Abstract
GSK1265744 (GSK744) is an integrase strand-transfer inhibitor that has been formulated as a long-acting (LA) injectable suitable for monthly to quarterly clinical administration. GSK744 LA was administered at two time points 4 weeks apart beginning 1 week before virus administration, and macaques were challenged weekly for 8 weeks. GSK744 LA, at plasma concentrations achievable with quarterly injections in humans, protected all animals against repeated low-dose challenges. In a second experiment, macaques were given GSK744 LA 1 week before virus administration and challenged repeatedly until infection occurred. Protection decreased over time and correlated with the plasma drug levels. With a quarterly dosing schedule in humans, our results suggest that GSK744 LA could potentially decrease adherence problems associated with daily preexposure prophylaxis (PrEP).
The global HIV-1 pandemic remains a public health problem of unprecedented proportions. There were 2.3 million new infections in 2012, with an estimated 35.3 million people already infected (1). Because an effective vaccine remains elusive, multiple clinical trials have been performed to evaluate the efficacy of various anti-retrovirals as preexposure prophylaxis (PrEP). The CAPRISA 004, the Chemoprophylaxis for HIV Prevention in Men (iPrEx), the PartnersPrEP, and the TDF2 studies have shown that topical or oral PrEP agents reduced the risk of HIV-1 infection, with efficacies ranging from 39 to 75% (2–5). The variability in efficacy likely resulted from adherence differences among the study populations. In the iPrEx study, the efficacy of daily oral emtricitabine and tenofovir disoproxil fumarate (FTC/TDF) increased from 44% in all participants assigned to treatment to 90% in those participants with detectable drug in plasma (3). In a follow-up pharmacokinetic (PK) study, intracellular drug concentrations achieved from two, four, or seven directly observed doses of oral FTC/TDF per week correlated with 76%, 96%, or 99% efficacy in the iPrEx study, respectively (6). In contrast, the FEM-PrEP and the VOICE PrEP trials were stopped because of futility, and in both studies plasma drug concentrations revealed that <30% of participants were adherent to the dosing regimen (7, 8).
We hypothesize that long-acting (LA) anti-retroviral formulations requiring infrequent dosing could improve adherence and thus PrEP efficacy. GSK1265744 (GSK744) is an analog of dolutegravir, a recently approved integrase strand-transfer inhibitor with a favorable efficacy and safety profile in several treatment trials (9–15). About 2600 patient years of exposure (median duration of 590 days, range from 1 to 1031) were accrued during phase 2b to 3b dolutegravir clinical trial programs. GSK744 is potent in vitro with a median inhibitory concentration (IC50) of 0.22 nM against HIV-1 BAL in human peripheral blood mononuclear cells (PBMCs) (16). However, GSK744 is highly protein-bound, there-by shifting up the target IC50 408-fold in the presence of human serum (17). In infected patients treated daily with 5 or 30 mg of oral GSK744 monotherapy for 10 days, mean plasma trough concentrations exceeded the protein-adjusted levels that would block 90% of infections (PAIC90) by 3.5- and 20-fold, respectively, resulting in a 2.2- to 2.3-log10 decrease in plasma HIV-1 RNA without the emergence of drug resistance mutations (18).
GSK744’s high potency, low aqueous solubility, slow metabolism, and high melting point permitted its formulation as a 200-mg/ml LA injectable product. In healthy volunteers, GSK744 LA yielded an apparent terminal-phase half-life (t1/2) ranging from 21 to 50 days, compared with about 40 hours for a single oral dose (16, 18). This increase resulted from a slower release of drug from the injected nanosuspension rather than a change in the metabolic elimination rate. Safety analyses across six GSK744 oral studies and two GSK744 LA studies, dosing a total of 245 participants (65 females, 180 males; median age of 32 years), revealed drug-related adverse events (dizziness and grade 1 rash) in only two participants, without any drug-related grade 3 or 4 adverse events or deaths (19). Dose-independent injection site reactions were common after GSK744 LA administration, but these were generally well tolerated and self-limited (19, 20). Although both oral and parenteral formulations of GSK744 continue to be evaluated in additional clinical trials, the safety data to date support further clinical development.
The goal of this work was to evaluate GSK744 LA as a PrEP agent in macaques to establish proof of concept. In anticipation of performing a low-dose intrarectal (IR) challenge experiment, we first conducted a PK study to identify a dosing regimen that could achieve clinically relevant plasma concentrations. Indian rhesus macaques (Macaca mulatta) were injected intramuscularly (IM) at two time points 4 weeks apart with 10 mg/kg (n = 8) or 30 mg/kg (n = 4; two injections of 15 mg/kg) of GSK744 LA, but the mean plasma GSK744 concentrations (Fig. 1A) were not always above 4× PAIC90, a level found to be important for the therapeutic effect in infected patients (18). A single intramuscular dose of 200, 400, or 800 mg of GSK744 LA in healthy volunteers revealed dose-proportional increases in drug exposure [AUC(0-∞)] (Fig. 1B) (8, 21). The 800-mg dose of GSK744 LA resulted in sustained mean plasma concentrations >4× PAIC90 for 16 weeks (Fig. 1B). Because of injection volume limits, the 800-mg dose was administered as a split injection (two of 400 mg), resulting in a supraproportional maximum concentration (Cmax) compared with the 200- and 400-mg doses. This finding suggested a faster release of GSK744 into the circulation as a consequence of dose splitting. However, only a dose-proportional increase in Cmax was observed when the dose was split in macaques. The elimination t1/2 of GSK744 LA was much shorter in macaques, 3 to 12 days, compared with humans, which is often the case for drugs in smaller mammals (22). The faster clearance of GSK744 in macaques required that a higher dose of GSK744 LA be used to maintain clinically relevant plasma concentrations throughout the dosing interval in the virus prevention study.
Fig. 1. GSK744 LA PK profile in rhesus macaques and humans.
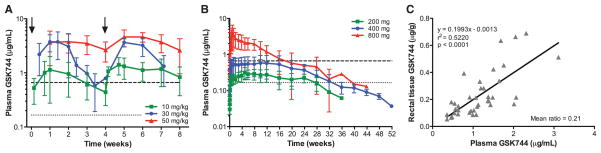
(A) GSK744 LA PKs were evaluated in rhesus macaques (n = 8) after intramuscular injections of 10 mg/kg into quadriceps as a single injection, 30 mg/kg (n = 4, two injections of 15 mg/kg) as a split injection, or 50 mg/kg (n = 8, four of 12.5 mg/kg) as a split dose with four injections, two per muscle. GSK744 LA was administered on weeks 0 and 4 (black arrows). (B) GSK744 LA single-dose PKs were evaluated in a phase 1 study, and the results were adapted here for comparison (16, 21). GSK744 LA was administered by intramuscular gluteal injection of 200 (n = 6), 400 (n = 14), or 800 (n = 6, two of 400 mg) mg to healthy human participants. Plasma GSK744 concentrations were assessed by liquid chromatography–mass spectrometry (HPLC-MS/MS) with a limit of quantitation (LOQ) >0.01 μg/ml. Means ± SDs are shown. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively. (C) Rectal tissue distribution of GSK744 was evaluated in macaques dosed with 10 or 30 mg/kg of GSK744 LA. The drug concentration from pinch mucosal biopsies was assessed each week in a subset (n = 4) of animals injected with 10 mg/kg. GSK744 concentration was assessed in all animals (n = 4) treated with 30 mg/kg of GSK744 LA at weeks 2, 4, and 7. Tissue concentrations were assessed by HPLC-MS/MS with LOQ > 0.05 μg/g. Each symbol represents the simultaneous plasma and rectal tissue concentrations of an individual macaque.
To understand drug distribution to the site of virus inoculation, we determined the levels of GSK744 in rectal biopsy tissues as compared with those observed in the plasma of macaques treated with 10 or 30 mg/kg of GSK744 LA. As expected, higher plasma concentrations correlated with higher tissue concentrations (Fig. 1C). The ratio of mean rectal tissue to plasma concentrations (T:P) was 0.21 (range from 0.08 to 0.54). The animals treated with GSK744 LA at 30 mg/kg were necropsied 3 weeks after the second dose, and various tissues were analyzed for GSK744 (table S1). The drug was consistently identified in various regions of the gastrointestinal tract (colon, ileum, jejunum, and duodenum) with T:P similar to that observed for rectal tissue. GSK744 was detected in all lymphoid tissues analyzed, including cervical, inguinal, mesenteric, and axillary lymph nodes as well as tonsil and spleen. The highest GSK744 concentration in tissue was found in the liver, which may be due to the uptake of nanoparticles by Kupffer cells (23). The muscle tissues at injection sites had the lowest GSK744 concentration, suggesting that the drug was largely released by the time of necropsy.
To evaluate the efficacy of GSK744 LA as PrEP, we used a repeat low-dose rectal challenge macaque model (24–28), which was developed to more closely mimic HIV-1 exposures in humans. Previously, using the same model, oral FTC/TDF demonstrated protection as observed in the iPrEx study among adherent volunteers (26). The earlier PK results from 10 and 30 mg/kg of GSK744 LA dosing indicated that a dose of 50 mg/kg would likely maintain plasma concentrations comparable to those in GSK744 LA-treated humans throughout the dosing period. Eight macaques were therefore injected IM with 50 mg/kg of GSK744 LA at two time points 4 weeks apart, starting 1 week before the first virus exposure (Fig. 2A), and an additional eight macaques remained untreated as controls. Both groups of macaques were challenged by nontraumatic inoculation of 1 ml of SHIV162P3 (50 TCID50) into the rectal vault through a sterile gastric feeding tube. The macaques were similarly challenged weekly for up to eight challenges or until infection was confirmed by real-time reverse transcription polymerase chain reaction amplification of viral gag sequences from plasma. GSK744 LA–treated animals remained aviremic as assessed by weekly measurements during the challenge period as well as the wash-out phase (Fig. 2B). In contrast, untreated macaques became infected during the challenge period, requiring a median of two challenges (range from 1 to 7; Fig. 2, B and C). GSK744 LA–treated macaques had a 28.2-fold [hazard ratio, 95% confidence interval (CI) 5.8, 136.8] lower risk of infection compared with untreated macaques (P < 0.0001, log-rank test).
Fig. 2. Monthly injection of GSK744 LA protects macaques against repeated SHIV exposure.
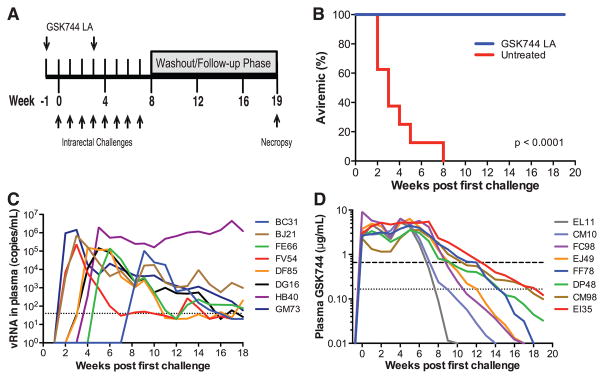
(A) Study design. Eight male macaques were injected IM in the quadriceps with 50 mg/kg (four of 12.5 mg/kg) of GSK744 LA at two time points, week –1 and 3. An additional eight male macaques were untreated and served as controls. All animals were challenged IR each week with 50 TCID50 of SHIV162P3 for up to eight exposures or until infection occurred. GSK744 LA–treated macaques were necropsied at week 19 for evaluation of proviral DNA in multiple tissues. (B) Kaplan-Meier plot of treated and untreated macaques remaining aviremic after serial SHIV challenges. (C) Viral loads of individual untreated control macaques. Dotted line represents the LOQ, >40 SHIV RNA copies per milliliter of plasma. (D) Plasma GSK744 concentrations in individual macaques throughout the course of study. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively. LOQ > 0.01 μg/ml.
As expected, dosing macaques with GSK744 LA at 50 mg/kg yielded mean drug exposure above 4× PAIC90 (Fig. 1A) throughout the period of virus challenge. The interanimal variability in PK (Fig. 2D) was anticipated on the basis of similar results observed in humans. All macaques, except EL11, maintained plasma drug concentrations >4× PAIC90 throughout the challenge period. GSK744 concentrations in the plasma of EL11 fell to 0.50 μg/ml for the last challenge (week 7), whereas the other macaques sustained plasma drug concentrations >4× PAIC90 through weeks 7 to 12. The t1/2 of GSK744 LA in monkeys was indeed shorter, ranging from 5 to 12 days. Nevertheless, the mean plasma GSK744 concentrations achieved in the protected macaques closely parallel plasma concentrations measured in humans administered a single, 800-mg intramuscular dose of GSK744 LA (fig. S1).
SHIV-specific antibodies in plasma were detected by means of an enzyme immunoassay in all untreated controls 1 to 3 weeks after viral RNA detection but were not detected in any GSK744 LA–treated macaques throughout the study (table S2). Proviral DNA was not identified in PBMCs from any drug-treated macaque throughout the study (table S2). Further virologic studies were performed to verify that the GSK744 LA–treated macaques were not locally infected despite lacking evidence of systemic viral dissemination. The macaques were necropsied 19 weeks after the first challenge, and tissues at the sites of virus inoculation and draining lymph nodes were analyzed for the presence of proviral DNA. To increase the sensitivity of the assay, we enriched rectum and colon tissues for mucosal mononuclear cells before analyzing the tissues for proviral DNA. No proviral DNA was detected in any of the samples, with the exception of the rectal mucosal mononuclear cells from EL11, where a positive signal below the limit of quantitation was observed (2 positive out of 20 wells assayed) (table S2). Although proviral DNA was detected at an extremely low level, a productive systemic infection was never established during the 9 weeks of follow-up when plasma GSK744 concentrations were below the limit of detection.
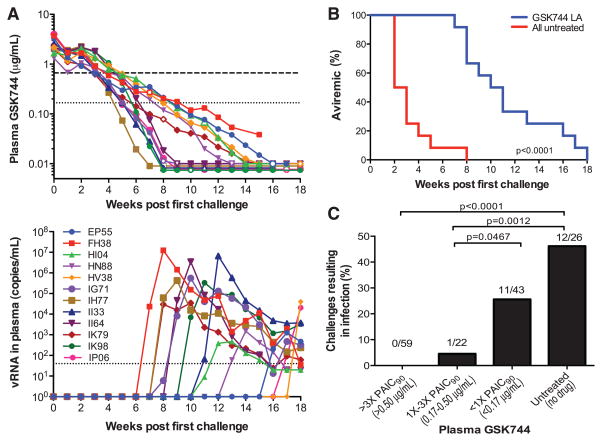
A follow-up experiment was then performed to determine the minimal drug level that affords protection against repeated low-dose IR SHIV challenges. Twelve macaques were injected IM with 50 mg/kg of GSK744 LA once 1 week before the first virus exposure. All macaques were challenged IR each week with 50 TCID50 of SHIV162P3 until systemic infection was detected. An additional four macaques remained untreated as controls. In anticipation of the long duration of the experiment, one control macaque began the series of virus challenges every 4 weeks. All control macaques became infected quickly, after 1 or 2 challenges, as evidenced by rising viremia (fig. S2). GSK744 LA–treated macaques remained aviremic throughout the initial phase of SHIV challenges, but as the plasma drug concentrations declined (Fig. 3A, top) they became infected, gradually and successively (Fig. 3A, bottom). Overall, the treated animals were infected after 6 to 17 (median of 10) virus challenges compared with 1 to 7 challenges (median of 2, based on data from untreated macaques from both experiments) for untreated controls (Fig. 3B). Thus, a single dose of GSK744 LA delayed infection by 5 to 10 challenges (median of 8) compared with untreated macaques. Assuming a 2-week eclipse phase between the start of infection and detection of viremia, we calculated plasma GSK744 levels that coincided with the start of SHIV infection (fig. S3). All infections occurred when the plasma drug concentrations were below 0.50 μg/ml, or ~3× PAIC90. A more detailed analysis was then carried out to calculate the percent of virus challenges that resulted in infection within various ranges of plasma GSK744 concentrations (Fig. 3C). The 12 GSK744 LA–treated macaques were collectively exposed to 59 SHIV challenges at plasma concentrations >3× PAIC90, and none resulted in infection. One of 22 challenges at plasma concentrations between 1× and 3× PAIC90 led to infection. As plasma GSK744 concentrations decreased below 1× PAIC90, 11 infections resulted from 43 virus challenges, yielding an infection rate of 25.6%. This value was lower than the 46.2% (12/26) infection rate observed in untreated macaques, but the difference was not statistically significant (P = 0.11, Fisher’s exact test). On the basis of the analysis shown in Fig. 3C, we determined that plasma GSK744 concentrations >3× PAIC90 conferred 100% protection, whereas concentrations ≥1× PAIC90 conferred ~97% protection (see legend for calculation). No signature integrase resistance-conferring mutations were identified in breakthrough viruses (table S3). This second experiment not only defined the correlate of protection but also confirmed the results of the first experiment by showing that sufficiently high but clinically achievable GSK744 concentrations could effectively protect all macaques from repeated IR SHIV challenges.
Fig. 3. Plasma GSK744 levels that protected against repeated SHIV exposures.
Twelve male macaques were injected IM with GSK744 LA at 50 mg/kg (four of 12.5 mg/kg) 1 week before the first virus exposure. Four macaques remained untreated as controls. All animals were challenged IR each week with 50 TCID50 of SHIV162P3 until infection was confirmed. (A) (Top) Plasma GSK744 concentrations from individual macaques. Open symbols correspond to first detection of viral RNA. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively. LOQ > 0.01 μg/ml. (Bottom) Viral loads of GSK744 LA–treated macaques. Dotted line represents the LOQ at >40 SHIV RNA copies per milliliter of plasma. (B) Kaplan-Meier plot demonstrating the delay of infection in GSK744 LA–treated macaques (n = 12) relative to untreated control macaques (n = 12; 4 from the current experiment and 8 from the first experiment). (C) Rate of SHIV infection within various ranges of GSK744 concentrations in plasma. The numbers above the bars represent the number of infections/number of challenges within a plasma concentration range. Plasma concentrations >3× PAIC90 yielded 100% protection, whereas concentrations >1× PAIC90 resulted in an infection rate of 1.2% (0/59 and 1/22). Compared with a 46.2% (12/26) infection rate in the placebo macaques, one can calculate a protective efficacy of ~97% in macaques with drug concentrations >1× PAIC90.
GSK744 LA appears to be a promising next-generation PrEP agent that has afforded high-level protection against repeated IR SHIV challenges in rhesus macaques (Figs. 2B and 3B). Plasma drug levels >3× PAIC90 provided 100% protection, whereas levels ≥1× PAIC90 provided ~97% protection (Fig. 3C). These plasma concentrations can be readily achieved in humans with quarterly 800-mg IM injections of GSK744 LA (Fig. 1B and fig. S1). Given that the half-life of GSK744 LA is 3 to 12 days in macaques whereas the half-life is 21 to 50 days in humans, we anticipate a longer-lasting protective effect in humans. Nevertheless, the proof will have to be demonstrated in future clinical trials. Optimistically, toxicity issues not withstanding, GSK744 LA has the potential to achieve in preventing HIV-1 infection what a long-acting contraceptive has achieved in preventing unintended pregnancies (29). It also stands to reason that the protective efficacy of GSK744 LA could approximate the high efficacy (>90%) observed in high-risk participants who were most adherent to daily oral FTC/TDF as PrEP (3, 6). These considerations, coupled with a favorable drug safety profile, have placed GSK744 LA on track for phase 2 (safety) clinical trials as well as a phase 3 (efficacy) study in men who have sex with men. Follow-up macaque experiments to protect against intra-vaginal and intravenous SHIV or SIV challenges could establish the proof of concept to move into efficacy trials in other populations at high risk for HIV-1 infection.
Supplementary Material
Acknowledgments
This work was funded in part by NIH grants R0-AI100724 and 1DP1-DA033263 and Tulane National Primate Research Center grant 2P51-OD11104-52. We thank D. A. Margolis, A. Rinehart, J. C. Pottage Jr., Y. Huang, K. Meyers, and N. Padte for helpful discussions; J. Blanchard for veterinary services; M. Boente-Carrera, L. Tsai, and F. Yu for technical assistance; and G. Bowers, Y. L. Yueh, G. Tabolt, and P. Savina for PK, analytical, and statistical support. W.R.S., L.M., and S.F. are full-time employees of and hold shares in GlaxoSmithKline, Z.H. is a full-time employee of and holds shares in GlaxoSmithKline and serves on the ViiV Healthcare Board, and D.D.H. is a paid consultant to GlaxoSmithKline. The GenBank accession numbers for integrase sequences described in table S3 are KJ415285 to KJ415297.
Footnotes
References and Notes
- 1.UNAIDS. 2013 UNAIDS Report on the Global AIDS Epidemic. 2013 www.unaids.org/en/resources/documents/2013/name,85053,en.asp.
- 2.Abdool Karim Q, et al. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, et al. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, et al. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, et al. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 6.Anderson PL, et al. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Damme L, et al. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrazzo J, et al. paper presented at the 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 3 to 6 March 2013.. [Google Scholar]
- 9.van Lunzen J, et al. Lancet Infect Dis. 2012;12:111–118. doi: 10.1016/S1473-3099(11)70290-0. [DOI] [PubMed] [Google Scholar]
- 10.Stellbrink HJ, et al. AIDS. 2013;27:1771–1778. doi: 10.1097/QAD.0b013e3283612419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raffi F, et al. Lancet. 2013;381:735–743. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 12.Raffi F, et al. Lancet Infect Dis. 2013;13:927–935. doi: 10.1016/S1473-3099(13)70257-3. [DOI] [PubMed] [Google Scholar]
- 13.Walmsley SL, et al. N Engl J Med. 2013;369:1807–1818. doi: 10.1056/NEJMoa1215541. [DOI] [PubMed] [Google Scholar]
- 14.Cahn P, et al. Lancet. 2013;382:700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 15.Eron JJ, et al. J Infect Dis. 2013;207:740–748. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spreen WR, Margolis DA, Pottage JC., Jr Curr Opin HIV AIDS. 2013;8:565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns BA, et al. J Med Chem. 2013;56:5901–5916. doi: 10.1021/jm400645w. [DOI] [PubMed] [Google Scholar]
- 18.Spreen W, et al. HIV Clin Trials. 2013;14:192–203. doi: 10.1310/hct1405-192. [DOI] [PubMed] [Google Scholar]
- 19.Lou Y, et al. paper presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Denver, CO. 10 to 13 September 2013.. [Google Scholar]
- 20.Spreen W, et al. paper presented at the 7th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Kuala Lumpur, Malaysia. 30 June to 3 July 2013.. [Google Scholar]
- 21.Spreen W, et al. paper presented at the 19th International AIDS Conference; Washington, DC. 22 to 27 July 2012.. [Google Scholar]
- 22.Mordenti J. J Pharm Sci. 1986;75:1028–1040. doi: 10.1002/jps.2600751104. [DOI] [PubMed] [Google Scholar]
- 23.Moghimi SM, Hunter AC, Murray JC. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 24.Subbarao S, et al. J Infect Dis. 2006;194:904–911. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 25.Subbarao S, et al. J Med Primatol. 2007;36:238–243. doi: 10.1111/j.1600-0684.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 26.García-Lerma JG, et al. PLOS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hessell AJ, et al. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García-Lerma JG, et al. Sci Transl Med. 2010;2:14ra4. doi: 10.1126/scitranslmed.3000391. [DOI] [PubMed] [Google Scholar]
- 29.Winner B, et al. N Engl J Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.