Abstract
BACKGROUND
It is unclear whether high-density lipoprotein (HDL) cholesterol concentration plays a causal role in atherosclerosis. A more important factor may be HDL cholesterol efflux capacity, the ability of HDL to accept cholesterol from macrophages, which is a key step in reverse cholesterol transport. We investigated the epidemiology of cholesterol efflux capacity and its association with incident atherosclerotic cardiovascular disease outcomes in a large, multiethnic population cohort.
METHODS
We measured HDL cholesterol level, HDL particle concentration, and cholesterol efflux capacity at baseline in 2924 adults free from cardiovascular disease who were participants in the Dallas Heart Study, a probability-based population sample. The primary end point was atherosclerotic cardiovascular disease, defined as a first nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization or death from cardiovascular causes. The median follow-up period was 9.4 years.
RESULTS
In contrast to HDL cholesterol level, which was associated with multiple traditional risk factors and metabolic variables, cholesterol efflux capacity had minimal association with these factors. Baseline HDL cholesterol level was not associated with cardiovascular events in an adjusted analysis (hazard ratio, 1.08; 95% confidence interval [CI], 0.59 to 1.99). In a fully adjusted model that included traditional risk factors, HDL cholesterol level, and HDL particle concentration, there was a 67% reduction in cardiovascular risk in the highest quartile of cholesterol efflux capacity versus the lowest quartile (hazard ratio, 0.33; 95% CI, 0.19 to 0.55). Adding cholesterol efflux capacity to traditional risk factors was associated with improvement in discrimination and reclassification indexes.
CONCLUSIONS
Cholesterol efflux capacity, a new biomarker that characterizes a key step in reverse cholesterol transport, was inversely associated with the incidence of cardiovascular events in a population-based cohort.
A low level of high-density lipoprotein (HDL) cholesterol is a major independent risk factor for atherosclerotic cardiovascular disease.1 However, in randomized, controlled trials, high-dose niacin or inhibitors of cholesteryl ester transfer protein did not improve cardiovascular outcomes despite significantly increasing the HDL cholesterol level.2–5 Furthermore, genetic variants associated with HDL cholesterol levels are often not associated with cardiovascular disease.6 These observations suggest that HDL cholesterol may not be causally associated with cardiovascular disease, and they highlight the potential limitations of using the HDL cholesterol level to assess risk or responses to therapies targeted at HDL cholesterol.
HDL has numerous antiatherosclerotic actions that are not readily reflected by HDL cholesterol levels.7 A key function of HDL is to promote reverse cholesterol transport from the periphery to the liver, and the critical initial step in reverse cholesterol transport is cholesterol efflux from macrophages to HDL.8 Macrophage-specific cholesterol efflux capacity has been directly and causally linked to the prevention of atherosclerosis in animal models.8
The ability to assess the clinical relevance of reverse cholesterol transport in humans has been limited thus far. Recently, however, strategies to measure cholesterol efflux capacity have been used successfully in clinical studies, revealing inverse correlations between cholesterol efflux capacity and prevalent coronary artery disease, independently of the HDL cholesterol level.9,10 It is not known whether cholesterol efflux capacity is associated with incident cardiovascular events (i.e., events occurring after time of sample collection) in unselected persons from the population. It is also not known whether sex, race, adiposity, relative insulin sensitivity or resistance, or inflammation influences cholesterol efflux capacity. In a large, unselected, probability-based population cohort free from clinical cardiovascular disease at baseline, we investigated the epidemiology of cholesterol efflux capacity and evaluated the association of cholesterol efflux capacity with incident cardiovascular outcomes.
METHODS
STUDY DESIGN
The Dallas Heart Study is a multiethnic, population-based cohort study that includes residents of Dallas County.11 This random probability sample includes intentional oversampling of black persons to make up 50% of the cohort. Participants 30 to 65 years of age underwent fasting blood and urine collection as well as dual-energy x-ray absorptiometry to assess body composition, detailed cardiovascular phenotyping by means of electron-beam computed tomography and magnetic resonance imaging (MRI) of the heart, and MRI of the abdomen to evaluate body-fat distribution. Persons with a history of cardiovascular disease (self-reported history of myocardial infarction, stroke, arterial revascularization, heart failure, or arrhythmia) or niacin use were excluded, as were persons who died within 1 year after enrollment. Details of risk-factor assessments and other measurements are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
ASSESSMENT OF LIPID VARIABLES AND EFFLUX CAPACITY
Blood collected from all the participants at baseline by means of venipuncture was placed into EDTA tubes, stored at 4°C for less than 4 hours, and centrifuged, and plasma was removed and stored at −70°C. Plasma lipids, including HDL cholesterol, were measured as described previously.11 HDL particle concentration and size were measured by means of nuclear magnetic resonance spectroscopy (LipoScience).
Cholesterol efflux capacity was assessed by measuring the efflux of fluorescence-labeled cholesterol from J774 macrophages to apolipoprotein B–depleted plasma in study participants with the use of a previously described method.12 This assay primarily evaluates cholesterol efflux as mediated by ATP-binding cassette transporter A1 (ABCA1). The fluorescence-labeled reagent, termed boron dipyrromethene difluoride (BODIPY) cholesterol, was used because it is more amenable to use in a large number of samples than radiolabeled cholesterol (details of the assay protocol are provided in the Supplementary Appendix). For comparison, we performed a parallel assessment of efflux capacity with the use of radiolabeled cholesterol in a limited number of plasma samples.9
Cholesterol efflux capacity measured with the use of fluorescence-labeled cholesterol was moderately correlated with measurements performed with radiolabeled cholesterol (correlation coefficient for normalized cholesterol efflux, 0.54) (Fig. S1 in the Supplementary Appendix). The cholesterol efflux capacity did not change significantly when it was measured in samples obtained throughout a single day or 7 days apart (Fig. S2 in the Supplementary Appendix) or when samples underwent a freeze–thaw cycle (Fig. S3A and S3B in the Supplementary Appendix). However, as compared with 3-to-12-month storage at −70°C, parallel storage of plasma at −20°C reduced cholesterol efflux capacity measured with the use of either fluorescence-labeled cholesterol or radiolabeled cholesterol (Fig. S3C and S3D in the Supplementary Appendix). Measurements of cholesterol efflux capacity in this study were therefore performed with the use of the fluorescence-labeled cholesterol assay on plasma samples stored at −70°C.
CLINICAL END POINTS
The primary end point was a composite atherosclerotic cardiovascular disease outcome, defined as a first nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization (percutaneous coronary intervention or coronary-artery bypass grafting) or death from cardiovascular causes. A secondary end point, total cardiovascular disease, was defined as all the events included in the primary end point plus peripheral revascularization and hospitalization for heart failure or atrial fibrillation. All the end points were adjudicated by two cardiologists who were unaware of the measurements of cholesterol efflux capacity.13 The National Death Index was used to determine vital status for all the participants through December 31, 2010. Death from cardiovascular causes was defined according to the International Classification of Diseases, 10th Revision, codes I00 to I99.
STUDY OVERSIGHT
The first author designed the study, gathered the data with two of the coauthors, and analyzed the data with another author. The first author and the last two authors wrote the manuscript with contributions from all the authors, and the first author made the decision to submit it for publication. All the authors vouch for the data and analyses reported. Merck Sharp & Dohme, which provided partial support for the study, had no role in the design of the study, in the collection or analysis of the data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Written informed consent was obtained from all the participants. The protocol for this study was approved by the institutional review board at the University of Texas Southwestern Medical Center.
STATISTICAL ANALYSIS
Demographic and clinical variables were compared across quartiles of cholesterol efflux capacity with the use of the Jonckheere–Terpstra trend test. Cholesterol efflux capacity was compared across sex-specific and race-specific categories with the use of the Wilcoxon rank-sum test. Correlations with continuous markers were assessed with the use of nonparametric Spearman coefficients. The association between cholesterol efflux capacity and prevalent coronary-artery calcium (CAC) was assessed in logistic-regression models. Prevalent CAC was defined as a CAC value of 10 Agatston units or more and in linear regression models with the use of log(CAC+1).14
Cox proportional-hazards models were used to assess the association between cholesterol efflux capacity and the time to a first event for both atherosclerotic cardiovascular disease (the primary end point) and total cardiovascular disease (the secondary end point). Multivariable models included age, sex, race, presence or absence of diabetes, presence or absence of hypertension, status with regard to current smoking, body-mass index, total cholesterol level, log-transformed triglyceride level, and status with regard to a history of statin use. Serial adjustments were made for the HDL cholesterol level and HDL particle concentration. The prespecified analyses calculated the hazard of cardiovascular events associated with increasing sex-specific and race-specific quartiles of cholesterol efflux capacity. The proportional-hazards assumption was met for all models.
The contribution of cholesterol efflux capacity, over and above that of traditional risk factors, in the prediction of cardiovascular events was analyzed with the use of multiple metrics of biomarker performance,15 including discrimination (Harrell’s C-statistic),16 calibration (Hosmer–Lemeshow test), and reclassification (integrated-discrimination-improvement index17 and net reclassification index18). Sensitivity analyses were performed by analyzing cholesterol efflux capacity as a continuous variable; by investigating the association of cholesterol efflux capacity with each component of the primary end point separately; by using a hard cardiovascular outcome, defined as nonfatal myocardial infarction, nonfatal stroke, or death from myocardial infarction or stroke; and by eliminating exclusion criteria and including all the participants in the Dallas Heart Study in the analyses of the primary and secondary outcomes.
Two-sided P values of 0.05 or less were considered to indicate statistical significance. All statistical analyses were performed with the use of SAS software, version 9.3 (SAS Institute).
RESULTS
STUDY PARTICIPANTS
A total of 2971 persons underwent phenotyping by means of blood testing and imaging, of whom 2924 were included in the analysis of baseline characteristics. Of these 2924 participants, 161 had missing covariates and another 347 had incomplete follow-up for nonfatal end points, leaving 2416 participants to be included in the analysis of cardiovascular outcomes.
The median age of the participants at study entry was 42 years. A total of 57% of the participants were women, and 49% were black (Table 1). A low level of HDL cholesterol was present in 46% of the women (HDL cholesterol level, <50 mg per deciliter [1.30 mmol per liter]) and in 35% of the men (HDL cholesterol level, <40 mg per deciliter [1.05 mmol per liter]). As expected, the HDL cholesterol level was significantly higher in women than in men and significantly higher in blacks than in nonblacks (Table S1 in the Supplementary Appendix). The HDL particle concentration was significantly higher in women than in men and was similar in blacks and nonblacks.
Table 1.
Cholesterol Efflux Capacity and Traditional Risk Factors for Atherosclerotic Cardiovascular Disease.*
| Characteristic | All Participants (N = 2924) | Cholesterol Efflux Capacity | P Value for Trend | |||
|---|---|---|---|---|---|---|
| Quartile 1 (N = 731) | Quartile 2 (N = 731) | Quartile 3 (N = 731) | Quartile 4 (N = 731) | |||
| Cholesterol efflux capacity† | 0.21–3.93 | 0.21–0.83 | 0.84–0.99 | 1.00–1.18 | 1.19–3.93 | |
|
| ||||||
| Age (yr) | <0.001 | |||||
|
| ||||||
| Median | 42 | 42 | 41 | 43 | 44 | |
|
| ||||||
| Interquartile range | 36–51 | 35–50 | 35–50 | 35–51 | 37–52 | |
|
| ||||||
| Male sex (%) | 43 | 41 | 43 | 43 | 46 | 0.05 |
|
| ||||||
| Race (%)‡ | ||||||
|
| ||||||
| Black | 49 | 55 | 49 | 45 | 48 | 0.003 |
|
| ||||||
| White | 30 | 26 | 31 | 34 | 31 | 0.02 |
|
| ||||||
| Other | 21 | 19 | 20 | 21 | 21 | 0.31 |
|
| ||||||
| Hypertension (%) | 30 | 30 | 27 | 29 | 35 | 0.03 |
|
| ||||||
| Diabetes (%) | 10 | 11 | 9 | 12 | 11 | 0.65 |
|
| ||||||
| Current smoking (%) | 28 | 27 | 29 | 27 | 30 | 0.35 |
|
| ||||||
| Total cholesterol (mg/dl) | <0.001 | |||||
|
| ||||||
| Median | 177 | 170 | 173 | 178 | 185 | |
|
| ||||||
| Interquartile range | 154–202 | 148–195 | 151–200 | 155–204 | 162–211 | |
|
| ||||||
| Triglycerides (mg/dl) | 0.008 | |||||
|
| ||||||
| Median | 96 | 95 | 92 | 101 | 99 | |
|
| ||||||
| Interquartile range | 67–146 | 67–138 | 67–138 | 69–151 | 69–153 | |
|
| ||||||
| LDL cholesterol (mg/dl) | <0.001 | |||||
|
| ||||||
| Median | 104 | 101 | 100 | 104 | 110 | |
|
| ||||||
| Interquartile range | 83–126 | 80–121 | 82–125 | 82–127 | 88–133 | |
|
| ||||||
| Non-HDL cholesterol (mg/dl) | <0.001 | |||||
|
| ||||||
| Median | 127 | 121 | 123 | 128 | 133 | |
|
| ||||||
| Interquartile range | 103–154 | 101–147 | 101–152 | 102–155 | 108–163 | |
|
| ||||||
| LDL particle concentration (μmol/liter) | 0.15 | |||||
|
| ||||||
| Median | 1212 | 1200 | 1212 | 1206 | 1232 | |
|
| ||||||
| Interquartile range | 971–1491 | 982–1475 | 961–1466 | 972–1513 | 959–1530 | |
|
| ||||||
| HDL cholesterol (mg/dl) | <0.001 | |||||
|
| ||||||
| Median | 47 | 46 | 47 | 48 | 49 | |
|
| ||||||
| Interquartile range | 40–57 | 39–55 | 41–56 | 39–59 | 40–60 | |
|
| ||||||
| HDL particle concentration (μmol/liter) | <0.001 | |||||
|
| ||||||
| Median | 32.8 | 31.5 | 32.6 | 33.2 | 33.9 | |
|
| ||||||
| Interquartile range | 28.9–37.0 | 27.8–35.9 | 28.5–36.4 | 29.1–36.8 | 30.0–39.0 | |
|
| ||||||
| Lipoprotein(a) (nmol/liter) | 0.54 | |||||
|
| ||||||
| Median | 48 | 47 | 51 | 46 | 49 | |
|
| ||||||
| Interquartile range | 19–104 | 21–110 | 21–102 | 17–104 | 19–109 | |
|
| ||||||
| High-sensitivity CRP (mg/liter) | 0.53 | |||||
|
| ||||||
| Median | 2.7 | 2.7 | 2.7 | 2.7 | 2.8 | |
|
| ||||||
| Interquartile range | 1.1–6.6 | 1.1–6.8 | 1.1–6.8 | 1.1–6.2 | 1.2–6.8 | |
|
| ||||||
| Exercise (MET × min/wk) | 0.21 | |||||
|
| ||||||
| Median | 120 | 120 | 68 | 159 | 122 | |
|
| ||||||
| Interquartile range | 0–560 | 0–533 | 0–480 | 0–660 | 0–600 | |
|
| ||||||
| Alcohol intake (g/wk) | 0.002 | |||||
|
| ||||||
| Median | 3 | 2 | 4 | 4 | 4 | |
|
| ||||||
| Interquartile range | 0–39 | 0–30 | 0–39 | 0–39 | 0–59 | |
|
| ||||||
| Coronary-artery calcium >10 Agatston units (%)§ | 19 | 18 | 17 | 19 | 20 | 0.31 |
All continuous measures are reported as medians with interquartile ranges, and categorical measures as percentages. To convert values for cholesterol to millimoles per liter, multiply by 0.02586. To convert values for triglycerides to millimoles per liter, multiply by 0.01129. CRP denotes C-reactive protein, HDL high-density lipoprotein, LDL low-density lipoprotein, and MET metabolic equivalent.
Cholesterol efflux capacity is expressed as a percentage of efflux in the sample, normalized to a reference sample.
Race was self-reported.
A coronary-artery calcium level of more than 10 Agatston units indicates prevalent coronary calcium.14
ASSOCIATION OF EFFLUX CAPACITY WITH CARDIOVASCULAR RISK FACTORS AND SUBCLINICAL ATHEROSCLEROSIS
Increasing quartiles of cholesterol efflux capacity were not correlated with, or were only weakly correlated with, traditional cardiovascular risk factors other than lipid levels (Table 1). Traditional risk factors as well as self-reported weekly exercise activity and alcohol intake explained only 3% of the variance in cholesterol efflux capacity versus 35% of the variance in the HDL cholesterol level (Table S2 in the Supplementary Appendix). Cholesterol efflux capacity was similar in women and men but was significantly lower in blacks than in nonblacks (Table S1 in the Supplementary Appendix).
Correlations of cholesterol efflux capacity, HDL cholesterol level, and the HDL particle concentration with other lipoprotein-related variables, measures of adiposity and insulin resistance, and inflammatory markers are shown in Table 2. HDL cholesterol level had a strong positive correlation with several of these factors and had an inverse correlation with several others. In contrast, there was only a modest correlation between cholesterol efflux capacity and most of these variables. Cholesterol efflux capacity was also not associated with prevalent coronary-artery calcium (Table 1).
Table 2.
Correlation of HDL Cholesterol Level, HDL Particle Concentration, and Cholesterol Efflux Capacity with Lipoprotein and Metabolic Variables and Inflammatory Markers.*
| Variable | HDL Cholesterol | HDL Particle Concentration | Cholesterol Efflux Capacity |
|---|---|---|---|
| Lipoprotein variables | |||
| Cholesterol level | |||
| HDL | — | 0.52† | 0.07† |
| Total | 0.06 | 0.23† | 0.15† |
| LDL | −0.15† | 0.04 | 0.10† |
| Particle size | |||
| HDL | 0.72† | 0.17† | 0.02 |
| LDL | 0.62† | 0.19† | −0.01 |
| VLDL | −0.03 | 0.14† | 0.05† |
| Particle concentration | |||
| HDL | 0.52† | 0.15† | |
| LDL | −0.42† | −0.04 | 0.02 |
| VLDL | −0.46† | −0.04† | 0.06† |
| Triglycerides | −0.45† | 0.01 | 0.05 |
| VLDL triglycerides | −0.50† | 0.01 | 0.08† |
| Metabolic variables | |||
| Body-mass index | −0.23† | −0.05 | −0.02 |
| Waist-to-hip ratio | −0.38† | −0.13† | 0.02 |
| Truncal fat‡ | −0.20† | 0.03 | 0.004 |
| Abdominal fat§ | |||
| Visceral | −0.41† | −0.05 | 0.03 |
| Subcutaneous | −0.15† | 0.008 | −0.02 |
| Adiponectin | 0.38† | 0.21† | 0.05† |
| Leptin | 0.05† | 0.09† | −0.03 |
| HOMA-IR score | −0.29† | −0.08 | −0.05 |
| Inflammatory marker | |||
| High-sensitivity CRP | −0.10† | 0.02 | 0.008 |
| Interleukin-18 | −0.17† | −0.07† | 0.02 |
| Cystatin C | −0.17† | −0.12† | 0.04† |
Values are Spearman’s correlation coefficients. HOMA-IR denotes homeostasis model assessment of insulin resistance, and VLDL very-low-density lipoprotein.
P<0.05.
Truncal fat was measured with the use of dual-energy x-ray absorptiometry.
Abdominal fat was measured with the use of magnetic resonance imaging.
ASSOCIATION OF EFFLUX CAPACITY WITH CARDIOVASCULAR EVENTS
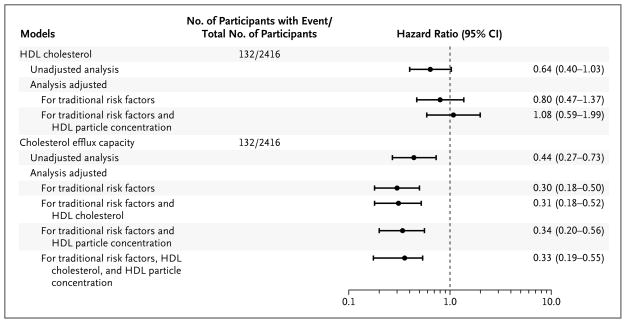
Among the 2416 participants with complete follow-up data, 132 had a primary atherosclerotic cardiovascular disease event during the median follow-up period of 9.4 years. Although there was a trend toward an inverse association of HDL cholesterol level with atherosclerotic cardiovascular disease in unadjusted models, it was diminished after adjustment for cardiovascular risk factors, and it was further attenuated with adjustment for HDL particle concentration (Fig. 1). HDL particle concentration was inversely associated with the primary end point of atherosclerotic cardiovascular disease in models adjusted for cardiovascular risk factors and HDL cholesterol level (adjusted hazard ratio for the fourth vs. first quartile of HDL particle concentration, 0.53; 95% confidence interval [CI], 0.31 to 0.89).
Figure 1. Atherosclerotic Cardiovascular Disease Events, According to Models Based on High-Density Lipoprotein (HDL) Cholesterol Level and Cholesterol Efflux Capacity.
Hazard ratios and 95% confidence intervals (CIs), derived from Cox proportional-hazards models, are shown for the comparisons of quartile 4 (highest) with quartile 1 (lowest) of cholesterol efflux capacity. A total of 132 participants had a primary end-point event of atherosclerotic cardiovascular disease, defined as a first nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization (percutaneous coronary intervention or coronary-artery bypass grafting) or death from cardiovascular causes. Traditional risk factors included age, sex, race, presence or absence of diabetes, presence or absence of hypertension, status with regard to current smoking, body-mass index, total cholesterol level, log-transformed triglyceride level, and status with regard to a history of statin use.
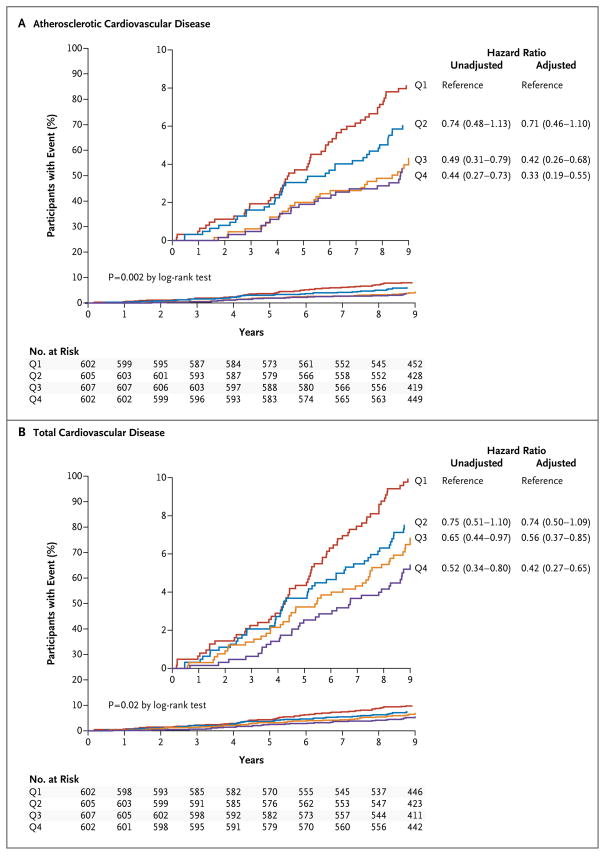
There was a graded inverse association between increasing quartiles of cholesterol efflux capacity and the primary end point of atherosclerotic cardiovascular disease (hazard ratio for the fourth vs. first quartile of cholesterol efflux capacity, 0.44; 95% CI, 0.27 to 0.73) (Table 3 and Fig. 2A). Adjustment for traditional risk factors, HDL cholesterol level, and HDL particle concentration did not attenuate the association (hazard ratio, 0.33; 95% CI, 0.19 to 0.55) (Table 3 and Fig. 1). Similar findings were obtained for the secondary end point of total cardiovascular disease (Table 3 and Fig. 2B). The hazard ratios were directionally consistent across all individual components of the composite primary end point, except for death from cardiovascular causes (Table 3). In well-calibrated models, the addition of cholesterol efflux capacity to traditional risk factors was associated with an improvement in all the risk-prediction indexes for the primary end point, including the C-statistic (from 0.827 to 0.841, P = 0.02), the integrated-discrimination-improvement index (0.02, P<0.001), and the net reclassification index (0.37; 95% CI, 0.18 to 0.56).
Table 3.
Cardiovascular Events and Hazard Ratios, According to Quartile of Cholesterol Efflux Capacity.*
| End Point | All Participants (N = 2416) | Cholesterol Efflux Capacity | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI)† | |||
|---|---|---|---|---|---|---|---|
| Quartile 1 (N = 602) | Quartile 2 (N = 605) | Quartile 3 (N = 607) | Quartile 4 (N = 602) | ||||
| no. of participants | |||||||
| Primary end point: atherosclerotic cardiovascular disease | 132 | 49 | 35 | 26 | 22 | 0.44 (0.27–0.73) | 0.33 (0.19–0.55) |
|
| |||||||
| Myocardial infarction‡ | 28 | 11 | 6 | 4 | 7 | — | — |
|
| |||||||
| Stroke‡ | 36 | 15 | 11 | 8 | 2 | — | — |
|
| |||||||
| Coronary revascularization‡ | 26 | 14 | 7 | 2 | 3 | — | — |
|
| |||||||
| Death from cardiovascular causes‡ | 42 | 9 | 11 | 12 | 10 | — | — |
|
| |||||||
| Secondary end points | |||||||
|
| |||||||
| Total cardiovascular disease | 172 | 58 | 44 | 39 | 31 | 0.52 (0.34–0.80) | 0.42 (0.27–0.65) |
|
| |||||||
| Hard atherosclerotic cardiovascular disease | 84 | 30 | 21 | 16 | 17 | 0.53 (0.29–0.97) | 0.40 (0.21–0.74) |
|
| |||||||
| Myocardial infarction | 30 | 12 | 6 | 5 | 7 | 0.59 (0.23–1.49) | 0.44 (0.17–1.18) |
|
| |||||||
| Stroke | 37 | 16 | 11 | 8 | 2 | 0.12 (0.03–0.54) | 0.11 (0.02–0.47) |
|
| |||||||
| Coronary revascularization | 48 | 25 | 10 | 6 | 7 | 0.28 (0.12–0.65) | 0.19 (0.08–0.45) |
|
| |||||||
| Death from cardiovascular causes | 46 | 9 | 15 | 12 | 10 | 1.11 (0.45–2.74) | 0.94 (0.37–2.37) |
Data are hazard ratios and 95% confidence intervals for the cholesterol efflux capacity in quartile 4 (highest quartile) as compared with quartile 1 (lowest quartile) for the listed end points, obtained from Cox proportional-hazards models. Atherosclerotic cardiovascular disease was defined as the composite of a first nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization (percutaneous coronary intervention or coronary-artery bypass grafting) or death from cardiovascular causes. Total cardiovascular disease was defined as the composite of the end points related to atherosclerotic cardiovascular disease and peripheral revascularization or hospitalization for heart failure or atrial fibrillation. Hard atherosclerotic cardiovascular disease was defined as the composite of fatal or nonfatal myocardial infarction or stroke.
The hazard ratio was adjusted for age, sex, race, presence or absence of diabetes, presence or absence of hypertension, status with regard to current smoking, body-mass index, total cholesterol level, log-transformed triglyceride level, status with regard to a history of statin use, HDL cholesterol level, and HDL particle concentration.
The components of the primary composite end point are summarized as mutually exclusive components without hazard ratios or confidence intervals, since these components do not represent true event rates. Total numbers of patients with these end-point events are also shown as secondary end points with hazard ratios and confidence intervals.
Figure 2. Kaplan–Meier Curves and Hazard Ratios for Cardiovascular Events, According to Quartile of Cholesterol Efflux Capacity.
Kaplan–Meier curves and hazard ratios and 95% confidence intervals are shown for quartiles (Q1 through Q4) of cholesterol efflux capacity, with the use of quartile 1 (lowest cholesterol efflux capacity) as the reference, derived from Cox proportional-hazards models. Each quartile had equal numbers of men and women, and equal numbers of blacks and nonblacks. A total of 132 participants had a primary end-point event of atherosclerotic cardiovascular disease (Panel A), and 172 had a secondary end-point event of total cardiovascular disease (Panel B). Total cardiovascular disease was defined as the composite of the end points related to atherosclerotic cardiovascular disease and peripheral revascularization or hospitalization for heart failure or atrial fibrillation. Adjusted models included traditional risk factors for atherosclerotic cardiovascular disease, HDL cholesterol level, and HDL particle concentration. The insets show the same data on enlarged y axes.
Sensitivity analyses modeling cholesterol efflux capacity as a continuous measure yielded consistent findings (fully adjusted hazard ratio for atherosclerotic cardiovascular disease per 1-SD increase in efflux capacity, 0.68 [95% CI, 0.55 to 0.84]; fully adjusted hazard ratio for total cardiovascular disease per 1-SD increase in efflux capacity, 0.79 [95% CI, 0.67 to 0.94]) (Table S3 in the Supplementary Appendix). Analyses that restricted the end point to the hard cardiovascular outcomes of nonfatal and fatal myocardial infarction and stroke did not alter the findings (Table 3, and Fig. S4 in the Supplementary Appendix). Eliminating all the exclusion criteria and including all the participants in the Dallas Heart Study, with adjustment for history of cardiovascular disease in the multivariable analyses, also did not alter the findings (data not shown).
There were no interactions according to age, sex, or race for the association between cholesterol efflux capacity and the primary end point of atherosclerotic cardiovascular disease (P>0.10 for interaction in all cases) (Fig. S5 in the Supplementary Appendix). Subgroup analyses that were stratified according to cardiovascular risk (with the use of the pooled-cohort equations from the 2013 cholesterol guidelines of the American College of Cardiology–American Heart Association)1 showed consistent findings across the risk strata, with relative hazards of the primary end point of atherosclerotic cardiovascular disease (for the fourth vs. the first quartile of cholesterol efflux capacity) of 0.25 (95% CI, 0.09 to 0.69) in the low-risk cohort (estimated 10-year risk, <7.5%) and 0.36 (95% CI, 0.19 to 0.69) in the high-risk cohort (estimated 10-year risk, =7.5 (P=0.83 for interaction).
DISCUSSION
In this study, we found that a functional property of HDL, namely cholesterol efflux capacity, was inversely associated with incident atherosclerotic cardiovascular disease in a population-based cohort free from cardiovascular disease at baseline. This association persisted after multivariate adjustment, suggesting that HDL function is associated with cardiovascular risk by means of processes distinct from those reflected by the HDL cholesterol level, HDL particle concentration, or traditional cardiovascular risk factors.
Reverse cholesterol transport, a process by which cholesterol is transferred from peripheral tissues and cells to the liver for biliary secretion, is believed to contribute to protection from atherosclerosis.19 A critical step in reverse cholesterol transport is cholesterol efflux from macrophages by HDL. Studies in genetically modified mice have shown that macrophage-specific cholesterol efflux and reverse cholesterol transport are inversely correlated with atherosclerotic lesion size and are more accurate predictors of changes in atherosclerosis severity than HDL cholesterol level.8 Cholesterol efflux by HDL is also required for signaling by the lipoprotein in endothelial cells, which underlies the capacity of HDL to activate endothelial nitric oxide synthase and to promote endothelial repair and induce angiogenesis.20,21 Impaired cholesterol efflux capacity has also been correlated with increased platelet reactivity in vitro.22,23 Thus, HDL cholesterol efflux may have multiple atheroprotective functions.
In previous studies, reduced cholesterol efflux capacity has been correlated with prevalent coronary artery disease.9,10 However, the cross-sectional design of these studies does not allow for the determination of the relationship between the baseline HDL function and the development of cardiovascular disease. One recent study involving participants recruited from a cardiac catheterization laboratory suggested a positive, rather than inverse, association between cholesterol efflux capacity at the time of catheterization and prospective cardiovascular events,10 but the interpretation of these findings is complicated by the use of a convenience population in that study, which included participants with prevalent cardiovascular disease (index-event bias).24
In our study, there was little or no correlation between cholesterol efflux capacity and most traditional cardiovascular risk factors, CAC, or markers of inflammation. We also found that whereas a low level of HDL cholesterol was associated with indexes of adiposity and insulin resistance, mirroring prior findings,25 there was no relationship between measures of adiposity or insulin resistance and cholesterol efflux capacity. This suggests that the processes that govern cholesterol efflux capacity are independent of those underlying the link between adiposity or insulin resistance and HDL cholesterol level. This finding may be due to the young age of our study sample (median age, 42 years) and the exclusion of participants with cardiovascular disease, or it may indicate that cholesterol efflux capacity reflects a biologic process not captured by traditional risk factors.
Our use of fluorescence-labeled cholesterol, which primarily measures ABCA1-mediated efflux,12 provided the ability to perform high-throughput measurements at low cost without the need for a radioisotope. Studies in animals suggest that ABCA1-mediated efflux is an important mode of efflux by which the severity of atherosclerosis is modulated.8,26,27 In addition, in humans with similar levels of HDL cholesterol, differences in macrophage-specific cholesterol efflux are due only to ABCA1-mediated efflux, not to other transporters.28 Thus, it is plausible that the examination of ABCA1-mediated efflux with the use of fluorescence-labeled cholesterol provides a selective assessment of the mode of efflux of greatest relevance in assessing the severity of atherosclerosis and its clinical consequences. However, there is no established standard for the measurement of macrophage-specific cholesterol efflux capacity, and there is greater experience with assessment by means of radiolabeled cholesterol than by means of fluorescence-labeled cholesterol.
Several limitations of our study merit comment. First, our cohort was relatively young and at low overall cardiovascular risk, characteristics that accounted for the modest number of first events of atherosclerotic cardiovascular disease. Second, the race and ethnic-group distribution of our study sample, with oversampling of blacks, does not reflect the general population distribution. Third, the levels of HDL cholesterol in our study sample were mostly within the normal range, so the data are not informative for persons with a high or a low HDL cholesterol level. Finally, measurements of apolipoprotein A-I were not available for correlation with cholesterol efflux capacity.
In conclusion, we found that cholesterol efflux capacity was inversely associated with incident atherosclerotic cardiovascular disease in a population-based cohort that was free from cardiovascular disease at baseline. This association persisted after adjustment for traditional cardiovascular risk factors, HDL cholesterol level, and HDL particle concentration.
Supplementary Material
Acknowledgments
Funded by the Donald W. Reynolds Foundation and others.
The views expressed in this article are those of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute (NHLBI), the National Institutes of Health (NIH), the American Heart Association, or Merck Sharp & Dohme.
Supported by grants from the Donald W. Reynolds Foundation, the National Center for Advancing Translational Sciences of the NIH (UL1TR001105), the NHLBI (K08HL1813), and the American Heart Association (12SDG11900066) and by a research grant from the Investigator Studies Program of Merck Sharp & Dohme.
We thank the Dallas Heart Study staff and the study participants for contributions to this study, and Teresa Eversole, Sharon Reimold, and Susan Matulevicius for guidance in completing this study.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Stone NJ, Robinson J, Lichtenstein AH, et al. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. Erratum, Circulation 2014;129:Suppl 2:S46–S48. [DOI] [PubMed] [Google Scholar]
- 2.Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. Erratum, N Engl J Med 2012;367:189. [DOI] [PubMed] [Google Scholar]
- 3.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 5.The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 6.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. Erratum, Lancet 2012;380:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XM, Tang WH, Mosior MK, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–705. doi: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M, et al. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J Lipid Res. 2011;52:2332–40. doi: 10.1194/jlr.D018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maroules CD, Rosero E, Ayers C, Peshock RM, Khera A. Abdominal aortic atherosclerosis at MR imaging is associated with cardiovascular events: the Dallas Heart Study. Radiology. 2013;269:84–91. doi: 10.1148/radiol.13122707. [DOI] [PubMed] [Google Scholar]
- 14.Jain T, Peshock R, McGuire DK, et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J Am Coll Cardiol. 2004;44:1011–7. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 15.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–722. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenson RS, Brewer HB, Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assanasen C, Mineo C, Seetharam D, et al. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest. 2005;115:969–77. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saddar S, Carriere V, Lee WR, et al. Scavenger receptor class B type I is a plasma membrane cholesterol sensor. Circ Res. 2013;112:140–51. doi: 10.1161/CIRCRESAHA.112.280081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calkin AC, Drew BG, Ono A, et al. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation. 2009;120:2095–104. doi: 10.1161/CIRCULATIONAHA.109.870709. [DOI] [PubMed] [Google Scholar]
- 23.Murphy AJ, Bijl N, Yvan-Charvet L, et al. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med. 2013;19:586–94. doi: 10.1038/nm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khera AV, Rader DJ. Cholesterol efflux capacity: full steam ahead or a bump in the road? Arterioscler Thromb Vasc Biol. 2013;33:1449–51. doi: 10.1161/ATVBAHA.113.301519. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. Errata, Circulation 2005;112(17):e297, e298. [DOI] [PubMed] [Google Scholar]
- 26.Baldán A, Pei L, Lee R, et al. Impaired development of atherosclerosis in hyper-lipidemic Ldlr−/− and ApoE−/− mice transplanted with Abcg1−/− bone marrow. Arte-rioscler Thromb Vasc Biol. 2006;26:2301–7. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 27.Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1−/− bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2308–15. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 28.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.