Abstract
Objective
To assess changes in total adipose tissue (TAT), subcutaneous (SAT), visceral (VAT), and intermuscular (IMAT) by whole-body MRI before surgery, at 12 months and 24 months post-surgery in a subset of participants of the Longitudinal Assessment of Bariatric Surgery-2.
Design and Methods
From 0 to 12 months, n=20F and 3M; from 12 to 24 months, n=42F and 7M. Paired t-tests and GLM repeated measures examined changes in TAT, SAT, VAT, and IMAT at 12 and 24 months, with sex and age as covariates.
Results
Changes from 0 to 12 months, included weight (−41.9±12.1kg; −36%), TAT (−33.5±9.6kg; −56%), SAT (−29.2±8.2kg; −55%), VAT (−3.3±1.6kg; −73%), and IMAT (−0.99±0.68kg; −50%), all p<0.001. In females, from 12 to 24 months, despite relative weight stability (−1.8±6.5kg, −2%; p=0.085), VAT (−0.5±0.7kg; −30%; p<0.001) and IMAT (−0.2±0.4kg; −14%; p=0.012) decreased further. In males from 12 to 24 months, weight increased (5.1±5.2kg; 6%; p=0.04) with no significant changes in TAT or sub-depots.
Conclusions
Bariatric surgery continues to induce favorable changes in body composition, i.e., persistent adipose tissue loss at 24 months in the absence of further significant weight loss.
Keywords: adipose tissue depot, body composition, LABS-2, MRI, obesity surgery
Introduction
Bariatric surgery procedures result in significant and rapid weight loss of which fat mass or adipose tissue mass accounts for some 80% of the weight lost (1). Insofar as different adipose tissue depots may contribute differently to obesity related comorbidities (e.g., insulin resistance and cardiovascular disease), it remains unclear how adipose tissue depots respond to bariatric surgery (2). Most studies to date have focused on visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) findings from a single abdominal slice, with the assumption that the selected slice is an appropriate surrogate measure of total VAT and total SAT. In some instances, studies lacked baseline data and reported on post-surgery measures only (3), the interval between pre- and post-surgery measures was variable (1 month to 25 months follow-up) (3, 4), or there is a lack of agreement in findings with respect to short and longer-term reductions in the different AT depots (4, 5). Lacking are studies that have characterized whole body SAT, VAT and intermuscular AT (IMAT) with measures acquired before surgery and at intervals following bariatric surgery.
Significant changes in adipose tissue depots have been reported by CT (6, 7, 8) and MRI (9, 10) at 3 months to 12 months post-surgery. At 12 weeks post gastric banding, losses were observed in abdominal subcutaneous fat (−20%), VAT (−15%) proportional to VAT at baseline (10), hepatic fat (−19%) (10). Weiss et al (6) reported reductions of 35% in VAT and 32% in SAT from a single abdominal slice at 6 months post-surgery. In this study, laparoscopic bariatric surgery procedures included Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric band (LB), duodenal switch (DS), and sleeve gastrectomy (SG). Carroll et al (7) calculated total VAT volume (by CT) from 8 abdominal slices that decreased by 22% at 6 months post-LB surgery. Using a single abdominal slice at 3 and 12 months post-LB surgery, VAT decreased by 20% and 34%, respectively (9). Korner et al, (3) using whole body MRI in post-surgery weight stable female patients (at 19 to 25 months post-LB and RYGB surgery), found that VAT was 43% lower compared to non-surgery height, weight, and age matched controls. Clearly, there are reductions in all measured adipose tissue depots; however, the degree of change is highly variable based on these published studies.
Obesity comorbidities such as diabetes, hyperlipidemia, hypertension, and obstructive sleep apnea ameliorate in a significant portion of bariatric surgery (1) patients post-surgery compared to lifestyle modifications or pharmacological therapy. A clear understanding of the quantitative changes that occur in adipose tissue depots would be a first step to understand mechanisms underlying the benefits of bariatric surgery.
The aim of this study was to quantify TAT and sub-depots, namely SAT, VAT, and IMAT, using whole body MRI prior to bariatric surgery and to describe changes in these sub-depots at 12 months and 24 months following surgery. We hypothesized that the distribution of TAT in SAT, VAT, and IMAT is different from before (T0) to 12 months (T12) and 24 months (T24) with weight loss following bariatric surgery. A secondary aim was to compare sub-depots in surgery patients at T12 and T24 to healthy non-surgery controls.
Methods and procedures
Surgery Participants
Between November 2006 and February 2009, participants (n=64) enrolled in the Longitudinal Assessment of Bariatric Surgery 2 (LABS-2) at the Weill Cornell Medical College and the University of Pittsburgh Medical Center sites were invited to participate in this ancillary study (11, 12, 13). Due to a delay in recruiting relative to LABS-2, recruitment was extended through December 2009, during which an additional 41 Non-LABS-2 participants were enrolled for a total of 105 ancillary study participants, 53 from Weill Cornell and 52 from Pittsburgh. 5 patients enrolled in the study did not proceed to have surgery performed due to health insurance coverage issues.
Measurements for the Weill Cornell participants were acquired at St. Luke’s-Roosevelt Hospital. Baseline measures were collected on average 1.3 wk prior to surgery (T0) (Range: 0 to 11 wk). Postoperative measures were collected approximately 12 months later (T12) (Range: 10.6 to 17.7 mo) and 24 months later (T24). Surgery types included: Roux-en-Y gastric bypass (RYGB), laparoscopic adjustable gastric band (LB), biliopancreatic diversion (BPD), duodenal switch (DS), and sleeve gastrectomy (SG). Exclusions for entry into the study included pregnancy, claustrophobia, abnormal thyroid or cortisol levels, and self-reported use of medications known to influence body composition (such as diuretics and corticosteroids) at time of entry.
Non-Surgical Controls
The surgical group was compared at one and two years post-surgery to a non-surgical control group from a study whose design and primary findings were previously published (14). The multiethnic control group included 207 women and 87 men with mean BMI 26.0±5.2 kg/m2, age 41.7±14.0 years, and weight 73.0±16.4 kg.
Body Composition Measures
Subjects reported in the early morning after an 8 hour fast. Subjects were weighed in a hospital gown to the nearest 0.1 kg (Weight Tronix, New York, NY; and BWB 800 Tanita Corp.-Pittsburgh) and height to the nearest 0.5 cm using a stadiometer (Holtain; Crosswell, Wales-New York; and Perspective Enterprises, Portage, MI-Pittsburgh).
Fat-free mass and fat mass were measured using a modified 3-compartment model (Silva 2004): Fat-3C (kg)=2.122*(BW/D)−0.779*TBW−1.356*BW, where BW is body weight in kg, D is body density derived from BodPod, and TBW is total body water in kg. TBW was measured using a D2O (~1 g/kg) oral dose administered after a fasting venous blood sample was taken from an antecubital vein. After three hours, a second fasting blood sample was obtained. Body density was measured using BOD POD (Cosmed, Chicago, IL; software version 2.3) (15, 16). Further details regarding body composition assessments in this cohort were recently published (17).
Magnetic Resonance Imaging
Total AT (TAT) including total SAT, VAT, and IMAT were measured by using whole-body multislice MRI as previously described (18, 19). Subjects at both sites (New York and Pittsburgh) were placed on a 1.5T scanner platform (GE, 6X Horizon, Milwaukee, WI) with their arms extended above their heads. The protocol involved the acquisition of ≈40 axial images, 10 mm in thickness and at 40mm intervals across the whole body. SliceOmatic 4.2 image analysis software (Tomovision, Montreal, CA) was used to analyze images on a PC workstation. Estimates of MRI volume were converted to mass by using the assumed density of 0.92 kg/L for AT(20). All scans were read by the same technician at the New York Obesity Nutrition Research Center. The technical errors for 3 repeated readings of the same scan by the same observer for SAT, VAT, and IMAT volumes in our laboratory were 0.96%, 1.97%, and 0.65%, respectively.
Statistical Analysis
Descriptive statistics, expressed as means ± SD, were calculated for subject baseline characteristics and for changes from T0 to T12 and from T12 to T24. The paired t-test was used to test the null hypothesis that the mean change from T0 to T12 and from T12 to T24 was equal to zero. Analyses were performed for males and females separately. T-tests were used to compare TAT, SAT, IMAT, and VAT between surgery group and controls. General linear models verified statistical significant differences in TAT, SAT, VAT, and IMAT between the post-bariatric surgery group and the control group after adjusting for age, sex, height, and weight.
General linear models were used to determine the contribution of age and sex in accounting for differences in TAT, SAT, VAT, and IMAT in the surgery patients. Subjects who had T0 and T12 measurements (N=23) or T12 and T24 (N=49) measurements were included in the analyses. Tests for preferential VAT loss were performed based on the allometric model described by Hallgreen and Hall (21). Regression models were used to investigate the relation of VAT and IMAT to TAT after weight loss. Data were analyzed using SPSS for Windows v15 (SPSS, Chicago, IL). Statistical significance was set at p< 0.05, two-tailed.
Results
Baseline
Baseline characteristics of the total study cohort (n=100) who had bariatric surgery are presented in Table 1 by sex and surgery type. Prior to surgery, 71 participants had body sizes that extended beyond the MRI field of view and did not have an MRI performed. A total of 29 subjects had an MRI at T0, from which 23 (3 M, 20 F) had an MRI at T12; 49 (7 M, 42 F) had MRI at T12 and 24 months (T24) (81% RYGB, 19% other); and 17 (1M, 16 F) had MRI at T0, T12, and T24. From the main study cohort, subjects with an MRI at T0 weighed less (p<0.0001) and had a lower body mass index (BMI; p<0.0001) than those with no MRI at T0 (Table 1). Mean %Fat for women and men at T0 was 47% and 54%, respectively and mean BMI was 45.6 kg/cm2. All males and 70% of the females had RYGB surgery.
Table 1.
Pre-surgery characteristics of study participants
| N | Mean ± SD | Males | Females | |
|---|---|---|---|---|
|
| ||||
| Demographic characteristics | 100 | 17% | 83% | |
| Race | ||||
| Caucasian | 68 | 12 (70%) | 56 (67.5%) | |
| African American | 16 | 1 (5.9%) | 15 (18.1%) | |
| Hispanics | 15 | 4 (23.5%) | 11 (13.3%) | |
| Asian | 1 | - | 1 (1.2%) | |
| Surgery type | ||||
| RYGB | 72 | 14 (82.4%) | 58 (69.9%) | |
| LB | 11 | 1 (5.9%) | 10 (12%) | |
| BPD/DS | 4 | - | 4 (4.8%) | |
| SG | 13 | 2 (11.8%) | 11 (13.3%) | |
| Age (y) | 100 | 44.2 ± 11.6 | 45.5 ± 14.2 | 43.9 ± 11.0 |
| Height (cm) | 100 | 167.6 ± 8.3 | 178.2 ± 6.0 | 165.4 ± 7.0 |
| Weight (kg) | 100 | 128.6 ± 22.5 | 144.6 ± 21.5 | 125.4 ± 21.4 |
| Fit in MRI | 29 | 115.8 ± 13.2 | 130.0 ± 1.4 | 114.1 ± 13.0 |
| Did not fit in MRI | 71 | 133.9 ± 23.4 | 147.7 ± 22.5 | 130.5 ± 22.5 |
| BMI (kg/m2) | 100 | 45.7 ± 6.7 | 45.4 ± 5.8 | 45.8 ± 6.8 |
| Fit in MRI | 29 | 42.1±4.4 | 41.2 ± 0.8 | 42.2 ± 4.7 |
| Did not fit in MRI | 71 | 47.2±6.9 | 46.4 ± 6.0 | 47.4 ± 7.1 |
| % Fat (3C) †† | 97 | 52.5 ± 6.1 | 46.8 ± 8.5 | 53.7 ± 4.7 |
| Body composition† | ||||
| TAT (kg) | 29 | 59.5 ± 8.0 | 55.5 ± 5.5 | 59.9 ± 8.2 |
| SAT (kg) | 29 | 52.8 ± 7.7 | 46.3 ± 6.1 | 53.5 ± 7.6 |
| VAT (kg) | 29 | 4.7 ± 1.8 | 7.2 ± 2.5 | 4.5 ± 1.8 |
| IMAT (kg) | 29 | 2.0 ± 0.6 | 2.3 ± 0.3 | 2.0 ± 0.6 |
RYGB Roux-en-Y gastric bypass, LB laparoscopic adjustable gastric band, BPD biliopancreatic diversion, DS duodenal switch, SG sleeve gastrectomy
%FAT (3C) = 2.122*(bodpod weight/density)−0.779*0.9937*TBW−1.356*bodpod weight
97 subjects completed the densitometry measurement for the 3C model
29 subjects had whole body MRIbefore surgery
TAT total adipose tissue, SAT subcutaneous adipose tissue, VAT visceral adipose tissue, IMAT intermuscular adipose tissue
Table 2 shows baseline characteristics of subjects who had MRI at T0 and T12 (N=23) or at T12 and T24 (N=49). Graphical comparisons between the two groups showed that those who were larger at T0 and did not fit in MRI had a similar weight loss pattern to those who fit in MRI. Percentage fat based on a three compartment model (%Fat-3C), was higher for males and females who did not fit in the MRI at T0.
Table 2.
Pre-surgery characteristics of participants who had MRI at T0 and T12, and those who had MRI at T12 and T24.
| N=23* | N=49* | |||
|---|---|---|---|---|
|
| ||||
| Male (N=3) | Female (N=20) | Male (N=7) | Female (N=42) | |
| Age | 45.0 ± 20.2 | 41.6 ± 8.1 | 42.3 ± 16.8 | 43.2 ± 10.0 |
| Height (cm) | 177.7± 2.7 | 164.2 ± 7.6 | 178.1 ± 6.5 | 165.8 ± 6.3 |
| Weight (kg) | 130.0 ± 1.4 | 113.9± 14.2 | 141.5 ± 14.9 | 124.2 ± 19.3 |
| BMI (kg/m2) | 41.2 ± 0.8 | 42.3 ± 4.9 | 44.7 ± 5.4 | 45.1 ± 5.7 |
| % Fat (3C) | 41.6 ± 4.3 | 51.7 ± 4.4† | 50.1 ± 4.6 | 53.7 ± 4.5†† |
| Surgery Type | N (%) | N (%) | N (%) | N (%) |
| RYGB | 3 (100%) | 16 (80%) | 6 (85.7%) | 33 (78.6%) |
| LB | - | - | - | 1 (2.4%) |
| BPD/DS | - | 2 (10%) | - | 5 (11.9%) |
| SG | - | 2 (10%) | 1 (14.3%) | 3 (7.1%) |
Values are mean ± SD;
N=18 females;
N=42 females
%FAT (3C) = 2.122*(weight/density)−0.779*0.9937*TBW−1.356*weight
T0 pre-surgery, T12 12 months, T24 24 months, RYGB Roux-en-Y gastric bypass, LB laparoscopic adjustable gastric band, BPD biliopancreatic diversion, DS duodenal switch, SG sleeve gastrectomy
Changes in total adipose tissue and sub-depots
Pre-surgery (T0) to 12 months post-surgery (T12)
Body weight, BMI, and body composition characteristics at T0 and T12 by surgery type are shown in Table 3. All males and 80% of females had RYGB. Females in the RYGB group weighed the most at baseline (115 kg, SD 14.9) and weighed less (69.8 kg, SD 11.1) at T12, compared to females who had LB and SG. Among males and females, absolute reductions occurred in body weight, TAT, and all TAT sub-depots from T0 to T12 (females p<0.001, males p<0.01) (Table 4).
Table 3.
Adipose tissue distribution by surgery procedure before surgery (T0) and 12 months post-surgery (T12).
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| RYGB (N=16) | LB (N=2) | SG (N=2) | RYGB (N=3) | |||||
|
| ||||||||
| T0 | T12 | T0 | T12 | T0 | T12 | T0 | T12 | |
| Weight (kg) | 115.0 ± 14.9 | 69.8 ± 11.1 | 107.8 ± 16.1 | 88.4 ± 12.1 | 110.8 ± 10.8 | 81.6 ± 8.1 | 130.0 ± 1.4 | 82.4 ± 4.9 |
| BMI (kg/m2) | 43.4 ± 4.8 | 26.2 ± 3.4 | 38.3 ± 0.8 | 31.4 ±1.1 | 37.5 ± 3.7 | 27.5 ± 3.0 | 41.2 ± 0.82 | 26.2 ± 1.2 |
| % Fat (3C)* | 52.4 ± 4.1† | 31.0 ± 6.2 | 50.0 ± 1.9 | 44.0 ± 1.6 | 48.6 ± 9.1 | 40.1†† | 41.6 ± 4.3 | 18.7 ± 7.3 |
| TAT (kg) | 60.9 ± 8.8 | 25.1 ± 7.9 | 57.8 ± 9.9 | 41.4 ± 4.3 | 54.6 ± 13.9 | 28.2 ± 10.3 | 55.5 ± 5.5 | 18.3 ± 4.3 |
| SAT (kg) | 54.5 ± 8.0 | 22.9 ± 7.3 | 52.1 ± 7.7 | 37.4 ± 4.2 | 50.9 ± 13.9 | 26.7 ± 10.2 | 46.3 ± 6.1 | 16.3 ± 3.5 |
| VAT (kg) | 4.4 ± 1.5 | 1.2 ± 0.7 | 3.9 ± 1.7 | 2.5 ± 0.5 | 2.0 ± 0.5 | 0.3 ± 0.2 | 6.9 ± 0.7 | 1.1 ± 0.7 |
| IMAT (kg) | 2.0 ± 0.7 | 1.0 ± 0.4 | 1.7 ± 0.5 | 1.5 ± 0.4 | 1.8 ± 0.5 | 1.2 ± 0.1 | 2.3 ± 0.3 | 0.9 ± 0.2 |
| %SAT | 89.3 ± 2.6 | 91.3 ± 3.2 | 90.4 ± 2.1 | 90.3 ± 0.7 | 93.0 ± 1.8 | 94.3 ± 1.8 | 83.2 ± 3.0 | 89.3 ± 2.8 |
| %VAT | 7.3 ± 2.2 | 4.6 ± 2.4 | 6.6 ± 1.8 | 6.0 ± 0.7 | 3.6 ± 0.1 | 1.2 ± 0.1 | 12.6 ± 2.8 | 5.7 ± 2.3 |
| %IMAT | 3.3 ± 0.9 | 4.1 ± 1.5 | 3.0 ± 0.3 | 3.8 ± 1.4 | 3.4 ± 1.9 | 4.5 ± 2.0 | 4.2 ± 0.4 | 5.0 ± 0.6 |
RYGB Roux-en-Y gastric bypass, LB laparoscopic adjustable gastric band, SG sleeve gastrectomy
TAT total adipose tissue, SAT subcutaneous adipose tissue, VAT visceral adipose tissue, IMAT intermuscular adipose tissue
Percentages are sub-depots as percentage of total adipose tissue: %SAT=(SAT/TAT)*100, %VAT=(VAT/TAT)*100, %IMAT=(IMAT/TAT)*100
%FAT (3C) = 2.122*(weight/density)−0.779*0.9937*TBW−1.356* weight
All values are mean ± SD
N=14 females
N=1 female
Table 4.
Changes in adipose tissue distribution at 12 months post-surgery and compared to controls.
| Surgery Females (N=20)
| |||||||
|---|---|---|---|---|---|---|---|
| T0 | T12 | Changes† | P-value† | Percent change† | Control Females (N=207) | P-value‡ | |
| Weight (kg) | 113.9 ± 14.2 | 72.8 ± 12.2 | −41.1 ± 12.8 | <0.001 | −36.1% | 68.7 ± 16.3 | 0.28 |
| TAT (kg) | 60.0 ± 9.0 | 27.1 ± 9.0 | −32.9 ± 9.9 | <0.001 | −54.9% | 25.9 ± 11.9 | 0.68 |
| SAT (kg) | 53.9 ± 8.1 | 24.8 ± 8.3 | −29.1 ± 8.5 | <0.001 | −54.0% | 22.1 ± 8.7 | 0.58 |
| VAT (kg) | 4.1 ± 1.6 | 1.3 ± 0.8 | −2.9 ± 1.3 | <0.001 | −69.8% | 1.49 ± 1.2 | 0.36 |
| IMAT (kg) | 2.0 ± 0.6 | 1.1 ± 0.4 | −0.9 ± 0.7 | <0.001 | −46.8% | 1.0 ± 0.7 | 0.92 |
|
| |||||||
| Surgery Males (N=3) | Controls Males (N=87) | ||||||
|
| |||||||
| Weight (kg) | 130.0 ± 1.4 | 82.4 ± 4.9 | −47.6 ± 4.3 | 0.003 | −36.6% | 83.1 ± 11.2 | 0.92 |
| TAT (kg) | 55.5 ± 5.5 | 18.3 ± 4.3 | −37.2 ± 7.1 | 0.012 | −67.0% | 20.7 ± 7.3 | 0.59 |
| SAT (kg) | 46.3 ± 6.1 | 16.3 ± 3.4 | −29.9 ± 7.0 | 0.018 | −64.6% | 17.5 ± 5.7 | 0.72 |
| VAT (kg) | 6.9 ± 0.7 | 1.1 ± 0.7 | −5.8 ± 0.8 | 0.006 | −84.1% | 2.4 ± 1.7 | 0.23 |
| IMAT (kg) | 2.3 ± 0.3 | 0.9 ± 0.2 | −1.4 ± 0.3 | 0.012 | −60.9% | 0.8 ± 0.5 | 0.69 |
Values are mean ± SD
TAT total adipose tissue, SAT subcutaneous adipose tissue, VAT visceral adipose tissue, IMAT intermuscular adipose tissue Changes and percent change are for pre-surgery (T0) to 12 months post-surgery (T12).
Baseline (T0) versus 12 months post-surgery (T12)
12 months (T12) versus Controls
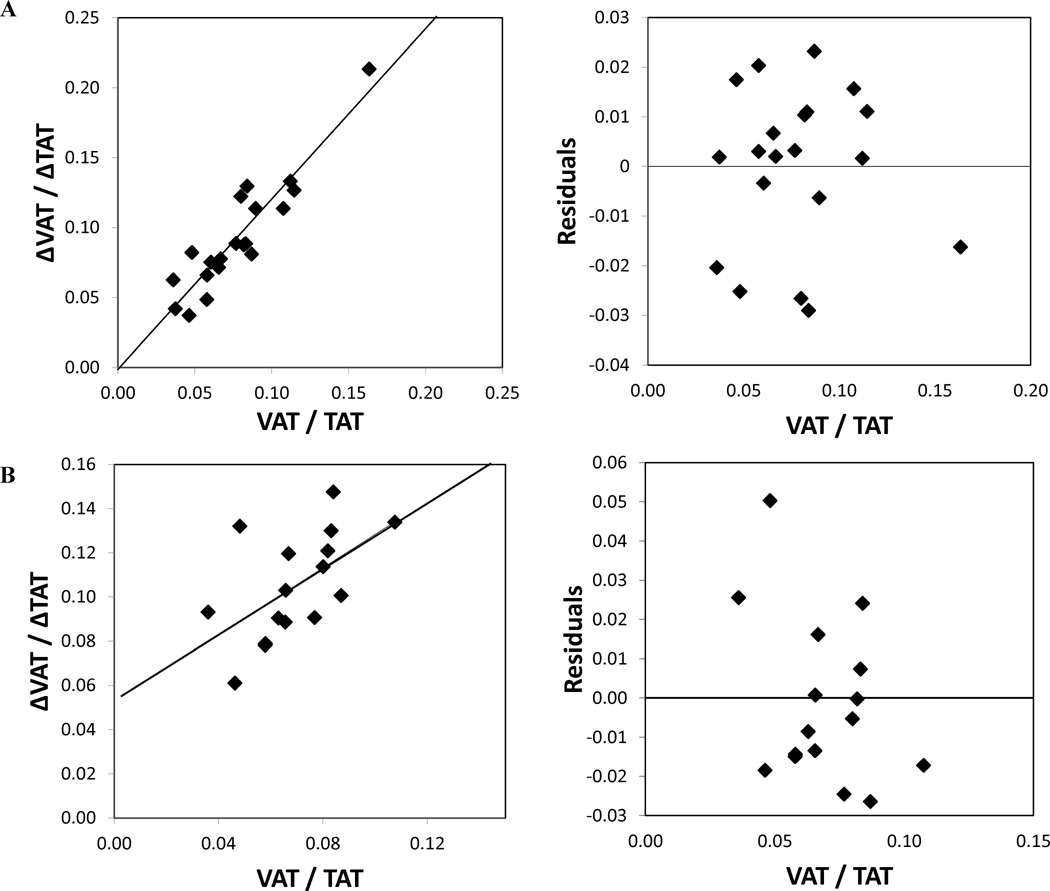
General linear models by sex adjusted for age showed significant losses in TAT (−35.1kg; p<0.001), SAT (−29.54kg; p<0.001), and IMAT. With respect to VAT, controlling for TAT, males had greater VAT at T0 compared to females (6.9kg vs. 4.14kg) and males lost more VAT at T12 (−5.8kg, −84%) compared to females (−2.9kg, −70%; p=0.002). Males had greater IMAT than females at T0.Using GLM, for subjects who had MRI at T0 and T12, sex was a significant determinant of VAT (p=0.002), such that males lost significantly more VAT than females. Age was a significant predictor of SAT (p=0.039) such that older subjects had higher SAT. The change in VAT relative to TAT from T0 to T12 showed k=1.3± 0.03 (95% CI 1.20–1.33) when the Hallgreen and Hall model (19) was applied (Figure 1A).
Figure 1.
(A) The left panel shows change in VAT from baseline to 12 months relative to the change in TAT from baseline to 12 months (ΔVAT/ΔTAT) versus the baseline visceral fat mass divided by the total fat mass (VAT/TAT). The line represents the best fit allometric relationship to the weight loss data with k = 1.3 ± 0.03 and R2 = 0.99. The right panel shows residuals of the best fit allometric model versus baseline VAT to TAT ratio. (B) The left panel shows change in VAT from baseline to 24 months relative to the change in TAT from baseline to 24 months (ΔVAT/ΔTAT) versus the baseline visceral fat mass divided by the total fat mass (VAT/TAT). The line represents the best fit allometric relationship to the weight loss data with k = 1.5 ± 0.06 and R2 = 0.98 The right panel shows residuals of the best fit allometric model versus baseline VAT to TAT ratio.
T12 to 24 months post-surgery (T24)
Body composition characteristics at T12 and T24 by surgery type are shown in Table 5. In females, those who had RYGB or SG, lost more weight and fat than females who had LB or BPD/DS. In males, those who had RYGB lost more weight and fat than males who had SG.
Table 5.
Adipose tissue distribution by surgery type at 12 and 24 months post-surgery.
| Females (n=42) | Males (n=7) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| RYGB (N=33) | LB (N=5) | BPD/DS (N=1) | SG (N=3) | RYGB (N=6) | SG (N=1) | |
|
T12
| ||||||
| Weight (kg) | 80.3 ± 17.9 | 98.4 ± 18.2 | 88.4 | 83.2 ± 3.9 | 90.7 ± 10.1 | 100.5 |
| BMI | 29.0 ± 5.7 | 34.6 ± 4.3 | 33.0 | 30.4 ± 0.7 | 28.3 ± 3.4 | 33.2 |
| % Fat (3C)* | 35.1 ± 8.6 | 47.5 ± 3.5 | 46.7 | 43.2 ± 2.7 | 26.5 ± 8.9 | 34.7 |
| TAT (kg) | 32.6 ± 13.8 | 47.8 ± 10.9 | 37.9 | 36.8 ± 1.4 | 26.7 ± 8.5 | 38.2 |
| SAT (kg) | 29.9 ± 12.6 | 43.3 ± 10.5 | 36.2 | 33.6 ± 0.7 | 22.8 ± 6.0 | 33.9 |
| VAT (kg) | 1.6 ± 1.4 | 2.8 ± 0.5 | 1.1 | 2.0 ± 1.8 | 2.3 ± 1.8 | 3.2 |
| IMAT (kg) | 1.1 ± 0.6 | 1.7 ± 0.5 | 0.6 | 1.2 ± 0.2 | 1.6 ± 0.9 | 1.1 |
| %SAT | 91.8 ± 3.4 | 90.4 ± 1.2 | 95.7 | 91.6 ± 5.1 | 86.7 ± 5.9 | 88.7 |
| %VAT | 4.6 ± 2.8 | 6.0 ± 1.4 | 2.9 | 5.3 ± 4.7 | 7.5 ± 4.7 | 8.4 |
| %IMAT | 3.5 ± 1.4 | 3.6 ± 1.1 | 1.5 | 3.1 ± 0.5 | 5.8 ± 1.7 | 2.9 |
|
| ||||||
|
T24
| ||||||
| Weight (kg) | 78.8 ± 19.2 | 95.2 ± 21.1 | 84.0 | 81.2± 14.5 | 94.3 ± 12.5 | 115.1 |
| BMI | 28.7 ± 6.3 | 33.9 ± 4.9 | 32.2 | 30.0± 3.3 | 29.8 ± 4.6 | 38.0 |
| % Fat (3C)* | 35.9 ± 9.2† | 45.5 ± 6.6 | 35.9 | 40.6± 10.9 | 24.7 ± 13.4†† | 42.7 |
| TAT (kg) | 30.7 ± 14.1 | 46.1 ± 13.8 | 34.0 | 35.4± 11.5 | 29.1 ± 10.4 | 51.7 |
| SAT (kg) | 28.7 ± 13.2 | 42.2 ± 13.1 | 33.2 | 32.6 ± 11.3 | 24.6 ± 7.2 | 46.8 |
| VAT (kg) | 1.1 ± 1.2 | 2.4 ± 0.6 | 0.4 | 1.6 ± 1.8 | 2.7± 2.6 | 3.4 |
| IMAT (kg) | 0.9 ± 0.4 | 1.5 ± 0.6 | 0.5 | 1.2 ± 0.3 | 1.9 ± 1.2 | 1.5 |
| %SAT | 93.8 ± 3.2 | 91.3 ± 1.7 | 97.6 | 92.2 ± 5.5 | 86.2 ± 8.0 | 90.5 |
| %VAT | 3.1 ± 2.6 | 5.4 ± 1.4 | 1.1 | 4.4 ± 5.1 | 7.8 ± 5.9 | 6.7 |
| %IMAT | 3.1 ± 1.1 | 3.3 ± 0.9 | 1.3 | 3.4± 0.5 | 6.0 ± 2.2 | 2.8 |
Values are mean ± SD;
N=32 females;
N=5 males
RYGB Roux-en-Y gastric bypass, LB laparoscopic adjustable gastric band, BPD bilio pancreatic diversion, DS duodenal switch, SG sleeve gastrectomy
TAT total adipose tissue, SAT subcutaneous adipose tissue, VAT visceral adipose tissue, IMAT intermuscular adipose tissue
Percentages adipose tissue sub-depots as percentage of total adipose issue: %SAT=SAT/TAT*100, %VAT=VAT/TAT*100, %IMAT=IMAT/TAT*100
%FAT (3C) = 2.122*(weight/density)−0.779*0.9937*TBW−1.356*weight
The pattern of weight change from T12 to T24 differed by sex (Table 6). In females (n=42), despite a small and not statistically reliable change in weight (−1.8±6.5kg; p=0.085), there were further reductions in VAT (−30.1%; −0.5±0.7kg; p=0.001) and IMAT (−13.6%; −0.2±0.4kg; p=0.012), with non-statistically reliable changes in TAT (−5.5%, −1.9±6.3kg; p=0.059. In males (n=7), body weight increased (+5.5%; +5.1±5.2kg; p=0.039) with no significant change in TAT (+14.4%; p=0.078) and no changes in sub-depots (+13.8% SAT, +14.8% VAT, +17.9% IMAT; Table 6).
Table 6.
Adipose tissue depots at 12 and 24 months post-surgery and in Controls
| Surgery Females (N=42)
| |||||||
|---|---|---|---|---|---|---|---|
| T12 | T24 | Changes† | P-value† | Percent change† | Controls | P-value‡ | |
| Weight (kg) | 82.8 ± 17.9 | 81.0 ± 19.3 | −1.8 ± 6.5 | 0.085 | −2.2% | 68.7 ± 16.3 | <0.0001 |
| TAT (kg) | 34.9 ± 13.6 | 33.0 ± 14.4 | −1.9 ± 6.3 | 0.059 | −5.4% | 25.9 ± 11.9 | 0.001 |
| SAT (kg) | 31.9 ± 12.4 | 30.7 ± 13.4 | −1.2 ± 5.6 | 0.171 | −3.7% | 22.1 ± 8.7 | <0.0001 |
| VAT (kg) | 1.8 ± 1.3 | 1.2 ± 1.3 | −0.5 ± 0.7 | <0.001 | −30.1% | 1.49 ± 1.2 | 0.21 |
| IMAT (kg) | 1.2 ± 0.6 | 1.0 ± 0.5 | −0.2 ± 0.4 | 0.012 | −13.6% | 1.0 ± 0.7 | 0.71 |
| Surgery Males (N=7) | |||||||
| Weight (kg) | 92.1 ± 9.9 | 97.2 ± 13.9 | 5.1 ± 5.2 | 0.039 | 5.6% | 83.1 ± 11.2 | 0.002 |
| TAT (kg) | 28.4 ± 8.9 | 32.4 ± 12.8 | 4.0 ± 5.0 | 0.078 | 14.1% | 20.7 ± 7.3 | <0.0001 |
| SAT (kg) | 24.4 ± 6.9 | 27.7 ± 10.7 | 3.4 ± 4.6 | 0.099 | 13.8% | 17.5 ± 5.7 | <0.0001 |
| VAT (kg) | 2.5 ± 1.7 | 2.8 ± 2.4 | 0.4 ± 1.2 | 0.461 | 14.8% | 2.4 ± 1.7 | 0.52 |
| IMAT (kg) | 1.5 ± 0.8 | 1.8 ± 1.1 | 0.3 ± 0.5 | 0.158 | 17.9% | 0.8 ± 0.5 | <0.0001 |
Values are mean ± SD
TAT total adipose tissue, SAT subcutaneous adipose tissue, VAT visceral adipose tissue, IMAT intermuscular adipose tissue
12 months (T12) versus 24 months post-surgery (T24)
24 months (T24) versus Control
From T12 to T24, the analyses revealed significant sex differences in change in TAT (p=0.025), SAT (p=0.05), VAT (p=0.011), and IMAT (p=0.007) for repeated measures over time, with males gaining AT in all depots and females showing AT losses. Being older (age) was associated with increased IMAT (p=0.046). In females, the change in VAT relative to TAT from T0 to T24 showed k=1.5 ± 0.06 (95% CI 1.35–1.62) when the Hallgreen and Hall model (19) was applied (Figure 1B).
Adipose tissue depots in surgery compared to non-surgical controls
At one year post-surgery, TAT, SAT, IMAT, and VAT were not different from non-surgical controls (Table 4). At T24, compared to non-surgical controls, BMI, weight, TAT, and SAT were higher (p<0.05) in the bariatric surgery females while IMAT and VAT did not differ. In males (T24), BMI, weight, TAT, SAT, and IMAT were higher than controls whereas VAT was not different (p=0.52) (Table 6). At T12, after adjusting for age, height, weight, and sex, the surgery group had less VAT (B −1.385, p=0.014) than controls whereas TAT, SAT, and IMAT did not differ. Females had more TAT (B 11.785, p<0.001), SAT (B 12.194, p<0.001), and IMAT (B 0.421, p< 0.001) than males, with no significant group by sex interactions.
At T24, adjusting for age, height, weight, and sex, the surgery group had more TAT (B 53.007, p<0.001), SAT (B 47.466, p<0.001), and IMAT (B 2.571, p<0.001) than non-surgery controls. Females in the surgery group had less VAT (B −3.752, p<0.001; group by sex interaction) than control females. In general, females had significantly lower VAT than males (B −0.868, p<0.001).
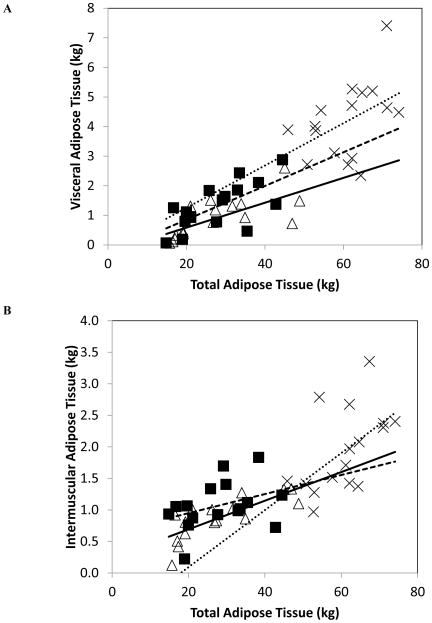
VAT and IMAT as a function of TAT in females from before to after surgery
We investigated the relationship of VAT and IMAT with TAT in the same women at T0, T12, and T24) to ascertain how VAT (Figure 2A) and IMAT (Figure 2B) levels might vary from before to after surgery when TAT is held constant. According to our regression models (Supplementary Table), a hypothetical 45 year old female weighing 90 kg with 45 kg TAT would have at T0 ~3.06 kg VAT and ~1.3 kg IMAT; at T12 (using the same age, weight and TAT parameters), this female would have ~2.42 kg VAT and ~1.45 kg IMAT, and at T24 ~1.68 kg VAT and ~1.21 kg IMAT.
Figure 2.
(A) Visceral adipose tissue (VAT) as a function of total adipose tissue (TAT) in females (N=16) at T0 (×), T12 (■), and T24 (△). Linear regression lines are shown for VAT at T0 (•••), T12 (–––), and T24 (⚊). At T0, the slope and intercept for VAT were not different from zero (p=0.083 and p=0.94, respectively). At T12, the slope for VAT was different from zero (p=0.005). The intercept for VAT was not different from zero (p=0.058). At T24, the slope for VAT was significantly different from zero (p<0.001) and the intercept was not different from zero. The data represent females on whom VAT was available at T0 and T12 and T24 (longitudinally). At T0: VAT = −0.175 +0.072 * TAT, (SEE=1.19 kg). At T12: VAT = −0.284 +0.057 * TAT, (SEE=0.61 kg). At T24: VAT = −0.253 + 0.042 * TAT, (SEE=0.50 kg). (B) Intermuscular adipose tissue (IMAT) as a function of total adipose tissue (TAT) in females (N=16) at T0 (×), T12 (■), and T24 (△). Linear regression lines are shown for IMAT at T0 (•••), T12 (–––), and T24 (⚊). At baseline, the slope for IMAT was not equal to zero (p=0.027) and the intercept was not different from zero (p=0.48). At T12, the slope and intercept (p=0.53) were not different from zero. At T24, the slope for IMAT was significantly different from zero (p<0.001), and the intercept was not significantly different from zero. The data represent females on whom IMAT was available at T0 and T12 and T24 (longitudinally). At T0: IMAT = −0.811 + 0.045 * TAT, (SEE=0.57 kg). At T12: IMAT = 0.645 + 0.015 * TAT, (SEE=0.37 kg). At T24: IMAT = 0.238 + 0.023 * TAT, (SEE= 0.22 kg).
Discussion
This study showed that bariatric surgery caused substantial and robust loss in total and regional adipose tissue at 12 and 24 months post-bariatric surgery, with continued losses between 12 and 24 months in women, when body weight change was not significant. These results indicate that bariatric surgery has an important effect on reducing adipose tissue depots even after body weight has begun to stabilize.
We found a remarkable 77% reduction in VAT at 12 months post-surgery in both men and women. Our findings are in agreement with Johansson (5), where in morbidly obese women at 12 months post-surgery, weight (−33.6%), VAT (−73.1%) and SAT (−53.2%) were significantly reduced.
Hallgreen and Hall (21) described an allometric relationship between VAT and fat mass (FM), where the change in VAT is primarily determined by changes in FM and is proportional to the initial ratio of VAT to FM (defined by the differential equation , where k is a dimensionless constant, k=1.3 ± 0.1). This relationship has been shown to hold true for different types of weight loss intervention and for both sexes equally (21). In the current study, VAT loss relative to TAT from T0 to T12 is in agreement with Hallgreen and Hall (21), where VAT was determined by the change in TAT and by the initial VAT to TAT ratio. Using inclusion criteria of weight loss of ≥5 kg and VAT and FM loss, we found that the value of k was 1.3±0.03 in both sexes. The greater absolute VAT loss in males compared to females at 12 months post-surgery is explained by a higher pre-surgery VAT to TAT ratio in males. As per Hallgreen and Hall model, the coefficients for men and women were the same indicating no sex differences in VAT loss after adjusting for the amount of TAT (or FM). However, from T0 to T24 in females, the value of k was 1.5, and was therefore greater than that proposed by the Hallgreen and Hall model, indicating increased VAT loss 2 years post-surgery in women. Remarkably, women who had bariatric surgery had significantly less VAT two years after surgery compared to BMI-matched controls.
These data emphasize the importance of longer-term longitudinal assessment of AT depots beyond the initial 12 month post-surgery period to better understand the effectiveness of bariatric surgery in mobilizing AT depots. Bariatric surgery studies have reported improvement or remission of metabolic complications of obesity in males and females 6 to 12 months post-surgery (22, 23). Males in the current study, by 24 months, showed significant gains in TAT and small gains in weight, VAT, SAT, and IMAT indicating unfavorable effects of weight regain that should be monitored more closely to avoid the development of obesity-related complications.
The strengths of this study include the longitudinal assessment of weight loss and specific adipose tissue depots measured by MRI (24). We report a novel finding of a preferential VAT loss in women beyond the initial 12 months post-surgery. The parent LABS trial (23) recently reported sustained weight loss at three years post-surgery in a large number of subjects with continued improvements in diabetes, hypertension, and dyslipidemia compared to pre-surgery levels. Although the parent trial did not collect measures of AT depots, it may well be that the sustained improvements in the observed risk factors are in part attributable to these reduced AT depots. Fat free mass, including skeletal muscle mass and bone mass, changes in the same cohort is being prepared for publication in a separate manuscript.
A limitation of this study was that not all participants fit in the MRI pre-surgery due the scanner field of view being less that that required to accommodate all individuals thereby limiting the sample size with pre-surgery values. The number of male participants was low, so our ability to derive stronger conclusions regarding sex differences across time was limited. A disproportionate number of participants had RYGB surgery compared to the other surgery types limited our ability to compare the effects of different surgery procedures due to inadequate sample sizes for most non-RYGB surgery types.
Conclusion
The energy restriction of bariatric surgery induces massive weight loss with continued adipose tissue mobilization from IMAT and VAT stores in women up to two years post-surgery. Males showed increases in all AT depots between 12 and 24 months post-surgery with weight regain. Studies seeking to understand weight loss and comorbidities after bariatric surgery must take into consideration all possible anatomical and AT depot pattern changes for more specific and efficient treatment in the clinical setting.
Supplementary Material
What is already known about this subject
Bariatric surgery induces marked weight loss with adipose tissue depot-specific losses.
Need for a better understanding of how adipose tissue sub-depots respond following bariatric surgery.
What this study adds
In women, between 12 and 24 months post-surgery when body weight is relatively stable or changes are non-significant, visceral and intermuscular adipose tissue depots continue to decrease.
Acknowledgments
Supported by National Institutes of Health Grants RO1-DK-72507; P30-DK-26687; UL1 TR000040. T. Toro-Ramos supported by a diversity supplement UO1DK094463. DG designed the study. IJ analyzed all MRI scans. GS, WY, SL, BG, JT, AC, AP contributed to recruitment, study procedures, and database management. TT-R, SL, and JT conducted the analyses. TT-R and DG wrote the paper. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Footnotes
Conflicts of interest statement
Dr. Gallagher reports grants from National Institutes of Health, during the conduct of the study; Dr. Courcoulas reports grants from NIH/NIDDK, during the conduct of the study; grants from Nutrisystem, grants from EndoGastric Solutions, grants from Covidien, personal fees from J&J Ethicon Scientific, outside the submitted work.
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Abate N, Chandalia M. Role of subcutaneous adipose tissue in metabolic complications of obesity. Metab Syndr Relat Disord. 2012;10:319–320. doi: 10.1089/met.2012.1502. [DOI] [PubMed] [Google Scholar]
- 3.Korner J, Punyanitya M, Taveras C, McMahon DJ, Kim HJ, Inabnet W, et al. Sex differences in visceral adipose tissue post-bariatric surgery compared to matched non-surgical controls. International journal of body composition research. 2008;6:93–99. [PMC free article] [PubMed] [Google Scholar]
- 4.Busetto L, Tregnaghi A, Bussolotto M, Sergi G, Beninca P, Ceccon A, et al. Visceral fat loss evaluated by total body magnetic resonance imaging in obese women operated with laparascopic adjustable silicone gastric banding. Int J Obes Relat Metab Disord. 2000;24:60–69. doi: 10.1038/sj.ijo.0801086. [DOI] [PubMed] [Google Scholar]
- 5.Johansson L, Roos M, Kullberg J, Weis J, Ahlstrom H, Sundbom M, et al. Lipid mobilization following Roux-en-Y gastric bypass examined by magnetic resonance imaging and spectroscopy. Obes Surg. 2008;18:1297–1304. doi: 10.1007/s11695-008-9484-0. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Appelbaum L, Schweiger C, Matot I, Constantini N, Idan A, et al. Short-term dynamics and metabolic impact of abdominal fat depots after bariatric surgery. Diabetes Care. 2009;32:1910–1915. doi: 10.2337/dc09-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll JF, Franks SF, Smith AB, Phelps DR. Visceral adipose tissue loss and insulin resistance 6 months after laparoscopic gastric banding surgery: a preliminary study. Obes Surg. 2009;19:47–55. doi: 10.1007/s11695-008-9642-4. [DOI] [PubMed] [Google Scholar]
- 8.Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath ML, Kow L, Slavotinek JP, Valentine R, Toouli J, Thompson CH. Abdominal adiposity and liver fat content 3 and 12 months after gastric banding surgery. Metabolism. 2009;58:753–758. doi: 10.1016/j.metabol.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Phillips ML, Lewis MC, Chew V, Kow L, Slavotinek JP, Daniels L, et al. The early effects of weight loss surgery on regional adiposity. Obes Surg. 2005;15:1449–1455. doi: 10.1381/096089205774859353. [DOI] [PubMed] [Google Scholar]
- 11.Belle SH, Berk PD, Chapman WH, Christian NJ, Courcoulas AP, Dakin GF, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9:926–935. doi: 10.1016/j.soard.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belle SH, Chapman W, Courcoulas AP, Flum DR, Gagner M, Inabnet WB, et al. Relationship of body mass index with demographic and clinical characteristics in the Longitudinal Assessment of Bariatric Surgery (LABS) Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2008;4:474–480. doi: 10.1016/j.soard.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Medicine and science in sports and exercise. 1995;27:1692–1697. [PubMed] [Google Scholar]
- 16.McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Medicine and science in sports and exercise. 1995;27:1686–1691. [PubMed] [Google Scholar]
- 17.Widen EM, Strain G, King WC, Yu W, Lin S, Goodpaster B, et al. Validity of bioelectrical impedance analysis for measuring changes in body water and percent fat after bariatric surgery. Obes Surg. 2014;24:847–854. doi: 10.1007/s11695-014-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Report of the task group on reference man. Pergamon Press; Oxford, United Kingdom: 1975. [Google Scholar]
- 21.Hallgreen CE, Hall KD. Allometric relationship between changes of visceral fat and total fat mass. Int J Obes (Lond) 2008;32:845–852. doi: 10.1038/sj.ijo.0803783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bluher S, Raschpichler M, Hirsch W, Till H. A case report and review of the literature of laparoscopic sleeve gastrectomy in morbidly obese adolescents: beyond metabolic surgery and visceral fat reduction. Metabolism. 2013;62:761–767. doi: 10.1016/j.metabol.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu HH, Li Y, Nagy TR, Goran MI, Nayak KS. Quantification of Absolute Fat Mass by Magnetic Resonance Imaging: a Validation Study against Chemical Analysis. International journal of body composition research. 2011;9:111–122. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.