-intensity RT. Oxidative stress markers were reduced in animals that ingested the B. virgilioides. As shown in Figure 5, we observed a significant reduction in plasma (55.68%, p <0.0001) and tissue (66.61%, p <0.0012) lipid peroxidation in TBVG rats as compared to TG rats. This finding indicates that B. virgilioides HEE effectively reduces oxidative stress in cellular lipid components.
Figure 5.

Effect of HEE on plasma and muscular lipid peroxidation induced by high-intensity exercise. (A) refers to plasma samples and (B) to muscular tissue from all animal groups: trained group (TG), trained Bowdichia virgilioides group (TBVG), control group (CG), and B. virgilioides group (BVG), each consisting of eight animals. The values represent the mean ± SD. Different letters indicate significant differences between groups (p <0.05). The statistical differences were determined using one-way ANOVA, followed by Bonferroni post-hoc test. All experiments were performed in triplicate.
Moreover, we also verified that B. virgilioides HEE efficiently prevented and/or reduced protein oxidation, as shown in Figure 6. Protein oxidation was reduced in BVG rats compared to that in CG rats, as well as in TBVG rats compared to that in TG rats. This reduction in plasma and tissue oxidation was approximately 62.83% (p <0.0005) and 54.97% (p <0.0197), respectively, in BVG and CG rats. Further, in the TBVG rats, the rate of oxidative was reduced 58.90% (p <0.0013) in the plasma and 52.75% (p <0.0059) in the muscular tissue, as compared to the TG rats.
Figure 6.
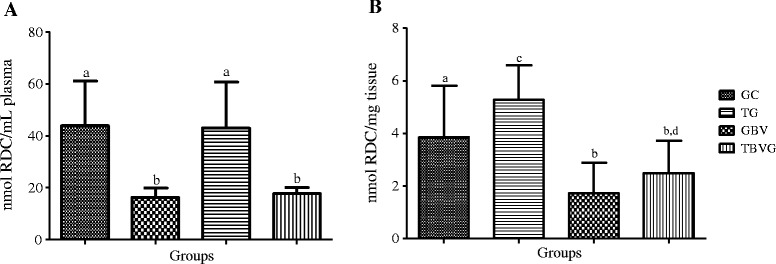
Effect of HEE on the oxidation induced by high intensity exercise. (A) refers to samples of plasma and (B) to muscular tissue from all animal groups: trained group (TG), trained Bowdichia virgilioides group (TBVG), Control group (CG) and Group Bowdichia virgilioides group (BVG), each consisting of eight animals. The values represent the mean ± standard deviation (SD). Different letters stand for significant differences between groups (p <0.05). The statistical differences were determined using one-way ANOVA followed by Bonferroni post-hoc test. All experiments were performed in triplicate.
Effect of B. virgilioides HEE on the prevention of tissue damage induced by high intensity RT
The results presented in Table 2 suggest that high-intensity RT induces muscular tissue damage (Group TG vs. CG). There was a significant increase (173.18%, p <0.0001) in plasma CK in the TG rats compared to the CG rats. We also observed that the consumption of HEE during training prevented an increase in markers of tissue damage in the TBVG as compared to the TG rats, including CK, ALT, and AST.
Table 2.
Serum concentrations of tissue damage enzymes in UI/L
| GROUPS | CK ± (SD) | LDH ± (SD) | ALT ± (SD) | AST ± (SD) |
|---|---|---|---|---|
| CG | 198.7 ± 35.21A | 23.61 ± 14.57A,B | 47.15 ± 27.62A | 128.9 ± 42.76A |
| BVG | 199.0 ± 72.13A, C | 8.75 ± 3.94B | 10.05 ± 7.84B | 92.95 ± 45.48A |
| TG | 542.0 ± 43.00B | 27.12 ± 17.19A | 37.10 ± 12.57A | 92.57 ± 23.90A |
| TBVG | 101.4 ± 80.75B, C | 9.25 ± 5.59B | 9.11 ± 4.44B | 30.03 ± 19.96B |
CK: creatine kinase, LDH: lactate dehydrogenase, ALT: alanine aminotransferase and AST: aspartate aminotransferase. Trained group (TG), trained Bowdichia virgilioides Group (TBVG), control group (CG), and Bowdichia virgilioides group (BVG). Values with different letters stand for significant differences (p <0.05). Data presented as means plus or minus standard deviation (SD). The statistical differences were determined using one-way ANOVA followed by Bonferroni post-hoc test. (n = 8, for all animal groups).
Discussion
In this study, we showed that intake of B. virgilioides HEE caused a moderate antioxidant effect in vitro, although a characteristic profile of substances with medium to high polarity was observed. In addition, we reported a significant reduction in markers of oxidative stress and muscle damage in resistance-trained rats treated with B. virgilioides HEE.
According to Wang and Huang [40], treating animals with polyphenol-based compounds can prevent lipid peroxide damage of cellular compounds. Similarly, Bansala et al. [41]. reported that products rich in polyphenols are effective in preventing both lipid peroxidation and protein oxidation in various animal tissues subjected to a high-intensity exercise protocol. This is likely due to the presence of amphipathic antioxidants, which increase their effects on cellular structures, neutralizing both intracellular and extracellular oxidizing agents [42]. Moreover, some polyphenols have significant antioxidant properties under low partial pressures of oxygen, a condition typical of skeletal muscles during intense exercise [43-45].
Phenols exhibit extensive diversity in structure and are characterized by one or more hydroxyl groups linked to an aromatic ring. They are subdivided into several categories, including simple phenols, phenolic acids (derived from benzoic and cinnamic acid), coumarins, flavonoids, stilbenes, condensed and hydrolysable tannins, lignans, and lignins, confirming the results of the phytochemical experiments [27,46]. It also confirms that these compounds are responsible for preventing lipid peroxidation, primarily due to their capacity to chelate transition metals and cellular oxidizing agents, especially those that interact with intracellular proteins [47,48].
When we assessed B. virgilioides HEE by using HPLC, we detected peaks with absorption spectra in UV/VIS range characteristic of phenolic compounds, including flavonoids. These absorption spectra showed variation between 250 nm and 350 nm [49]. Further, they were consistent with those reported by Im and colleagues [38], who described fingerprint characteristics of phenolic compounds of varying polarity. Data suggest that these molecules may be responsible for preventing the lipid peroxidation observed in our study. This is partly due to the ability of phenols to chelate transition metals, which inhibits cellular oxidizing agents [49]. Moreover, the compounds present in the extract are also capable of reducing lipid peroxidation, thereby neutralizing peroxyl radicals that originate from the lipid peroxidation cascade. The compounds present in B. virgilioides extract were also capable of reducing lipid peroxidation induced by AAPH and neutralizing peroxyl radicals, suggesting that they have an important role in the neutralization and sequestration of free radicals and chelation of transition metals, frequently acting in the initiation and propagation stages of oxidative stress [27]. This process may occur due to the phenols present in the B. virgilioides HEE, which were similar to those found by Dias et al. [50] in Abarema cochliacarpos, which has high antioxidant activity.
The high antioxidant activity of phenols increases with the degree of hydroxylation and depends on the rearrangement of functional groups around the nuclear structure of the molecule [51,45]. Thus, during reactions with free radicals, these compounds donate hydrogen with an unpaired electron, giving rise to another radical, which is stabilized by the rearrangement of electrons produced in the molecular resonance structure of the aromatic ring [52,53]. These studies show a significant correlation between high phenol content and antioxidant activity. Further, activity arises from the secondary metabolism in plants possessing these phenols, being primarily attributed to the hydroxyl groups attached to the aromatic ring [45,52-56]. Similar to extracts from other species, B. virgilioides HEE appear to have an antioxidant effect after the practice of intense RT.
RT programs such as the one adopted in this study are able to generate changes in muscle fibers owing to neural adaptations [57]. Considering that high-intensity RT causes tissue damage [42], animals subjected to the intensity of 75% of 1RM show muscular damage, as demonstrated by the increase in plasma CK compared to that in control animals that did not engage in RT. Such alterations may contribute to the development of morphological adjustments in skeletal muscles, including disruption of muscle fibers. However, one limitation of our study was the use of high-intensity RT for a period of four weeks. Thus, other studies should be made extending the period of the study.
Because high-intensity RT causes muscular damage [42], as shown by the increase in specific and non-specific markers in the serum. Numerous enzymes, such as lactate dehydrogenase from the cytoplasm of skeletal muscle fibers [58,59], AST from skeletal muscle and hepatocyte mitochondria, ALT from hepatocytes cytoplasm [60], and CK from skeletal muscles cytoplasm, increase in the serum as a result of decreased plasma membrane integrity. The animals that were treated with B. virgilioides HEE showed a significant reduction in all of these markers.
According to Clarkson and Hubal [61] and Deminice [19], tissue damage caused by intense exercise primarily depends on the intensity and type of exercise performed. This damage usually occurs in contractile muscle fibers and components of the cytoskeleton, causing rupture, widening, or lengthening of the Z-line, which is the contact point of contractile proteins and support the transmission of force when muscle fibers contract. Breakage of the sarcolemma may also occur [62]. The exact mechanisms involved in muscle damage induced by RT are still not fully understood [63]. However, the hypothesis that metabolic stress is associated with an increase in ROS, leading to oxidative stress, is becoming increasingly common in literature [64]. Mastaloudis et al. [65] stated that RT increases the metabolism of prostanoids, such as xanthine oxidase and nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) oxidase, oxidation of purine bases and proteins containing iron ions, and disturbs calcium (Ca2+) homeostasis. These events favor increased production of oxidizing agents, triggering damage to cells and tissues [66,67]. This evidence may seem conflicting, because Kerksick and colleagues [68] reported that each marker exhibits different responses to exercise. Regarding lipid peroxidation, these authors observed no increments following an exercise program, which may be justified depending on the length, volume, and intensity of the exercise adopted in both studies. Thus, our results showed tissue damage caused by the mechanical stress of exercise, as evidenced by the increase in serum levels of the enzyme markers as well as an increase in lipid peroxidation and protein oxidation in the trained group.
This study showed that a lower degree of muscular damage and oxidative stress was observed in rats subjected to a high-intensity RT protocol after ingestion of B. virgilioides HEE. Some authors state that tissue damage caused by oxidative stress during high-intensity RT can be lessened through supplementation with antioxidants, such as vitamins C, E, A, and products derived from medicinal plant, including polyphenols [42,43,69,38,36].
The results of this study are in agreement with those of Panza [42], who reported that the consumption of green tea can prevent oxidative stress, as well as muscular damage in individuals engaged in high-intensity RT. Green tea is a natural product rich in polyphenols, which are excellent antioxidants capable of neutralizing the deleterious effects of free radicals and other oxidizing agents produced during physical exercise. The phenol level B. virgilioides HEE was moderate, and thus, showed a moderate antioxidant activity in terms of reducing DPPH free radicals.
Consuming B. virgilioides HEE significantly reduced lipid peroxidation in the plasma and muscles of exercised animals treated with HEE. Nevertheless, we also found that there was a reduction in the level of oxidized proteins in animals treated with HEE compared to that in those who only exercised. These data suggest that HEE can prevent or reduce muscular oxidative stress caused by high-intensity RT and minimize or prevent muscular tissue damage caused by oxidative stress. There was a reduction in plasma CK content in animals treated with HEE compared to that in the group that only engaged in exercise.
Conclusion
Our study showed that the B. virgilioides HEE reduced some markers of oxidative stress and tissue damage caused by high-intensity RT for a period of four weeks. We also propose that the intake of B. virgilioides HEE during and/or after RT may act as an important adjuvant in the reestablishment of muscular function.
Acknowledgments
The authors would like to acknowledge CAPES and FAPITEC for their funding support for this study. We also thank teacher Abilio Borghi and EDITAGE (™) for the assistance with the grammar review of the manuscript.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JLS was responsible for the study design, execution of biochemical analysis, statistical analysis and writing of the manuscript. CAL, SSA, READ, and ECVA were responsible for biochemical analysis. ACM and CSE provided critical intellectual input in the preparation of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Jymmys Lopes dos Santos, Email: jymmyslopes@yahoo.com.br.
Rafaela Eugênia Arce Dantas, Email: acmarcal@yahoo.com.br.
Clésio Andrade Lima, Email: clesio_ufs@ibest.com.br.
Silvan Silva de Araújo, Email: silvan.ssa@gmail.com.
Elis Cristiane Valença de Almeida, Email: eliscristiane1@hotmail.com.
Anderson Carlos Marçal, Email: acmarcal@yahoo.com.br.
Charles dos Santos Estevam, Email: cse.ufs@gmail.com.
References
- 1.Powell KF, Caspersen CJ, Christenson GM. Physical activity, exercise and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 2.Raymond MJ, Bramley-Tzerefos RE, Jeffs KJ, Winter A, Holland AE. Systematic review of high-intensity progressive resistance strength training of the lower limb compared with other intensities of strength training in older adults. Arch Phys Med Rehabil. 2013;94:1458–1472. doi: 10.1016/j.apmr.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Feo P. Is high-intensity exercise better than moderate-intensity exercise for weight loss? Nutr Metabol Cardiovasc Dis. 2013;23:1037–1042. doi: 10.1016/j.numecd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Munn J, Herbert RD, Hancock MJ, Gandevia SC. Resistance training for strength: effect of number of sets and contraction speed. Med Sci Sports Exerc. 2005;37:1622–1626. doi: 10.1249/01.mss.0000177583.41245.f8. [DOI] [PubMed] [Google Scholar]
- 5.Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson’s disease: a preliminary study. Parkinsonism Relat D. 2009;15:752–757. doi: 10.1016/j.parkreldis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Pierce K, Rozenek R, Stone MH. Effects of high volume weight training on lactate, heart rate, and perceived exertion. J Strength Cond Res. 1993;7:211–215. [Google Scholar]
- 7.Barauna VG, Junior MLB, Costa Rosa LFBP, Casarini DE, Krieger JE, Oliveira EM. Cardiovascular adaptations in rats submitted to a resistance-training model. Clin Exp Pharmacol Physiol. 2005;32:249–254. doi: 10.1111/j.1440-1681.2005.04180.x. [DOI] [PubMed] [Google Scholar]
- 8.Ceci R, Reyes MBV, Duranti G, Dimauro I, Quaranta F, Pittaluga M, Sabatini S, Caserotti P, Parisi P, Parisi A, Caporossi D. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2014;2:65–72. doi: 10.1016/j.redox.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1(1):15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margonis K, Fatouros IG, Jamurtas ZA, Douroudos I, Nikolaidisb MG, Chatzinikolaou A, Mitrakou A, Mastorakos G, Papassotiriou I, Taxildaris K, Kouretas D, Chatzinikolaou A, Mitrakou A, Mastorakos G, Papassotiriou I, Taxildaris K, Kouretas D. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic Biol Med. 2007;43:901–910. doi: 10.1016/j.freeradbiomed.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Ver. 2010;8:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 14.Thiele JJ, Hsieh SN, Briviba K, Sies H. Protein oxidation in human stratum corneum: susceptibility of keratins to oxidation in vitro and presence of a keratin oxidation gradient in vivo. J Invest Dermatol. 1999;113:335–339. doi: 10.1046/j.1523-1747.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- 15.Todo-Bom A, Pinto AM. Exercício físico. Resposta imunoinflamatória. Rev Port Imunoalergologia. 2007;15:123–133. [Google Scholar]
- 16.Hawke TJ. Muscle stem cell and exercise training. Exerc Sport Sci Rev. 2005;33:63–68. doi: 10.1097/00003677-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Saxton JM, Claxton D, Winter E, Pockley G. Peripheral blood leucocyte functional responses to acute eccentric exercise in humans are influenced by systemic stress, but not by exercise-induced muscle damage. Clin Sci (Lond) 2003;104:69–77. doi: 10.1042/CS20020096. [DOI] [PubMed] [Google Scholar]
- 18.Vierck J, O’Reilly B, Hossner K, Antonio J, Byrne K, Bucci L, Dodson M. Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biol Int. 2000;24:263–272. doi: 10.1006/cbir.2000.0499. [DOI] [PubMed] [Google Scholar]
- 19.Stupka N, Lowther S, Chorneyko K, Bourgeois JM, Hogben C, Tarnopolsky MA. Gender differences in muscle inflammation after eccentric exercice. J Appl Physiol. 2000;89:2325–2332. doi: 10.1152/jappl.2000.89.6.2325. [DOI] [PubMed] [Google Scholar]
- 20.Deminice R, Rosa FT, Franco GS, Jordao AA, Freitas EL. Effects of creatine supplementation on oxidative stress and inflammatory markers after repeated-sprint exercise in humans. Nutrition. 2013;29:1127–1132. doi: 10.1016/j.nut.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Deyne PG. Application of passive stretch and its implications for muscle fibers. Phys Ther. 2001;81:819–827. doi: 10.1093/ptj/81.2.819. [DOI] [PubMed] [Google Scholar]
- 22.Belviranli M, Gökbel H, Okudan N, And Büyükbas S. Effects of grape seed polyphenols on oxidative damage in liver tissue of acutely and chronically exercised rats. Phytother Res. 2013;27:672–677. doi: 10.1002/ptr.4772. [DOI] [PubMed] [Google Scholar]
- 23.Araújo MB, Moura LP, Junior RCV, Junior MC, Dalia RA, Sponton AC, Ribeiro C, Mello MAR. Creatine supplementation and oxidative stress in rat liver. J Int Soc Sports Nutr. 2013;10(54):1550–2783. doi: 10.1186/1550-2783-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wawrzyniak A, Górnicka M, Hamułka J, Gajewska M, Drywień M, Pierzynowska J, Gronowska-Senger A. α-Tocopherol, ascorbic acid, and β-carotene protect against oxidative stress but reveal no direct influence on p53 expression in rats subjected to stress. Nutr Res. 2013;33:868–875. doi: 10.1016/j.nutres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Valko M, Leibfritz D, Moncol JAN, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Mcanulty SR, Mcanulty LS, Nieman DC, Dumke CL, Morrow JD, Utter AC, Henson DA, Proulx WR, George GL. Consumption of blueberry polyphenols reduces exercise-induced oxidative stress compared to vitamin C. Nutr Res. 2004;24:209–221. doi: 10.1016/j.nutres.2003.10.003. [DOI] [Google Scholar]
- 27.Morillas-Ruiz JM, Villegas GJA, López FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr. 2006;25:444–453. doi: 10.1016/j.clnu.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Jówko E, Sacharuk J, Balasinska B, Wilczak J, Charmas M, Ostaszewski P, Charmas R. Effect of a single dose of green tea polyphenols on the blood markers of exercise-induced oxidative stress in soccer players. Int J Sport Nutr Exerc Metab. 2012;22:486–496. doi: 10.1123/ijsnem.22.6.486. [DOI] [PubMed] [Google Scholar]
- 29.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 30.Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- 31.Sousa CMM, Silva HR, Vieira JR, Ayres MCC, Da Costa CLS, Araújo DS, Cavalcante LCD, Barros EDS, Araújo PBM, Brandão MS, Chaves MH. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim Nova. 2007;30:351–355. doi: 10.1590/S0100-40422007000200021. [DOI] [Google Scholar]
- 32.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 33.Lapenna D, Ciofani G, Pierdomenico SD, Giamberardino MA, Cuccurullo F. Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Rad Biol Med. 2001;31:331–335. doi: 10.1016/S0891-5849(01)00584-6. [DOI] [PubMed] [Google Scholar]
- 34.Tamaki T, Uchiyama S, Nakano S. A weightlifiting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Med Sci Sports Exerc. 1992;24:881–886. doi: 10.1249/00005768-199208000-00009. [DOI] [PubMed] [Google Scholar]
- 35.American College of Sport Medicine Position Stand Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 36.Branco ACSC, Diniz MFFM, Almeida RN, Santos HB, Oliveira KM, Ramalho JA, Dantas JG. Biochemical and hematological parameters of Wistar rats and Swiss mice in the professor thomas george animal laboratory. R Bras Ci Saúde. 2011;15:209–214. doi: 10.4034/RBCS/2011.15.02.11. [DOI] [Google Scholar]
- 37.Faure P, Lafond JL: Measurement of plasma sulphydryl and carbonyl groups as a possible indicator of protein oxidation.Birkhäuser Verlag 1995, 237–248.
- 38.Scherer R, Godoy HT. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009;112:654–658. doi: 10.1016/j.foodchem.2008.06.026. [DOI] [Google Scholar]
- 39.Im HW, Suh BS, Lee SE, Kozukue N, Ohnisi-Kameyama M, Levin CE, And Friedman M. Analysis of phenolic compounds by high-performance liquid chromatography and liquid chromatography/mass spectrometry in potato plant flowers, leaves, stems, and tubers and in home-processed potatoes. J Agric Food Chem. 2008;56:3341–3349. doi: 10.1021/jf073476b. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Huang Y. Effects of exercise intensity of lymphocyte apoptosis induced by oxidative stress in men. Eur J Appl Physiol. 2005;95:290–297. doi: 10.1007/s00421-005-0005-8. [DOI] [PubMed] [Google Scholar]
- 41.Bansala P, Paula P, Nayaka PG, Pannakalc ST, Zoud JH, Laatschd H, Priyadarsinie KL, Unnikrishnan MK. Phenolic compounds isolated from Pilea microphylla prevent radiation-induced cellular DNA damage. Acta Pharm Sin B. 2011;4:226–235. doi: 10.1016/j.apsb.2011.10.006. [DOI] [Google Scholar]
- 42.Panza VS, Wazlawik E, Schütz RG, Comin L, Hecht KC, Silva EL. Consumption of green tea favorably affects oxidative stress markers in weight-trained men. Nutrition. 2008;24:433–442. doi: 10.1016/j.nut.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Resnick AZ, Packer K. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:263–357. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez MC, Rosenfeld J, Tarnopolsky MA. Plasma malondialdehyde increases transiently after ischemic forearm exercise. Med Sci Sports Exerc. 2003;35:1859–1865. doi: 10.1249/01.MSS.0000093609.75937.70. [DOI] [PubMed] [Google Scholar]
- 45.Viitala PE, Newhouse IJ, LaVoie N, Gottardo C: Effects of antioxidant vitamin supplementation on resistance exercise induced peroxidation in trained and untrained participants.Lipids Health Dis 2004, 3:14. [DOI] [PMC free article] [PubMed]
- 46.Blainski A, Lopes GC, Mello JCP. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from limonium brasiliense L. Molecules. 2013;18:6852–6865. doi: 10.3390/molecules18066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compound. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- 48.Vaher M, Matso K, Levandi T, Helmja K, Kaljurand M. Phenolic compounds and the antioxidant activity of the bran, flour and whole grain of different wheat varieties. Procedia Chem. 2010;2:76–82. doi: 10.1016/j.proche.2009.12.013. [DOI] [Google Scholar]
- 49.Ugaz, OL. Investigacion Fitoquımica: Pontificia Universidad Cattolica del Peru, Lima-Fondo.Editorial 1994.
- 50.Dias AS, Lima ACB, Santos ALML, Rabelo TK, Serafini MR, Andrade CR, Fernandes XA, Moreira JCF, Gelain DP, Estevam CS, Araujo BS. Redox properties of Abarema cochliacarpos (Gomes) Barneby & Grime (Fabaceae) stem bark etanol extract and fractions. Formerly Nat Prod Lett. 2012;10:1–5. doi: 10.1080/14786419.2012.722083. [DOI] [PubMed] [Google Scholar]
- 51.Van Acker SA, Van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ, Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radical Biol Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 52.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Li HB, Wong CC, Cheng KW, Chen F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. Food Sci Technol. 2008;41:385–390. [Google Scholar]
- 55.Melo JG, Araújo TAS, Castro VTNA, Cabral DLV, Rodrigues MD, Nascimento SC, Amorim ELC, Albuquerque UP. Antiproliferative activity, antioxidant capacity and tannin content in plants of Semi-Arid Northeastern Brazil. Molecules. 2010;15(12):8534–8542. doi: 10.3390/molecules15128534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moritani T, Vries HÁ DE. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med Rehabil. 2002;58:115–130. [PubMed] [Google Scholar]
- 57.Silva LCN, Silva Júnior CA, Souza RM, Macedo AJ, Silva MV, Correia MTS. Comparative analysis of the antioxidant and DNA protection capacities of Anadenanthera colubrina, Libidibia ferrea and Pityrocarpa moniliformis fruits. Food Chem Toxicol. 2011;49:2222–2228. doi: 10.1016/j.fct.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 58.Rêgo Júnior NO, Fernandez LG, Castro RD, Silva LC, Gualberto AS, Pereira MLA. Bioactive compounds and antioxidant activity of crude extracts of brushwood vegetable species. Braz J Food Technol. 2011;14:50–57. doi: 10.4260/BJFT2011140100007. [DOI] [Google Scholar]
- 59.Araujo SS, Mesquita TRR, Santos RM, Oliveira JEL, Alves ARA: Anthropometric, functional, and metabolic profiles of soccer players.J Exerc Physiol Online 2012, 15:6.
- 60.Balogh N. Biochemical and antioxidant changes in plasma and erythrocytes of pentathlon horses before and after exercise. Vet Clin Pathol. 2001;30:214–218. doi: 10.1111/j.1939-165X.2001.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 61.Clarkson PM, Hubal ML. Exercise induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81(Suppl, 11):52–69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 62.Tee JC, Bosch AN, Lambert MI. Metabolic consequences of exercise -induced muscle damage. Sports Med. 2007;10:827–836. doi: 10.2165/00007256-200737100-00001. [DOI] [PubMed] [Google Scholar]
- 63.Nottle C, Nosaka K. The magnitude of muscle damage induced by downhill backward walking. J Sci Med Sport. 2005;3:264–273. doi: 10.1016/S1440-2440(05)80037-4. [DOI] [PubMed] [Google Scholar]
- 64.Aguilo A, Tauler P, Fuentespina E, Tur JA, Cordova A, Pons A. Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol Behav. 2005;84:1–7. doi: 10.1016/j.physbeh.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 65.Mastaloudis A, Yu TW, O’Donnell RP, Frei B, Dashwood RH, Traber MG. Endurance exercise results in DNA damage as detected by the comet assay. Free Radic Biol Med. 2004;36:966–975. doi: 10.1016/j.freeradbiomed.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 66.Oztürk R, Ayar-Kayali H, Tarhan L. Characterization of the antioxidant properties of seeds and skins in selected Turkish grapes. Asian J Chem. 2008;20:50–62. [Google Scholar]
- 67.Tongul B, Tarhan L. The effect of menadione-induced oxidative stress on the in vivo reactive oxygen species and antioxidant response system of Phanerochaete chrysosporium. Process Biochem. 2014;49:195–202. doi: 10.1016/j.procbio.2013.11.004. [DOI] [Google Scholar]
- 68.Kerksick CM, Kreider RB, Willoughby DS. Intramuscular adaptations to eccentric exercise and antioxidante supplementation. Amino Acids. 2010;39:219–232. doi: 10.1007/s00726-009-0432-7. [DOI] [PubMed] [Google Scholar]
- 69.Watson TA, Callister R, Taylor RD, Sibbritt DW, MacDonald-Wicks LK, Garg ML. Antioxidant restriction and oxidative stress in short-duration exhaustive exercise. Med Sci Sports Exerc. 2005;37:63–71. doi: 10.1249/01.MSS.0000150016.46508.A1. [DOI] [PubMed] [Google Scholar]


