Abstract
Long-distance animal migrations have important consequences for infectious disease dynamics. In some cases, migration lowers pathogen transmission by removing infected individuals during strenuous journeys and allowing animals to periodically escape contaminated habitats. Human activities are now causing some migratory animals to travel shorter distances or form sedentary (non-migratory) populations. We focused on North American monarch butterflies and a specialist protozoan parasite to investigate how the loss of migratory behaviours affects pathogen spread and evolution. Each autumn, monarchs migrate from breeding grounds in the eastern US and Canada to wintering sites in central Mexico. However, some monarchs have become non-migratory and breed year-round on exotic milkweed in the southern US. We used field sampling, citizen science data and experimental inoculations to quantify infection prevalence and parasite virulence among migratory and sedentary populations. Infection prevalence was markedly higher among sedentary monarchs compared with migratory monarchs, indicating that diminished migration increases infection risk. Virulence differed among parasite strains but was similar between migratory and sedentary populations, potentially owing to high gene flow or insufficient time for evolutionary divergence. More broadly, our findings suggest that human activities that alter animal migrations can influence pathogen dynamics, with implications for wildlife conservation and future disease risks.
Keywords: movement ecology, long-distance migration, infectious disease, virulence evolution, Danaus plexippus, neogregarine
1. Introduction
Each year, billions of animals migrate long distances to track seasonal changes in resources or climate. These animals comprise a significant portion of global biodiversity, and their migratory behaviours have large effects on ecosystem processes [1,2]. In recent decades, numerous migratory species have declined or altered their migratory behaviours in response to anthropogenic environmental change [3–5]; some populations now migrate shorter distances or have transitioned into year-round resident populations [6–8]. For instance, numerous bird species have shown a reduced migratory tendency linked to climate warming [6], or established new non-migratory populations owing to habitat loss or supplemental feeding by humans (e.g. at bird feeders) [9]. As one example, Spanish white storks now forego their traditional migration to Africa each winter and instead subsist on city landfills year-round [10]. Other species are showing similar behaviours, including European blackbirds, great crested grebes and grey-headed flying foxes [11–13]. Changes in migration behaviours could influence nutrient transfer in ecosystems, affect pest control and pollination, and, in particular, alter infectious disease dynamics [2,14,15].
Long-distance migration influences interactions between animals and pathogens. A crucial question is how the loss of migration and a shift towards sedentary behaviours will affect pathogen transmission and evolution [15]. In some cases, seasonal migration can cause hosts to encounter more diverse pathogen assemblages over heterogeneous habitats [16,17], acquire infections during periods of dense aggregations [18] and spread pathogens to geographically distant areas [19]. However, recent work suggests that migration more typically lowers infection risk for migrants by (i) allowing animals to periodically leave behind parasite-contaminated habitats (a process termed migratory escape [20]), (ii) weeding out infected individuals during strenuous, long-distance journeys (migratory culling [21,22]) and (iii) separating vulnerable juveniles from infectious adults (migratory allopatry [23]). Support for the role of migration in lowering infection risk comes from theoretical models [24] and field studies [25,26] (reviewed in [15]). Diminished migrations could enhance pathogen transmission via the loss of migratory escape or migratory culling. Further, reduced migration could allow more virulent pathogen strains to persist by increasing opportunities for pathogen transmission [27]; without the physical demands of migration, infected individuals could survive longer to transmit virulent strains. In sum, sedentary populations could support greater parasite prevalence and virulence than their migratory counterparts [28]. Pathogen dynamics have already shifted in response to changes in migratory patterns for some wildlife populations. For example, the breakdown of nomadic movements among fruit bats in Australia probably underpins Hendra virus spillover to horses and humans near sedentary bat colonies in urban centres [29].
Here, we ask whether a shift from migratory to resident behaviour alters the prevalence and virulence of a specialist protozoan pathogen in monarch butterflies (Danaus plexippus). Each autumn in North America, monarchs migrate up to 2500 km from their summer-breeding range in the eastern US and Canada to overwintering sites in central Mexico (figure 1) [31,32]. In Mexico, migratory monarchs cluster on trees in high-altitude forests in a semi-dormant and non-reproductive state. In spring, these same individuals mate and fly north to recolonize their breeding range over two to three generations [30]. Past work showed that long-distance migration annually reduces protozoan infection prevalence in North American monarchs through migratory escape and migratory culling [22].
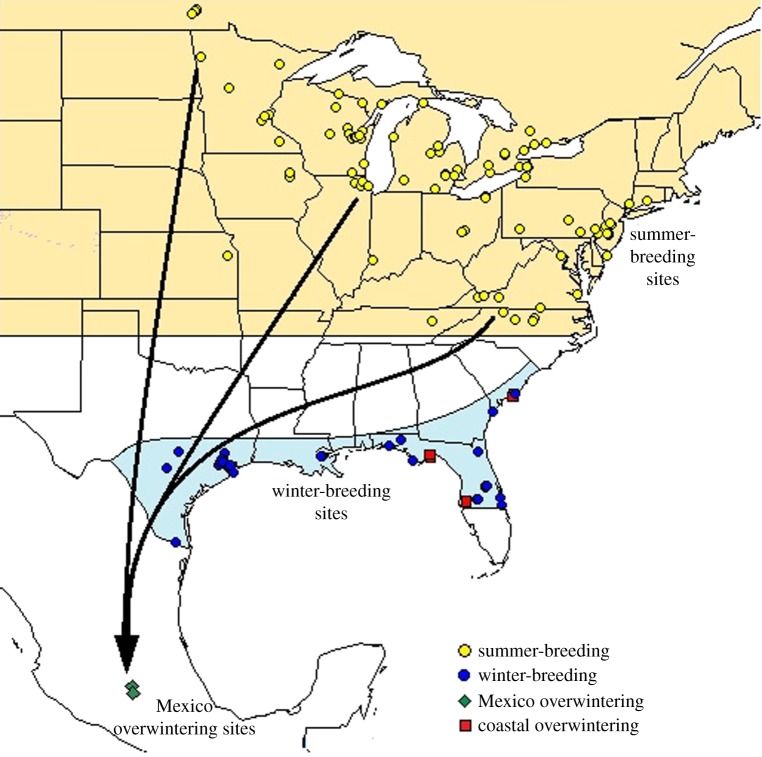
Figure 1.
Sampling locations (symbols) and major autumn migratory routes (arrows) in eastern North America. In autumn, migratory monarchs travel from the summer-breeding range (extending from the southern US into Canada) to overwintering sites in high-altitude fir forests in the transvolcanic mountains in central Mexico. In the spring, the same individuals fly north from Mexico into the southern US [30], where they lay eggs on milkweed to produce the next generation. Symbols show sampling locations used to compare infection prevalence for summer-breeding (yellow circles) and Mexico overwintering sites (green diamonds) of migratory monarchs. Also shown are winter-breeding sites (blue circles) for non-migratory monarchs sampled in locations where tropical milkweed grows year-round, and coastal overwintering sites where adults but no breeding activity are observed (red squares). Sample sizes for each source and year are provided in the electronic supplementary material.
In recent years, the population size of migratory monarchs in Mexico has severely declined [33,34] in response to deforestation of overwintering sites and intensive agricultural practices that reduce habitat for milkweeds in the USA [35,36]. To counter this decline, some conservation groups have encouraged the public to plant milkweed (monarch host plants) in gardens. Over 100 milkweed species are native to North America [37]; however, the most commercially available is an exotic species known as tropical milkweed (Asclepias curassavica). Tropical milkweed does not naturally senesce in the autumn like native milkweeds, and in areas with mild climates it continues to produce new foliage and flowers during autumn and winter [38]. Thus, tropical milkweed provides monarch larval food throughout the year, and reports of monarchs breeding during the winter—rather than migrating or overwintering—have become common in the southern US. These behaviours are almost exclusively restricted to sites where tropical milkweed is present [39]. Hereafter, we refer to such areas as winter-breeding sites, where monarch eggs and larvae occur between December and February (excluding south Florida, as noted in methods). Population dynamics at winter-breeding sites are not well understood, and the origin of immigrant monarchs into these areas is not known. However, a recent study involving cage experiments showed that exposure to milkweed in good condition can induce a percentage of autumn migratory monarchs to become reproductively active (and thus, unlikely to migrate) [38]. Although historical data are limited, a search of herbarium records suggests that tropical milkweed occurrence and monarch winter-breeding have become more frequent in the southern US in recent decades (electronic supplementary material). Considered altogether, while the number of migratory monarchs in Mexico has declined largely due to the loss of native milkweeds, the relative number of non-migratory monarchs has probably increased due to the year-round persistence of exotic milkweed in southern locations—leading to a net loss of migratory behaviour.
To investigate whether winter-breeding behaviours support greater pathogen prevalence in monarchs, we used a combination of field monitoring and citizen science data. We tested the prediction that resident monarchs at winter-breeding sites experience higher prevalence of infection with the protozoan Ophryocystis elektroscirrha (OE), compared with migratory monarchs at overwintering sites or in the summer-breeding range. Next, we experimentally tested whether virulence was greater among parasites collected from winter-breeding monarchs compared to parasites from migratory monarchs, as would be expected if year-round transmission favours the persistence of more virulent strains.
2. Material and methods
(a). Biology of the study system
Adult monarchs infected with the specialist protozoan OE emerge from their pupal cases covered with millions of dormant parasite spores on the outside of their bodies [40]. Transmission occurs when infected adults scatter parasite spores onto eggs or milkweed, and larvae ingest spores while feeding [41]. Larva-to-larva transmission does not occur; rather, spores from adults must be eaten by a larva to cause a new infection. Infected monarchs suffer from wing deformities, smaller body size, reduced flight performance and shorter adult lifespan [21,42]. Infections occur in all monarch populations examined to date, and populations with greater migratory propensity tend to have lower infection prevalence [43,44]. Previous studies of seasonal patterns suggest that parasite prevalence in eastern North American migratory monarchs is reduced annually by migratory culling and migratory escape [22].
(b). Measuring prevalence in migratory and winter-breeding monarchs
We used a combination of field sampling and citizen science data to quantify parasite infection in wild monarchs for two consecutive years (during 2011–2013) at multiple sites (figure 1; electronic supplementary material, table S1). We focused on four sources: (i) resident monarchs sampled at winter-breeding sites in the southern US; (ii) migratory monarchs sampled across their summer-breeding range (northern US and southern Canada); (iii) migratory monarchs sampled at Mexico overwintering sites; and (iv) migratory monarchs sampled at coastal overwintering sites in the southern US, where a small fraction of eastern North American monarchs overwinter with no breeding activity [45]. We collaborated with citizen scientists through the Monarch Health (MH) programme to quantify infection prevalence at 30 winter-breeding sites in the southern US between December and March (n = 571 monarchs sampled by 36 volunteers), and at 89 summer-breeding sites in the eastern US and Canada between June and October (n = 2566 samples from 69 volunteers). Our laboratory team sampled additional monarchs at five winter-breeding sites (n = 96 samples), and collaborated with J. W. McCord and others to sample five coastal overwintering sites in the southern US (n = 254 samples). Winter-breeding sites excluded southern Florida below 27.34° N latitude (Sarasota, FL, USA) where a distinct population of non-migratory monarchs that breeds year-round has long been established [46] and is known to harbour high infection prevalence [43]. Parasite samples from overwintering migratory monarchs at two sites in Michoacán, Mexico were obtained in collaboration with E. Rendón-Salinas, P. F. Jaramillo-López and WWF-Mexico (n = 2390 samples).
We tested monarchs for OE infection non-destructively by pressing transparent tape (1.27 cm2) against each adult monarch's abdomen and viewing samples at 63× magnification (as described in [43]). Citizen scientists through the MH programme collected similar samples and mailed these to our laboratory to be scored for the presence or absence of infection. Following [22], we classified samples with more than 100 spores as heavily infected, indicating an acute infection acquired as a larva; in contrast, samples with less than 100 spores can result from passive spore transfer between adult monarchs [41,47]. Data for each sample included date, sex, location and monarch collection stage (adult or larva reared to adulthood). Additional protocols for the MH programme are described in [22] and at www.monarchparasites.org.
(c). Virulence experiment
We experimentally tested for variation in parasite virulence using isolates collected from wild migratory and resident monarchs. From each of three sources (winter-breeding, summer-breeding and Mexico overwintering), we chose 17–20 parasite isolates representing temporally and geographically dispersed samples (electronic supplementary material, table S2). Before the experiment, isolates were passed through one monarch generation in the laboratory to obtain viable stocks and a second generation to clone isolates through single-spore inoculations (following [48,49]).
We randomly assigned monarch larvae from five outbred lineages (half-sib families) to infection by one of 57 parasite clones (10 monarchs per clone). Host lineages were the grand-progeny of wild, uninfected monarchs collected from east-central Texas in April 2012 (representing spring migrants). Larvae were orally inoculated at the second-instar stage following [48]. Control larvae (n = 80) were treated similarly but without parasites. Larvae that consumed the inoculum (10 spores per leaf) were transferred to individual 0.47 l plastic containers with mesh lids and reared to the adult stage under ambient light at 27–30°C and 32–49% RH. We re-supplied stalks of swamp milkweed (Asclepias incarnata) and cleaned containers daily. Treatment groups remained blind to experimenters. We recorded pupal mass and signs of OE infection during development, following [49]. After adult eclosion, we recorded sex and tested monarchs with no signs of infection using the tape method described above to verify the absence of infection. Adults were held in individual glassine envelopes at 12°C. We recorded adult longevity (number of days until death), used in prior studies as an inverse measure of OE virulence; shorter adult longevity indicates higher parasite virulence [27,48]. Deceased monarchs were stored at −20°C and quantitative parasite load (a measure of parasite replication) was obtained for infected monarchs by vortexing each abdomen for 5 min in deionized water and using a counting chamber to estimate the total number of spores per butterfly [48].
(d). Data analysis
We tested for differences in infection across monarch sources (winter-breeding, summer-breeding, Mexico overwintering and coastal overwintering) in R v. 3.0.3 [50] using two approaches. First, we examined predictors of individual monarch infection status (infected/uninfected) using generalized linear mixed models (GLMM) with a binomial error distribution and logit link in package lme4. Factors included source, year, collection stage (adult or larva) and sex. Site was a random effect nested within source population. The analysis excluded 151 samples with missing data. We completed model averaging of top models (ΔAICc < 10) with the package AICcmodavg [51]. Second, we analysed site-level prevalence based on the proportion of samples per site that were heavily infected; sites with fewer than eight samples were excluded from these analyses. Because Moran's I-tests and variograms of prevalence data indicated spatial autocorrelation among summer-breeding (I = 0.194, p = 0.0002) and winter-breeding sites (I = 0.279, p = 0.01), we accommodated spatial structure in our site-level prevalence analyses. Specifically, we tested the main effect of source population on prevalence per site using a generalized least-squares (GLS) model with a Gaussian spatial correlation structure in the package nlme [52]. The Gaussian structure substantially reduced spatial dependence among sites and improved model fit as evaluated by AIC. We also included a variance structure (varIdent), after observing unequal variance in residuals among sources, to allow for heterogeneity without transforming prevalence values [52]. In the GLS model, prevalence per site was calculated across the entire study period (2011–2013). Finally, we used a linear mixed model (package nlme) in a third analysis, which also examined site-level prevalence and accounted for spatial proximity of sites. For this analysis, we assigned sites to sub-regions nested within source and examined effects of source and year (further described in the electronic supplementary material).
Analyses for the virulence experiment were completed in SPSS v. 22, using a series of general linear models with three response variables: adult longevity (an inverse measure of virulence; log10-transformed), parasite load (a measure of within-host replication; log10-transformed) and pupal mass (to indicate parasite effects on host body size). In each analysis, predictor variables were parasite source (winter-breeding, summer-breeding or Mexico overwintering) and monarch sex as fixed factors, and monarch lineage and parasite isolate nested within source as random effects. We used Tukey HSD post-hoc tests to examine differences among source means. Pearson correlations tested associations among the three response variables, with the expectation that parasite load would correlate negatively with adult longevity and pupal mass.
3. Results
(a). Field infection prevalence
Across all field samples (n = 5877), 16% of monarchs were heavily infected with OE, with sharp differences in infection measures among sources (figure 2; electronic supplementary material, figure S2). Across years, infection frequency was five to nine times higher among non-migratory (winter-breeding) monarchs compared with migratory monarchs sampled in Mexico or at coastal overwintering sites. Infection frequency at winter-breeding sites was also more than three times higher than for migratory monarchs sampled at summer-breeding sites (figure 2). Analysis of individual-level infection status (using binomial GLMMs) supported significant effects of source, year and collection stage (electronic supplementary material, tables S3 and S4). In particular, winter-breeding monarchs were far more likely to be infected than monarchs at any other source sampled (significant at the p < 0.005 level, as reported in electronic supplementary material, table S3), whereas infection levels among the three migratory sources did not differ significantly. Wild monarchs captured as adults were less likely to be infected compared with those captured as larvae/pupae (electronic supplementary material, figure S3). Monarchs were more likely to be infected in 2012–2013 than in the previous year, 2011–2012, and infection probability was slightly higher for males than females (but n.s.).
Figure 2.
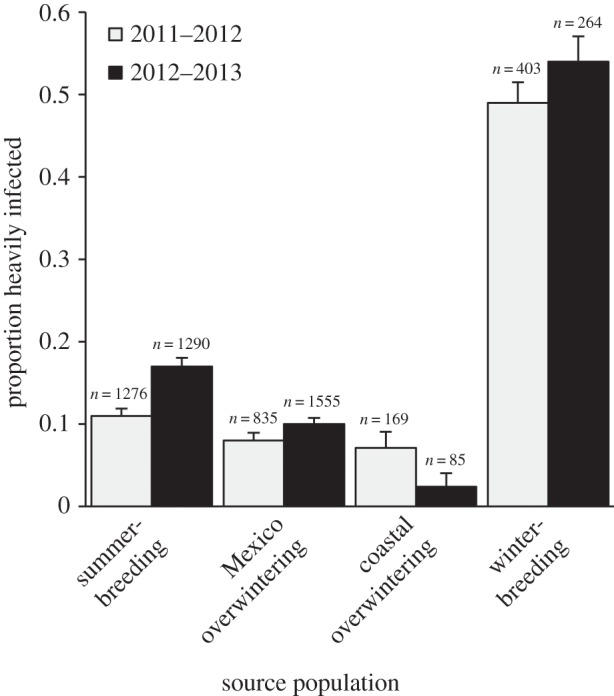
Proportions of monarchs heavily infected with OE parasites across sources and years of sampling. Comparison of means in the GLS model showed that prevalence was significantly higher among non-migratory monarchs sampled at winter-breeding sites in the southern coastal US (50.8% infected on average) compared with migratory monarchs sampled at Mexico overwintering sites (9.3% infected; t19 = −5.08, p < 0.00001) or coastal overwintering sites (5.5% infected; t21 = −5.03, p < 0.00001). Prevalence at winter-breeding sites was also higher than for migratory monarchs sampled at summer-breeding sites (14.1% infected; t70 = −4.36, p < 0.0001). Proportions shown are averaged across all samples (regardless of sample size per site) within each source (site locations shown in figure 1). Error bars represent standard error.
Large differences in infection between sources persisted when we further analysed data at the site level (infection prevalence) and accounted for spatial dependence among sites. Congruent with the previous analysis, the GLS model showed infection prevalence varied significantly among sources (F3,74 = 9.54, p < 0.0001), explaining 69% of the variance in infection among sites (see figure 2 legend for additional details). A linear mixed model for site-level prevalence yielded similar results, with strong effects of source population (electronic supplementary material).
(b). Parasite virulence experiment
Survival of experimental monarchs to the adult stage was within the range observed for prior studies in our laboratory (82.3% for inoculated monarchs, n = 570; 87.5% for control monarchs, n = 80). Of the inoculated individuals that survived to adulthood, 95.8% became heavily infected with OE; no control monarchs were infected. Adult longevity was lower for infected monarchs (7.9 days ± 0.1 s.e.m.) relative to uninfected monarchs (20.9 days ± 0.4 s.e.m.), while pupal mass was similar for infected (1.30 g ± 0.007 s.e.m.) and uninfected individuals (1.31 g ± 0.017 s.e.m.).
Among infected monarchs, measures of adult longevity (figure 3), parasite load and pupal mass were similar across parasite source populations. Adult longevity, an inverse measure of virulence, did not depend on source population (F2,437 = 0.24, p = 0.79). Adult longevity did covary with sex, such that infected males lived on average 0.7 days longer than infected females (F1,438 = 6.20, p = 0.013). Adult longevity also varied significantly among parasite isolates nested within source population (F54,383 = 1.77, p = 0.001; figure 3), with average longevity per isolate ranging from 6.0 to 10.7 days, depending on monarch lineage (F4,435 = 8.15, p < 0.001).
Figure 3.
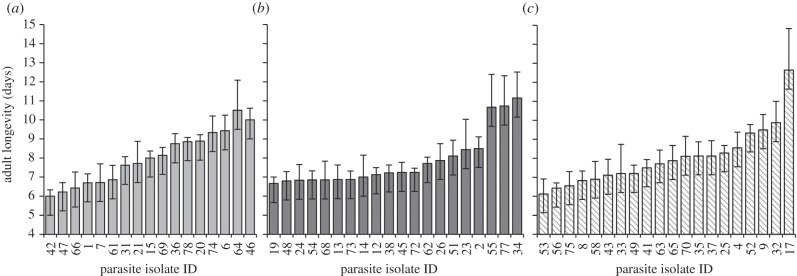
Monarch longevity in days for adults heavily infected with OE. Shorter longevity indicates higher parasite virulence. Averages for parasite clones are shown, representing three sources: (a) summer-breeding sites, 17 clones; (b) Mexico overwintering sites, 20 clones; and (c) winter-breeding sites, 20 clones. Origins of parasite isolates are noted in electronic supplementary material. Error bars represent standard error.
Quantitative parasite load was similar across source populations, with an average of 106.1 spores (±104.7 s.e.m.) per infected monarch. Parasite load differed significantly among isolates (F54,384 = 2.55, p < 0.001) and monarch lineages (F4,436 = 4.857, p = 0.001), but did not depend on sex. As observed in previous studies, monarchs with higher parasite loads lived shorter lives (Pearson r = −0.492, n = 451, p < 0.001). Counter to our expectations, pupal mass was positively correlated with spore load (Pearson r = 0.183, n = 446, p < 0.001). Pupal mass was higher for males (1.36 g ± 0.01 s.e.m.) than for females (1.26 g ± 0.01 s.e.m.; F1,502 = 124.30, p < 0.001). Pupal mass also varied significantly across monarch lineages (F4,499 = 28.28, p < 0.001), but did not depend on parasite source population (F2,433 = 0.58, p = 0.57).
4. Discussion
Non-migratory monarchs sampled in tropical milkweed gardens showed markedly higher infection rates compared with migratory monarchs. This difference suggests that loss of the traditional migration leads to greater infection risk, probably owing to the absence of processes by which migration removes infected individuals and interrupts parasite transmission. Despite higher infection rates at winter-breeding locations, we found no evidence that parasites isolated from winter-breeding monarchs were more virulent than parasites from migratory monarchs. Instead, virulence and replication (quantitative parasite load) varied more widely among parasite isolates within each source population than between source populations.
High prevalence of infection at winter-breeding sites could occur through several mechanisms, including the absence of migratory culling and migratory escape. There is ample evidence for migratory culling in monarchs; heavily infected monarchs fly less well [21] and experience shorter lifespans [42], leading to the disproportionate removal of infected monarchs during strenuous migratory journeys. Consistent with this idea, OE prevalence has been shown to decline during the long-distance autumn migration and is lowest when monarchs reach Mexico, at the end of their journey [22]. By contrast, infected monarchs that remain in the US to breed during the winter bypass the physical demands of migration and overwintering, and can reproduce shortly after eclosing. Prior studies on monarchs also offer support for migratory escape. Infection rates within the migratory monarchs' breeding range are lowest early in the breeding season and increase later in the season [22]; this suggests that successive cycles of host breeding allow infectious parasite stages to accumulate [41]. Migratory monarchs leaving behind contaminated patches during the autumn will experience a temporary reprieve from parasite transmission and return to parasite-free host plants in the spring. By contrast, continuous breeding allows OE transmission cycles to continue uninterrupted. High larval monarch density could be another factor increasing infection rates in winter-breeding monarchs. We have noted average larval densities of up to 10 eggs/larvae per milkweed plant at winter-breeding sites (D. Satterfield 2013, personal observation), several times higher than in the summer-breeding range [22]. Past field and experimental work showed that parasite transmission and host susceptibility increase with monarch larval density [53].
Because winter-breeding sites are distributed along the southern US coast, whereas summer-breeding sites occur farther north, latitudinal differences could confound comparisons of non-migratory versus migratory behaviours. However, a previous study using citizen science data showed that infection prevalence was low for migratory monarchs breeding in the southern US, which occurs as migrants return from Mexico in the spring. Our findings from monarchs overwintering in the coastal southern US also provide evidence that migratory and breeding behaviours, rather than geography, affect parasite transmission most strongly. A small fraction of the eastern monarch population overwinters, but does not breed, in the southern US, while the vast majority of their conspecifics overwinter in Mexico [45]. Results here showed that these coastal overwintering adults (not exhibiting breeding activity) experienced low infection prevalence, similar to that of Mexico overwintering monarchs and much lower than winter-breeding monarchs at neighbouring locations. This finding has two important implications: migration from summer-breeding grounds to southern coastal areas can produce some level of migratory culling; and year-round breeding enabled by the planting of tropical milkweed is the primary driver of the high parasite transmission reported in parts of the southern US.
In addition to large variation among regional sources, our findings demonstrate wide variation in infection prevalence among local sites. For instance, across all winter-breeding sites, prevalence ranged from 0 to 100%. In the GLMM analyses, variance explained by the model (R2) more than doubled when site was included as a random effect (electronic supplementary material, table S4). Other site-level factors not measured in this study, such as host density, local host genotypes, temperature, precipitation and patch size, probably play a role in infection dynamics. Sensitivity to environmental variables at local spatial scales is common in host–pathogen systems [54–56] and merits additional study. Importantly, prevalence differences among migratory and non-migratory monarchs remained strong despite heterogeneity at smaller scales.
Multiple studies to date on monarch parasites and other host–pathogen systems show that greater transmission opportunities tend to favour increased pathogen virulence [27,49,57]. Contrary to this trend, parasite isolates from winter-breeding sources were not more virulent than parasites from migratory sources. Our finding was consistent with another recent study showing no differences in the virulence of OE strains from eastern migratory versus south Florida resident monarchs [58]. We expected that highly virulent strains would be selectively removed among migratory monarchs, especially those sampled at overwintering sites in Mexico, whereas such selection would be relaxed at winter-breeding sites. We found high heterogeneity in virulence among parasite isolates within each source, suggesting that genetic variation for virulence exists, as found in earlier work [27,49]. One explanation for the lack of evolutionary divergence is that the parasite has experienced too few transmission cycles to produce a response to selection, especially because many winter-breeding study sites were established only recently (D. Satterfield 2013, personal observation).
Another explanation for lack of parasite differentiation (as suggested by Sternberg et al. [58]) is that high gene flow of parasites between resident and migratory monarchs constrains evolutionary divergence. During the autumn, migratory monarchs probably pass through locations inhabited by winter-breeding monarchs [59]. Moreover, migratory monarchs might lay eggs at winter-breeding sites in the spring when they travel north from Mexico to reproduce in the Gulf coast states [60]. If migrants oviposit on milkweed previously visited by winter-breeding butterflies, their progeny would be exposed to the same parasite strains as winter-breeding monarchs. A corollary to this scenario is that tropical milkweed patches that support winter-breeding monarchs are likely to create sources of infection that increase parasite prevalence across the entire migratory monarch range. Other host–parasite systems demonstrate this possibility. As one example, sedentary salmon reared in aquaculture enclosures can become infested with sea lice. Farmed salmon pens are often located along migratory routes where wild juvenile salmon enter the sea from their freshwater hatching grounds. As a result, wild juvenile salmon become infected with sea lice during a stage when they are highly vulnerable, decreasing wild salmon survival [61,62]. In a similar way, infection of migratory monarchs passing through the southern US each spring could offset the effects of migration in lowering parasite prevalence during the autumn. In support of this idea, OE prevalence has increased nearly threefold in eastern migratory monarchs since 2002 (S. Altizer & J. de Roode 2014, unpublished data). Future work is needed to investigate the extent to which migratory and non-migratory monarchs transmit OE and share habitat.
Our study indicates that by planting exotic tropical milkweed in southern coastal areas, humans are providing a consistent resource that allows monarchs to forego long-distance migration, breed year-round and suffer high parasite transmission. Such human-provided resources have altered pathogen transmission in other wildlife hosts by encouraging more sedentary behaviour and higher host aggregations around food sources. For example, supplemental feeding of elk during winter in the Greater Yellowstone Ecosystem increased host exposure to brucellosis and gastrointestinal nematodes [63,64]. Importantly, without considering infectious disease, winter-breeding monarchs could represent a reserve population to augment the numbers of eastern migratory monarchs in the face of steep declines. However, because these same winter-breeding monarchs support high parasite transmission, their potential role as a source of infection for migratory monarchs during seasonal periods of mixing is cause for concern. The widespread decline of migratory monarchs in North America has been widely publicized [33], with most attention focused on habitat loss as a major cause [35,36]. Shifts towards year-round breeding on tropical milkweed, resulting in high rates of OE infection, could pose an additional emerging threat to the long-term viability of migratory monarchs.
Year-round breeding in the southern US may be relatively new as a widespread phenomenon. While records of winter-breeding occurred anecdotally in earlier decades (i.e. five reports from 1939 to 1960, excluding south Florida; electronic supplementary material), winter-breeding appears to have become more common in recent years (95 reports from 2002 to 2010 [39]). Winter-breeding behaviours could also be enhanced by milder winters associated with global climate change. In the future, monarch activity along the Gulf and southern Atlantic coasts could increasingly resemble that of south Florida, where a long-established resident monarch population (not sampled in this study) experiences consistently high infection rates (more than 70%) and breeds year-round [43,46]. An online herbarium search shows a modest temporal increase in the frequency of records of tropical milkweed, but quantitative data are lacking (see the electronic supplementary material). Recent commercial demand for milkweed has stimulated tropical milkweed sales, often to the exclusion of native milkweeds [65]. To reduce monarch winter-breeding and its associated disease risk, gardeners and land managers need wider access to native milkweeds (which naturally senesce in the autumn), especially in coastal areas with mild winters.
In conclusion, we provide evidence that transitioning from migratory to non-migratory behaviours coupled with a shift to year-round breeding on introduced host plants dramatically increases the prevalence of a debilitating parasite for North American monarchs. Our results add to a growing number of studies that show migratory species worldwide are shifting the timing and spatial patterns of movement in response to human activities. Such changes can have consequences for disease transmission and virulence evolution. While sedentary behaviour could reduce parasitism for some animal populations, the loss of migratory behaviours will probably increase infection rates for many species. As more animal species are predicted to shift or reduce their migrations in the future, we expect pathogens that have been historically regulated by host migration to pose greater threats to wildlife and human health.
Supplementary Material
Acknowledgements
We thank Monarch Health volunteers for generously contributing samples, especially Victor Madamba, Shirley Brown, Fitz Clarke, Marty and Gene Webb, Jane and Jessica Arnold, Mary Kennedy, Diane Rock, Sondra Cabell, Donna Zemba, Valerie and Joel Evanson, Donna Mitchell, Ilse Gebhard and Russ Schipper, Deb Marcinski, Jim Ellis and Jessica Miller. We thank Johanna Blakeslee, Jennifer Kukharchuk, Han Nguyen, Alexa Fritzsche McKay, Kelly Nail, Wendy Caldwell, Billy McCord, Dawn and Arin Satterfield, Nik Bauchat, Harlen and Altus Aschen, Meagan Weathers, Michael Maudsley and Amy Wright for field and laboratory assistance. We thank collaborators at WWF-Mexico and Universidad Nacional Autónoma de México, including Pablo F. Jaramillo López, Eduardo Rendón Salinas, Diana López and Servando Rodriguez Mejia, for sampling monarchs at overwintering sites. We thank Andrew Davis, Andrew Park, Lincoln Brower, Alyssa Gehman and members of the Altizer lab for help with data analyses and comments on the manuscript. D.A.S. collected field data, processed and coordinated citizen science data, conducted the virulence experiment, ran statistical analyses, participated in the design of the study and drafted the manuscript. J.C.M. contributed to the design of the experiment, suggested and interpreted statistical analyses, and revised the manuscript. S.A. conceived of the study and helped to design the experiment, coordinated citizen scientists, collected field data, assisted with statistical analyses, offered important revisions and helped to draft the manuscript. All authors approved final submission.
Ethics statement
Authorization for interstate movement of monarchs was obtained from USDA APHIS permit P525P-11-04112.
Data accessibility
Data available in Dryad (doi:10.5061/dryad.s4dv0).
Funding statement
Financial support was provided by a National Science Foundation (NSF) grant no. (DEB-0643831) to S.A.; a grant from the International Programs of the US Forest Service through the University of Minnesota to SA; and an NSF Dissertation Improvement Grant (DEB-1406862) and NSF Graduate Research Fellowship to D.S.
Conflict of interests
We have no competing interests.
References
- 1.Dingle H. 1996. Migration: the biology of life on the move. New York, NY: Oxford University Press. [Google Scholar]
- 2.Bauer S, Hoye BJ. 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344, 54–62. ( 10.1126/science.1242552) [DOI] [PubMed] [Google Scholar]
- 3.Wilcove DS, Wikelski M. 2008. Going, going, gone: is animal migration disappearing? PLoS Biol. 6, e188 ( 10.1371/journal.pbio.0060188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser ME, Perdeck AC, Van Balen JH, Both C. 2009. Climate change leads to decreasing bird migration distances. Glob. Change Biol. 15, 1859–1865. ( 10.1111/j.1365-2486.2009.01865.x) [DOI] [Google Scholar]
- 5.Both C, Bouwhuis S, Lessells CM, Visser ME. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83. ( 10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 6.Fiedler W, Bairlien F, Köppen U. 2004. Using large-scale data from ringed birds for the investigation of effects of climate change on migrating birds: pitfalls and prospects. Adv. Ecol. Res. 35, 49–67. ( 10.1016/S0065-2504(04)35003-8) [DOI] [Google Scholar]
- 7.Pulido F, Berthold P. 2010. Current selection for lower migratory activity will drive the evolution of residency in a migratory bird population. Proc. Natl Acad. Sci. USA 107, 7341–7346. ( 10.1073/pnas.0910361107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones JD, Kauffman MJ, Monteith KL, Scurlock BM, Albeke SE, Cross PC. 2014. Supplemental feeding alters migration of a temperate ungulate. Ecol. Appl. 24, 1769–1779. ( 10.1890/13-2092.1) [DOI] [PubMed] [Google Scholar]
- 9.Luniak M. 2004. Synurbization—adaptation of animal wildlife to urban development. In Proc. 4th Int. Urban Wildl. Sympos (eds WW Shaw, LK Harris, L Vandruff), pp. 50–55. Tucson, AZ: School of Natural Resources, University of Arizona. [Google Scholar]
- 10.Tortosa FS, Caballero JM, Reyes-López J. 2002. Effects of rubbish dumps on breeding success in the White Stork in southern Spain. Waterbirds 25, 39–43. ( 10.1675/1524-4695(2002)025[0039:EORDOB]2.0.CO;2) [DOI] [Google Scholar]
- 11.Evans KL, Newton J, Gaston KJ, Sharp SP, McGowan A, Hatchwell BJ. 2012. Colonisation of urban environments is associated with reduced migratory behaviour, facilitating divergence from ancestral populations. Oikos 121, 634–640. ( 10.1111/j.1600-0706.2011.19722.x) [DOI] [Google Scholar]
- 12.Adriaensen F, Ulenaers P, Dhondt AA. 1993. Ringing recoveries and the increase in numbers of European great crested grebes Podiceps cristatus. Ardea 81, 59–70. [Google Scholar]
- 13.Van Der Ree R, McDonnell MJ, Temby I, Nelson J, Whittingham E. 2006. The establishment and dynamics of a recently established urban camp of flying foxes (Pteropus poliocephalus) outside their geographic range. J. Zool. 268, 177–185. ( 10.1111/j.1469-7998.2005.00005.x) [DOI] [Google Scholar]
- 14.Gresh T, Lichatowich J, Schoonmaker P. 2000. An estimation of historic and current levels of salmon production in the Northeast Pacific ecosystem: evidence of a nutrient deficit in the freshwater systems of the Pacific Northwest. Fisheries 25, 15–21. () [DOI] [Google Scholar]
- 15.Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331, 296–302. ( 10.1126/science.1194694) [DOI] [PubMed] [Google Scholar]
- 16.Waldenström J, Bensch S, Kiboi S, Hasselquist D, Ottosson U. 2002. Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Mol. Ecol. 11, 1545–1554. ( 10.1046/j.1365-294X.2002.01523.x) [DOI] [PubMed] [Google Scholar]
- 17.Figuerola J, Green A. 2000. Haematozoan parasites and migratory behaviour in waterfowl. Evol. Ecol. 14, 143–153. ( 10.1023/A:1011009419264) [DOI] [Google Scholar]
- 18.Krauss S, Stallknecht DE, Negovetich NJ, Niles LJ, Webby RJ, Webster RG. 2010. Coincident ruddy turnstone migration and horseshoe crab spawning creates an ecological ‘hot spot’ for influenza viruses. Proc. R. Soc. B 277, 3373–3379. ( 10.1098/rspb.2010.1090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricklefs R, Fallon S, Latta S, Swanson B, Bermingham E. 2005. Migrants and their parasites. In Birds of two worlds: the ecology and evolution of migration, pp. 210–221. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 20.Loehle C. 1995. Social barriers to pathogen transmission in wild animal populations. Ecology 76, 326–335. ( 10.2307/1941192) [DOI] [Google Scholar]
- 21.Bradley CA, Altizer S. 2005. Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecol. Lett. 8, 290–300. ( 10.1111/j.1461-0248.2005.00722.x) [DOI] [Google Scholar]
- 22.Bartel RA, Oberhauser KS, de Roode JC, Altizer SM. 2011. Monarch butterfly migration and parasite transmission in eastern North America. Ecology 92, 342–351. ( 10.1890/10-0489.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krkošek M, Gottesfeld A, Proctor B, Rolston D, Carr-Harris C, Lewis MA. 2007. Effects of host migration, diversity and aquaculture on sea lice threats to Pacific salmon populations. Proc. R. Soc. B 274, 3141–3149. ( 10.1098/rspb.2007.1122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall RJ, Altizer S, Bartel RA. 2014. Greater migratory propensity in hosts lowers pathogen transmission and impacts. J. Anim. Ecol. 83, 1068–1077. ( 10.1111/1365-2656.12204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folstad I, Nilssen AC, Halvorsen O, Andersen J. 1991. Parasite avoidance: the cause of post-calving migrations in Rangifer? Can. J. Zool. 69, 2423–2429. ( 10.1139/z91-340) [DOI] [Google Scholar]
- 26.Akbar H, et al. 2012. Characterizing pneumocystis in the lungs of bats: understanding pneumocystis evolution and the spread of pneumocystis organisms in mammal populations. Appl. Environ. Microbiol. 78, 8122–8136. ( 10.1128/AEM.01791-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Roode JC, Altizer S. 2010. Host–parasite genetic interactions and virulence–transmission relationships in natural populations of monarch butterflies. Evolution 64, 502–514. ( 10.1111/j.1558-5646.2009.00845.x) [DOI] [PubMed] [Google Scholar]
- 28.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341, 514–519. ( 10.1126/science.1239401) [DOI] [PubMed] [Google Scholar]
- 29.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P. 2011. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B 278, 3703–3712. ( 10.1098/rspb.2011.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malcolm SB, Cockrell B, Brower L. 1993. Spring recolonization of eastern North America by the monarch butterfly: successive brood or single sweep migration? In Biology and conservation of the monarch butterfly (eds SB Malcolm, MP Zalucki), pp. 253–267. Los Angeles, CA: Natural History Museum of Los Angeles. [Google Scholar]
- 31.Urquhart FA, Urquhart NR. 1978. Autumnal migration routes of the eastern population of the monarch butterfly (Danaus p. plexippus L.; Danaidae; Lepidoptera) in North America to the overwintering site in the Neovolcanic Plateau of Mexico. Can. J. Zool. 56, 1759–1764. ( 10.1139/z78-240) [DOI] [Google Scholar]
- 32.Brower LP, Malcolm SB. 1991. Animal migrations: endangered phenomena. Am. Zool. 31, 265–276. ( 10.1093/icb/31.1.265) [DOI] [Google Scholar]
- 33.Brower LP, Taylor OR, Williams EH, Slayback DA, Zubieta RR, Ramírez MI. 2012. Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv. Diver. 5, 95–100. ( 10.1111/j.1752-4598.2011.00142.x) [DOI] [Google Scholar]
- 34.Rendón-Salinas E, Tavera-Alonso G. 2012. Monitoreo de la superficie forestal ocupada por las colonias de hibernación de la Mariposa Monarca en diciembre de 2013. Mexico City, Mexico: WWF.
- 35.Brower LP, Castilleja G, Peralta A, Lopez-Garcia J, Bojorquez-Tapia L, Diaz S, Melgarejo D, Missrie M. 2002. Quantitative changes in forest quality in a principal overwintering area of the monarch butterfly in Mexico, 1971–1999. Conserv. Biol. 16, 346–359. ( 10.2307/3061361) [DOI] [Google Scholar]
- 36.Pleasants JM, Oberhauser KS. 2013. Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv. Diver. 6, 135–144. ( 10.1111/j.1752-4598.2012.00196.x) [DOI] [Google Scholar]
- 37.Woodson REJ. 1954. The North American species of Asclepias L Annals of the Missouri Botanical Garden41, 1–211 ( 10.2307/2394652) [DOI] [Google Scholar]
- 38.Batalden R, Oberhauser K. In press. Potential changes in eastern North American monarch migration in response to an introduced milkweed, Asclepias curassavica. In Monarchs in a changing world: biology and conservation of an iconic insect. Ithaca, NY: Cornell University Press. [Google Scholar]
- 39.Howard E, Aschen H, Davis AK. 2010. Citizen science observations of monarch butterfly overwintering in the southern United States. Psyche 2010, 1–6. ( 10.1155/2010/689301) [DOI] [Google Scholar]
- 40.McLaughlin RE, Myers J. 1970. Ophryocystis elektroscirrha sp. n., a neogregarine pathogen of the monarch butterfly Danaus plexippus (L.) and the Florida queen butterfly D. gilippus berenice Cramer1. J. Euk. Microbiol. 17, 300–305. ( 10.1111/j.1550-7408.1970.tb02375.x) [DOI] [Google Scholar]
- 41.Altizer S, Oberhauser K, Geurts K. 2004. Transmission of the protozoan parasite Ophryocystis elektroscirrha in monarch butterfly populations: implications for prevalence and population-level impacts. In The monarch butterfly: biology and conservation (eds KS Oberhauser, MJ Solensky), pp. 203–218. Ithaca, NY: Cornell University Press. [Google Scholar]
- 42.Altizer SM, Oberhauser KS. 1999. Effects of the protozoan parasite Ophryocystis elektroscirrha on the fitness of monarch butterflies (Danaus plexippus). J. Invert. Pathol. 74, 76–88. ( 10.1006/jipa.1999.4853) [DOI] [PubMed] [Google Scholar]
- 43.Altizer SM, Oberhauser KS, Brower LP. 2000. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecol. Entomol. 25, 125–139. ( 10.1046/j.1365-2311.2000.00246.x) [DOI] [Google Scholar]
- 44.Altizer S, de Roode JC. 2014. In press. Monarchs and their debilitating parasites: immunity, migration and medicinal plant use. In Monarchs in a changing world: biology and conservation of an iconic insect. Ithaca, NY: Cornell University Press. [Google Scholar]
- 45.Urquhart FA. 1960. The monarch butterfly. Toronto, Canada: University of Toronto Press. [Google Scholar]
- 46.Brower LP. 1961. Studies on the migration of the monarch butterfly I. Breeding populations of Danaus plexippus and D. gilippus berenice in south central Florida. Ecology 42, 76–83. ( 10.2307/1933269) [DOI] [Google Scholar]
- 47.De Roode JC, Chi J, Rarick RM, Altizer S. 2009. Strength in numbers: high parasite burdens increase transmission of a protozoan parasite of monarch butterflies (Danaus plexippus). Oecologia 161, 67–75. ( 10.1007/s00442-009-1361-6) [DOI] [PubMed] [Google Scholar]
- 48.De Roode JC, Gold LR, Altizer S. 2007. Virulence determinants in a natural butterfly-parasite system. Parasitology 134, 657–668. ( 10.1017/S0031182006002009) [DOI] [PubMed] [Google Scholar]
- 49.De Roode JC, Yates AJ, Altizer S. 2008. Virulence-transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. Proc. Natl Acad. Sci. USA 105, 7489–7494. ( 10.1073/pnas.0710909105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 51.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 52.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 53.Lindsey E, Mehta M, Dhulipala V, Oberhauser K, Altizer S. 2009. Crowding and disease: effects of host density on response to infection in a butterfly–parasite interaction. Ecol. Entomol. 34, 551–561. ( 10.1111/j.1365-2311.2009.01107.x) [DOI] [Google Scholar]
- 54.Pierce AA, de Roode JC, Altizer S, Bartel RA. 2014. Extreme heterogeneity in parasitism despite low population genetic structure among monarch butterflies inhabiting the Hawaiian Islands. PLoS ONE 9, e100061 ( 10.1371/journal.pone.0100061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Real LA, Biek R. 2007. Spatial dynamics and genetics of infectious diseases on heterogeneous landscapes. J. R. Soc. Interface 4, 935–948. ( 10.1098/rsif.2007.1041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brownstein JS, Skelly DK, Holford TR, Fish D. 2005. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia 146, 469–475. ( 10.1007/s00442-005-0251-9) [DOI] [PubMed] [Google Scholar]
- 57.Fraser C, Hollingsworth TD, Chapman R, Wolf F, Hanage WP. 2007. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc. Natl Acad. Sci. USA 104, 17 441–17 446. ( 10.1073/pnas.0708559104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sternberg ED, Li H, Wang R, Gowler C, de Roode JC. 2013. Patterns of host-parasite adaptation in three populations of monarch butterflies infected with a naturally occurring protozoan disease: virulence, resistance, and tolerance. Am. Nat. 182, E235–E248. ( 10.1086/673442) [DOI] [PubMed] [Google Scholar]
- 59.Knight A, Brower L. 2009. The influence of eastern North American autumnal migrant monarch butterflies (Danaus plexippus L.) on continuously breeding resident monarch populations in southern Florida. J. Chem. Ecol. 35, 816–823. ( 10.1007/s10886-009-9655-z) [DOI] [PubMed] [Google Scholar]
- 60.Knight A, Brower LP, Williams EH. 1999. Spring remigration of the monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae) in north-central Florida: estimating population parameters using mark-recapture. Biol. J. Linn. Soc. 68, 531–556. ( 10.1006/bijl.1999.0351) [DOI] [Google Scholar]
- 61.Costello MJ. 2009. How sea lice from salmon farms may cause wild salmonid declines in Europe and North America and be a threat to fishes elsewhere. Proc. R. Soc. B 276, 3385–3394. ( 10.1098/rspb.2009.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krkos˘ek M, Lewis MA, Morton A, Frazer LN, Volpe JP. 2006. Epizootics of wild fish induced by farm fish. Proc. Natl Acad. Sci. USA 103, 15 506–15 510. ( 10.1073/pnas.0603525103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hines AM, Ezenwa VO, Cross P, Rogerson JD. 2007. Effects of supplemental feeding on gastrointestinal parasite infection in elk (Cervus elaphus): preliminary observations. Vet. Parasitol. 148, 350–355. ( 10.1016/j.vetpar.2007.07.006) [DOI] [PubMed] [Google Scholar]
- 64.Cross PC, Edwards WH, Scurlock BM, Maichak EJ, Rogerson JD. 2007. Effects of management and climate on elk brucellosis in the Greater Yellowstone Ecosystem. Ecol. Appl. 17, 957–964. ( 10.1890/06-1603) [DOI] [PubMed] [Google Scholar]
- 65.Borders B, Lee-Mäder E. 2014. Milkweeds: a conservation practitioner's guide. Portland, OR: Xerces Society for Invertebrate Conservation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available in Dryad (doi:10.5061/dryad.s4dv0).