Abstract
Sleep and circadian rhythms modulate or control daily physiological patterns with importance for normal metabolic health. Sleep deficiencies associated with insufficient sleep schedules, insomnia with short-sleep duration, sleep apnea, narcolepsy, circadian misalignment, shift work, night eating syndrome and sleep-related eating disorder may all contribute to metabolic dysregulation. Sleep deficiencies and circadian disruption associated with metabolic dysregulation may contribute to weight gain, obesity, and type 2 diabetes potentially by altering timing and amount of food intake, disrupting energy balance, inflammation, impairing glucose tolerance and insulin sensitivity. Given the rapidly increasing prevalence of metabolic diseases, it is important to recognize the role of sleep and circadian disruption in the development, progression, and morbidity of metabolic disease. Some findings indicate sleep treatments and countermeasures improve metabolic health, but future clinical research investigating prevention and treatment of chronic metabolic disorders through treatment of sleep and circadian disruption is needed.
Keywords: sleep, circadian misalignment, insufficient sleep, sleep deficiency, insulin sensitivity, energy balance, shift work, type 2 diabetes, metabolic syndrome, obesity, glucose tolerance, circadian disruption, sleep apnea
Introduction
The prevalence of chronic metabolic disorders such as obesity and Type 2 diabetes (T2D) has increased rapidly over the past 30 years reaching world-wide epidemic proportions (1). As such, diseases of metabolic dysregulation are now a major public health burden. In parallel with the increased prevalence of obesity and metabolic disorders, nightly sleep duration in the United States has decreased with reports of over 50% of Americans sleeping ≤7 hours/night (2). Furthermore, U shaped associations between body mass index and sleep duration have been reported (3). These findings have brought to light the hypothesis that sleep deficiencies may have causal mechanisms contributing, at least in part, to the rapidly increasing prevalence of metabolic disorders.
Supporting this hypothesis, sleep and circadian rhythms are known to modulate or control daily patterns in human physiology with importance for normal metabolic function. Daily patterns of energy expenditure (4), hormones, and lipids involved in energy metabolism (e.g., leptin, ghrelin, PYY, glucose, insulin, glucocorticoids, catecholamines, fatty acids, triglycerides) are regulated by sleep and circadian rhythms (4-8). Disruption of sleep and circadian rhythms is increasingly evident as a contributing factor to impaired physiological function and disease processes, especially with respect to metabolic dysregulation (9-12). Sleep deficiency occurs as a result of a number of untreated sleep problems including insufficient sleep schedules, insomnia, sleep apnea, periodic limb movement disorder, narcolepsy, shift work and shift work disorder, night eating syndrome and sleep-related eating disorder (13-15). Metabolic disorders associated with sleep deficiency include unwanted weight gain, obesity, and T2D (Fig. 1) (16-19). We are now beginning to understand how sleep and circadian disruption contributes to metabolic dysregulation and how treatment of sleep and circadian problems may improve metabolic treatment outcomes.
Figure 1.
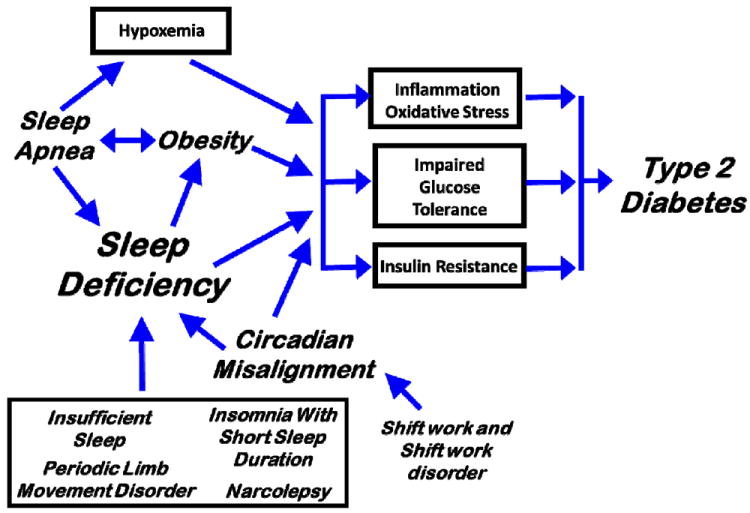
Model of the hypothesized relationships between sleep and circadian disorders and type 2 diabetes. Sleep deficiency can result from several sleep and circadian disorders including insufficient sleep, periodic limb movement disorder (PLMD), insomnia and narcolepsy. By working at times that overlap with the normal sleep opportunity, shift work and shiftwork disorder can induce circadian misalignment contributing to sleep deficiency. There is a bidirectional relationship between obesity and sleep apnea and apnea results in both hypoxemia and sleep deficiency. Sleep deficiency is also associated with increased risk of obesity. Sleep deficiency, circadian misalignment, obesity and hypoxia are associated with increased inflammation, oxidative stress, impaired glucose tolerance and insulin resistance. Thus, sleep problems likely increase type 2 diabetes risk through multiple pathways.
Insufficient Sleep
More than 50% of Americans report regularly sleeping less than 7h per night (2). The International Classification of Sleep Disorders, 3rd edition (ICSD-3)(20), describes insufficient sleep syndrome as failure to obtain sufficient nocturnal sleep necessary to maintain alert wakefulness. Symptoms include daytime sleepiness with uncontrolled need for sleep or lapses into sleep, short sleep duration for the age, need of an alarm clock or being awakened by another, and longer sleep duration without such measures on free days (e.g., weekends or vacation). The sleep pattern is persistent for at least three months and reversal of the sleep problem occurs with sleep extension. Insufficient sleep is associated with the risk of multiple health problems including inflammation, depression, stress, anxiety, obesity, diabetes, cardiovascular disease and fatigue related accidents (18, 21-23).
With regards to metabolic disruption, findings from a meta-analysis (24), indicated the relative risk of developing T2D increased 2% for every year of follow-up in subjects reporting insufficient sleep. Similarly, findings from a meta-analysis including 603,519 adults and 29,502 children, showed an increased risk of obesity in adults who sleep less than 5h per night and children who sleep less than 10h per night (25). Although these reports indicate an association between insufficient sleep and metabolic diseases, causal directionality cannot be inferred based on such cross-sectional designs.
Several controlled laboratory intervention studies have investigated mechanisms of how insufficient sleep may contribute to metabolic dysregulation. With respect to weight gain, Spiegel et al. (26) reported that two nights of 4h versus 10h sleep opportunities increased daytime ghrelin and reduced daytime leptin levels in 12 healthy young men. These hormonal changes were associated with increased hunger and appetite. Importantly, food intake was kept the same between conditions in this study, thus the impact of insufficient sleep on food intake was not determined. Recently, Markwald et al. (4) reported that 5 nights of insufficient sleep (5h per night), modeling a 5 day work-week, increased food intake and total daily energy expenditure. Increased food intake during the insufficient sleep schedule exceeded energy expenditure contributing to weight gain. Women showed more eating restraint when sleep was adequate, whereas both men and women overate during insufficient sleep. In this study, 24h leptin and Peptide-YY (PYY) levels increased and 24h ghrelin levels decreased. As food intake was ad-libitum, changes in appetitive hormones likely reflected the increased food intake during insufficient sleep. Comparable findings of increased food intake and altered appetitive hormones during insufficient sleep have been reported (27, 28). Together, these studies identified increased hunger and food intake associated with increased energy expenditure as potential mechanisms by which insufficient sleep contributes to obesity.
Glucose metabolism is also disturbed during insufficient sleep. Findings from a seminal study on this topic showed that six nights of a 4h per night sleep opportunity reduced glucose clearance by 40% and the acute insulin response to glucose by 30% during an intravenous glucose tolerance test (IVGTT) (29). This outcome is similar to differences between young glucose tolerant and older glucose intolerant adults. Findings from a similar study (16) indicated that seven nights of 5h sleep opportunity in healthy men decreased insulin sensitivity assessed by the hyperinsulinemic euglycemic clamp. In a longer duration study (30), 14 days of a 5.5h per night sleep opportunity reduced oral glucose tolerance and IVGTT insulin sensitivity (Si) in healthy subjects. Insufficient sleep of four nights of a 4h per night sleep opportunity has also been shown to decrease cellular adipose insulin signaling (30%) and whole body Si (16%) (31). Collectively, these controlled laboratory studies support the hypothesis that insufficient sleep can contribute to impaired metabolism. If persistent, such metabolic changes could contribute to or worsen T2D. Additional research is needed to improve our understanding of potential mechanisms underlying impaired metabolism associated with insufficient sleep and to investigate the potential benefit of increasing sleep in short sleepers with metabolic disorders.
Circadian Misalignment
Altering the timing of sleep and wakefulness such that sleep occurs during the daytime and wakefulness occurs at night results in circadian misalignment. One common cause of circadian misalignment is shift work in which shifts occur during the biological night, a time normally reserved for sleeping. Other common causes include insufficient sleep, social jet-lag, and night time light exposure. Circadian misalignment has been shown to alter neuroendocrine physiology with the potential for negative health consequences such as obesity and diabetes (5, 8, 9). Findings from several studies suggest that circadian misalignment may contribute to impaired glucose tolerance and reduced insulin sensitivity (8, 32, 33). In studies where meals occur during the biological night, findings show increased glucose or insulin levels (8, 33).
Research findings have shown that short-term circadian misalignment in humans to elevate postprandial blood glucose levels. These findings likely resulted from reduced pancreatic β-cell compensation or reduced insulin sensitivity (8). Long-term blood glucose dysregulation from chronic circadian misalignment may be due to differences in the circadian timing of central and peripheral circadian clocks (8, 33), which has been demonstrated in animals (34, 35), but has yet to be tested in humans. Findings from a genome wide association study indicate a possible link between circadian rhythm regulation, glucose homeostasis and melatonin signaling in the pancreas, supporting the idea that uncoupling of peripheral circadian clocks may contribute to metabolic dysregulation in humans (36). Furthermore, altered cortisol secretion relative to the feeding-fasting, wakefulness-sleep cycle during circadian misalignment may contribute to insulin resistance (8).
Weight gain in shift workers has been implicated as one potential mechanism contributing to T2D development (37). One mechanism through which this could occur is through changes in appetitive hormones reported to occur during circadian misalignment. In humans, leptin levels are typically low during wakefulness and higher during sleep. Circadian misalignment has been reported to reduce circulating leptin levels (8, 33), potentially contributing to increased appetite and weight gain. Additionally, the circadian timing of food intake can contribute to weight gain. Findings from a study that fed mice a high-fat diet (60% fat) exclusively during either the light or dark phase showed that mice fed during the light phase when they would normally be sleeping gained significantly more weight than mice fed during the dark phase when they would normally be awake (38). Similarly, mice fed a high-fat diet but with restricted feeding to their habitual wake time (dark cycle) were protected from obesity and hyperinsulinemia as compared to mice that freely consumed a similar amount of high-fat calories at unrestricted times (39). Findings from another study investigating circadian disruption in mice showed exposure to constant light increased the duration of food intake and increased the respiratory exchange ratio during habitual sleep time, indicating a higher relative carbohydrate to fat oxidation ratio (40). Such findings suggest that food consumption during inappropriate circadian times can contribute to weight gain (38). Thus, the metabolic effect of calories consumed is dependent upon the biological time of food intake. Such findings have important implications for risk of weight gain and obesity in shift workers who tend to consume more calorie-dense (41) and carbohydrate-rich (42) foods at night during the typical sleep time.
Insomnia
The prevalence of insomnia in the general population varies widely raging from ~8 to 40% (43-45). Such findings are complicated by the definition of insomnia used. For example, about 8 to 10% of the population suffers from chronic insomnia whereas about 20 to 30% suffer from symptoms of insomnia (43-45). Insomnia is defined in the ICSD-3 (20) as a complaint of difficulty three or more times per week, for three or more months, of initiating or maintaining sleep, or earlier awakening than desired, with associated daytime functioning complaints (e.g. fatigue or daytime sleepiness, cognitive impairment and accidents, mood or behavioral problems, dissatisfaction with sleep). Findings from studies investigating associations between insomnia and metabolic dysregulation are mixed (46-50). Most that did not show associations between insomnia and blood glucose regulation did not investigate insomnia with short sleep duration or did not stratify on sleep duration (46, 48, 50). Instead, findings from the Penn Sleep Study indicated that subjects with insomnia and short sleep duration (≤ 6h per night) had the highest odds ratio (2.95 [1.2-7.0 95% CI]) of developing T2D (47). Supporting this outcome, findings from another study (49) showed insomnia with fragmented sleep in diabetic patients was associated with increased fasting insulin and reduced insulin sensitivity assessed by Homeostasis Model Assessment (HOMA). Contradictory findings from one observational study showed increased insulin sensitivity assessed by HOMA in patients with insomnia who were short-sleepers compared to patients with insomnia with ≥ 6-h sleep/night (51). But, this increased insulin sensitivity was accompanied by reduced beta-cell function assessed by HOMA-B. Chronically, reduced beta-cell function could contribute to increased T2D risk. Small sample sizes and use of only one single overnight PSG to define short-sleep limit these findings. Together, these outcomes highlight the importance of differentiating insomnia with and without short sleep duration as insomnia with short sleep duration may negatively impact glycolytic regulation. It has been proposed that different diagnoses and treatments be identified for insomnia with and without short sleep duration as these two conditions may have different causal mechanisms (44). Thus, those with short-sleep duration may particularly benefit metabolically from insomnia treatment (44). In all, the efficacy of treating patients diagnosed with insomnia with short sleep duration for metabolic outcomes remains to be established and prospective interventional studies are needed.
Obstructive Sleep Apnea (OSA)
Over 18 million Americans are affected by the respiratory sleep disorder OSA. Symptoms of OSA include daytime sleepiness and fatigue, and sleep related loud snoring or gasping for air, and reduced or cessation of airflow during sleep. The number of hypopneas (shallow breathing) and apneas (cessation of breathing), and the number of respiratory effort related arousals that occur per hour define the severity of the disorder (20). Apnea events typically last between 10-30 seconds but can extend to minutes (52). Apneas often result in reduced arterial oxygen saturation and frequent arousals from sleep. Obesity is a risk factor for both OSA and diabetes. With respect to OSA, approximately 50% of obese patients are thought to have apnea. Continuous positive airway pressure (CPAP), oral appliances and surgery are commonly used OSA therapies. Unfortunately, poor compliance is common for OSA therapies and likely contributes to deterioration of OSA associated sleep and metabolic problems.
Findings from studies consistently show an association between OSA and T2D severity independent of obesity and other potential confounding factors (53-56). Overall, data supporting an association between OSA and disturbed glycemic regulation is strong. Importantly, many patients may not present with clinical symptoms of OSA (56). Thus undiagnosed OSA may be a contributing factor to metabolic dysregulation.
Adequate treatment of OSA appears to improve glycemic regulation. For example, findings from a study of newly diagnosed OSA patients showed improvements in glycated hemoglobin (HbA1c) in subjects that used CPAP for ≥ 4 nights per week for six months, highlighting the importance of CPAP compliance (57). Findings from another study, utilizing CPAP versus sham in patients with impaired glucose tolerance, but not T2D, showed CPAP did not improve results of an oral glucose tolerance test (OGTT) (58). More than eight weeks of CPAP may thus be needed to induce OGTT improvements in non-diabetic patients. However, with each hour of use, CPAP improved the insulin sensitivity index by 4.2% from baseline, further implicating CPAP compliance for improving glycemic regulation. In a CPAP versus sham trial (59), three months of CPAP in Metabolic Syndrome (MetS) patients improved cholesterol, triglycerides, HbA1c, and resolved MetS in 20% of the patients. Interestingly, resolution of MetS did not result from a single common MetS component. In contrast to most studies, CPAP use lead to reduced body mass index (BMI), likely contributing to resolution of MetS in this study. Whereas some trials provide evidence that CPAP therapy may improve blood glucose regulation (57, 59-61), findings are mixed (58, 62-64). Variable study populations and protocols (i.e. severity/duration of OSA and T2D diagnosis, CPAP versus sham, and CPAP duration/compliance) are likely major contributors to differing outcomes on the effectiveness of CPAP to improve blood glucose regulation.
Importantly, findings from an epidemiological study indicated that diabetes is also associated with central sleep apnea (cessation of respiratory effort) and periodic Cheyne Stokes breathing (65). Central sleep apnea and periodic breathing may be associated with diabetic autonomic neuropathy in diabetic patients (66). Like obstructive sleep apnea, central sleep apnea and periodic breathing may contribute to metabolic dysregulation in diabetic patients.
Dual therapy with CPAP and T2D medications has been investigated in a few studies with respect to treatment outcomes. A retrospective analysis of T2D patients taking a variety of blood glucose regulating medications while using CPAP (61) showed that HbA1c decreased by 0.5% over an average of 134 days of treatment. This clinically significant improvement indicates that CPAP has the potential to further benefit blood glucose regulation beyond treatment with T2D medications. Alternatively, findings from a study of T2D patients with newly diagnosed OSA and taking blood glucose medications showed 41 days of CPAP did not benefit HbA1c (62). The relatively short CPAP treatment duration may have been too short to observe benefits in glycemic regulation as measured by HbA1c. In a more controlled trial, men with established T2D on various treatment regimens underwent three months of CPAP or sham in addition to their T2D therapies (63). Findings showed HbA1c and insulin sensitivity were similar between treatment conditions suggesting CPAP may not improve blood glucose regulation over and above T2D treatment regimens in men with established T2D. However, low CPAP compliance potentially reduced its efficacy in this study, but as noted by the authors, analysis of only good compliers did not change the outcome. As the authors suggested, in patients with established T2D as in this study, β-cell function may be too impaired for CPAP to benefit blood glucose regulation on top of T2D treatments. Thus, patients with higher functioning β-cells may be most responsive to the addition of CPAP. In all, the findings from the few studies examining dual CPAP and T2D therapies for improved glycemic regulation are mixed. The studies done to date are limited and more research is required to determine the metabolic benefits of combined medication and apnea treatment.
Narcolepsy
Narcolepsy occurs in 1 out 2000 people and is defined in the ICSD-3 (20) as (a) Type 1, daytime sleepiness with uncontrolled need for sleep or lapses into sleep, cataplexy and cerebral spinal fluid (CSF) hypocretin-1 deficiency, or as (b) Type 2, daytime sleepiness with uncontrolled need for sleep or lapses into sleep, without cataplexy or CSF hypocretin-1 deficiency, but with two or more sleep onset REM periods on a daytime Multiple Sleep Latency Test performed in a sleep disorders center.
Findings from a retrospective study at the Max-Planck Institute demonstrated patients with narcolepsy had higher BMI compared to the general population (67) and such findings are supported by others (68-70). As treated and untreated patients had similar BMI, it was hypothesized that elevated BMI resulted from the pathophysiology of narcolepsy itself (e.g., loss of orexin peptide) or disease behavior such as reduced physical activity. More recently, blood glucose regulation and T2D was investigated in two studies of patients with narcolepsy using BMI matched controls (71, 72). Findings from both of these studies showed that narcoleptic patients did not have elevated risk of developing T2D or blood glucose dysregulation independent of BMI. Given that associations between narcolepsy and elevated BMI and morbidity have been reported (67-70, 73), weight gain and metabolic regulation in narcoleptic patients should be monitored. Future research is needed to understand mechanisms and test prevention of weight gain in patients with narcolepsy.
Periodic Limb Movement Disorder
Periodic Limb Movement Disorder (PLMD) is a sleep related movement disorder that affects more than 10 million American adults. The disorder is characterized by stereotypic movements of the limbs during sleep often associated with brief arousals and therefore sleep disturbance. Daytime consequences include impaired cognition, behavioral and physical functioning (20). Patients with Restless Legs Syndrome (RLS) present with PLMD, and RLS and PLMD appear to be common in patients with diabetes (74-77). Little is known about whether PLMD is associated with metabolic dysregulation in the absence of frank diabetes and therefore this represents an important research need. Also, it is unknown whether treatment of PLMD in patients with diabetes improves metabolic function.
Shift Work and Shift Work Disorder
Shift work refers to work schedules that occur during times typically reserved for sleep. Such schedules are especially common in health care, transportation, manufacturing and public services (78). In industrialized countries, shift workers make up 20-25% of the total work force (79). Findings consistently show years of shift work negatively impact sleep duration, sleep quality, performance and health outcomes (78). Shift Work Disorder (SWD) is defined in the ICSD-3 (20) as excessive sleepiness or insomnia accompanied by a reduction of total sleep time, which is associated with a recurring work schedule that overlaps the usual time for sleep during the previous three months. The prevalence of SWD is estimated at 10-38% of shift workers (15, 80, 81).
Findings from epidemiological studies consistently show shift work is associated with obesity (82-85) and T2D (86). However, findings from studies controlling for sleep duration indicate shift work is not consistently associated with MetS (87). Based on self-reported T2D, and controlling for gender and BMI, findings indicate shift workers have increased odds of developing T2D relative to individuals having never worked shift work (88). Similar findings were reported in another study that controlled for education, smoking, physical activity, alcohol consumption and insomnia symptoms (89). Findings from a study comparing rotating shift work versus day work controls indicated rotating shift work is an independent risk factor for developing T2D (90). Overall, the current literature indicates that shift workers are at increased risk of developing T2D. Whereas some studies have investigated specific causal mechanisms including circadian misalignment, this and the effectiveness of treatments for shift workers represents an important research area requiring further investigation.
Night Eating Syndrome and Sleep-Related Eating Disorder
Two eating disorders that are associated with sleep or altered circadian timing and may contribute to metabolic dysfunction are night eating syndrome and sleep-related eating disorder. Night eating syndrome is defined by consumption of a large part of the total daily caloric intake in the evening and nighttime hours (at least 25% of caloric intake after the evening meal or nocturnal awakenings with food ingestion ≥ two time per week) and a reduction of caloric intake in the morning, for at least three months (91, 92). This eating pattern is associated with a later timing of the internal clock (93), which may contribute to the later eating pattern which is also observed during insufficient sleep schedules (4). Later sleep schedules are also reported to be associated with greater evening food intake and higher BMI (94, 95). Sleep-related eating disorder is defined in the ICSD-3 (20) by dysfunctional eating after an arousal from the primary sleep episode. This disorder is thought to be a form of sleep-walking (96-98) with partial or complete arousal from sleep and consumption of food or inedible or toxic substances (e.g., cleaning fluids). Patients are often not aware of their eating behavior until the next morning upon seeing a mess in the kitchen or on their bed clothes. Obesity is often, but not always, associated with night eating syndrome and sleep-related eating disorder (99). Limited research has been conducted to determine whether these disorders are associated with metabolic dysfunction beyond obesity. However, night eating syndrome does appear to be more prevalent than other eating disorders (e.g., binge eating disorder) in patients with T2D (100).
Conclusion
Several common sleep problems including insufficient sleep schedules, insomnia, sleep apnea, narcolepsy, shift work and shift work disorder are known to cause sleep deficiencies that likely contribute to metabolic diseases (Fig. 1). Importantly, bi-directional relationships exist between metabolic dysfunction and some of these sleep problems, most notably for OSA and obesity and T2D. Evidence for the treatment of sleep and circadian problems to improve metabolic health is emerging, with treatment of sleep apnea showing the most evidence thus far. To optimize public health recommendations based around sleep deficiency and metabolic dysfunction, a better understanding of the physiological mechanisms by which sleep problems contribute to metabolic disorders is needed. While the metabolic consequences of some sleep disorders like OSA have been more thoroughly investigated, less is known about others. Notably, associations of circadian misalignment, PLMD, Night Eating Syndrome, and Sleep-Related Eating Disorder with T2D represent important unmet research needs. Additionally, the metabolic consequence of circadian disruption represents an emerging clinically relevant area of investigation. For example, how the circadian timing of food intake in humans influences metabolic health in patients with primary circadian rhythm sleep-wake disorders such as delayed sleep phase is unknown. Specific gaps in our understanding of physiological mechanisms by which sleep and circadian disruption contribute to metabolic dysregulation include: 1) How does insufficient sleep and circadian misalignment contribute to reduced insulin sensitivity at a tissue specific level?; 2) What are the causal mechanisms and metabolic consequences of insomnia with short-sleep duration?; 3) What are specific causal mechanisms of weight gain in narcolepsy?; 4) Are the mechanisms of circadian misalignment that lead to reduced insulin sensitivity different from the mechanisms of insufficient sleep at a whole body and tissue specific level? 5) Do sleep and circadian treatments improve metabolic health when combined with other lifestyle and treatment regimens? Given the low success of current recommendations and lifestyle interventions focusing on diet and physical activity, future treatment approaches should include new and combined approaches including those focusing on optimal sleep and treatment of sleep disorders. Healthy sleep and circadian function are increasingly becoming recognized as important for metabolic health as are sufficient physical activity and good nutrition.
Acknowledgments
Kenneth P. Wright Jr. has received grant support from NIH R01 HL109706, R21 DK092624.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Christopher M. Depner and Ellen R. Stotharddeclare that they have no conflict of interest. Kenneth P. Wright Jr. has been a Past Chair Scientific Advisory Board—fatigue and maritime work schedules for Northwestern University American Waterways Project; Past Chair Scientific Advisory Board—company developed sleep monitoring system for the general public for Zeo, Inc.; past consultant for Takeda Pharmaceuticals; has received honoraria from the University of Chicago and Associated Professional Sleep Societies; payment from Potomac Center for Medical Education for shift work sleep disorders in hospitals; and has received grant support for Philips, Inc.
Contributor Information
Christopher M. Depner, Email: christopher.depner@colorado.edu.
Ellen R. Stothard, Email: ellen.Stothard@colorado.edu.
Kenneth P. Wright, Jr., Email: kenneth.wright@colorado.edu.
References Cited
Papers of particular interest, published recently, have been highlighted as:
-
*
Of importance
- 1.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32(Suppl 7):S120–6. doi: 10.1038/ijo.2008.247. [DOI] [PubMed] [Google Scholar]
- 2.Sleep in America Poll. National Sleep Foundation; 2008. [Google Scholar]
- 3.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110(14):5695–700. doi: 10.1073/pnas.1216951110. This controlled laboratory study modeled a work week of insufficent sleep with ad-libitum feeding and measured 24 hour energy expenditure using whole room calorimetry. The findings indicate that insufficinet sleep contributes to weight gain via excessive food intale associated with incresed energy expoenditure. The findings also indicate potential benefit of recovery sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen J, Wright KP., Jr Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep. 2009;2010(2):9–18. doi: 10.2147/NSS.S7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel K, Tasali E, Leproult R, Scherberg N, Van Cauter E. Twenty-four-hour profiles of acylated and total ghrelin: relationship with glucose levels and impact of time of day and sleep. J Clin Endocrinol Metab. 2011;96(2):486–93. doi: 10.1210/jc.2010-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18(5):716–38. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 8*.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–8. doi: 10.1073/pnas.0808180106. This study employs a circadian research protocol called forced desynchrony to disassociate sleep and circadian modulation of cardiometabolic measures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markwald RRWJ, Kenneth P. Circadian Misalignment and Sleep Disruption in Shift Work: Implications for Fatigue and Risk of Weight Gain and Obesity. In: Shiromani P, Horvath Tamas, Redline Susan, Van Cauter Eve, editors. Sleep loss and obesity : intersecting epidemics. New York: Springer; 2012. pp. 101–18. [Google Scholar]
- 10.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627–31. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985) 2005;99(5):2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 13.Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1460–83. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Jr, Vitiello MV, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1484–501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright KP, Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17(1):41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59(9):2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 18.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165(1):25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997;45(1):1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 20.International Classification of Sleep Disorders. American Academy of Sleep Medicine. (3) 2014 [Google Scholar]
- 21.Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, Chrousos GP. Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf) 1999;51(2):205–15. doi: 10.1046/j.1365-2265.1999.00763.x. [DOI] [PubMed] [Google Scholar]
- 22.Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students. J Psychosom Res. 1997;42(6):583–96. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 23.Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep. 1994;17(1):84–93. doi: 10.1093/sleep/17.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 27.Calvin AD, Carter RE, Adachi T, Macedo PG, Albuquerque FN, van der Walt C, et al. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest. 2013;144(1):79–86. doi: 10.1378/chest.12-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1(5):266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 30.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94(9):3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157(8):549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014 doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43. doi: 10.1126/scitranslmed.3003200. This study describes metabolic alterations in response to extended (3 weeks) sleep restriction combined with circadian misalignment, as well as subsequent recovery sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105(39):15172–7. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barclay JL, Husse J, Bode B, Naujokat N, Meyer-Kovac J, Schmid SM, et al. Circadian desynchrony promotes metabolic disruption in a mouse model of shiftwork. PLoS One. 2012;7(5):e37150. doi: 10.1371/journal.pone.0037150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41(1):89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 37.Kivimaki M, Batty GD, Hublin C. Shift work as a risk factor for future type 2 diabetes: evidence, mechanisms, implications, and future research directions. PLoS Med. 2011;8(12):e1001138. doi: 10.1371/journal.pmed.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17(11):2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coomans CP, van den Berg SA, Houben T, van Klinken JB, van den Berg R, Pronk AC, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27(4):1721–32. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 41.Lowden A, Moreno C, Holmback U, Lennernas M, Tucker P. Eating and shift work - effects on habits, metabolism and performance. Scand J Work Environ Health. 2010;36(2):150–62. doi: 10.5271/sjweh.2898. [DOI] [PubMed] [Google Scholar]
- 42.Debry G, Girault P, Lefort J, Thiebault J. Survey of the food habits of workers on shift work. Bull Inst Natl Sante Rech Med. 1967;22(6):1169–202. [PubMed] [Google Scholar]
- 43.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–54. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 46.Chedraui P, San Miguel G, Villacreses D, Dominguez A, Jaramillo W, Escobar GS, et al. Assessment of insomnia and related risk factors in postmenopausal women screened for the metabolic syndrome. Maturitas. 2013;74(2):154–9. doi: 10.1016/j.maturitas.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 47*.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32(11):1980–5. doi: 10.2337/dc09-0284. Findings from this study provide the strongest evidence that there are differences between insomnia with short-sleep duration versus insomnia with normal sleep duration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Troxel WM, Buysse DJ, Matthews KA, Kip KE, Strollo PJ, Hall M, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33(12):1633–40. doi: 10.1093/sleep/33.12.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–6. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keckeis M, Lattova Z, Maurovich-Horvat E, Beitinger PA, Birkmann S, Lauer CJ, et al. Impaired glucose tolerance in sleep disorders. PLoS One. 2010;5(3):e9444. doi: 10.1371/journal.pone.0009444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasisht KP, Kessler LE, Booth JN, 3rd, Imperial JG, Penev PD. Differences in insulin secretion and sensitivity in short-sleep insomnia. Sleep. 2013;36(6):955–7. doi: 10.5665/sleep.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–89. [PubMed] [Google Scholar]
- 53.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–13. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papanas N, Steiropoulos P, Nena E, Tzouvelekis A, Maltezos E, Trakada G, et al. HbA1c is associated with severity of obstructive sleep apnea hypopnea syndrome in nondiabetic men. Vasc Health Risk Manag. 2009;5:751–6. doi: 10.2147/vhrm.s7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ronksley PE, Hemmelgarn BR, Heitman SJ, Hanly PJ, Faris PD, Quan H, et al. Obstructive sleep apnoea is associated with diabetes in sleepy subjects. Thorax. 2009;64(10):834–9. doi: 10.1136/thx.2009.115105. [DOI] [PubMed] [Google Scholar]
- 56.Schober AK, Neurath MF, Harsch IA. Prevalence of sleep apnoea in diabetic patients. Clin Respir J. 2011;5(3):165–72. doi: 10.1111/j.1752-699X.2010.00216.x. [DOI] [PubMed] [Google Scholar]
- 57.Steiropoulos P, Papanas N, Nena E, Tsara V, Fitili C, Tzouvelekis A, et al. Markers of glycemic control and insulin resistance in non-diabetic patients with Obstructive Sleep Apnea Hypopnea Syndrome: does adherence to CPAP treatment improve glycemic control? Sleep Med. 2009;10(8):887–91. doi: 10.1016/j.sleep.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Weinstock TG, Wang X, Rueschman M, Ismail-Beigi F, Aylor J, Babineau DC, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35(5):617–25B. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59*.Sharma SK, Agrawal S, Damodaran D, Sreenivas V, Kadhiravan T, Lakshmy R, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365(24):2277–86. doi: 10.1056/NEJMoa1103944. Findings that CPAP resolved MetS in 20% of subjects show strong potential benefit from CPAP. Future studies need to confirm and extend these initial findings to address critiques raised by some researchers. [DOI] [PubMed] [Google Scholar]
- 60.Barcelo A, Barbe F, de la Pena M, Martinez P, Soriano JB, Pierola J, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63(11):946–50. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- 61.Hassaballa HA, Tulaimat A, Herdegen JJ, Mokhlesi B. The effect of continuous positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 2005;9(4):176–80. doi: 10.1007/s11325-005-0033-y. [DOI] [PubMed] [Google Scholar]
- 62.Dawson A, Abel SL, Loving RT, Dailey G, Shadan FF, Cronin JW, et al. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4(6):538–42. [PMC free article] [PubMed] [Google Scholar]
- 63.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62(11):969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29(4):720–7. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 65.Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26(3):702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 66.Aurora RN, Punjabi NM. Obstructive sleep apnoea and type 2 diabetes mellitus: a bidirectional association. Lancet Respir Med. 2013;1(4):329–38. doi: 10.1016/S2213-2600(13)70039-0. [DOI] [PubMed] [Google Scholar]
- 67.Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355(9211):1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- 68.Poli F, Plazzi G, Di Dalmazi G, Ribichini D, Vicennati V, Pizza F, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32(11):1491–7. doi: 10.1093/sleep/32.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251(2):85–9. doi: 10.1007/s004060170057. [DOI] [PubMed] [Google Scholar]
- 70.Heier MS, Jansson TS, Gautvik KM. Cerebrospinal fluid hypocretin 1 deficiency, overweight, and metabolic dysregulation in patients with narcolepsy. J Clin Sleep Med. 2011;7(6):653–8. doi: 10.5664/jcsm.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Beitinger PA, Fulda S, Dalal MA, Wehrle R, Keckeis M, Wetter TC, et al. Glucose tolerance in patients with narcolepsy. Sleep. 2012;35(2):231–6. doi: 10.5665/sleep.1628. Findings strengthened by use of BMI matched controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72*.Engel A, Helfrich J, Manderscheid N, Musholt PB, Forst T, Pfutzner A, et al. Investigation of insulin resistance in narcoleptic patients: dependent or independent of body mass index? Neuropsychiatr Dis Treat. 2011;7:351–6. doi: 10.2147/NDT.S18455. Findings strengthened by use of BMI matched controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jennum P, Ibsen R, Knudsen S, Kjellberg J. Comorbidity and mortality of narcolepsy: a controlled retro- and prospective national study. Sleep. 2013;36(6):835–40. doi: 10.5665/sleep.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopes LA, Lins Cde M, Adeodato VG, Quental DP, de Bruin PF, Montenegro RM, Jr, et al. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care. 2005;28(11):2633–6. doi: 10.2337/diacare.28.11.2633. [DOI] [PubMed] [Google Scholar]
- 75.Merlino G, Fratticci L, Valente M, Del Giudice A, Noacco C, Dolso P, et al. Association of restless legs syndrome in type 2 diabetes: a case-control study. Sleep. 2007;30(7):866–71. doi: 10.1093/sleep/30.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuellar NG, Ratcliffe SJ. A comparison of glycemic control, sleep, fatigue, and depression in type 2 diabetes with and without restless legs syndrome. J Clin Sleep Med. 2008;4(1):50–6. [PMC free article] [PubMed] [Google Scholar]
- 77.Rizzi M, Barrella M, Kotzalidis GD, Bevilacqua M. Periodic Limbic Movement Disorder during Sleep as Diabetes-Related Syndrome? A Polysomnographic Study. ISRN Endocrinol. 2011;2011:246157. doi: 10.5402/2011/246157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akerstedt T, Wright KP., Jr Sleep Loss and Fatigue in Shift Work and Shift Work Disorder. Sleep Med Clin. 2009;4(2):257–71. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Painting, firefighting, and shiftwork. IARC Monogr Eval Carcinog Risks Hum. 2010;98:9–764. [PMC free article] [PubMed] [Google Scholar]
- 80.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 81.Flo E, Pallesen S, Mageroy N, Moen BE, Gronli J, Hilde Nordhus I, et al. Shift work disorder in nurses--assessment, prevalence and related health problems. PLoS One. 2012;7(4):e33981. doi: 10.1371/journal.pone.0033981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishizaki M, Morikawa Y, Nakagawa H, Honda R, Kawakami N, Haratani T, et al. The influence of work characteristics on body mass index and waist to hip ratio in Japanese employees. Ind Health. 2004;42(1):41–9. doi: 10.2486/indhealth.42.41. [DOI] [PubMed] [Google Scholar]
- 83.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58(11):747–52. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Lorenzo L, De Pergola G, Zocchetti C, L’Abbate N, Basso A, Pannacciulli N, et al. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord. 2003;27(11):1353–8. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 85.van Amelsvoort LG, Schouten EG, Kok FJ. Duration of shiftwork related to body mass index and waist to hip ratio. Int J Obes Relat Metab Disord. 1999;23(9):973–8. doi: 10.1038/sj.ijo.0801028. [DOI] [PubMed] [Google Scholar]
- 86.Guo Y, Liu Y, Huang X, Rong Y, He M, Wang Y, et al. The effects of shift work on sleeping quality, hypertension and diabetes in retired workers. PLoS One. 2013;8(8):e71107. doi: 10.1371/journal.pone.0071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Canuto R, Garcez AS, Olinto MT. Metabolic syndrome and shift work: a systematic review. Sleep Med Rev. 2013;17(6):425–31. doi: 10.1016/j.smrv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 88.Monk TH, Buysse DJ. Exposure to shift work as a risk factor for diabetes. J Biol Rhythms. 2013;28(5):356–9. doi: 10.1177/0748730413506557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puttonen S, Viitasalo K, Harma M. The relationship between current and former shift work and the metabolic syndrome. Scand J Work Environ Health. 2012;38(4):343–8. doi: 10.5271/sjweh.3267. [DOI] [PubMed] [Google Scholar]
- 90.Suwazono Y, Sakata K, Okubo Y, Harada H, Oishi M, Kobayashi E, et al. Long-term longitudinal study on the relationship between alternating shift work and the onset of diabetes mellitus in male Japanese workers. J Occup Environ Med. 2006;48(5):455–61. doi: 10.1097/01.jom.0000214355.69182.fa. [DOI] [PubMed] [Google Scholar]
- 91.Birketvedt GS, Florholmen J, Sundsfjord J, Osterud B, Dinges D, Bilker W, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA. 1999;282(7):657–63. doi: 10.1001/jama.282.7.657. [DOI] [PubMed] [Google Scholar]
- 92.Allison KC, Lundgren JD, O’Reardon JP, Geliebter A, Gluck ME, Vinai P, et al. Proposed diagnostic criteria for night eating syndrome. Int J Eat Disord. 2010;43(3):241–7. doi: 10.1002/eat.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goel N, Stunkard AJ, Rogers NL, Van Dongen HP, Allison KC, O’Reardon JP, et al. Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms. 2009;24(1):85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baron KG, Reid KJ, Horn LV, Zee PC. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite. 2013;60(1):246–51. doi: 10.1016/j.appet.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–81. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 96.Winkelman JW. Clinical and polysomnographic features of sleep-related eating disorder. J Clin Psychiatry. 1998;59(1):14–9. doi: 10.4088/jcp.v59n0104. [DOI] [PubMed] [Google Scholar]
- 97.Brion A, Flamand M, Oudiette D, Voillery D, Golmard JL, Arnulf I. Sleep-related eating disorder versus sleepwalking: a controlled study. Sleep Med. 2012;13(8):1094–101. doi: 10.1016/j.sleep.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 98.Winkelman JW, Johnson EA, Richards LM. Sleep-related eating disorder. Handb Clin Neurol. 2011;98:577–85. doi: 10.1016/B978-0-444-52006-7.00037-X. [DOI] [PubMed] [Google Scholar]
- 99.Gallant AR, Lundgren J, Drapeau V. The night-eating syndrome and obesity. Obes Rev. 2012;13(6):528–36. doi: 10.1111/j.1467-789X.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 100.Allison KC, Crow SJ, Reeves RR, West DS, Foreyt JP, Dilillo VG, et al. Binge eating disorder and night eating syndrome in adults with type 2 diabetes. Obesity (Silver Spring) 2007;15(5):1287–93. doi: 10.1038/oby.2007.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


