Abstract
While many studies focus on how animals use public information, the dynamics of information spread and maintenance within groups, i.e. the ‘ecology of information’, have received little attention. Here we use fruitflies trained to lay eggs on specific substrates to implement information into groups containing both trained and untrained individuals. We quantify inter-individual interactions and then measure the spread of oviposition preference with behavioural tests. Untrained individuals increase their interactive approaches in the presence of trained individuals, and the oviposition preference transmission is directly proportional to how much trained and untrained individuals interact. Unexpectedly, the preference of trained individuals to their trained oviposition substrate decreases after interactions with untrained individuals, leading to an overall informational loss. This shows that social learning alone is not enough to support informational stability.
Keywords: social learning, social interaction, information, Drosophila, oviposition
1. Introduction
Within the last 20 years, a consistent volume of research has been devoted to understanding how animals use social information, i.e. how they learn socially from other animals to perform fundamental biological functions. Much of the existing research on social learning (usually defined as ‘learning that is influenced by observation of, or interaction with, another animal or its products’ [1]) has addressed its underlying neural structures, or investigated its genetic, physiological and behavioural bases [2–5]. In behavioural ecology, social learning is often studied from an individual-to-group perspective. Many studies have explored the adaptive consequences of individual public information use within social contexts, whether receiving public information increases fitness of individuals. Several studies have also investigated the social learning mechanisms and the influence of social dynamics on the spread of information within groups (reviewed in [6–9]). Only a few studies have focused on the real object of social learning—the information itself—and how the information exchange influences behaviours of both informed and uninformed individuals during and after the transfer process. The ‘ecology of information’ [10], defined here as the dynamics of information transfer and maintenance within animal groups, with their ecological and evolutionary causes and consequences, has indeed received little attention.
Social learning has been studied in several taxa, ranging from insects to humans, including fish, birds and mammals. Most evidence of social learning in insects comes from studies of Hymenoptera [11,12], but there has been little research in other insect groups. Recent evidence shows that even non-eusocial insects such as Drosophila fruitflies can also copy the behaviour of conspecifics [13–16]. While it is generally accepted that information can pass from an animal a (the social demonstrator, i.e. an informed individual) to an animal b (the social learner, i.e. an uninformed individual) via direct or indirect interactions, there is a general lack of attention to the informational transfer itself. In particular, while most studies implicitly define social learning as a unidirectional, intransitive process, it is a matter of fact that social information can flow not only from a demonstrator to a learner but also from a learner to a demonstrator. Investigating social learning as a mutual exchange of information might open novel interesting scenarios connecting behaviour, ecology and evolution.
In this study, we use a well-studied animal model, the fruit fly Drosophila melanogaster, to explore how the dynamics of information spread may influence the behavioural ecology of groups and populations. In D. melanogaster, individuals aggregate on decaying fruit, on which most of the reproductive functions occur, i.e. mating and egg-laying. These aggregations, although to some extent ephemeral, allow individuals to interact. In a previous study, we showed that D. melanogaster can copy the behaviour of conspecifics [14]. In particular, oviposition site preference can spread from trained female flies to untrained naive ones by direct interactions. Here we analyse the frequency and pattern of interactions among flies. Using a video-tracking system, we quantify interactions within mixed groups of trained and untrained females and determine the impact of interactions on the social transmission of oviposition site preference. We then compare fly behaviour in mixed groups to two control groups composed of either only trained or only untrained females. Finally, we combine the video-tracking of interactions with the test of oviposition choice in both trained and untrained flies. Our results highlight the link between individual behaviour, group composition and the general outcome of the transfer of information about oviposition site preference.
2. Material and methods
(a). Fly stocks and maintenance
Flies came from a D. melanogaster strain collected in central France in 2009 and had been raised since then in the laboratory on standard axenic medium in a 12 L : 12 D cycle at 21°C. The experiments were performed at 23°C in constant light. Five-day-old female flies kept with males of the same age were sexed under ice anaesthesia 6 h before the beginning of each experiment.
(b). General procedure
The experiment consisted of three treatments: untrained (UT, N = 19), trained (T, N = 20) and untrained + trained (UT + T, N = 49). The UT treatment groups were composed of 12 untrained naive female flies. The T treatment groups were composed of 12 trained flies. The UT + T treatment groups were composed of eight trained flies and four naive untrained flies. In each treatment group, four flies were partially wing-clipped and the other eight were left intact in order to differentiate untrained from trained flies. Note that control groups of the UT and T treatments were also composed of intact and partially wing-clipped flies. We did not find any behavioural difference between partially wing-clipped and intact flies in these treatments. The experiment included three phases: (i) a conditioning phase in which some females were conditioned to prefer either banana- or strawberry-flavoured egg-laying medium (trained females), (ii) an interaction phase during which we video-tracked the interactions among trained and/or untrained females, and (iii) a test phase during which both trained and untrained females were tested for oviposition site choice; each group's oviposition site preference was quantified by comparing the number of eggs laid on each medium.
(c). Conditioning phase
Some individuals were conditioned to prefer one of two egg-laying media (strawberry or banana), immediately before they were allowed to interact in the transmission phase. We call these ‘trained’ individuals. To perform the conditioning, we introduced groups of four or eight females into a 120 × 50 × 90 mm plastic cage and left them for 8 h with the choice between two oviposition media (3 ml contained in 30 mm diameter Petri dishes with 20 g l−1 of sucrose, 10 g l−1 of agar and 6 ml l−1 of artificial banana or strawberry flavours, la Gazignaire SA). One of the two media also contained quinine (3 g l−1), an aversive gustatory stimulus. In the T treatment, the two subgroups of eight and four flies were separately trained to avoid the same flavoured oviposition medium before being placed together during the interaction phase. In T and UT + T treatments, 50% of the replicates had quinine in the banana-flavoured medium and 50% had quinine in the strawberry-flavoured medium. During the 8 h conditioning phase, untrained individuals were placed in tubes with standard axenic medium. In the UT treatment, the two subgroups of eight and four flies were kept in different tubes. Thus, at the beginning of each interaction phase and in all treatments, the groups of 12 flies were always composed of two subgroups that had never previously met. This allowed us to control for potential behavioural bias when flies interacted with other ‘unknown’ flies.
(d). Interaction phase
In each treatment, the two subgroup of flies were introduced together in a semi-opaque white polyoxymethylene (Delrin) arena (diameter 100 mm; height 3 mm) covered with transparent Plexiglas for 4 h (design based on previous work by Simon & Dickinson [17]). Our experimental design allowed us to simultaneously track four groups of 12 flies over the 4 h. The tracking apparatus consisted of four synchronized firewire cameras (Guppy pro, Allied Vision Technologies), each filming one interaction arena which was backlit by a 150 × 150 mm IR backlight (R&D vision). We used vision software to analyse spatial data (open-source C-trax 0.3.7 [18]) that allowed us to collect 10 positions per second for each fly over 4 h video experiments. Tracking corrections were made post C-trax analysis with Fixerrors toolbox 0.2.11 using Matlab software v. 7.11.0 to suppress swaps between individuals.
(e). Test phase
After filming the flies for 4 h during the interaction phase, we gently removed them from the arena and introduced them into a plastic cage containing both flavoured oviposition media (with no quinine) to test for their oviposition preference. During this last 4 h phase, we maintained groups of 12 flies together in the T and UT treatments. In the UT + T treatment, untrained and trained flies were tested in two different cages to quantify their respective oviposition site preference. At the end of the test phase, we counted the number of eggs laid on each medium and calculated the proportion of eggs laid on the ‘right’ medium (the one that the trained flies had been conditioned to prefer). On average, groups of flies (N = 143) laid 90 ± 5 eggs on the Petri dishes during this phase.
(f). Social interactions analysis
We developed an automated code using the R software (v. 3.0.3, R Development Core Team 2014, http://www.r-project.org/) to identify the interactions among individuals based on the proximity between flies and the duration of contact between them (code available upon request). The first 15 min of video recording was not included in the analysis to remove the impact of initial disturbance on the flies. We first calculated the distances among all individuals in the arena at each position, and we then detected the interactions by setting up a spatial and temporal constraint to the data. Criteria for defining an interaction (body contact) were satisfied if (i) the distance between the centres of two individuals was smaller than or equal to 1.1 mean body lengths of the individuals and (ii) the time spent at this minimum threshold distance lasted at least five time frames (0.5 s), independent of the fly's orientation. These parameters were chosen after repeated careful direct observation of different videos and distribution of distance among flies. Instantaneous fly speed was calculated using the distance moved by a fly over four time frames (0.4 s).
(g). Statistical analysis
All analyses were done using R software. We calculated the total number of interactions for each video and compared the values among T, UT and T + UT treatments using a Student's t-test. The same statistic was used to test for the occurrence of assortative interactions between fly types, using the proportion of interactions that took place among trained flies, untrained flies and between trained and untrained flies, weighting for their frequency. Assortative interactions would be considered when the proportion of interactions between two types of flies deviate from a hypergeometric distribution (probability of T–T interactions = 0.42; probability of UT–UT interactions = 0.1; probability of UT–T interactions = 0.48). Comparison of the locomotion velocity of the different type of flies among treatments was done using Student's t-test. The relationships between flies' interaction during the transmission phase and the proportion of eggs laid on the right medium (performance) during the test phase in the T and UT + T treatments were analysed using a generalized linear model (GLM) for proportional data with binomial error distribution and ‘Probit’ link function.
3. Results
During the test phase, untrained flies tended to preferentially lay eggs on the oviposition medium preferred by the trained flies with whom they interacted in the UT + T treatment (proportion of eggs laid on the right medium during test phase by untrained flies: mean = 0.63 ± 0.052, N = 49; Wilcoxon signed-rank test on the number of eggs laid on the right versus wrong medium: z = −2.59, p = 0.009).
The total number of interactions per group was similar in groups of all trained or all untrained flies (t = −0.41, p = 0.68; figure 1), suggesting that the ‘internal state’ of the fly (trained versus untrained) does not affect social interactions. However, in the mixed groups of the UT + T treatment, we observed a significant increase in the total number of interactions among flies compared with the T or UT treatments (UT + T versus T flies: p = 0.021; UT + T versus UT flies: p = 0.011; figure 1). In the UT + T treatment, untrained flies were disproportionately more engaged into social interactions than were trained individuals compared with expected values (figure 2). This was not due to wing-clipped versus non-clipped flies as the proportion of interaction between these types of flies in the T or the UT treatment did not deviate from random association (proportion of interaction between wing-clipped and non-clipped flies: UT: 0.485 ± 0.01; T: 0.49 ± 0.013). The social context thus had a strong impact on the behaviour of the flies. Interestingly untrained fly locomotion, defined as the average instant speed with which they moved during the interaction phase, was strongly higher in the UT + T treatment (mean = 0.284 ± 0.012) compared with the UT treatment (mean = 0.18 ± 0.016; t = 4.61, p < 0.001; figure 3), whereas trained fly speed did not change between T (mean = 0.230 ± 0.017) and UT + T treatment (mean = 0.236 ± 0.008, t = −0.313, p = 0.75; figure 3).
Figure 1.
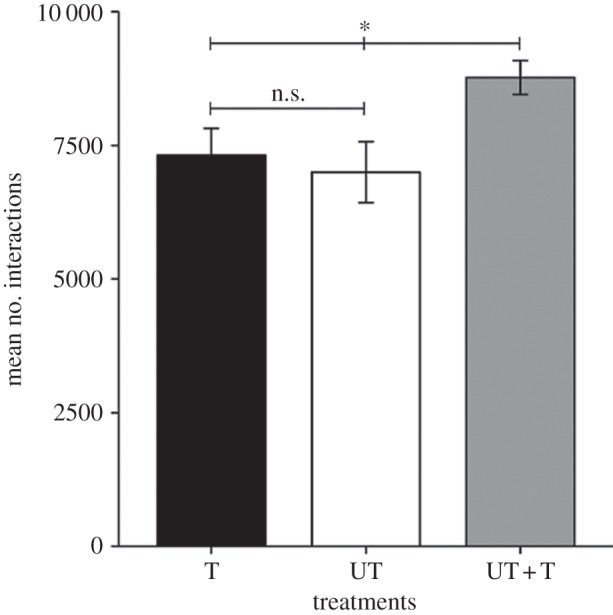
Total number of interactions in UT + T treatment differs from control treatments. The mean number of interactions and its standard error for each treatment type are presented: all trained individuals (T; N = 19), all untrained individuals (UT; N = 20) and eight trained + four untrained individuals (UT + T; N = 49). *p < 0.05, t-test.
Figure 2.
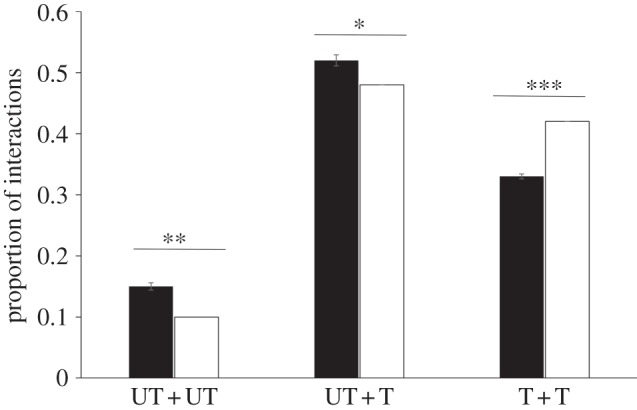
Proportion of interactions among trained and untrained flies during transmission phase in UT + T treatment. Black bars represent the observed proportion of interactions between untrained and untrained flies, untrained and trained flies or trained and trained flies (N = 49). Error bars are standard errors of the mean. White bars present the theoretical probability of encounter assuming hypergeometric distribution. ***p < 10−3, **p < 0.01, *p < 0.05, t-test.
Figure 3.
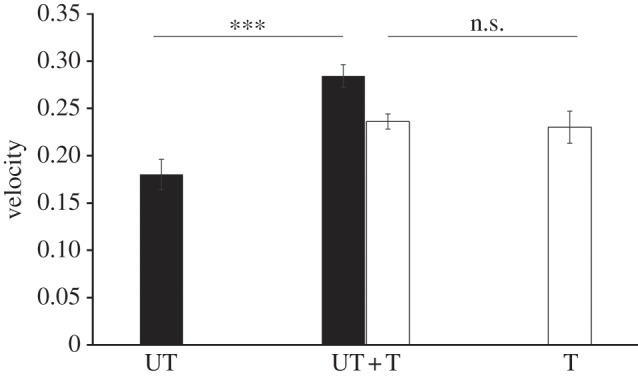
Untrained fly velocity is higher in UT + T treatment compared with control treatment. Fly velocity (mm s−1) during the transmission phase for untrained (black bars) and trained flies (white bars) depending on the treatment. Error bars represent standard error of the mean. ***p < 10−3, t-test.
We then asked whether the number of interactions between trained and untrained flies in the UT + T treatment affected their behavioural decision during the subsequent oviposition test phase. Untrained flies performed better after more interactions with trained flies. However, trained flies performed more poorly after more interactions with untrained flies (performance UT flies: z = 7.725, p < 0.001; performance T flies: z = −23.06, p < 0.001; figure 4). This translates into a surprising exchange of information between trained and untrained flies during interactions: information does not just move from trained to untrained flies, but is transferred in both directions. The number of interactions between trained flies in the T treatment had no influence on their performance (z = 0.84, p = 0.399).
Figure 4.
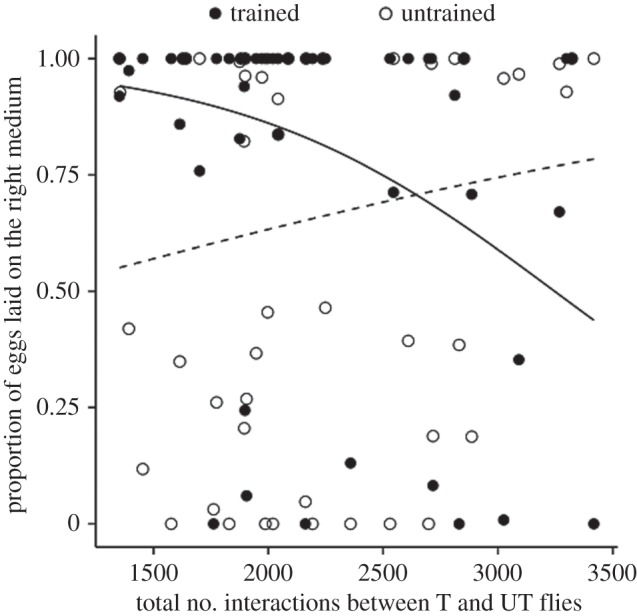
Interactions between T and UT flies influence their performance during test phase. In UT + T treatment, the proportion of eggs laid on the right medium (performance) by untrained flies is positively influenced by the number of interactions with trained flies. On the contrary, trained flies decrease their performance when interactions with untrained flies increase. Regression lines of the generalized proportional models are shown for T (solid line) and UT (dotted line) flies (N = 49).
4. Discussion
In our study, we investigated how interactions correlate with informational transfer. Our results show (i) that the transfer of social information in Drosophila mimics a mutual exchange of information between trained and untrained flies, and (ii) that the number of social interactions depends on the composition of groups. This study confirms that social structure matters and might affect many evolutionary processes such as cooperation, host–pathogen interactions and information exchange (see [19] for a review).
Social learning is usually treated as if it was a unidirectional transfer of information from a social demonstrator (like our trained flies) to a social learner (like our untrained flies). Nevertheless, we found here that the performance of trained flies was affected by the rate of previous interactions with untrained flies, suggesting that informational exchange is in fact bidirectional. We propose that, during interaction events between untrained and trained flies, untrained flies acquire olfactory information carried by trained flies, and at the same time trained flies receive conflicting information from ‘odourless’ untrained flies. The absence of banana–strawberry cue may be seen as new information for trained individuals. This may potentially mimic a form of backward blocking [20] which has been described in vertebrates but also in invertebrates such as honeybees [21]. An individual is first trained with a compound reinforced stimulus (AB+), and then exposed to A alone in a second phase. If backward blocking occurs, the effects of B in a third test phase are reduced by the subject's experience with A in the second phase. Couvillon et al. [21] showed that honeybees trained to collect sucrose solution from targets labelled with a combination of different odours and then exposed to a single odorant tended to decrease their response to the other odorant in a following test.
As suggested recently by Giurfa [22], this social transmission event may underlie simple associative processes where the fruit odorant may act as conditioned stimulus whereas mated fly carrying it may act as a positive unconditioned stimulus. When untrained flies interact with trained ones, the former face simultaneously the conditioned and the unconditioned stimuli, leading to positive association and increased preference. By contrast, trained flies face the unconditioned stimulus alone, or associated with any other potential odorant adhered to untrained flies, and show later a weaker response. This may be caused by information interference or an unconditioned stimulus postexposure effect, which have been found to affect the conditioned response in conditioned flavour preference [23,24]. It may also explain why in the T and UT treatments we did not observe any correlation between the number of interactions during the interaction phase and the following proportion of eggs laid on the correct medium. As all flies probably transferred and received a similar quantity of information, the informational exchange did not produce any visible effect on their performance. This dual information exchange may potentially allow keeping track of any outside option in particular under heterogeneous environmental conditions.
Previous studies on social networks have demonstrated that heterogeneous groups composed of different strains or genotypes show specific dynamics that are not seen in the respective homogeneous settings [25]. This may either be due to genetic differences in how much strains signal and perceive information, or indicate group level, competitive responses related to the perception of within-group genetic heterogeneity [26–28]. Here we extend these findings by showing that not only genetic heterogeneity, but also diversity in individuals' experience may lead to variation in social dynamics. In particular, we suspect that trained flies may carry useful information related to the oviposition preference, like for example some phenotypical traits linked with individual fitness. Untrained flies might detect odorants on trained flies that also exhibit fitness-related information about oviposition preference. The resulting two-level information possibly triggers an increase of interest in untrained flies, which finally determines their increase in activity level that can be observed during the interaction phase.
Interestingly, our findings also suggest that trained flies could modulate the information flow within groups or, in other words, that trained individuals might be able to decide whether to retain or transmit information. For an untrained, naive fly, engaging in social interactions would provide beneficial information. However, for a trained fly, increasing social interactions would increase information transfer but decrease its own information level. Transferring information about oviposition sites could be adaptive for a trained fly, as larval survival depends on a balance between resource exploitation and larval competition [29–31]. When few larvae feed on a natural resource such as decaying fruit, they cannot optimally exploit the medium, and thus fail to challenge the development of bacterial and fungal competitors. On the other hand, when larval density on food sources is high, there is a deleterious effect of inter-individual competition. A relationship between information transfer and quality of the resource should therefore be adaptive. A trained fly would be expected to share its information about a suboptimal substrate that cannot be exploited by few larvae (such as its own offspring alone), while signalling an already optimal substrate would only trigger disadvantageous competition. Clearly, more work is required to better understand the adaptive value of information transfer.
Our study opens perspectives about the mechanisms underlying social learning and transmission of information, at both individual and group level. First, it raises the question of what benefit trained individuals acquire by signalling or not their personal information. Then, most importantly, it shows that the dynamics of the information exchange may strongly affect the spread and the maintenance of information within groups. Investigating how the social structure of the networks evolves with time, particularly in organisms such as gregarious, non-social insects, may provide further understanding on population dynamics in the face of discontinuous environments, and will shed light on the importance of non-genetic transmission of information in the evolution of complex systems.
Acknowledgements
We thank S. Wardrop, Barbara Taborsky and two anonymous reviewers for their most valuable comments on previous versions of the manuscript.
Data accessibility
All data are deposited in the Dryad repository doi:10.5061/dryad.mv5dh.
Funding statement
This project was funded by an ANR Programme Blanc (ANR 12 BSV7 0013 02) to F.M. and C.S. C.S is funded by the University of Strasbourg Institute for Advanced Study (USIAS) and the Fyssen Foundation. M.B., C.P. and S.T. are funded by an ANR Programme Blanc (ANR 12 BSV7 0013 02) to F.M. and C.S.
References
- 1.Heyes CM, Galef BG. 1996. Social learning in animals: the roots of culture. Amsterdam, The Netherlands: Elsevier Science. [Google Scholar]
- 2.Gariépy J-F, Watson KK, Du E, Xie DL, Erb J, Amasino D, Platt ML. 2014. Social learning in humans and other animals. Front. Neurosci. 8, 58 ( 10.3389/fnins.2014.00058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee V, Harris L. 2013. How social cognition can inform social decision making. Front. Neurosci. 7, 259 ( 10.3389/fnins.2013.00259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoppitt W, Laland KN. 2013. Social learning: an introduction to mechanisms, methods, and models. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Kurvers R, van Oers K, Nolet BA, Jonker RM, van Wieren SE, Prins HHT, Ydenberg RC. 2010. Personality predicts the use of social information. Ecol. Lett. 13, 829–837. ( 10.1111/j.1461-0248.2010.01473.x) [DOI] [PubMed] [Google Scholar]
- 6.Kendal R, Coolen I, Laland K. 2009. Adaptive trade-offs in the use of social and personal information. In Cognitive ecology II (eds Dukas R, Ratcliffe JM.), pp. 249–272. Chicago, IL: University of Chicago Press. [Google Scholar]
- 7.Kendal RL, Galef BG, van Schaik CP. 2010. Social learning research outside the laboratory: how and why? Learn. Behav. 38, 187–194. ( 10.3758/lb.38.3.187) [DOI] [PubMed] [Google Scholar]
- 8.Allen J, Weinrich M, Hoppitt W, Rendell L. 2013. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485–488. ( 10.1126/science.1231976) [DOI] [PubMed] [Google Scholar]
- 9.Atton N, Hoppitt W, Webster MM, Galef BG, Laland KN. 2012. Information flow through threespine stickleback networks without social transmission. Proc. R. Soc. B 279, 4272–4278. ( 10.1098/rspb.2012.1462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt KA, Dall SRX, Van Gils JA. 2010. The ecology of information: an overview on the ecological significance of making informed decisions. Oikos 119, 304–316. ( 10.1111/j.1600-0706.2009.17573.x) [DOI] [Google Scholar]
- 11.Gruter C, Leadbeater E. 2014. Insights from insects about adaptive social information use. Trends Ecol. Evol. 29, 177–184. ( 10.1016/j.tree.2014.01.004) [DOI] [PubMed] [Google Scholar]
- 12.Leadbeater E, Chittka L. 2007. Social learning in insects—from miniature brains to consensus building. Curr. Biol. 17, R703–R713. ( 10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- 13.Sarin S, Dukas R. 2009. Social learning about egg-laying substrates in fruitflies. Proc. R. Soc. B 276, 4323–4328. ( 10.1098/rspb.2009.1294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battesti M, Moreno C, Joly D, Mery F. 2012. Spread of social information and dynamics of social transmission within Drosophila groups. Curr. Biol. 22, 309–313. ( 10.1016/j.cub.2011.12.050) [DOI] [PubMed] [Google Scholar]
- 15.Mery F, Varela SAM, Danchin E, Blanchet S, Parejo D, Coolen I, Wagner RH. 2009. Public versus personal information for mate copying in an invertebrate. Curr. Biol. 19, 730–734. ( 10.1016/j.cub.2009.02.064) [DOI] [PubMed] [Google Scholar]
- 16.Durisko Z, Anderson B, Dukas R. 2014. Adult fruit fly attraction to larvae biases experience and mediates social learning. J. Exp. Biol. 217, 1193–1197. ( 10.1242/jeb.097683) [DOI] [PubMed] [Google Scholar]
- 17.Simon JC, Dickinson MH. 2010. A new chamber for studying the behavior of Drosophila. PLoS ONE 5, e8793 ( 10.1371/journal.pone.0008793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. 2009. High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451–457. ( 10.1038/nmeth.1328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurvers R, Krause J, Croft DP, Wilson ADM, Wolf M. 2014. The evolutionary and ecological consequences of animal social networks: emerging issues. Trends Ecol. Evol. 29, 326–335. ( 10.1016/j.tree.2014.04.002) [DOI] [PubMed] [Google Scholar]
- 20.Shanks DR. 1985. Forward and backward blocking in human contingency judgment. Q. J. Exp. Psychol. B 37, 1–21. ( 10.1080/14640748508402082) [DOI] [Google Scholar]
- 21.Couvillon PA, Nagrampa JA, Bitterman ME. 1994. Learning in honeybees (Apis mellifera) as a function of sucrose concentration—analysis of the retrospective effect. J. Comp. Psychol. 108, 274–281. ( 10.1037/0735-7036.108.3.274) [DOI] [Google Scholar]
- 22.Giurfa M. 2012. Social learning in insects: a higher-order capacity? Front. Behav. Neurosci. 6, 57 ( 10.3389/fnbeh.2012.00057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawa K, Ishii K. 2012. Conditioned flavor preference and the US postexposure effect in the house musk shrew (Suncus murinus). Front. Psychol. 3, 242 ( 10.3389/fpsyg.2012.00242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boakes RA, Albertella L, Harris JA. 2007. Expression of flavor preference depends on type of test and on recent drinking history. J. Exp. Psychol. 33, 327–338. ( 10.1037/0097-7403.33.3.327) [DOI] [PubMed] [Google Scholar]
- 25.Weng LL, Menczer F, Ahn YY. 2013. Virality prediction and community structure in social networks. Sci. Rep. 3, 2522 ( 10.1038/srep02522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krupp JJ, Kent C, Billeter J-C, Azanchi R, So AKC, Schonfeld JA, Smith BP, Lucas C, Levine JD. 2008. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18, 1373–1383. ( 10.1016/j.cub.2008.07.089) [DOI] [PubMed] [Google Scholar]
- 27.Kent C, Azanchi R, Smith B, Formosa A, Levine JD. 2008. Social context influences chemical communication in D. melanogaster males. Curr. Biol. 18, 1384–1389. ( 10.1016/j.cub.2008.07.088) [DOI] [PubMed] [Google Scholar]
- 28.Schneider J, Dickinson MH, Levine JD. 2012. Social structures depend on innate determinants and chemosensory processing in Drosophila. Proc. Natl Acad. Sci. USA 109, 17 174–17 179. ( 10.1073/pnas.1121252109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohlfs M. 2005. Clash of kingdoms or why Drosophila larvae positively respond to fungal competitors. Front. Zool. 2, 2 ( 10.1186/1742-9994-2-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulliam R, Caraco T. 1984. Living in groups: is there an optimal group size? In Behavioral ecology: an evolutionary approach, 2nd edn (eds Krebs JR, Davies NB.), pp. 122–147. Oxford: Blackwell Scientific Publications. [Google Scholar]
- 31.Rohlfs M, Hoffmeister T. 2003. An evolutionary explanation of the aggregation model of species coexistence. Proc. R. Soc. Lond. B 270, S33–S35. ( 10.1098/rsbl.2003.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are deposited in the Dryad repository doi:10.5061/dryad.mv5dh.


