Abstract
Climate change is altering global patterns of precipitation and temperature variability, with implications for parasitic diseases of humans and wildlife. A recent study confirmed predictions that increased temperature variability could exacerbate disease, because of lags in host acclimation following temperature shifts. However, the generality of these host acclimation effects and the potential for them to interact with other factors have yet to be tested. Here, we report similar effects of host thermal acclimation (constant versus shifted temperatures) on chytridiomycosis in red-spotted newts (Notophthalmus viridescens). Batrachochytrium dendrobatidis (Bd) growth on newts was greater following a shift to a new temperature, relative to newts already acclimated to this temperature (15°C versus 25°C). However, these acclimation effects depended on soil moisture (10, 16 and 21% water) and were only observed at the highest moisture level, which induced greatly increased Bd growth and infection-induced mortality. Acclimation effects were also greater following a decrease rather than an increase in temperature. The results are consistent with previous findings that chytridiomycosis is associated with precipitation, lower temperatures and increased temperature variability. This study highlights host acclimation as a potentially general mediator of climate–disease interactions, and the need to account for context-dependencies when testing for acclimation effects on disease.
Keywords: Batrachochytrium dendrobatidis, chytrid fungus, global warming
1. Introduction
Most studies of climate change impacts on ecological communities, including how climate influences species interactions, have focused on potential effects of multi-decadal shifts in mean temperature or precipitation (e.g. [1]). However, climate change is also projected to cause changes in the magnitude and frequency of extreme weather events, including variability in temperature on multiple timescales [2–5]. The importance of short-term climate variability to species interactions remains an important open question in ecology [6], and recent studies suggest that temperature shifts on diurnal, daily and weekly timescales influence host–parasite interactions in ways that can be overlooked by constant-temperature experiments [7–9]. Furthermore, organisms are influenced by multiple climatic factors simultaneously, yet to our knowledge no study has experimentally investigated interactive effects of moisture and temperature variability on species interactions in general or on parasitism in particular. We sought to fill this gap by testing for interactive effects of temperature variability (i.e. a sudden shift in temperature) and moisture on chytridiomycosis, a parasitic infection of amphibians.
Chytridiomycosis, caused by the aquatic chytrid fungus Batrachochytrium dendrobatidis (Bd), is arguably the most damaging known wildlife pandemic, having contributed to hundreds of amphibian declines and extinctions worldwide in the last few decades [10]. Experiments with Bd in vitro indicate that water is necessary for zoospore movement, survival and colonization (i.e. sporangium formation), suggesting that at least a thin film of water must be present for Bd to infect new hosts or to spread across an individual host's skin [11,12]. Consistent with these observations, numerous field studies have identified correlations between Bd infection and moisture-related variables including precipitation, humidity, aquatic connectivity and host water usage [12–17]. However, others have found a negative or no relationship between Bd and moisture in certain host species [18,19]. Experimental measurements of how moisture influences Bd infection in vivo would help to resolve the mechanisms underlying these differences.
Bd growth is also highly temperature-dependent, with growth rates in vitro increasing with temperature until approximately 23°C [11]. Temperature-dependence of infection in vivo, however, varies among host species with higher infection levels typically occurring at lower temperatures, probably because of positive temperature-dependence of host immune responses [8,20]. Furthermore, host resistance to Bd can also be affected by variability in temperature. In a recent study, Cuban treefrogs (Osteopilus septentrionalis) were less resistant to Bd when exposed to random daily temperature fluctuations or following an abrupt shift in temperature, relative to frogs held at a constant temperature [8], consistent with previous findings that disease-associated frog declines occurred at higher rates following years with elevated month-to-month temperature variability [2]. These patterns were predicted a priori by Raffel et al. [20], who postulated that ectothermic hosts would be more susceptible to infection following a shift in temperature, because their immune systems have not yet physiologically adjusted for improved performance at the new temperature, whereas parasites are thought to acclimate more quickly because of their smaller size and faster metabolisms [8]. This prediction derives from the Beneficial Acclimation Hypothesis of thermal biology, which states that organisms should respond adaptively following a temperature shift to improve their physiological performance at the new temperature [21]. However, the generality of thermal acclimation effects on Bd infection in host species other than Cuban treefrogs, and the potential for these effects to interact with moisture, have yet to be experimentally tested.
We selected juvenile red-spotted newts to test whether thermal acclimation effects on Bd infection interact with moisture because newts (i) have an extended terrestrial juvenile stage that is sensitive to both temperature and soil moisture [22,23], (ii) experience high Bd prevalence in the wild [24,25], and (iii) experience seasonal changes in immune parameters consistent with lags in beneficial acclimation [20]. We conducted an experiment in 60 replicate temperature-controlled incubators, crossing three soil moisture levels with two host acclimation temperatures (15°C or 25°C) and two temperatures for exposure to Bd (15°C or 25°C). Thus, half the newts were switched to a new temperature just prior to Bd exposure and were not yet thermally acclimated to this ‘exposure temperature’. We predicted that newts would be more susceptible to Bd infection following temperature shifts (in either direction), based on field observations showing newt immune parameters lagged following temperature shifts [20]. We also predicted that infection would be more severe at higher moisture levels. Finally, we postulated that decreased soil moisture would reduce the effects of both temperature and host thermal acclimation on Bd infection, either (i) by limiting Bd's ability to establish an infection at all or (ii) by inducing drying stress in the host [22], thus decreasing the overall effectiveness of (potentially temperature-dependent) host immune responses.
2. Material and methods
(a). Animal collection and maintenance
Larval red-spotted newts (Notophthalmus viridescens) were collected from a pond in Clarke County, GA, USA and transported to the University of South Florida. They were maintained in 40 l aquarium tanks in 10 l of artificial spring water [26] at a density of less than 30 newts per tank. Sand was added to one end of the aquarium, to provide a dry surface where newts could emerge from the water when they metamorphosed. Larval newts were fed zooplankton (primarily copepods) collected from a Bd-free pond on the University of South Florida (USF) campus. After metamorphosis, newts were maintained on moist sandy soil, collected from the USF Botanical Gardens. Soil and sand were autoclaved before and after use to remove any potential amphibian parasites. Juvenile newts were fed springtails, which were cultured on a wet charcoal substrate sprinkled weekly with dried milk. Prior to the experiment, all newts were warmed to 28°C for 1 day and then 32°C for 4 days, to ensure clearance of any potential existing Bd infection from their collection site. Newts were then maintained at 25°C for 1 day and 20°C for another day before starting the experiment, to avoid cold-shocking newts in the 15°C treatment.
During the experiment, newts were held in individual vented deli containers (350 ml total volume) with moist soil (described below). Each container contained a sterile live oak leaf for the newt to use as a cover item. Newts were fed 5–10 temperate white springtails (Collembola sp.) weekly and transferred to a fresh container of moistened soil every two weeks (see below).
(b). Experimental design
To ensure true replication of temperature treatments, we used 60 individual incubators with individual thermostats. Each incubator contained two newts in individual containers, one to be exposed to Bd and one to act as an uninfected control. Newts were acclimated to one of two temperatures (15°C or 25°C) for a four-week acclimation period. Temperature was controlled independently for each replicate using a customized incubator with a Plexiglass window in the lid to provide light, as described by Raffel et al. [8] and in the electronic supplementary methods. At the end of this period, half of the incubators were switched to the other temperature, and one newt in each incubator was inoculated with Bd. Detailed methods for Bd inoculate preparation and newt exposures are provided in the electronic supplementary methods. Mortality was then assessed daily for six weeks, and each newt was swabbed with a sterile swab on days 14 and 28 post-exposure for quantification of Bd abundance (details in the electronic supplementary methods). During this Bd exposure period, temperature treatments were crossed with one of three moisture levels (described below) and assigned to both the infected and control newts in each incubator. Treatments were assigned at random for a total of five replicates of each Moisture × Temperature × Acclimation combination.
(c). Controlling soil moisture
All soil for the experiment was collected from the USF Botanical Gardens, in an area with soil classified as Candler fine sand [27], and autoclaved prior to use to kill any potential amphibian parasites. During the acclimation period (first four weeks), all newts were given soil with a medium moisture level (16% water by mass). During the Bd exposure period (following the temperature shift), however, newts in each incubator were assigned to one of three moisture treatments. We used the methods of Rohr & Palmer [28] to create and maintain soil moisture treatments. Briefly, soil was thoroughly dried at 100°C and divided into three equal portions, and deionized water was added to each portion to generate soil with 10, 16 and 21% water (quantified as gravimetric water content, i.e. per cent of water + soil mass) for our ‘Dry’, ‘Medium’ and ‘Wet’ soil moisture treatments, respectively. The 21% gravimetric water content treatment was saturated soil that had a film of water on the surface. Each batch of soil was thoroughly mixed to reduce variation in soil moisture among replicates of the same moisture treatment. Each newt container received 120 ml of soil, corresponding to approximately 160 g of dry (0% moisture) soil. Thus, each container contained approximately 20.8, 32.0 or 43.2 ml water in the dry, medium or wet treatments, respectively. This procedure was repeated every other week to reapply the same soil moisture treatment with fresh soil for each newt. Given that there should be more evaporative water loss from the warmer terraria, we weighed each terrarium once the moisture treatment was applied and added enough deionized water each week to return the water that was lost to evaporation. This ensured that our temperature and moisture treatments were, for the most part, independent and that there was never any overlap in actual moisture levels among the different moisture treatments [28].
(d). Statistical analyses
All analyses were conducted using R statistical software [29]. We analysed Bd abundance on infected newts (zoospore genome equivalents (GEs) with abundance = 0 for uninfected newts) using a zero-inflated negative-binomial generalized linear model (function ‘zeroinfl’ in package ‘pscl’ [30]) as described by Raffel et al. [24]. This model includes a zero-inflation component that models infection as a binomial process (uninfected versus infected) and a count component that models infection intensity as a negative-binomial process [31]. To reduce the number of interacting parameters, we simplified this model by using a single parameter for the zero-inflation component, meaning that all the negative counts had the same probability of being true negatives in the model [8,24]. This model provided a better fit than a regular negative-binomial model (function ‘glm.nb’ in package ‘MASS' [32]), based on both ease of model convergence and Akaike's information criterion (AIC = 641.9 versus 647.0, respectively). This finding is consistent with results of previous studies [8,24] and supports the idea that Bd infection operates as a two-step process, i.e. (i) the probability of initial infection by a given zoospore, followed by (ii) the rate of Bd development and spread across the skin of an individual host. As in Raffel et al. [8], we focused on swabs taken at 14 days post-exposure for the analysis of treatment effects on Bd abundance because the timing of later swabs (15–28 days post-exposure, electronic supplementary material, figure S2) was confounded with infection-induced mortality. We tested for all main effects and interactions among treatments (moisture level, exposure temperature and acclimation status). There were no detectable differences in Bd load between the Dry and Medium treatments, i.e. no significant main effect of moisture or interactions between moisture and either temperature or acclimation (all p > 0.05), so these moisture levels were pooled into a single Dry treatment level for subsequent analyses of Bd load. This increased the statistical power of the full analysis and made it easier to interpret regression coefficients for main and interactive effects of moisture.
Each incubator contained one Bd+ and one Bd− newt to allow a direct assessment of mortality due to infection. We therefore tested for mortality effects of Bd exposure, moisture, exposure temperature and acclimation using a generalized linear mixed effects model with binomial errors (function ‘glmer’ in package ‘lme4’ [33]), with mortality at the end of the experiment (42 days post-exposure) as the response. This approach allowed us to control for the random effect of incubator on newt mortality and avoid pseudoreplication of the temperature and moisture treatments. As with the Bd load analysis, there were no detectable differences in mortality between the Dry and Medium moisture treatments, so we again pooled these moisture levels into a single Dry treatment level for subsequent mortality analyses. We lacked the sample size to test for three- and four-way interactions among these predictors for a binomial analysis (only five newts in some treatment combinations), so we limited this analysis to all possible main effects and two-way interactions.
The significance of all factors was assessed using likelihood ratio tests, excluding higher level terms for marginal predictors. This testing procedure is analogous to Type II sums of squares for normal general linear models.
3. Results
At 14 days post-exposure, Bd abundance on infected newts was significantly higher at the colder exposure temperature and with higher soil moisture (electronic supplementary material, table S1; figure 1). There were also highly significant interactive effects between Acclimation status × Moisture × Temperature, with a stronger acclimation effect on Bd load in the Wet treatment (Moisture × Acclimation: 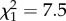 , p = 0.006). This effect was greater at 15°C than at 25°C (Moisture × Temperature × Acclimation:
, p = 0.006). This effect was greater at 15°C than at 25°C (Moisture × Temperature × Acclimation: 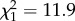 , p = 0.003; figure 1; electronic supplementary material, table S1). Both the Moisture × Acclimation and Moisture × Temperature × Acclimation interactions indicated lower Bd loads in newts that had been acclimated to the exposure temperature, relative to newts shifted to a new temperature immediately prior to exposure. However, this acclimation effect was greater following a decrease than an increase in temperature, with the difference between acclimated and unacclimated newts being approximately 60 000 zoospore GE at 15°C but only approximately 25 000 GE at 25°C (figure 1a).
, p = 0.003; figure 1; electronic supplementary material, table S1). Both the Moisture × Acclimation and Moisture × Temperature × Acclimation interactions indicated lower Bd loads in newts that had been acclimated to the exposure temperature, relative to newts shifted to a new temperature immediately prior to exposure. However, this acclimation effect was greater following a decrease than an increase in temperature, with the difference between acclimated and unacclimated newts being approximately 60 000 zoospore GE at 15°C but only approximately 25 000 GE at 25°C (figure 1a).
Figure 1.
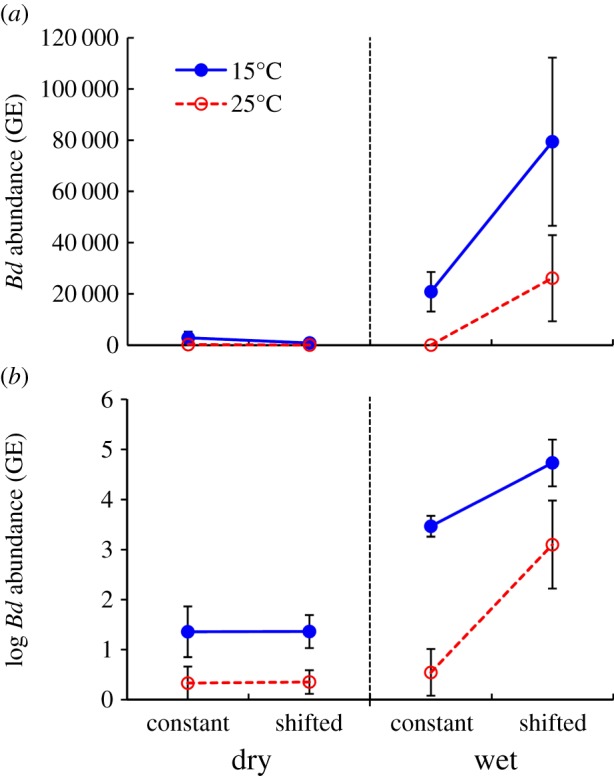
Batrachochytrium dendrobatidis (Bd) load in newts at 14 days post-exposure was influenced by soil moisture (dry versus wet; dry = combined Dry and Medium treatments), exposure temperature (filled circles: 15°C; open circles: 25°C) and host acclimation status (constant versus shifted temperature). There were significant interactions between these variables, indicating a stronger effect of acclimation in the cold and wet treatments (electronic supplementary material, table S1). Bd abundance is quantified as the zoospore GEs per skin swab and includes uninfected newts (GE = 0). (a) Mean Bd abundance. (b) Mean log-transformed Bd abundance, log(N + 1). N = 59; error bars, s.e. (Online version in colour.)
Mortality was higher for Bd-exposed newts than the control newts and for newts at the highest moisture level than those in the lower moisture treatments, with an interactive effect of the Bd-exposure and moisture treatments (figure 2; electronic supplementary material, table S2). Newts exposed to Bd experienced nearly 10-fold higher mortality than control newts at the highest moisture level, but there was no significant difference in mortality between Bd-exposed and control newts at the lower moisture levels (Infection × Moisture: 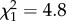 , p = 0.029; figure 2; electronic supplementary material, table S2). There were no significant main or interactive effects of temperature or acclimation status on newt mortality (electronic supplementary material, table S2).
, p = 0.029; figure 2; electronic supplementary material, table S2). There were no significant main or interactive effects of temperature or acclimation status on newt mortality (electronic supplementary material, table S2).
Figure 2.
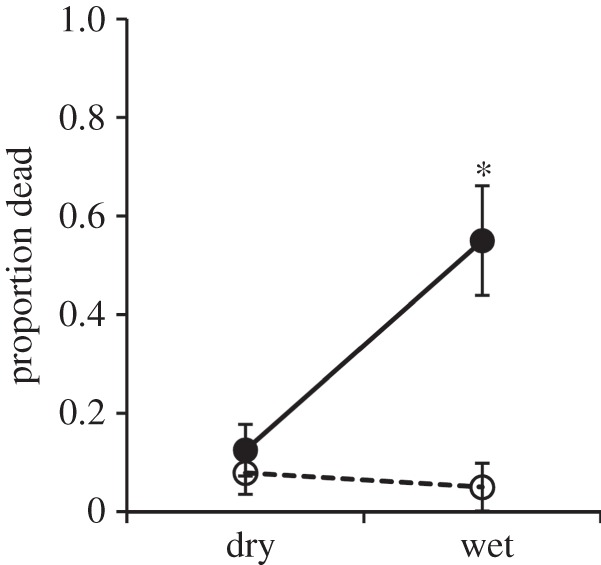
Batrachochytrium dendrobatidis (Bd) exposure (filled circles: Bd+; open circles: Bd−) and soil moisture level (dry versus wet; dry = combined Dry and Medium treatments) had interactive effects on the proportion of newts dead at 42 days post-exposure (electronic supplementary material, table S2), with significant mortality due to infection only occurring in the wet moisture treatment, as indicated by the asterisk. N = 118; error bars, s.e.
4. Discussion
Our study revealed a significant synergistic effect of temperature and moisture on Bd infection in red-spotted newts, with Bd abundance only reaching high levels (greater than 10 000 zoospore equivalents [34]) in newts maintained at the highest moisture level and the cooler temperature treatment, and elevated mortality only occurring with high moisture. These results are consistent with previous studies showing that Bd loads frequently correlate with precipitation patterns in the field, probably because Bd is an aquatic pathogen requiring water for zoospore dispersal [11–13]. Our results are also consistent with previous experiments showing greater mortality due to infection and Bd abundance on frogs at lower temperatures [10,35,36], despite Bd growth in culture generally increasing with temperature up to a maximum growth temperature of approximately 23°C [11,37,38]. This might be because amphibian immune responses are stronger at higher temperatures, such that the temperature-dependence of infection depends on the relative rates at which parasite infectivity and host resistance increase with temperature [8]. Our results show that newt resistance to Bd is greater at 25°C than 15°C; this is consistent with observed increases in immune parameters and decreases in Bd with temperature in wild newts [20,24].
Our results also supported Raffel et al.'s [20] prediction that newts would be more susceptible to Bd following a sudden temperature shift, relative to newts already acclimated to the new temperature, because of a delay in beneficial acclimation of the immune system. We found evidence of beneficial acclimation regardless of the direction of the temperature shift (i.e. 15 to 25°C or 25 to 15°C), consistent with field patterns showing lower-than-expected levels of immune parameters for newts in the field following both temperature increases and decreases [20]. We also found a greater acclimation effect following a decrease in temperature, relative to an increase, consistent with acclimation effects on Bd infection in Cuban treefrogs [8] and seasonal patterns of immune parameters in adult newts [20]. These similarities are striking given that newts are taxonomically and ecologically dissimilar from Cuban treefrogs, suggesting that acclimation effects on Bd infection might be widespread in amphibians. Further work with other ectothermic hosts will be needed to determine whether thermal acclimation of the immune system continues to exhibit consistent patterns across host species.
The Temperature Variability Hypothesis described by Rohr & Raffel [2] postulates that delays in host acclimation following temperature shifts could drive increased infection rates in fluctuating temperature environments, assuming that parasite acclimation responses occur more rapidly than those of their hosts [8]. This study adds support for host acclimation effects postulated by this hypothesis. Whether and how quickly the pathogen acclimates to new temperatures remain uncertain, though a recent study of Bd growth in culture suggests that the pathogen's thermal history has important effects on the temperature-dependence of zoospore production and population growth rates [38]. These results highlight the complexity of fluctuating temperature effects on infectious disease, which are further complicated by differential host and parasite responses to variation at different temporal ([7,8], e.g. diurnal [39]) and spatial (e.g. behavioural fever [40]) scales. Predicting effects of altered climate variability on Bd infection in amphibians will thus require a better understanding of both host and parasite responses to temperature fluctuations on multiple scales.
It is not surprising that higher soil moisture levels would drive higher Bd growth rates on juvenile newts, given that Bd is an aquatic fungus [10]. However, the Dry and Medium moisture treatments yielded similar Bd growth rates, despite a nearly two-fold difference in moisture between these treatments. We propose that the observed increase in Bd growth on newts in the Wet treatment was driven by the presence of the visible film of liquid water on the soil surface, which probably facilitated zoospore dispersal to the hosts' skin during initial infection and the subsequent spread of infection across the skin [11,12]. Below the soil's saturation point, there might have been too narrow a film of water for zoospores to disperse, making these treatments qualitatively different from the Wet treatment. An alternative possibility is that excess moisture induces physiological changes in terrestrial amphibians that cause them to be more susceptible to infection. For example, excessive water might cause immune suppression via physiological stress [41], or induce changes in skin morphology that allow easier penetration by Bd zoospores. However, microscopic examination of the skin of control newts from this study revealed no obvious effects of soil moisture on skin morphology, so any such effects would need to have been at the cellular level.
In addition to demonstrating that soil moisture can increase Bd growth on a terrestrial amphibian, our results have potentially important implications for newt survival in populations harbouring Bd. Infected newts in the Dry or Medium moisture treatments maintained low Bd zoospore levels and rates of mortality indistinguishable from uninfected control newts over a six-week period. Newts living in forest habitats likely only intermittently experience conditions as moist as in the Wet treatment (i.e. rainy days), and even then should be able to seek relatively dry microhabitats [23]. This type of behavioural response could be similar to ‘behavioural fever’, in which infected hosts move to warmer microhabitats to rid themselves of a temperature-dependent infection [40]. Consistent with this idea, prior work showed that Bd-infected boreal toads selected dry microhabitats at a greater rate than uninfected toads [36], and both boreal toads and Panamanian golden frogs had lower Bd infection levels and/or mortality when provided the option of a dry microhabitat instead of being forced to live in shallow water [36,42]. Similarly, Longo et al. [18] provided infected frogs a choice between moist and dry conditions but did not directly measure behavioural responses to Bd exposure, instead showing that frogs huddled together in wet microhabitats under drought conditions are more likely to die from Bd. Regardless of behavioural responses to infection, however, our results suggest that terrestrial newts might be capable of tolerating Bd infection for extended periods (i.e. more than six weeks), assuming that they can avoid wet microhabitats. Based on a recent study showing that amphibians can acquire improved resistance to Bd following repeated exposures [35], adult newts could plausibly return from their terrestrial juvenile stage with substantially improved resistance to Bd, possibly helping to explain reports of widespread Bd infection in aquatic adult newts despite no evidence for Bd-induced pathology or population declines in this species [24,25].
In conclusion, we found that newts were less resistant to Bd infection following an abrupt temperature shift, relative to newts already acclimated to the new temperature, with a stronger effect following a temperature decrease than an increase. These results are consistent with numerous field studies showing increased Bd prevalence and abundance at lower temperatures, and with previous studies of thermal acclimation effects on amphibian immunity and resistance to Bd. However, these acclimation effects were only observed in infected newts maintained on water-saturated soil, which caused elevated levels of Bd growth on newts and infection-induced mortality. These results have potentially broad relevance for amphibian diseases, many of which are water-transmitted and might also experience interactive effects of moisture and temperature variability. Further studies into the range of microhabitats available to amphibians and the extent to which amphibians select microhabitats to mitigate disease risk will be needed to determine the full conservation implications of these results. Nevertheless, this study contributes to our understanding of how temperature variability and moisture influence Bd infection in amphibians, and highlights the potential complexity of environmental effects on infectious diseases.
Supplementary Material
Acknowledgements
We would like to thank M. Mancao, E. Sites, N. Pradeep, N. Eustace, M. Chawdry, M. Martin, K. Post, A. Tapolyai, M. Frangione, A. Drennen, D. Gill, A. Congelosi, J. Guzy, A. Deyle and K. McCoy for their assistance with conducting the experiment and processing samples.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.2vr4c.
Funding statement
This research was financially supported by National Science Foundation (NSF; DEB-0809487) and US Department of Agriculture (NRI 2008–00622 and 2008–01785) grants to J.R.R., a US Environmental Protection Agency STAR (R83–3835) grant to J.R.R. and T.R.R, an EPA CAREER (no. 83518801) grant to J.R.R. and an NSF grant to T.R.R. and P. T. Johnson (IOS-1121529).
References
- 1.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. ( 10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 2.Rohr JR, Raffel TR. 2010. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc. Natl Acad. Sci. USA 107, 8269–8274. ( 10.1073/pnas.0912883107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074. ( 10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 4.Screen JA. 2014. Arctic amplification decreases temperature variance in northern mid- to high-latitudes. Nat. Clim. Change 4, 577–582. ( 10.1038/nclimate2268) [DOI] [Google Scholar]
- 5.Huntingford C, Jones PD, Livina VN, Lenton TM, Cox PM. 2013. No increase in global temperature variability despite changing regional patterns. Nature 500, 327–331. ( 10.1038/nature12310) [DOI] [PubMed] [Google Scholar]
- 6.Jiang L, Morin PJ. 2007. Temperature fluctuation facilitates coexistence of competing species in experimental microbial communities. J. Anim. Ecol. 76, 660–668. ( 10.1111/j.1365-2656.2007.01252.x) [DOI] [PubMed] [Google Scholar]
- 7.Paaijmans KP, Blanford S, Bellb AS, Blanford JI, Read AF, Thomas MB. 2010. Influence of climate on malaria transmission depends on daily temperature variation. Proc. Natl Acad. Sci. USA 107, 15 135–15 139. ( 10.1073/pnas.1006422107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. 2013. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 3, 146–151. ( 10.1038/nclimate1659) [DOI] [Google Scholar]
- 9.Hamilton PT, Richardson JML, Govindarajulu P, Anholt BR. 2012. Higher temperature variability increases the impact of Batrachochytrium dendrobatidis and shifts interspecific interactions in tadpole mesocosms. Ecol. Evol. 2, 2450–2459. ( 10.1002/ece3.369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilpatrick AM, Briggs CJ, Daszak P. 2010. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol. Evol. 25, 109–118. ( 10.1016/j.tree.2009.07.011) [DOI] [PubMed] [Google Scholar]
- 11.Piotrowski JS, Annis SL, Longcore JE. 2004. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96, 9–15. ( 10.2307/3761981) [DOI] [PubMed] [Google Scholar]
- 12.Kriger KM. 2009. Lack of evidence for the drought-linked chytridiomycosis hypothesis. J. Wildlife Dis. 45, 537–541. ( 10.7589/0090-3558-45.2.537) [DOI] [PubMed] [Google Scholar]
- 13.Holmes I, McLaren K, Wilson B. 2014. Precipitation constrains amphibian chytrid fungus infection rates in a terrestrial frog assemblage in Jamaica, West Indies. Biotropica 46, 219–228. ( 10.1111/btp.12093) [DOI] [Google Scholar]
- 14.Liu X, Rohr JR, Li YM. 2013. Climate, vegetation, introduced hosts and trade shape a global wildlife pandemic. Proc. R. Soc. B 280, 20122506 ( 10.1098/rspb.2012.2506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossack BR, Lowe WH, Ware JL, Corn PS. 2013. Disease in a dynamic landscape: host behavior and wildfire reduce amphibian chytrid infection. Biol. Conserv. 157, 293–299. ( 10.1016/j.biocon.2012.09.013) [DOI] [Google Scholar]
- 16.Sapsford SJ, Alford RA, Schwarzkopf L. 2013. Elevation, temperature, and aquatic connectivity all influence the infection dynamics of the amphibian chytrid fungus in adult frogs. PLoS ONE 8, e82425 ( 10.1371/journal.pone.0082425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrell VCK, Engbrecht NJ, Pessier AP, Lannoo MJ. 2014. Drought reduces chytrid fungus (Batrachochytrium dendrobatidis) infection intensity and mortality but not prevalence in adult crawfish frogs (Lithobates areolatus). J. Wildlife Dis. 50, 56–62. ( 10.7589/2013-01-016) [DOI] [PubMed] [Google Scholar]
- 18.Longo AV, Burrowes PA, Joglar RL. 2010. Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Dis. Aquat. Organ. 92, 253–260. ( 10.3354/dao02054) [DOI] [PubMed] [Google Scholar]
- 19.Woodhams DC, Alford RA. 2005. Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conserv. Biol. 19, 1449–1459. ( 10.1111/j.1523-1739.2005.004403.x) [DOI] [Google Scholar]
- 20.Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ. 2006. Negative effects of changing temperature on amphibian immunity under field conditions. Funct. Ecol. 20, 819–828. ( 10.1111/j.1365-2435.2006.01159.x) [DOI] [Google Scholar]
- 21.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis, p. 320 Oxford, UK: Oxford University Press. [Google Scholar]
- 22.Rohr JR, Madison DM. 2003. Dryness increases predation risk in efts: support for an amphibian decline hypothesis. Oecologia 135, 657–664. [DOI] [PubMed] [Google Scholar]
- 23.Roe AW, Grayson KL. 2008. Terrestrial movements and habitat use of juvenile and emigrating adult eastern red-spotted newts, Notophthalmus viridescens. J. Herpetol. 42, 22–30. ( 10.1670/07-040.1) [DOI] [Google Scholar]
- 24.Raffel TR, Michel PJ, Sites EW, Rohr JR. 2010. What drives chytrid infections in newt populations? Associations with substrate, temperature, and shade. Ecohealth 7, 526–536. ( 10.1007/s10393-010-0358-2) [DOI] [PubMed] [Google Scholar]
- 25.Groner ML, Relyea RA. 2010. Batrachochytrium dendrobatidis is present in northwest Pennsylvania, USA, with high prevalence in Notophthalmus viridescens. Herpetol. Rev. 41, 462–465. [Google Scholar]
- 26.Cohen LN, Neimark H, Eveland LK. 1980. Schistosoma mansoni: response of cercariae to a thermal gradient. J. Parasitol. 66, 362–364. ( 10.2307/3280843) [DOI] [PubMed] [Google Scholar]
- 27.Doolittle JA, Schellentrager G, Ploetz S. 1989. Soil survey of Hillsborough County, Florida. National Cooperative Soil Survey, p. 168 Washington, DC: Soil Conservation Service, United States Department of Agriculture. [Google Scholar]
- 28.Rohr JR, Palmer BD. 2013. Climate change, multiple stressors, and the decline of ectotherms. Conserv. Biol. 27, 741–751. ( 10.1111/cobi.12086) [DOI] [PubMed] [Google Scholar]
- 29.R Development Core Team. 2014. R: a language and environment for statistical computing, version 3.1.1 Vienna, Austria: R Foundation for Statistical Computing; (http://www.r-project.org) [Google Scholar]
- 30.Jackman S.2008. Package ‘pscl’: classes and methods for R developed in the Political Science Computational Laboratory, Stanford University. R package version 1.03. ( http://www.r-project.org)
- 31.Zeileis A, Kleiber C, Jackman S. 2008. Regression models for count data in R. J. Stat. Softw. 27, 1–25. [Google Scholar]
- 32.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 33.Bates D, Maechler M, Dai B.2008. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375–28. ( http://www.r-project.org)
- 34.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl Acad. Sci. USA 107, 9689–9694. ( 10.1073/pnas.0914111107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahon TA, et al. 2014. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511, 224–227. ( 10.1038/nature13491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy PJ, St-Hilaire S, Corn PS. 2011. Temperature, hydric environment, and prior pathogen exposure alter the experimental severity of chytridiomycosis in boreal toads. Dis. Aquat. Organ. 95, 31–42. ( 10.3354/dao02336) [DOI] [PubMed] [Google Scholar]
- 37.Woodhams DC, Alford RA, Briggs CJ, Johnson M, Rollins-Smith LA. 2008. Life-history trade-offs influence disease in changing climates: strategies of an amphibian pathogen. Ecology 89, 1627–1639. ( 10.1890/06-1842.1) [DOI] [PubMed] [Google Scholar]
- 38.Voyles J, Johnson LR, Briggs CJ, Cashins SD, Alford RA, Berger L, Skerratt LF, Speare R, Rosenblum EB. 2012. Temperature alters reproductive life history patterns in Batrachochytrium dendrobatidis, a lethal pathogen associated with the global loss of amphibians. Ecol. Evol. 2, 2241–2249. ( 10.1002/ece3.334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodhams DC, Alford RA, Marantelli G. 2003. Emerging disease of amphibians cured by elevated body temperature. Dis. Aquat. Organ. 55, 65–67. ( 10.3354/dao055065) [DOI] [PubMed] [Google Scholar]
- 40.Rowley JJL, Alford RA. 2013. Hot bodies protect amphibians against chytrid infection in nature. Sci. Rep. 3, 1–4. ( 10.1038/srep01515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rollins-Smith LA, Ramsey JP, Pask JD, Reinert LK, Woodhams DC. 2011. Amphibian immune defenses against chytridiomycosis: impacts of changing environments. Integr. Comp. Biol. 51, 552–562. ( 10.1093/icb/icr095) [DOI] [PubMed] [Google Scholar]
- 42.Bustamante HM, Livo LJ, Carey C. 2010. Effects of temperature and hydric environment on survival of the Panamanian golden frog infected with a pathogenic chytrid fungus. Integr. Zool. 5, 143–153. ( 10.1111/j.1749-4877.2010.00197.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.2vr4c.


