Abstract
Amniotes, tetrapods that evolved the cleidoic egg and thus independence from aquatic larval stages, appeared ca 314 Ma during the Coal Age. The rapid diversification of amniotes and other tetrapods over the course of the Late Carboniferous period was recently attributed to the fragmentation of coal-swamp rainforests ca 307 Ma. However, the amniote fossil record during the Carboniferous is relatively sparse, with ca 33% of the diversity represented by single specimens for each species. We describe here a new species of reptilian amniote that was collected from uppermost Carboniferous rocks of Prince Edward Island, Canada. Erpetonyx arsenaultorum gen. et sp. nov. is a new parareptile distinguished by 29 presacral vertebrae and autapomorphies of the carpus. Phylogenetic analyses of parareptiles reveal E. arsenaultorum as the closest relative of bolosaurids. Stratigraphic calibration of our results indicates that parareptiles began their evolutionary radiation before the close of the Carboniferous Period, and that the diversity of end-Carboniferous reptiles is 80% greater than suggested by previous work. Latest Carboniferous reptiles were still half as diverse as synapsid amniotes, a disparity that may be attributable to preservational biases, to collecting biases, to the origin of herbivory in tetrapods or any combination of these factors.
Keywords: carboniferous, diversification, evolution, parareptile
1. Introduction
Phylogenetic studies of the past three decades confirm the basal dichotomy of amniotes into synapsids (i.e. mammals and their fossil relatives) on the one hand and reptiles (e.g. squamates, crocodiles, birds and their fossil relatives; a.k.a. ‘sauropsids') on the other hand [1–3]. Whereas the timing of the origin and basal amniote dichotomy during the Carboniferous Period is highly debated [4,5], the oldest unequivocal amniote fossils are known from the Joggins Formation of Nova Scotia, Canada, and are generally regarded to be 313–316 million years (Ma) in age [5].
Among the 10 tetrapod taxa described from the Joggins formation, only two amniote species are recognized as valid [5,6]. The amniote fossil record continues to be sparse at succeeding Carboniferous localities, with the exception of the Early Kasimovian (ca 305 Ma) amniote-rich fauna at Garnett, Kansas [7,8]. Nevertheless, reviews of the alpha taxonomy of Carboniferous amniotes revealed that the early diversification of synapsids outpaced that of reptiles, with the result that, by the end of the Carboniferous, synapsid species outnumbered reptiles approximately 2 to 1 [9,10]. An interesting corollary of the disparity in diversity between these two great clades of amniotes is that Carboniferous reptiles are substantially smaller animals than contemporaneous synapsids [9].
A more dramatic dichotomy exists within early Reptilia itself: all Carboniferous species of this group are classified as members of Eureptilia [3], whereas contemporaneous members of the sister group Parareptilia have yet to be identified. Instead, a single ghost taxon has been reconstructed for Parareptilia, inferred to extend from the Permian Period down to the origin of amniotes [11]. We describe here the first Carboniferous parareptile, based on a nearly complete, articulated skeleton from Prince Edward Island, Canada. The early appearance and the phylogenetic relationships of this new parareptile have dramatic implications for our understanding of the early diversification of reptiles and amniotes.
2. Material
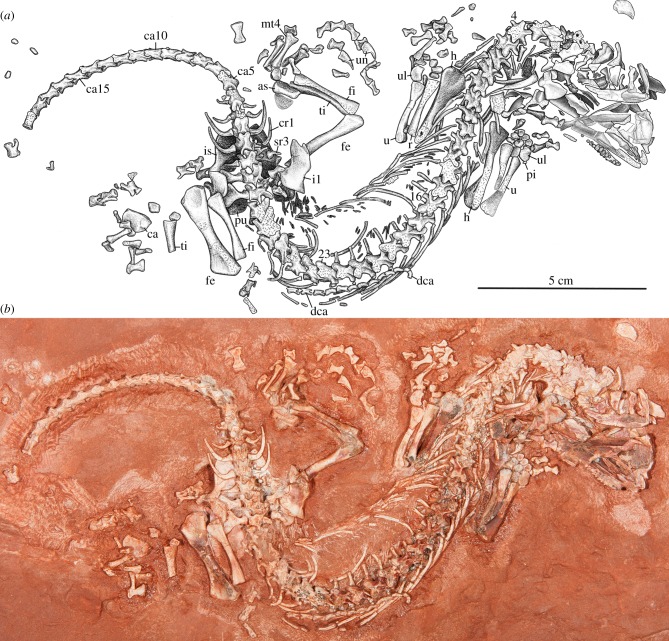
The study material (figure 1) comprises a nearly complete, articulated skeleton preserved on a sandstone slab and reposited in the collections of the Royal Ontario Museum, Toronto, as ROM 55402. The fossil was prepared mechanically by use of air scribes and pin vices.
Figure 1.
Erpetonyx arsenaultorum n. gen. et sp., ROM 55402, holotype. (a) Interpretive drawing of skeleton in dorsal aspect. (b) Photograph of skeleton in dorsal aspect. as, astragalus; ca, calcaneum; ca5, caudal vertebra 5; ca15, caudal vertebra 15; cr1, caudal rib 1; dca, distal caudal vertebra; fe, femur; fi, fibula; h, humerus; il, ilium; is, ischium; mt, metatarsal; pi, pisiform; pu, pubis; r, radius; sr3, sacral rib 3; ti, tibia; u, ulna; ul, ulnare; un, unguals. Arabic numerals identify cervical vertebrae. (Online version in colour.)
The presence of plicidentine and the similarity of the dorsal vertebrae of ROM 55402 to those described for the Early Permian parareptile Delorhynchus cifellii [12] strongly suggested parareptilian affinities for the Prince Edward Island reptile. For our phylogenetic analysis, we used an augmented version of the characters and data matrix of Reisz et al. [12]. Although a recent study has resurrected the hypothesis that turtles are parareptiles [13], the problem of turtle origins is beyond the scope of our study—the relationships of a Late Carboniferous reptile and its implications—so we did not include turtle taxa (e.g. Odontochelys and/or Proganochelys) in our analysis. We recoded some taxa for certain characters (see the electronic supplementary material for data matrix), and performed a heuristic analysis, set to collapse branches with a maximum length of zero, in PAUP 4.0a134 [14]. We also conducted a Bremer decay analysis using the heuristic algorithm in PAUP, by relaxing parsimony a single step at a time and generating strict consensus trees until resolution was completely lost in the ingroup. We also conducted a bootstrap analysis (1000 replicates).
In addition to the traditional parsimony analysis, a Bayesian analysis was also conducted on the dataset in order to assess its robusticity to different methods of phylogeny estimation [15]. The Bayesian analysis of the morphological data matrix was conducted using MrBayes v. 3.1.2 [16], and employed the Mk model (datatype = standard) with the addition of a gamma shape parameter (rates = variable) to allow for rates of change to vary across characters, as recommended by Müller & Reisz [3]. The analysis was run for 3 000 000 generations (Markov Chain Monte Carlo (MCMC): four chains, two simultaneous independent runs), with a tree sampled every 100 generations, with the first 10% of the sampled trees being discarded as the ‘burn-in’. The majority-rule consensus tree with nodal clade credibility values is presented in the electronic supplementary material.
The results of our analyses indicate that the new Prince Edward Island reptile is closely related to bolosaurid parareptiles. Accordingly, we resurrect the name Bolosauria, which was erected as an ordinal name by Kuhn [17] to contain the family Bolosauridae Cope, 1878, and define it as a branch-based group: Bolosaurus striatus Cope, 1878 [18] and all species related more closely to it than to Procolophon trigoniceps Owen, 1876 [19].
3. Systematic palaeontology
Parareptilia Olson, 1947 [20].
Bolosauria Kuhn, 1959 [17].
Erpetonyx arsenaultorum n. gen. et sp.
(a). Etymology
The genus name is from classical Greek ερπετον, ‘crawler’, and ονυσ, ‘claw’. The specific epithet honours the Arsenault family of Prince County, Prince Edward Island, Canada, who discovered and collected the specimen.
(b). Material
ROM 55402, holotype, a nearly complete, mostly articulated skeleton (figure 1a,b).
(c). Locality and horizon
ROM 55402 was collected from a locality at Cape Egmont in southwestern Prince Edward Island, Canada. The Egmont Bay Formation crops out along the western part of the island, and is regarded to be latest Pennsylvanian (Stephanian) in geological age on the basis of plant body fossils and pollen [21]. Thus, the fossil is Gzhelian in age (303.7 to 298.9 Ma) [22].
(d). Diagnosis
A small, basal parareptile that possesses 29 presacral vertebrae (viz. five cervicals and 24 dorsals), relatively small carpal bones (the radiale and the pisiform are ca one-half the size of the ulnare and the fifth distal carpal, respectively), a femoral distal end with an epicondylar axis at 45° to the shaft, a fourth metatarsal with a relatively broad distal end, and well-developed unguals with prominent flexor tubercles.
4. Description
ROM 55402 (figure 1) comprises a nearly complete articulated skeleton that is preserved largely in dorsal view, and spanning ca 20 cm across a sandstone block. The skull, both manus, the left pes and the right hind limb are disarticulated to varying degrees. Much of the skull roof, dorsal portions of most of the presacral vertebrae and numerous trunk ribs have been damaged by weathering. The tail, partly disarticulated and missing an estimated 15 caudal vertebrae, is otherwise well preserved. The well-ossified nature of the autopodia and the closure of neurocentral sutures in the sacral and presacral vertebrae indicate that the skeleton is that of an adult animal.
(a). Skull
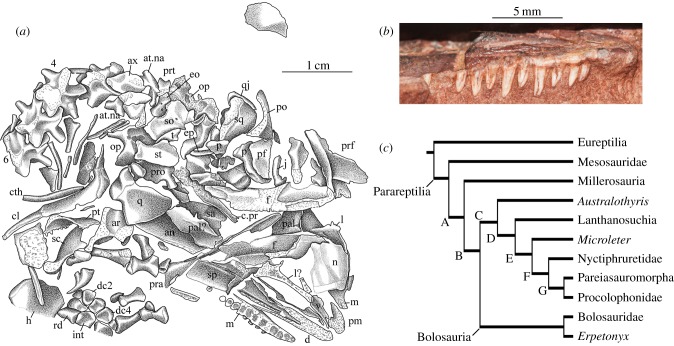
The skull of ROM 55402 is compressed obliquely and was partly disarticulated prior to burial (figures 1 and 2a). Roughly half of the skull bones, particularly those of the dermal skull roof, are weathered, such that either their surficial details or their outlines, or both, are indistinct. The immediately identifiable dermal bones are the premaxilla, the maxilla, the frontal, the lacrimal, the prefrontal and the supratemporal. A large, partially moulded surface anteriorly represents the impressions of the ventral surface of the nasals.
Figure 2.
Erpetonyx arsenaultorum n. gen. et sp., ROM 55402, holotype. (a) Interpretive drawing of skull. (b) Photograph of right maxillary dentition in lateral view. (c) Results of phylogenetic analysis; tree length = 557, consistency index (CI) = 0.3429, CI excluding informative characters = 0.3405, retention index = 0.6355, rescaled CI = 0.2179. Bootstrap/Bremer support values: Bolosauria: 39/3; Parareptilia: 13/1; clade A: 33/2; clade B (Procolophonomorpha): 32/2; clade C: 11/2; clade D (Ankyramorpha): 12/2; clade E: 19/2; clade F: 44/1; clade G: 15/1. Bolosauria is diagnosed by the following unambiguous synapomorphies: postparietal small (character 9, state 1); transverse flange of pterygoid dentition present (character 70, state 1); anterior caudal ribs elongate and extend posteriorly to end of next vertebra (character 132, state 0); greater trochanter of femur present (character 156, state 1); maxillary tooth positions number 15 or fewer (character 167, state 0). Anatomical abbreviations: an, angular; ar, articular; at.na, atlantal neural arch; ax, axis; cl, clavicle; cth, cleithrum; d, dentary; dc2, distal carpal 2; dc4, distal carpal 4; c.pr, cultriform process of parasphenoid; eo, exoccipital; ep, epipterygoid; f, frontal; int, intermedium; j, jugal; l, lacrimal; m, maxilla; n, nasal; op, opisthotic; p, parietal; pal, palatine; pf, postfrontal; pm, premaxilla; po, postorbital; pra, prearticular; pro, prootic; prf, prefrontal; prt, proatlas; pt, pterygoid; q, quadrate; qj, quadratojugal; rd, radiale; sa, surangular; sc, scapula; so, supraoccipital; sp, splenial; sq, squamosal; st, supratemporal; t, tabular; v, vomer. Arabic numerals identify cervical vertebrae. (Online version in colour.)
The premaxilla is preserved only as a section through the left element. Both maxillae are present, but preserved and/or exposed to varying degrees. The anterior end of the left maxilla is exposed in medial aspect, and shows that the maxilla formed the posteroventral corner of the external naris. The dorsal portion of the right maxilla has been eroded down to a spindle-shaped alveolar region anteriorly, exposing the bases of 13 teeth. Two (missing) teeth could have fit into the space between the last and penultimate maxillary teeth preserved, such that the maxilla featured at least 15 tooth positions.
The exposed bases of the anterior-most seven teeth show the simple infolding that is indicative of the plicidentine that is seen in Colobomycter pholeter and several other parareptiles [23,24]. Infolding extends down the length of the largest teeth (figure 2b). The teeth are slightly recurved, sharply tipped conical structures that lack cutting edges. There is neither a caniniform tooth nor caniniform region. The teeth gradually diminish in both length and basal diameter as one progresses posteriorly.
The left lacrimal is preserved in articulation with the left maxilla, and is exposed here mostly in posteromedial view, where it forms the anterolateral corner of the orbit (figure 2a). The facial portion extends anteriorly, but is mostly obscured by the nasal impression; it is not clear how far anteriorly the lacrimal extended.
Both frontals are preserved as a motley of heavily weathered bone and impression. Each frontal is least six times longer than it is wide. The middle third of the lateral margin is faintly concave and represents the dorsal margin of the orbit. There is a distinct posterolateral process, which bears a shallow shelf for the reception of the parietal. Closely associated with the left frontal is the left prefrontal, which consists of a slightly curved antorbital flange and a tongue-shaped facial process.
An irregularly shaped dermal bone, with what appears to be a small posterior ‘hornlet’, is identified here as the right supratemporal (figure 2a). Whereas the medial and posterior margins are well preserved, the anterior and lateral margins of this bone are heavily weathered (yielding an unnaturally straight lateral or anterolateral margin). The well-preserved posterior margin consists of a faintly sinusoidal free edge from which projects a small, narrow, tongue-like projection or ‘hornlet’. Where the dorsal surface is well preserved, it is smooth and featureless.
Various fragments of relatively thick bone that are clearly dermal roofing elements, but are not informative morphologically, are tentatively identified as the parietal, the postfrontal, the postorbital, the squamosal, the postparietal and the tabular bones.
The palatal bones are not well exposed. The anterior ends of the slender, paired vomers are preserved anteriorly in close association with the premaxilla. The left palatine is preserved in articulation with the left lacrimal in its expected position, partly exposed between the two separated frontal bones. The only other unequivocal palatal bone is a denticulated sheet of bone positioned posteriorly that, judging from the relative thinness of the bone and its distinctive curvature, probably represents parts of the base of the quadrate ramus and the pterygoid transverse process. The palatal denticles are tiny, simple cones. As in Feeserpeton and many other parareptiles [25], the denticles extend onto the quadrate ramus of the pterygoid. There are fragments of thin, smoothly finished bone elsewhere that may represent fragments of palatal elements, but these are uninformative.
The dorsal columella of an epipterygoid projects from beneath fragments of parietal bone. A remarkably robust right quadrate is preserved in its expected position, in the posterolateral corner of the skull, with the condyle directed posteriorly and the anterolateral surface of the dorsal lamella facing upwards. The quadrate condyle is a knuckle-like block of bone, slightly broader transversely than anteroposteriorly long, with a saddle-shaped articulating surface for the articular. The dorsal lamella is a trapezoidal plate that is roughly as tall as it is broad. In its general morphology, the quadrate of ROM 55402 resembles that of Belebey vegrandis [26].
Most braincase elements are present, but overlying dermal bone fragments preclude full descriptions (figure 2a). The parabasisphenoid is identifiable because of its prominent cultriform process, which is present as a slender trough formed of thin, low plates of bone. Although narrowing anteriorly, its anterior-most tip is missing; what is present indicates a long process, which is roughly equal in length to the body of the braincase, and probably extended the entire length of the interpterygoid vacuity. The main body of the parabasisphenoid is poorly preserved, bearing a weathered right clinoideus process and a partly exposed, poorly ossified retractor pit.
What is exposed of the prootics largely conforms with the general morphology of this element in other early reptiles. An exception is a ventral concavity in the anterior end of the right prootic, which possibly formed the dorsal half of a foramen that was completed ventrally by the parabasisphenoid, and may represent the opening for the hyomandibular branch of the facial nerve [27].
Immediately posterior to the prootics are the opisthotics, with their deep, U-shaped excavations of unfinished bone representing the posterior portions of the membranous labyrinths. A stubby paroccipital process extends laterally from the main body of the right opisthotic. Between the opisthotics lies the broad, plate-like supraoccipital, identifiable by the embayment in its ventral margin forming the dorsal portion of the foramen magnum. Only the ventral portion of the left exoccipital is preserved, as a slightly curved, hourglass-shaped bone.
The mandible is represented by the dentary, splenial and articular of the right ramus and the angular, the surangular and the prearticular of the left ramus (figure 2a). The dentary is worn down by weathering and is identifiable by its position and its outline, but it is not preserved well enough for description. Closely appressed to the dentary is the better preserved splenial; it has a smooth lingual surface and does not contribute to the mandibular symphysis. The angular and the surangular are low, elongate and slightly curved bones; the dorsal margin of the latter bone is a low, weakly convex edge. The prearticular is a slender, slightly curved element with a dorsoventrally expanded posterior end. The articular is preserved upside-down with respect to the skull and rotated slightly more than 90° anticlockwise, such that both the posterior-most end of the ventral edge of the mandible and the pterygoideus process are exposed in ventral view. It is an irregularly shaped bone with a well-developed retroarticular process, the ventral surface of which is damaged. The insertion surface for the pterygoideus musculature is visible as a slightly sigmoidal depression between the planed-down ventral extremities of the articular.
(b). Postcranial axial skeleton
The backbone is preserved as a sinusoidal, largely articulated series of 65 vertebrae, plus 10 disarticulated caudal vertebrae (figure 1). We estimate that an ‘arc’ between the two articulated caudal series would accommodate about 25 vertebrae, suggesting that—minus the 10 disarticulated caudals—about 15 caudal vertebrae from the middle to distal part of the tail are not preserved. Nearly all neural spines have been weathered down to low stumps.
There are 29 presacral vertebrae. Among non-mesosaurian parareptiles, presacral vertebrae number 26 or fewer [28–30]. As is typical for early amniotes, there is no morphological distinction between the last cervical vertebra and the first dorsal vertebra, but rib morphology suggests that there are five cervical vertebrae. A proatlas, portions of the atlas and the axis are present, but not morphologically informative. Beginning with the third cervical vertebra, the presacral neural arches exhibit a distinctive hourglass-shaped outline in dorsal aspect. The presacral neural arches are relatively narrow; the breadth across the postzygapophyses of the fifth cervical is ca 62% of the total anteroposterior length of the arch.
Twenty-four vertebrae form the dorsal series (figure 1). The neural arches gradually increase in both breadth and length posteriorly down the column, such that the 16th dorsal (two-thirds of the way down the dorsal series) is approximately 25% broader and longer than an anterior dorsal vertebra. All dorsal neural arches feature the conspicuous hourglass-shaped organization seen in the posterior cervicals. The hourglass shape seen in dorsal aspect is enhanced by oblique, paired excavations in the dorsolateral surfaces of the arch, positioned between the transverse process and a ridge extending from the posterior part of the prezygapophysis to the midpoint of the dorsal midline of the arch. Because of the tight articulation of the vertebrae, none of the zygopophyseal facets is fully visible, but they appear to be subcircular in outline, and inclined very weakly from the horizontal—those of the prezygapophyses facing slightly medially, perhaps as much as 10°, whereas those of the postzygapophyses tilt slightly laterally in complementary fashion.
There are three sacral vertebrae. As is typical of early terrestrial tetrapods [31,32], the first sacral has a neural arch morphology that is transitional to that seen in the preceding dorsal vertebra and the succeeding caudals in that the prezygapophyses are relatively broad, matching the broad postzygapophyses of the dorsal vertebra, but with the postzygapophyses forming a distinctly narrow posterior end to the first sacral neural arch, commensurate with the relatively narrow arches of the anterior-most caudals. There are three pairs of sacral ribs. The first and second ribs are flared laterally and similar in size, but the third sacral rib is a finger-like projection that had only a touch contact with the iliac blade.
Forty-three caudal vertebrae are preserved (figure 1). The base of the tail forms a tightly articulated series up to, and including, caudal 20, and there is an articulated series of 13 distal caudals preserved adjacent to presacrals 19–25. If the latter series is preserved in normal position with respect to the proximal articulated series (i.e. the base of the tail), we estimate that approximately 25 caudal vertebrae would arc along this ‘missing’ part of the tail. Ten caudals of intermediate size, including several single disarticulated caudals, two pairs of caudals and a loose articulated series of three caudals, are scattered loosely in this area, indicating that about 15 caudal vertebrae are not preserved. Altogether, at least 58 caudal vertebrae were present, a number closely comparable to that described for other early reptiles [29,32,33].
(c). Appendicular skeleton
The appendicular bones are preserved in close association with those of the axial skeleton (figure 1). However, except for the left ilium, they are mostly overlain either by the vertebral column, or by neighbouring elements. Most elements of the pectoral girdle are present, but they are not well exposed. What is visible of each suggests a morphology very similar to that of other early terrestrial reptiles [28,33].
Elements of the pectoral limb are well preserved and exposed for the most part. The humerus is roughly the same relative size and morphology as this element in other early reptiles. However, there is little development of a trochlea and a capitulum for articulation with the ulna and the radius, respectively, suggesting more freedom of movement at the elbow compared with early sprawling reptiles, such as Captorhinus [34]. The radius and the ulna are just under 80% of the length of the humerus. The olecranon process of the ulna is not well developed, being little more than a nubbin.
Neither manus is complete and both are disarticulated to varying degrees. The right carpus is preserved fully articulated as a mosaic of irregular tile-like polygons. The radiale, the pisiform, and the first and fifth distal carpals are unusually small for an early reptile. The elements distal to the carpus are not articulated well enough to provide a good understanding of the structure of the digits in either manus. Apart from the right fifth metatarsal, none of the metacarpals remains in articulation with its respective distal carpal. Some 12 non-ungual phalanges and a single unequivocal ungual (less than 40% of the 34 phalanges that are expected, assuming a plesiomorphic phalangeal formula of 2–3–4–5–3 [2]) are all that remain of the manual digits. The non-terminal phalanges are dorsoventrally compressed, gently waisted bow ties of bone. The ungual is a distinctly claw-like element which has a relatively broad, elliptical articulating head, a prominent flexor tubercle (roughly the same relative size as in the synapsid Dimetrodon [35]), and an acute tip.
All of the pelvic girdle elements are present in varying degrees of exposure and completeness, and are closely associated with each other and with neighbouring bones (figure 1). Only the left ilium is fully exposed (in medial aspect). It exhibits a relatively deep, posterodorsally directed iliac blade; there is no anterodorsal process. The pubes are largely obscured by overlying elements, but what is visible indicates that these bones are relatively flat and very short, subrectangular elements. The ischia are slightly better exposed than the pubes, revealing that each ischium is a flat tongue of bone that closely resembles the ischia of other early reptiles [36,37]. The ischium is primitive in being substantially longer than the pubis.
The femur closely resembles that of other early reptiles, except that the distal end features a strong, ca 45° angle formed by the condyles with respect to the long axis of the bone (this angle ranges from 10 to 30° in most early reptiles [35–38]). The tibia and the fibula are slender, slightly bowed rods of bone that are ca 75% the length of the femur. The pedes are almost entirely disarticulated; neither tarsus is intact, and only the astragalus on the left side and the calcaneum on the right side remain of the proximal tarsals. The astragalus is a rectangular (or transversely compressed, L-shaped) plate that is nearly twice as long as it is broad. The calcaneum is a D-shaped plate of bone, thickest along its straight, medial edge, and it thins gradually laterally. Five robust, polygonal distal tarsals are preserved. Three on the left side are semi-articulated; two smaller, irregular bones appear to be (because of their associations with distal tarsal 4 and the metatarsals) distal tarsals 3 and 5. The metatarsals are gently to moderately waisted rods of bone. The longest is interpreted here to be the fourth metatarsal; it is about 66% of the total length of the fibula. Apart from the relatively broad distal end of the fourth metatarsal, the morphology of the metatarsus is consonant with what has been described and illustrated for other early, terrestrial reptiles. A total of 21 pedal phalanges, including four unguals, are preserved. The left pes, with 12 non-terminal phalanges, preserves the majority of these. The non-terminal phalanges have hourglass-shaped outlines in dorsal aspect. Whereas the non-terminal toe bones are dorsoventrally compressed, and thus most of them are preserved in dorsal or ventral aspect, the unguals are mediolaterally compressed, and all are preserved on their sides. The ungual of the fourth digit is preserved on its lateral surface, and has a distinctly claw-shaped profile, with a remarkably robust, polygonal proximal end giving rise to a dorsoventrally narrow, weakly curving distal tip, accentuated by the slightly more convex dorsal margin of the entire element. A shallow groove runs the length of the medial surface of the claw-like tip. The ventral surface of the proximal portion bears a broadly rounded flexor tubercle that, commensurate with the position of the end of the longest pedal digit, is the largest among the preserved unguals. An equally well-preserved ungual lies close by and presumably belongs to a more medial digit. It is slightly longer, and has a more acute profile as a result of having a less robust proximal portion and smaller flexor tubercle. The pedal unguals are the longest phalanges present, and if correctly associated with their respective penultimate phalanges, the pedal unguals are ca 33% longer than the penultimate pedal phalanges.
5. Discussion
Our parsimony analysis (figure 2c) positions Erpetonyx arsenaultorum as the sister taxon of Bolosauridae, which is represented in our analysis by the genera Belebey and Eudibamus. We attach the name Bolosauria to the clade of Erpetonyx, Belebey and Eudibamus (see Material section for phylogenetic analysis and the electronic supplementary material for list of characters and data matrix). Support for Bolosauria and Bolosauridae is moderate, with Bremer support values of 3 and 4, respectively; all clades except Pareiasauridae collapse with the addition of six extra steps. The Bayesian analysis also recovered Bolosauria, but the overall phylogeny differs in the transposition of Bolosauria and Australothyris, and in the placement of Mesosauridae at the base of Eureptilia (see the electronic supplementary material).
A bolosaurian identification for Erpetonyx arsenaultorum, together with its latest Carboniferous age, has important implications for early parareptile diversification and reptilian evolution. Prior to the discovery of ROM 55402, the oldest parareptilian fossils were known from the Asselian Stage of the Permian Period of New Mexico, USA [39], and phylogenetic reconstructions required a long ghost taxon for Parareptilia to extend from the Asselian (ca 295 Ma) down to the latest Moscovian Stage (ca 314 Ma) of the Carboniferous period (figure 3).
Figure 3.
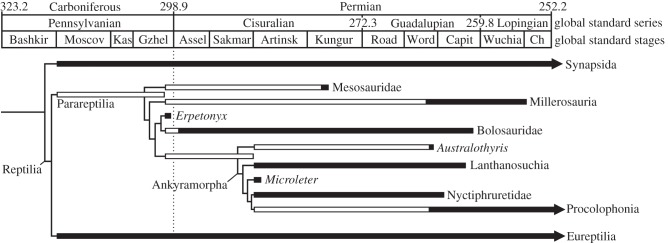
Temporal calibration of Reptilia composed of parareptilian phylogeny shown in figure 2c. Black bars are known ranges, open bars are ghost taxa and ghost lineages. Timescale from International Commission on Stratigraphy [22].
The inclusion of Erpetonyx arsenaultorum in a phylogenetic analysis of Parareptilia results in bolosaurs being positioned closer to the base of the parareptilian tree (as the basal-most members of Procolophonomorpha; sensu Modesto et al. [40]) and the Richards Spur taxon Microleter mckinsieorum moving deeper into the tree with respect to previous phylogenetic studies of Parareptilia [12,14,25,40]. The sister-group relationship between Erpetonyx arsenaultorum and Bolosauridae necessitates the extension of four parareptilian ghost lineages or ghost taxa (for Mesosauridae, Millerosauria, Bolosauridae and the clade of Australothyris smithi and Ankyramorpha) from the Early Permian down into the Gzhelian Stage of the Carboniferous Period. This results in a fivefold increase (from one to five lineages) for the diversity of parareptiles at the end of the Carboniferous, and an 80% increase (from five to nine lineages) for the diversity of reptiles at the end of the Carboniferous (see electronic supplementary material, figure S4).
Our results are consonant with previous work by Reisz [10], who reported that Carboniferous species of reptiles were outnumbered ca 2 to 1 by contemporaneous synapsids. Owing to lack of phylogenetic resolution among Palaeozoic reptiles at the time, Reisz [10] relied on comparisons of group diversity (sensu Norell [41]), i.e. direct counts of taxic occurrences. Our phylogenetic results, when calibrated stratigraphically and compared with stratigraphic calibrations of eureptilian [3] and synapsid [42,43] trees, confirm that reptilian diversification during the Carboniferous was outpaced by that of synapsids, but only during the Gzhelian Stage (303.4–299.0 Ma), with synapsids numbering 17 species per lineages versus reptiles numbering only 9.
Recently, Sahney et al. [44] inferred that the fragmentation and collapse of coal-swamp forests ca 307 Ma promoted diversification of post-Moscovian (latest Carboniferous) tetrapods, including the amniotes. Sahney et al. [44] inferred a vicariant mode of diversification for Coal Age tetrapods resulting from coal-forest fragmentation. If this is the case, we should expect to see similar diversity in closely related groups. Because sister taxa are the same age [41], a comparison of the numbers of species in sister taxa is an appropriate test [45]. If reptiles and synapsids (sister taxa) responded vicariantly to coal-forest fragmentation, they should show similar taxic diversity, particularly because both reptiles and synapsids are found at the same localities (indicating that both groups were equally widespread and thus susceptible to fragmentation). Our examination of Carboniferous amniote phylogenetic diversity provides a more complete and detailed timeline of diversification in Carboniferous taxa than provided by Sahney et al. [44]. For instance, there is evidence for a single species each for synapsids and reptiles during the Moscovian Stage (311.7–307.2 Ma), and for the late Kasimovian (ca 305 Ma)—a time when coal-swamp forests were reduced to ‘tiny wet spots' [44]—our study indicates that reptiles and synapsids were, indeed, equally diverse (8 reptilian species versus 10 synapsids). Accordingly, late Kasimovian amniote diversity patterns are consonant with the hypothesis of Sahney et al. [44]. Amniote diversity patterns change during the succeeding Gzhelian Stage, however, and by the end of the Carboniferous—and more than 4.5 Ma after coal forests were little more than isolated refugia—synapsids outnumbered reptiles ca 2 to 1 (17 synapsid species versus 9 reptiles).
If fragmentation of the Carboniferous coal swamps drove the diversification of amniotes and other tetrapods during the Kasimovian Stage, what might account for the disparity seen between synapsid and reptilian diversity by the end of the Gzhelian Stage? Our results do not reject the possibility that coal-forest fragmentation drove amniote diversification during the Gzhelian Stage [44]. However, preservational bias may be responsible for the low diversity of Gzhelian reptiles, for such diversity rests entirely upon Erpetonyx as the sole datum point for Reptilia in this 4.8-Myr-long stage. Previous workers [43] had regarded Hamilton Quarry, which preserves the (previous) youngest Carboniferous reptile (Spinoaequalis schultzei) as 303 Ma, or Gzhelian in age. As recognized by Sahney et al. [44], this locality is actually late Kasimovian in age (=early Virgilian Age of North American Carboniferous system). Thus, the greater diversity of Gzhelian synapsids is partly a reflection of the astonishingly poor fossil record of reptiles during this stage. Gzhelian amniotes may have been subject to a strong collecting bias, in which the larger synapsid fragmentary remains were more easily discovered and deemed sufficiently significant to be collected and described, whereas very small, fragmentary reptilian remains either were much more difficult to find at Gzhelian localities, or were deemed unworthy to receive attention and a formal name, thereby failing to enter the scientific literature. To examine this possibility, we used the body–mass–distribution methodology of Brown et al. [46], who concluded that the smaller members of the dinosaurian fauna of the Upper Cretaceous Dinosaur Park Formation of Alberta, Canada, were subjected to preservational bias. Our results (see the electronic supplementary material) confirm that Carboniferous reptiles cluster at the small end of the size spectrum and, on average, exhibit greater skeletal completeness than the larger contemporaneous synapsids. Erpetonyx, the sole Gzhelian reptile represented by a body fossil, was dwarfed by its larger synapsid contemporaries.
Finally, another possibility is the strong correlation between the increase in synapsid diversity and the origin of herbivory. The oldest herbivores are known from Gzhelian Stage, with both synapsids and diadectomorphs evolving this novel feeding strategy [42]. The adoption of high-fibre herbivory by two synapsid lineages may have fostered further diversification among synapsids, and represent a pattern that was superimposed over the older vicariant mode promoted by coal-forest fragmentation. Reptiles are exclusively represented by small animals in the Carboniferous, mainly insectivorous forms or carnivorous species, which could not readily prey on the much larger herbivores. In strong contrast, the Synapsida of the latest Carboniferous include several predators of relatively large size that would have been able to prey on diadectomorphs and herbivorous synapsids [42].
The discovery of the first Carboniferous parareptile is critical to our understanding of the early evolution of amniotes because it shows that reptiles were undergoing a dramatic diversification throughout the Carboniferous since the initial appearance of amniotes ca 311 Ma. Erpetonyx arsenaultorum is the first Carboniferous reptile to be described in nearly two decades. We anticipate that future field and laboratory studies on Palaeozoic reptiles will help to test hypotheses of preservational bias and vicariance that operated on the earliest fully terrestrial tetrapods.
Supplementary Material
Acknowledgement
We thank the family of M. Arsenault for collecting the specimen and K. Seymour for procuring the specimen for the ROM via the Louise Hawley Stone Charitable Trust. We thank N. Wong Ken, for additional preparation and for the illustration shown in figure 1, and O. Haddrath (ROM), for assistance with the Bayesian analysis.
Funding statement
This research was supported by Discovery Grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada to S.P.M., R.R.R. and D.C.E. The first author was also supported by a New Opportunities Fund Award from the Canadian Foundation for Innovation (CFI), and by a grant from the Nova Scotia Research and Innovation Trust.
References
- 1.Gauthier J, Kluge AG, Rowe T. 1988. The early evolution of the Amniota. In The phylogeny and classification of the tetrapods (ed. Benton MJ.), pp. 103–155. Oxford, UK: Clarendon Press. [Google Scholar]
- 2.Laurin M, Reisz RR. 1995. A reevaluation of early amniote phylogeny. Zool. J. Linn. Soc. 113, 165–223. ( 10.1111/j.1096-3642.1995.tb00932.x) [DOI] [Google Scholar]
- 3.Müller J, Reisz RR. 2006. The phylogeny of early eureptiles: comparing parsimony and Bayesian approaches in the investigation of a basal fossil clade. Syst. Biol. 55, 503–511. ( 10.1080/10635150600755396) [DOI] [PubMed] [Google Scholar]
- 4.Reisz RR, Müller J. 2004. Molecular timescales and the fossil record: a paleontological perspective. Trends Genet. 20, 237–241. ( 10.1016/j.tig.2004.03.007) [DOI] [PubMed] [Google Scholar]
- 5.Müller J, Reisz RR. 2005. Four well-constrained calibration points from the vertebrate fossil record for molecular clock estimates. Bioessays 27, 1069–1075. ( 10.1002/bies.20286) [DOI] [PubMed] [Google Scholar]
- 6.Reisz RR, Modesto SP. 1996. Archerpeton anthracos from the Joggins Formation of Nova Scotia: a microsaur, not a reptile. Can. J. Earth Sci. 33, 703–709. ( 10.1139/e96-053) [DOI] [Google Scholar]
- 7.Reisz RR. 1981. A diapsid reptile from the Pennsylvanian of Kansas. Spec. Publ. Mus. Nat. Hist. Univ. Kansas 7, 1–74. [Google Scholar]
- 8.Reisz RR, Heaton MJ, Pynn BR. 1982. Vertebrate fauna of Late Pennsylvanian Rock Lake shale near Garnett, Kansas: Pelycosauria. J. Paleontology 56, 741–750. [Google Scholar]
- 9.Reisz RR. 1972. Pelycosaurian reptiles from the Middle Pennsylvanian of North America. Bull. Mus. Comp. Zool. 144, 27–62. [Google Scholar]
- 10.Reisz RR. 2003. Cotylosaur phylogeny and the initial diversification of amniotes. J. Vert. Paleontol. 23(3 Suppl.), 1–24. ( 10.1080/02724634.2003.10010538) [DOI] [Google Scholar]
- 11.Tsuji LA, Müller J, Reisz RR. 2010. Microleter mckinzieorum gen. et sp. nov. from the Lower Permian of Oklahoma: the basalmost parareptile from Laurasia. J. Syst. Paleont. 8, 245–255. ( 10.1080/14772010903461099) [DOI] [Google Scholar]
- 12.Reisz RR, MacDougall MJ, Modesto SP. 2014. A new species of the parareptile genus Delorhynchus, based on articulated skeletal remains from Richards Spur, lower Permian of Oklahoma. J. Vert. Paleontol. 34, 1033–1043. ( 10.1080/02724634.2013.829844) [DOI] [Google Scholar]
- 13.Lyson TR, Bever GS, Bhullar B-AS, Joyce WG, Gauthier JA. 2010. Transitional fossils and the origin of turtles. Biol. Lett. 6, 830–833. ( 10.1098/rsbl.2010.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swofford DL. 2014. Phylogenetic analysis using parsimony (and other methods). Sunderland, MA: Sinauer Associates. [Google Scholar]
- 15.Brooks DR, et al. 2007. Quantitative phylogenetic analysis in the 21st century. Revista Mexicana de Biodiversidad 78, 225–252. [Google Scholar]
- 16.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. ( 10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 17.Kuhn O. 1959. Die Ordnungen der fossilen ‘Amphibien’ und ‘Reptilien’. N. Jahr. Geol. Palaont., Monat. 1959, 337–347. [Google Scholar]
- 18.Cope ED. 1878. Descriptions of extinct Batrachia and Reptilia from the Permian formation of Texas. Proc. Am. Phil. Soc. 17, 505–530. [Google Scholar]
- 19.Owen R. 1876. Catalogue of the fossil Reptilia of South Africa. London, UK: British Museum. [Google Scholar]
- 20.Olson EC. 1947. The family Diadectidae and its bearing on the classification of reptiles. Fieldiana Geol. 11, 1–53. [Google Scholar]
- 21.van de Poll HW. 1998. Lithostratigraphy of the Prince Edward Island redbeds. Atl. Geol. 25, 23–35. [Google Scholar]
- 22.International Commission on Stratigraphy. 2014. International chronostratigraphic chart See www.stratigraphy.org (accessed 13 July 2014).
- 23.Modesto SP, Reisz RR. 2008. New material of Colobomycter pholeter, a small parareptile from the Lower Permian of Oklahoma. J. Vert. Paleontol. 28, 677–684. ( 10.1671/0272-4634(2008)28[677:NMOCPA]2.0.CO;2) [DOI] [Google Scholar]
- 24.MacDougall MJ, LeBlanc ARH, Reisz RR. 2014. Plicidentine in the Early Permian parareptile Colobomycter pholeter, and its phylogenetic and functional significance among coeval members of the clade. PLoS ONE 9, e96559 ( 10.1371/journal.pone.0096559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDougall MJ, Reisz RR. 2012. A new parareptile (Parareptilia, Lanthanosuchoidea) from the Early Permian of Oklahoma. J. Vert. Paleontol. 32, 1018–1026. ( 10.1080/02724634.2012.679757) [DOI] [Google Scholar]
- 26.Reisz RR, Müller J, Tsuji LA, Scott D. 2007. The cranial osteology of Belebey vegrandis (Parareptilia: Bolosauridae), from the Middle Permian of Russia, and its bearing on reptilian evolution. Zool. J. Linn. Soc. 151, 191–214. ( 10.1111/j.1096-3642.2007.00312.x) [DOI] [Google Scholar]
- 27.Langston W, Jr, Reisz RR. 1981. Aerosaurus wellesi, new species, a varanopseid mammal-like reptile (Synapsida: Pelycosauria) from the Lower Permian of New Mexico. J. Vert. Paleontol. 1, 73–96. ( 10.1080/02724634.1981.10011881) [DOI] [Google Scholar]
- 28.Gow CE. 1972. The osteology and relationships of the Millerettidae (Reptilia: Cotylosauria). J. Zool. Lond. 167, 219–264. ( 10.1111/j.1469-7998.1972.tb01731.x) [DOI] [Google Scholar]
- 29.Berman DS, Reisz RR, Scott D, Henrici AC, Sumida SS, Martens T. 2001. Early Permian bipedal reptile. Science 290, 969–972. ( 10.1126/science.290.5493.969) [DOI] [PubMed] [Google Scholar]
- 30.Reisz RR, Scott D. 2002. Owenetta kitchingorum, sp. nov., a small parareptile (Procolophonia: Owenettidae) from the Lower Triassic of South Africa. J. Vert. Paleontol. 22, 244–256. ( 10.1671/0272-4634(2002)022[0244:OKSNAS]2.0.CO;2) [DOI] [Google Scholar]
- 31.Watson DMS. 1914. Procolophon trigoniceps, a cotylosaurian reptile from South Africa. Proc. Zool. Soc. 84, 735–748. ( 10.1111/j.1469-7998.1914.tb07060.x) [DOI] [Google Scholar]
- 32.Heaton MJ, Reisz RR. 1980. A skeletal reconstruction of the Early Permian captorhinid reptile Eocaptorhinus laticeps (Williston). J. Paleontol. 54, 136–143. [Google Scholar]
- 33.Modesto SP. 2010. The postcranial skeleton of the aquatic parareptile Mesosaurus tenuidens from the Gondwanan Permian. J. Vert. Paleontol. 30, 1378–1395. ( 10.1080/02724634.2010.501443) [DOI] [Google Scholar]
- 34.Holmes R. 1977. The osteology and musculature of the pectoral limb of small captorhinids. J. Morph. 152, 101–140. ( 10.1002/jmor.1051520107) [DOI] [PubMed] [Google Scholar]
- 35.Maddin HC, Reisz RR. 2007. The morphology of the terminal phalanges in Permo-Carboniferous synapsids: an evolutionary perspective. Can. J. Earth Sci. 44, 267–274. ( 10.1139/e06-076) [DOI] [Google Scholar]
- 36.Carroll RL. 1969. A Middle Pennsylvanian captorhinomorph, and the interrelationships of primitive reptiles. J. Paleontol. 43, 151–170. [Google Scholar]
- 37.Holmes RB. 2003. The hind limb of Captorhinus aguti and the step cycle of basal amniotes. Can. J. Earth Sci. 40, 515–526. ( 10.1139/e02-039) [DOI] [Google Scholar]
- 38.deBraga M. 2003. The postcranial skeleton, phylogenetic position, and probable lifestyle of the Early Triassic reptile Procolophon trigoniceps. Can. J. Earth Sci. 40, 527–556. ( 10.1139/e02-082) [DOI] [Google Scholar]
- 39.Lucas SG, Berman DS, Henrici AC, Hunt AP. 2005. Bolosaurus from the Lower Permian at Arroyo de Agua, New Mexico and its biostratigraphic significance. In The Permian of central New Mexico (eds Lucas SG, Morales M.), pp. 125–127. Bull. New Mexico Mus. Nat. Hist. Sci.31. [Google Scholar]
- 40.Modesto SP, Scott DM, Reisz RR. 2009. A new parareptile with temporal fenestration from the Middle Permian of South Africa. Can. J. Earth Sci. 46, 9–20. ( 10.1139/E09-001) [DOI] [Google Scholar]
- 41.Norell MA. 1992. Taxic origin and temporal diversity: the effect of phylogeny. In Extinction and phylogeny (eds Novacek MJ, Wheeler QD.), pp. 89–118. New York, NY: Columbia University Press. [Google Scholar]
- 42.Fröbisch J, Schoch RR, Müller J, Schindler T, Schweiss D. 2011. A new basal sphenacodontid synapsid from the Late Carboniferous of the Saar-Nahe Basin, Germany. Acta Palaeont. Pol. 56, 113–120. ( 10.4202/app.2010.0039) [DOI] [Google Scholar]
- 43.Reisz RR, Fröbisch J. 2014. The oldest caseid synapsid from the late Pennsylvanian of Kansas, and the evolution of herbivory in terrestrial vertebrates. PLoS ONE 9, e94518 ( 10.1371/journal.pone.0094518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahney S, Benton MJ, Falcon-Lang HJ. 2010. Rainforest collapse triggered Carboniferous tetrapod diversification in Euramerica. Geology 38, 1079–1082. ( 10.1130/G31182.1) [DOI] [Google Scholar]
- 45.Brooks DR, McLennan DA. 2002. The nature of diversity: an evolutionary voyage of discovery. Chicago, IL: Chicago University Press. [Google Scholar]
- 46.Brown CM, Evans DC, Campione NE, O'Brien LJ. 2012. Evidence for taphonomic size bias in the Dinosaur Park Formation (Campanian, Alberta), a model Mesozoic terrestrial alluvial-paralic system. Palaeogeogr. Palaeoclimatol. Palaeoecol. 372, 108–122. ( 10.1016/j.palaeo.2012.06.027) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.