Abstract
Large-scale central facilities such as Diamond Light Source fulfil an increasingly pivotal role in many large-scale scientific research programmes. We illustrate these developments by reference to energy-centred projects at the University of Nottingham, the progress of which depends crucially on access to these facilities. Continuing access to beamtime has now become a major priority for those who direct such programmes.
Keywords: central facilities, research programmes, structural information, metal-organic frameworks, gas cell studies
1. Introduction
Although we made some earlier use of the Daresbury Synchrotron Radiation Source (SRS) [1,2], our sustained use of synchrotron facilities began in 1997 with the inauguration of the pioneering small-molecule single-crystal SRS Station 9.8. Our single-crystal work on Stations 9.8 and 16.2SMX (e.g. [3–5]) continued until the closure of the SRS in 2008. Since October 2008, we have been users of the new Diamond Light Source, primarily of the small-molecule single-crystal Beamline I19 but increasingly of the high-resolution powder diffraction Beamline I11. We have recently used both these beamlines not only to pursue ab initio structure determinations of new materials [6] but also to perform in situ gas cell studies [7,8]. Beamline I19 has hosted our high-pressure crystallographic studies, initially on mononuclear coordination complexes in Experimental Hutch 1 [9,10], but more recently on metal-organic framework (MOF) materials in Experimental Hutch 2. Complementary experiments to investigate the structures and properties of MOFs [11,12] have employed facilities at the ISIS Neutron Centre adjacent to Diamond, and the Institut Laue-Langevin (ILL) and the European Synchrotron Radiation Facility (ESRF), both located in Grenoble.
2. Research on metal-organic framework energy materials
In this section, we highlight examples to illustrate two of our main areas of current activity, namely hydrogen storage and carbon capture.
(a). Hydrogen storage
Over the past 15 years our approach to MOFs has evolved from generating and identifying new structural types and motifs [13] to the design, synthesis and characterization of highly promising energy materials (figure 1). Central to these studies has been the search for materials capable of storing hydrogen (H2) which has undoubted attractions as a fuel because of its abundance, high energy density and low environmental impact, the only waste product from its combustion being water. Despite these advantages, the development of a viable system to store H2 for automotive applications presents a series of challenges involving the weight of the storage system, the operating temperature and the distance that the vehicle can travel before requiring refuelling. A storage system achieving 5.5 wt% H2 storage (US DOE 2015 target [14]) is considered viable for transport, but because this criterion includes the weight of the container and the necessary control systems, the capacity of the storage medium must be correspondingly higher. As an important class of crystalline coordination polymers which consist of metal centres bridged by organic linkers, MOFs show considerable promise for H2 storage, in part because of their high surface areas and tuneable properties. An important feature of MOFs is the predominance of light atoms (e.g. C, N, O) in the organic linker, which means that a MOF with a heavier element as the metal centre does not render the material impractically heavy overall: as a result, a very wide range of metal centres and thereby MOF structures can be investigated.
Figure 1.
Representations of a copper paddlewheel MOF (NOTT-112) designed for high-capacity hydrogen storage. (a) Differently sized cages indicated by coloured spheres and (b) tiling of the cages shown according to the same colouring scheme.
Two key aims of the research are to develop MOFs with increased H2 storage capacity and to develop systems which can store H2 at or near ambient temperature, which are desirable targets for any storage medium. Our work has shown that the pore environment can be controlled by factors such as cation exchange, allowing the design of pore gates to control the movement of gas; a key discovery was that it was possible to combine high H2 storage capacity and high binding energy, showing how potentially viable onboard H2 storage could be achieved [12,15,16].
(b). Carbon capture
According to the Tyndall Centre for Climate Change Research at the University of East Anglia, UK, global CO2 emissions from the consumption of fossil fuels increased to 36 billion tonnes in 2013, while the same year saw the average atmospheric concentration of CO2 exceed (albeit briefly) 400 ppm for the first time since measurements began [17]. With increased CO2 emissions being widely implicated in anthropogenic global warming and associated climate disruption, there is a pressing need for technologies to capture at least some of the CO2 emissions. The existing technology involves the use of amine solutions to capture carbon dioxide but this incurs certain penalties: there is an environmental penalty through the use of monoethanolamine (MEA) which is toxic, flammable and highly corrosive, while a large energy penalty arises from the heating required to drive off the carbon dioxide and regenerate the scrubbing solutions [18,19].
Having synthesized a polycrystalline product by a hydrothermal reaction between biphenyl-3,3′,5,5′-tetracarboxylic acid and Al(NO3)3⋅9H2O in water containing HNO3, we turned to Beamline I11 at Diamond for the collection of high-resolution powder diffraction data, from which the structure of NOTT-300(Al) could be solved and subsequently achieve a satisfactory Rietveld refinement. The structure features Al(III) ions bound to six O donors: four arise from carboxylate groups, two from bridging hydroxyl groups. Corner-sharing [AlO4(OH)2] octahedra are linked into chains through two mutually cis-μ2-OH groups. These chains are bridged by tetracarboxylate ligands, giving a porous three-dimensional framework structure with one-dimensional channels. Desolvated NOTT-300(Al) shows excellent selectivity for CO2 (and SO2) versus other gases including N2, CH4, CO, Ar, O2 and H2 (figure 2). A combined study of CO2-loaded NOTT-300(Al) using powder X-ray diffraction, inelastic neutron scattering and modelling showed that hydroxyl groups within the pores bind CO2. There are two types of hydrogen bond, Al–OH⋅⋅⋅O−C−O (I) and C−H⋅⋅⋅O−C−O (II), and five interactions in total. This array of supramolecular hydrogen bonds provides an explanation for the high selectivity of NOTT-300(Al) towards CO2 and for the ease with which it can be recovered [8]. By elucidating and understanding the dynamics of the CO2 capture mechanism, we are well-placed to design better carbon-capture systems.
Figure 2.

Illustration of the selective capture of CO2 from a gas mixture by a MOF represented by the green framework.
3. Collaboration between Nottingham and Diamond
The School of Chemistry at The University of Nottingham possesses a suite of equipment for X-ray diffraction studies, including three single-crystal diffractometers: one of these is equipped with dual-wavelength copper/molybdenum microfocus sources while two others have advanced copper X-ray sources. In excess of 1000 samples, covering broad ranges of both chemistry and sample quality, are examined on these systems each year. Despite this, we regularly apply for an allocation of single-crystal data collections at the EPSRC National Crystallography Service (NCS) based at the University of Southampton, which allows us to access their high-intensity rotating anode X-ray sources to enable the study of samples that are beyond the capabilities of our own instrumentation. For samples that exceed even these capabilities, the NCS provides transparent access to Beamline I19 at Diamond (figure 3). We have also gained access to Diamond beamlines through our peer-reviewed proposals using the Block Allocation Group (BAG), direct access and rapid access routes, principally but not exclusively for structural studies on MOF energy materials. Our collaboration with Diamond was highlighted by one of us (S.Y.) winning the inaugural 2011 Diamond Young Scientist Award.
Figure 3.
Relationship of Diamond to local and EPSRC national facilities for single-crystal structure analysis. (online version in colour.)
4. Continuing and future activities
Our principal interaction with Diamond will continue to centre on the characterization of important newly synthesized materials from single-crystal samples which are challenging because of pervasive factors such as sample size, limited crystallinity, poor ordering and high solvent content. We anticipate that structural results from Beamline I19 will continue to feature prominently in our high-profile publications, as they have done in the recent past [6–8,11,15,20–29].
We are keenly interested in exploiting upcoming developments on I19 such as robotic handling and an enhanced detector system capable of high throughput, as we believe these will bring major benefits to our research programmes. The availability of a new generation of photon-counting area detectors, such as the Pilatus detector developed by Dectris, has revolutionized macromolecular crystallography. They have brought about a step-change in the speed at which single-crystal diffraction data can be collected, as they can be operated in a continuous, shutterless scan, unlike the more conventional CCD detector. The Pilatus detector also has a significantly greater dynamic range, allowing extremely weak reflections to be recorded on the same image as strong reflections without the need for sets of retaken images (acquired with either shorter exposure times or attenuation of the incident beam). These advantages can be readily applied to small-molecule crystallography and a Pilatus detector will be a key feature of the forthcoming upgrade to Beamline I19. As data collections become much faster, a greater number of samples can be run and sample changes will need to be performed more efficiently to make best use of limited beam time. The use of a robotic sample changer, therefore, will become extremely important and new methods for storing air-sensitive samples, or samples vulnerable to solvent loss, will need to be implemented so that crystals can be stored for extended periods of time without degradation. This will be particularly important for MOF systems which can deteriorate rapidly once removed from their mother liquor and mounted on a sample loop. The Nottingham group has been involved in the commissioning of the robotic sample changer for these more challenging systems.
Other work on Beamline I19 will involve in situ gas cell studies of single crystals and will include the development of suitable sample cells. For example, we have determined the locations of CO2 molecules within a hydroxyl-functionalized MOF material NOTT-300(Ga) which is isostructural with NOTT-300(Al) described above. In its solvated form NOTT-300(Ga)-solv crystallizes in the chiral space group I4122 and shows a three-dimensional open-framework structure constructed from helical 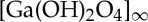 chains bridged by tetracarboxylate ligands (figure 4). The Ga(III) ion is octahedrally coordinated via six O donors, four of them from carboxylate groups and two O donors from μ2-OH bridging hydroxyl groups arranged in a mutually cis configuration. This form of connectivity leads to the formation of a square one-dimensional pore channel running through the framework along the c-axis. The structural results allow us to rationalize the high binding energy and high CO2 uptake capacity observed for this material.
chains bridged by tetracarboxylate ligands (figure 4). The Ga(III) ion is octahedrally coordinated via six O donors, four of them from carboxylate groups and two O donors from μ2-OH bridging hydroxyl groups arranged in a mutually cis configuration. This form of connectivity leads to the formation of a square one-dimensional pore channel running through the framework along the c-axis. The structural results allow us to rationalize the high binding energy and high CO2 uptake capacity observed for this material.
Figure 4.
Views of single-crystal structures for NOTT-300(Ga)-solv (a,d), NOTT-300(Ga) (b,e), NOTT-300(Ga)⋅2.35CO2 (c,f). The guest molecules (DMF, water, CO2) in the pore channels are highlighted in the ball-stick model (Ga green, C grey; O red, H white, N blue). The carbon atom of CO2II is highlighted in orange. The hydrogen bond interaction between the guest molecule and free OH group is highlighted in purple. The independent electrostatic dipole interactions between CO2 molecules are highlighted in cyan and green.
Beamline I19 has played a pivotal role in the recent development and exploitation of high-pressure crystallographic methods and it has already contributed to landmark high-pressure investigations of well-known MOFs [30–35]. We have begun studies of the behaviour of our own more flexible MOFs, several of which possess outstanding gas storage or separation properties, at pressures of up to 100 kbar. The unique facilities of EH2, including the high brightness of the mirror-focused X-ray beam, have already allowed us to collect data from some challenging samples. The heavy-duty diffractometer housed in EH2 has an integral motorized sample stage which allows a crystal contained within the diamond-anvil cell to be placed accurately within the X-ray beam. This accurate centring, coupled with the mechanical stiffness of the diffractometer, allows smaller, focused, beam sizes to be used, which reduces background and allows more weakly scattering samples, such as our MOFs, to be studied.
Another broad area of study will involve the application of powder X-ray diffraction techniques. These will involve the characterization of energy materials, their ab initio structure determination from high-resolution powder diffraction data, in situ gas cell studies on polycrystalline samples and long duration experiments. The successful determination of the structure of NOTT-300(Al) from data collected on Beamline I11 [8] exploited a highly effective alternative technique when we faced insuperable difficulties obtaining suitable single crystals of this material. NOTT-300(Al) was an attractive candidate for structure solution from powder data, as it possessed high symmetry (tetragonal, space group I4122) and had a relatively modest unit cell volume of ca 2600 Å3, but materials with lower symmetry and/or larger unit cells will present greater challenges even if they exhibit comparable crystallinity.
By contrast, powder diffraction offers a number of distinct advantages over single crystal methods for in situ gas cell studies: these include the smaller particle sizes which lead to more rapid equilibration following a change in gas pressure or composition, allowing improved time resolution; a large sample volume which is favourable for counting statistics and therefore rapid data collection; the ability to study materials that undergo phase transitions that might destroy or degrade a single crystal; and a simpler, more open diffraction geometry which facilitates the use of the gas cell (figure 5) [36].
Figure 5.
Beamline I11 gas pressure system and capillary sample cells: (a) the gas supply and control system which has a working pressure range from 0 mbar to 100 bar; (b) the low-pressure cell; (c) the low-pressure gas cell mounted on the I11 goniometer, with the red arrow indicating the direction of a cooling stream from an open-flow cryostat or heating by a hot-air blower; (d) a view of the high-pressure cell, with the inset showing an expanded view of a MOF sample in a sapphire capillary (arrowed). (Online version in colour.)
Our aim in such experiments is typically to obtain high-resolution powder diffraction data on porous framework materials as a function of temperature and of the loading of environmentally significant gases such as CO2, SO2 and NO2. These data allow us to identify preferred binding sites within the pore and thereby understand the mechanisms and processes through which these porous framework materials capture, retain and release the gases, leading to advances in the design and development of new materials for CO2 storage, toxic gas removal and energy storage.
We are interested in continuing our research using the existing facilities on Beamline I11 for high-resolution and fast time-resolved studies, but we also regard the new Long Duration Experiment facility on I11 as highly relevant. This facility [37] is designed for experiments which require extended periods (weeks or months) to take to completion but need only occasional monitoring. It will allow the investigation of relatively slow changes which do not occur within the timespan of a standard experiment. Our interests will focus on the long-term behaviour and stability of our new materials for gas capture and storage, under both ambient (storage/resting) and in operando conditions. The ability to monitor the stability and storage capacity of these materials through their life cycle will allow us to assess the viability of gas storage and carbon-capture devices based around them. Where otherwise promising materials fail to exhibit sufficient long-term stability, we will establish the mechanisms responsible for this and design the necessary stability into the next generation of materials. The facility will also be of benefit to the development of fuel cells where important information on the development of phases over time can only be obtained via long-term experiments. An active area of research for fuel cells is through the use of solid oxide (SOFC) and proton exchange membrane (PEMFC) designs. Synchrotron X-ray powder diffraction is already being used in fuel cell research to study the SOFC anode and cathode materials, and other potential new materials. However, more progress could be made through the study of active fuel cells over extended periods so that changes in structure and composition that occur during operation can be more fully understood.
There is also increasing interest in the development of materials that undergo a phase change, through dissolution or melting, for heat storage and release in both domestic and industrial applications. Although much of the research in this area is in the development of new materials with increased levels of released energy density, the new I11 facility will provide much-needed information on the evolution of energy-storage efficiency of the materials over many cycles and how the long-term chemical stability can be attained.
The new I11 facility will also be of benefit for studies on the long-term structural changes in materials owing to radiation exposure, high-temperature or catalytic damage in the nuclear or catalysis industries. The disposal and storage of waste (containment and spent ion-exchange materials) produced by nuclear reactors is a pressing example of a long-term issue which could be better understood by obtaining data from extended studies on I11.
Although we have focused on work carried out on Beamlines I11 and I19, other beamlines have supported users studying MOF materials. One example is Beamline B18 (Core EXAFS) which employs X-ray absorption spectroscopy to provide element-specific data on the local geometry and chemical nature of absorbing atoms. The technique has the advantage that it is equally applicable to ordered crystalline materials and extensively disordered, amorphous, liquid or gaseous samples. It supports a broad range of research programmes which require information on the local structure and electronic state of active components, fluids, amorphous and crystalline solids, surfaces and biomaterials. As part of this portfolio B18 has supported users looking at MOF materials, including some based on MIL-53 [38,39], where X-ray absorption near edge structure (XANES) spectroscopy at the metal K-edges was used to establish the oxidation states of the metal centres. Such techniques are poised to exert a major influence on dynamic structural studies over the next decade and will continue to be developed and expanded at Diamond.
5. The expanding role of Diamond
Central facilities now play a crucial role in many large, important projects, and this represents a major change in outlook and culture over the past 15–20 years. Prior to this, for example, non-macromolecular single crystal studies using synchrotron radiation were rare and depended on gaining occasional access to beamlines dedicated to other applications [1,2,40].
Such a situation clearly could not support any expansion in synchrotron chemical crystallography, but the inauguration in 1997 of Station 9.8 at the Daresbury SRS [41] marked a watershed. Not only did it provide superb facilities for single-crystal X-ray structural analysis, it also recognized the (future) importance and wide relevance of such a dedicated resource accessible by peer review. The same role, greatly enhanced and diversified, is now fulfilled by Beamline I19 at Diamond.
Large energy-centred research programmes in Nottingham with greater than £15 m of funding depend directly on access to these facilities, particularly at Diamond, but we are in no sense unique in this regard: for example, major research efforts based in Cardiff, Liverpool, St Andrews and Sheffield are among those benefitting from regular beamtime at Diamond.
6. Conclusion
Continuing access to beamtime has now become a major priority for multidisciplinary research programmes, because the results drive (and sometimes redirect) the development of projects and accelerate progress. They also accelerate the progress of the programmes. One indication of this need is the significant number of Programme Mode and BAG awards on beamlines such I11 and I19, offering beamtime for an extended period of 2 years.
Acknowledgements
Collaborators. Nottingham—Neil Champness, Robert Mokaya, Xiang Lin, Yan Yong, Chenrong Tan, Junhua Jia, Ilich Ibarra, Stephen Argent, Wenbin Yang, Alex Greenaway, Jessica Gould, Chris Morris, Mathew Savage, Harprit Athwal, Claire Wilson, William Lewis; Diamond—Sarah Barnett, Harriott Nowell, Julia Parker, Claire Murray; Daresbury—Pierre Rizkallah, Simon Teat, John Warren; ISIS—William I. F. David, Samantha Callear, Anibal Cuesta-Ramirez, Pascal Manuel. Facilities. Diamond, ESRF, ISIS, SRS.
Funding statement
EPSRC, European Research Council, Royal Society Merit Award, Wolfson Foundation, The University of Nottingham, Marie Curie Fellowships, Marie Curie EST Fumassec, INTAS, CVCP ORS Awards, Leverhulme Trust, Dorothy Hodgkin Postgraduate Awards, Royal Society.
References
- 1.Andrews SJ, Blake AJ, Franklin KR, Harding MM, Helliwell JR, Lowe BM, McMeeking R, Papiz MZ. 1988. Piperazine silicate (EU19): the structure of a very small crystal determined with synchrotron radiation. Acta Crystallogr. B 44, 73–77. ( 10.1107/S0108768187009820) [DOI] [Google Scholar]
- 2.Clegg W, Birkby SL, Banister AJ, Rawson JM, Wait ST, Rizkallah P, Harding MM, Blake AJ. 1994. Structures of [PhCNSSN]2[Pt(mnt)2] and [p-ClC6H4CNSSN)2Cl][Pt(mnt)2] (mnt = Maleonitriledithiolato Ligand). Acta Crystallogr. C 50, 28–33. ( 10.1107/S0108270193006651) [DOI] [Google Scholar]
- 3.Withersby MA, Blake AJ, Champness NR, Cooke PA, Hubberstey P, Realf AL, Teat SJ, Schröder M. 2000. Engineering of co-ordination polymers of trans-4,4′-azobis(pyridine) and trans-1,2-bis(pyridin-4-yl)ethene: a range of interpenetrated network motifs. J. Chem. Soc. Dalton Trans. 2000, 3261–3268. ( 10.1039/b006543i) [DOI] [Google Scholar]
- 4.Khlobystov AN, Brett MT, Blake AJ, Champness NR, Gill PMW, O’Neil D, Teat SJ, Wilson C, Schröder M. 2003. Stereoselective association of binuclear metallacycles in coordination polymers. J. Am. Chem. Soc. 125, 6753–6761. ( 10.1021/ja029048y) [DOI] [PubMed] [Google Scholar]
- 5.Tei L, et al. 2003. Coordination chemistry of a new cofacial binucleating macropolycycle derived from 1,4,7-triazacyclononane. Inorg. Chem. 42, 8690–8701. ( 10.1021/ic0345065) [DOI] [PubMed] [Google Scholar]
- 6.Yang S, et al. 2012. A partially-interpenetrated metal-organic framework for selective hysteretic sorption of carbon dioxide. Nat. Mater. 11, 710–716. ( 10.1038/nmat3343) [DOI] [PubMed] [Google Scholar]
- 7.Yang W, et al. 2012. Selective CO2 uptake and inverse CO2/C2H2 selectivity in a dynamic bi-functional metal-organic framework. Chem. Sci. 3, 2993–2999. ( 10.1039/c2sc20443f) [DOI] [Google Scholar]
- 8.Yang S, et al. 2012. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nat. Chem. 4, 887–894. ( 10.1038/nchem.1457) [DOI] [PubMed] [Google Scholar]
- 9.Wong HLS, Allan DR, Champness NR, McMaster J, Schröder M, Blake AJ. 2013. Bowing to the pressure of π … π interactions: bending of phenyl rings in a palladium(II) thioether crown complex. Angew. Chem. 52, 5093–5095. ( 10.1002/anie.201209845) [DOI] [PubMed] [Google Scholar]
- 10.Allan DR, et al. 2014. High pressure studies of six palladium and platinum thioether dihalide complexes. Acta Crystallogr. B 70, 469–486. ( 10.1107/S2052520614008786) [DOI] [PubMed] [Google Scholar]
- 11.Yang S, et al. 2013. Irreversible network transformation in a dynamic porous host catalysed by sulphur dioxide. J. Am. Chem. Soc. 135, 4954–4957. ( 10.1021/ja401061m) [DOI] [PubMed] [Google Scholar]
- 12.Yang S, Callear SK, Ramirez-Cuesta TAJ, David WIF, Sun J, Blake AJ, Champness NR, Schröder M. 2011. Pore with gate: modulating hydrogen storage in metal organic framework materials via cation exchange. Faraday Discuss. 151, 19–36. ( 10.1039/c1fd00006c) [DOI] [PubMed] [Google Scholar]
- 13.See for example, Blake AJ, Champness NR, Hubberstey P, Li W-S, Withersby MA, Schröder M. 1999. Inorganic crystal engineering using self-assembly of tailored building-blocks. Coord. Chem. Rev. 183, 117–138. ( 10.1016/S0010-8545(98)00173-8) [DOI] [Google Scholar]
- 14.US Department of Energy. Hydrogen storage. 2011. See www1.eere.energy.gov/hydrogenandfuelcells/storage/current_technology.html. (accessed 26 August 2011) [Google Scholar]
- 15.Yang S, Martin GSB, Titman JJ, Blake AJ, Allan DR, Champness NR, Schröder M. 2011. Pore with gate: enhancement of the isosteric heat of adsorption of dihydrogen via post-synthetic cation exchange. Inorg. Chem. 50, 9374–9384. ( 10.1021/ic200967b) [DOI] [PubMed] [Google Scholar]
- 16.Yang S, Lin X, Champness NR, Schröder M. 2010. Gas storage—pore with gate. WO 2010133891 Nottingham, UK: The University of Nottingham. [Google Scholar]
- 17.National Oceanic Atmospheric Administration. Trends in atmospheric carbon dioxide. See http://www.esrl.noaa.gov/gmd/ccgg/trends/weekly.html. [Google Scholar]
- 18.Villiers C, Dognon JP, Pollet R, Thuery P, Ephritikhine M. 2010. An isolated CO2 adduct of a nitrogen base: crystal and electronic structures. Angew. Chem. Int. Ed. 49, 3465–3468. ( 10.1002/anie.201001035) [DOI] [PubMed] [Google Scholar]
- 19.Rochelle GT. 2009. Amine scrubbing for CO2 capture. Science 325, 1652–1654. ( 10.1126/science.1176731) [DOI] [PubMed] [Google Scholar]
- 20.Yan Y, et al. 2010. Metal-organic polyhedral frameworks: high H2 adsorption capacities and neutron powder diffraction studies. J. Am. Chem. Soc. 132, 4092–4094. ( 10.1021/ja1001407) [DOI] [PubMed] [Google Scholar]
- 21.Blake AJ, Champness NR, Cowan AJ, Easun TL, Allan DR, Nowell H, George MW, Jia J, Sun X-Z. 2010. Photoreactivity examined through incorporation in metal-organic frameworks. Nat. Chem. 2, 688–694. ( 10.1038/nchem.681) [DOI] [PubMed] [Google Scholar]
- 22.Tan C, Yang S, Champness NR, Lin X, Blake AJ, Lewis W, Schröder M. 2011. High capacity gas storage by a 4,8-connected metal-organic polyhedral framework. Chem. Commun. 47, 4487–4489. ( 10.1039/c1cc10378d) [DOI] [PubMed] [Google Scholar]
- 23.Ibarra IA, et al. 2011. Highly porous and robust scandium-based metal-organic frameworks for hydrogen storage. Chem. Commun. 47, 8304–8306. ( 10.1039/c1cc11168j) [DOI] [PubMed] [Google Scholar]
- 24.Yan Y, Yang S, Blake AJ, Lewis W, Poirier E, Barnett SA, Champness NR, Schröder M. 2011. A mesoporous metal-organic framework constructed from a nanosized C3-symmetric linker and [Cu24(isophthalate)24] cuboctahedra. Chem. Commun. 47, 9995–9997. ( 10.1039/c1cc13170b) [DOI] [PubMed] [Google Scholar]
- 25.Yan Y, Suetin M, Bichoutskaia E, Blake AJ, Allan DR, Barnett SA, Schröder M. 2013. Modulating the packing of [Cu24(isophthalate)24] cuboctahedra in a triazole-containing metal-organic polyhedral framework. Chem. Sci. 4, 1731–1736. ( 10.1039/c3sc21769h) [DOI] [Google Scholar]
- 26.Yan Y, Yang S, Blake AJ, Schröder M. 2014. Studies on metal-organic frameworks of Cu(II) with isophthalate linkers for hydrogen storage. Acc. Chem. Res. 47, 296–307. ( 10.1021/ar400049h) [DOI] [PubMed] [Google Scholar]
- 27.Yan Y, Blake AJ, Lewis W, Barnett SA, Dailly A, Champness NR, Schröder M. 2011. Modifying cage structures in metal-organic polyhedral frameworks for H2 storage. Chemistry 17, 11162–11170. ( 10.1002/chem.201101341) [DOI] [PubMed] [Google Scholar]
- 28.Ibarra IA, et al. 2010. Structures and H2 adsorption properties of porous scandium metal-organic frameworks. Chemistry 46, 13671–13679. ( 10.1002/chem.201000926) [DOI] [PubMed] [Google Scholar]
- 29.Ibarra IA, Bayliss P, Pérez E, Yang S, Blake AJ, Nowell H, Allan DR, Poliakoff M, Schröder M. 2012. Near-critical water, a cleaner solvent for the synthesis of a metal-organic framework. Green Chem. 14, 117–122. ( 10.1039/c1gc15726d) [DOI] [Google Scholar]
- 30.Graham AJ, Tan J-C, Allan DR, Moggach SA. 2012. The effect of pressure on Cu-btc: framework compression vs. guest inclusion. Chem. Commun. 48, 1535–1537. ( 10.1039/c1cc16045a) [DOI] [PubMed] [Google Scholar]
- 31.Moggach SA, Graham AJ, Muszkiewicz A, Morrison CA. 2011. High pressure studies of metal organic framework materials. Int. J. Nanotechnol. 9, 18–22. ( 10.1504/IJNT.2012.044827) [DOI] [Google Scholar]
- 32.Fairen-Jimenez D, Moggach SA, Wharmby MT, Wright PA, Parsons S, Düren T. 2011. Opening the gate: framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 133, 8900–8902. ( 10.1021/ja202154j) [DOI] [PubMed] [Google Scholar]
- 33.Moggach SA, Bennett TD, Cheetham AK. 2009. The effect of pressure on ZIF-8: increasing pore size with pressure and the formation of a high-pressure phase at 1.47 GPa. Angew. Chem. Int. Ed. 48, 7087–7089. ( 10.1002/anie.200902643) [DOI] [PubMed] [Google Scholar]
- 34.Graham AJ, Allan DR, Muszkiewicz A, Morrison CA, Moggach SA. 2011. The effect of high pressure on MOF-5: guest-induced modification of pore size and content at high pressure. Angew. Chem. Int. Ed. 50, 11138–11141. ( 10.1002/anie.201104285) [DOI] [PubMed] [Google Scholar]
- 35.Bennett TD, Simoncic P, Moggach SA, Gozzo F, Macchi P, Keen DA, Tan J-C, Cheetham AK. 2011. Reversible pressure-induced amorphization of a zeolitic imidazolate framework (ZIF-4). Chem. Commun. 47, 7983–7985. ( 10.1039/c1cc11985k) [DOI] [PubMed] [Google Scholar]
- 36.Parker JE, Potter J, Thompson SP, Lennie AR, Tang CC. 2012. In situ gas supply system on the powder diffraction Beamline I11 at Diamond Light Source. Mater. Sci. Forum 706–709, 1707–1712. ( 10.4028/www.scientific.net/MSF.706-709.1707) [DOI] [Google Scholar]
- 37.Diamond Light Source. Design Concept: I11 Long Duration Experiment (LDE) Facility. See http://www.diamond.ac.uk/Home/Beamlines/I11/LDE_Upgrade
- 38.Munn AS, Clarkson GJ, Millange F, Dumont Y, Walton RI. 2013. M(II) (M = Mn, Co, Ni) variants of the MIL-53-type structure with pyridine-N-oxide as a Co-ligand. CrystEngComm 15, 9679–9687. ( 10.1039/c3ce41268g) [DOI] [Google Scholar]
- 39.Breeze MI, Clet G, Campo BC, Vimont A, Daturi M, Greneche J-M, Dent AJ, Millange F, Walton RI. 2013. Isomorphous substitution in a flexible metal-organic framework: mixed-metal, mixed-valent MIL-53 type materials. Inorg. Chem. 52, 8171–8182. ( 10.1021/ic400923d) [DOI] [PubMed] [Google Scholar]
- 40.Harding MM. 1996. Recording diffraction data for structure determination for very small crystals. J. Synchrotron Rad. 3, 250–259. ( 10.1107/S090904959600862X) [DOI] [PubMed] [Google Scholar]
- 41.Clegg W. 2000. Synchrotron chemical crystallography. J. Chem. Soc. Dalton Trans. 2000, 3223–3232. ( 10.1039/b004136j) [DOI] [Google Scholar]






