Abstract
Group size in both multicellular organisms and animal societies can correlate with the degree of division of labour. For ants, the task specialization hypothesis (TSH) proposes that increased behavioural specialization enabled by larger group size corresponds to anatomical specialization of worker brains. Alternatively, the social brain hypothesis proposes that increased levels of social stimuli in larger colonies lead to enlarged brain regions in all workers, regardless of their task specialization. We tested these hypotheses in acacia ants (Pseudomyrmex spinicola), which exhibit behavioural but not morphological task specialization. In wild colonies, we marked, followed and tested ant workers involved in foraging tasks on the leaves (leaf-ants) and in defensive tasks on the host tree trunk (trunk-ants). Task specialization increased with colony size, especially in defensive tasks. The relationship between colony size and brain region volume was task-dependent, supporting the TSH. Specifically, as colony size increased, the relative size of regions within the mushroom bodies of the brain decreased in trunk-ants but increased in leaf-ants; those regions play important roles in learning and memory. Our findings suggest that workers specialized in defence may have reduced learning abilities relative to leaf-ants; these inferences remain to be tested. In societies with monomorphic workers, brain polymorphism enhanced by group size could be a mechanism by which division of labour is achieved.
Keywords: division of labour, task specialization, brain anatomy, acacia ants, Pseudomyrmex spinicola
1. Introduction
Multicellularity and sociality are two of the major evolutionary transitions [1], and both transitions led to a great diversity in group size [2]. Group size is positively correlated with the degree of reproductive and non-reproductive division of labour in multicellular organisms (e.g. volvocine algae [3] and ants [4,5]). Individuals in a group can specialize on a set of tasks required for the efficient functioning of the group, leading to division of labour. Morphological differentiation can occur among ant castes, but most eusocial societies show division of labour with little morphological differentiation among individuals [5,6]. In ant species with morphologically similar workers (monomorphic ants), colony organization depends on task partitioning that emerges from body-size-independent behavioural specializations of the individuals [6]. Typically, when a colony has few individuals, all workers perform similar tasks; but as a colony grows, workers become increasingly more specialized in specific tasks. Theory predicts higher task specialization in larger colonies [5,7,8], but empirical studies to date have generated conflicting support [9–11].
Anatomical modifications of the brain and peripheral nervous system can underlie behavioural specialization [12], and because neural tissue is energetically costly, the relative size of brain regions is thought to correspond to each region's relative importance for behavioural performance [13,14]. For example, humans working in professions that require efficient spatial orientation (e.g. taxi drivers) have enlarged posterior hippocampi (a brain region involved in spatial memory) compared with humans working in professions that do not require efficient spatial orientation [15]. Workers of social insects specialized in tasks requiring different sensory or cognitive abilities may also have neuroanatomies that reflect their behavioural specialization. The brain also undergoes developmental changes during ageing in ants, especially in the integration centres of the brain—in insects called mushroom bodies (MBs) [16–18]—but there are also age-independent changes in the brain, which are primarily induced by experience [17].
Two hypotheses explain how group size could affect brain size and anatomy: the ‘task specialization hypothesis' (TSH) and the ‘social brain hypothesis' (SBH). The TSH proposes that, as group size increases, both task specialization and the relative size of brain regions required to perform those tasks also increase [19]. In contrast, the SBH proposes that living in larger social groups imposes greater requirements for cognitive processing, leading to larger brains in workers engaged in any task, and specially to enlarged integration centres in the brain, such as MBs [20–22]. This hypothesis assumes that individuals recognize other members of the society as individuals (i.e. using individual-level signals), rather than as members of a group (i.e. using group-level signals), which would increase the need for increased memory and learning as society size increases [19,23]. In social insects, the effect of social life on brain size and anatomy has been tested by comparing solitary and social species [24], and by comparing facultatively social species in their solitary and social phases [25]. Monomorphic ants that are obligate plant associates allow an accurate test of the TSH and the SBH, because workers show size-independent task specialization and colony size can be more accurately quantified in the field than for soil-nesting ant species.
We evaluated the TSH and the SBH in the monomorphic acacia ant (Pseudomyrmex spinicola; electronic supplementary material, figure S1). Acacia ants are obligate associates of acacia plants (genus Vachellia, formerly Acacia): they nest in hollow spines of the host tree, forage on food bodies (called Beltian bodies) and nectar produced by the plant, defend the tree against herbivores, and kill encroaching vegetation [26–28]. Workers show site fidelity and behavioural differences according to the site on the host plant where they work: workers at the base of the tree stand motionless with the head directed downwards (electronic supplementary material, figure S1) and show aggressive responses towards food. In contrast, workers on the leaves do not show aggressive responses towards food, are faster at manipulating brood and are less prone to attack intruders than workers on the trunk [27,29].
We examined the relationship between colony size, task specialization and brain anatomy to evaluate the TSH and the SBH. We first tested two assumptions of the TSH: as colony size increases, (i) site fidelity (leaves versus trunk) by workers and specialization on site-related tasks (foraging versus defence) increases, and (ii) workers specialized on different tasks show an increasing difference in reaction towards stimuli (e.g. food and intruders). Finally, we tested predictions regarding brain anatomy: as colony size increases, the SBH predicts that the total volume and relative size of the MBs increases for all workers, regardless of task specialization, whereas the TSH predicts task-dependent changes in the relative size of brain regions.
2. Material and methods
(a). Study site
We conducted the study at Parque Natural Metropolitano (8°59′ N, 79°32′ W), Panamá City, Panamá, between July and September, 2011. We studied colonies of P. spinicola ants living on acacia trees, Vachellia collinsii (formerly Acacia collinsii). Typically, each tree is inhabited by a single colony of ants with one queen.
(b). Task specialization
Site fidelity in P. spinicola is associated with behavioural differences in task specialization [29]. We measured task specialization as the percentage of workers that were found working on the same task as when they were originally marked on a previous day (detailed methods in the electronic supplementary material). To obtain those percentages, we marked and recounted after 24 h (i) workers foraging for Beltian bodies (electronic supplementary material, figure S1c) and (ii) workers defending the base of the trunk (electronic supplementary material, figure S1b), which stand motionless with the head directed towards the ground, as described previously [27]. From these worker counts, we calculated measures of foraging and defence specialization as the number of ants with a colour-mark re-sighted on the same location (trunk, leaves) where they were originally marked, divided by the total number of ants observed with that colour-mark (electronic supplementary material, table S1 lists sample sizes for each of the 17 experimental ant colonies).
(c). Colony size
Because colony size is correlated with tree size in obligate plant-associated ants [30], we used tree diameter and number of spines with an entrance hole as proxies for colony size. Using the records of marked ants (electronic supplementary material, table S1), we also estimated the number of workers outside the spines with the Petersen method of mark–recapture [31]. To assess the effect of colony size and task specialization on brain morphology, we summarized size-dependent traits in a single variable called ‘colony-size-related traits', which was defined as the first factor of a principal component analysis (electronic supplementary material, table S2) that included defence and foraging specialization, and numbers of outside workers and tree spines.
(d). Behavioural assays
To test whether differences in behaviour between leaf- and trunk-ants increased with colony size (second assumption of the TSH), we assessed the reactions of marked ants towards food and intruders. We recorded whether workers stored or discarded food, following Amador-Vargas [29]. We also recorded whether marked ants attacked or ignored intruder leaf-cutter ants (Atta colombica) that were placed on the tree. The TSH predicts that, as colony size increases, the probability of discarding food increases in trunk-ants and decreases in leaf-ants, and that trunk-ants are more likely to attack the Atta intruders, and leaf-ants more likely to ignore the intruders. Details of the behavioural assays and statistical models are provided in the electronic supplementary material.
(e). Brain anatomy
To obtain brain measurements of ants from colonies of different size, we collected leaf- and trunk-marked ants from eight colonies, and brought them alive to the laboratory for histological preparation (see the electronic supplementary material). By coding section images (figure 1a), brains were measured blind with respect to colony identity and type of ant. We obtained volumetric measurements of brains (leaf-ants, n = 29; trunk-ants, n = 34) from digital three-dimensional reconstructions of the histological sections using reconstruct software [32] (figure 1b). To evaluate how colony-size traits correlated with other non-neural morphological trait, we also measured the head area (figure 1c) of trunk- (n = 51) and leaf-ants (n = 43; see the electronic supplementary material).
Figure 1.
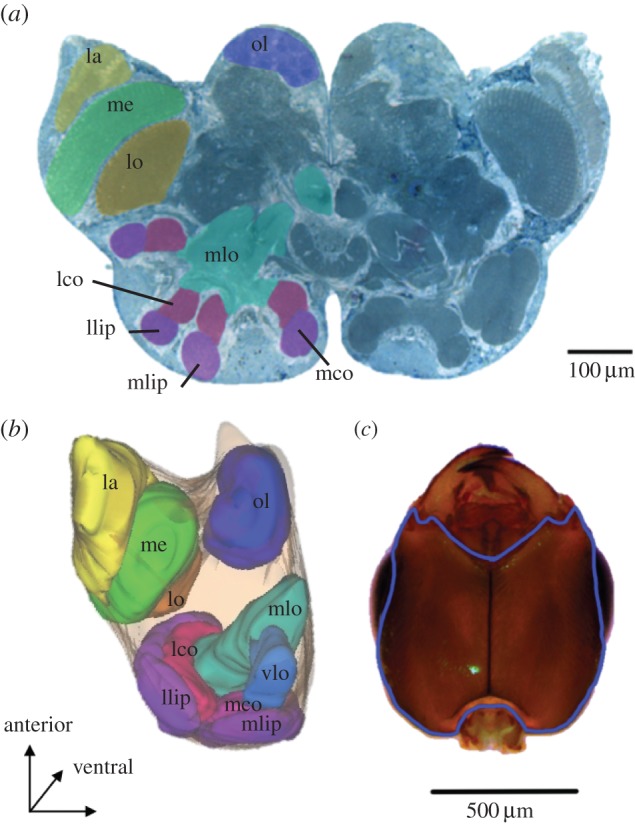
Acacia ant (Pseudomyrmex spinicola) transverse brain section and ventral view of head. (a) Brain section highlighting some of the measured neuropiles including: the lamina (la), lobula (lo) and medulla (me) of the optic lobe and the olfactory lobe (ol). In the mushroom bodies, we measured the lip and collar of the lateral (llip and lco) and medial calyces (mlip and mco); the vertical lobe (alpha; not visible) and medial lobe (mlo, beta). (b) Neuropile dimensions of brain sections were used to generate three-dimensional reconstructions of the brain regions to obtain volumetric measurements. Colours correspond to the neuropiles shown in the section; the vertical lobe (vlo), not visible in the two-dimensional section, is shown here. (c) Ventral view of the head showing in blue the contour used to calculate the head area, excluding eyes and mouthparts. (Online version in colour.)
We used generalized linear models to test for homogeneity of slopes between trunk- and leaf-marked ants on a regression of the colony-size-related traits (described above) and the relative brain-region volume (see the electronic supplementary material). To correct for multiple comparisons, we used false discovery rates [33] calculated using the q-value function of the package ‘qvalue’ in R [34]. We also report effect size as partial omega-squared (ωp2) with 95% CI, calculated with the package ‘boot’ in R [35].
3. Results
(a). Site fidelity and colony size
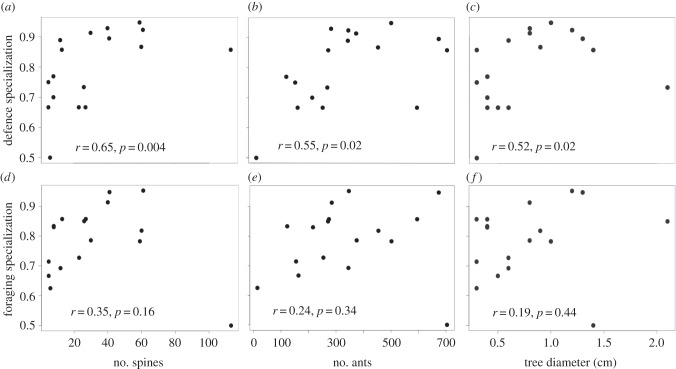
Observations support the assumption of the TSH that behavioural specialization increases with colony size. The proportion of trunk-ants re-sighted on the trunk was strongly correlated with the estimators of colony size: number of workers (figure 2a), spines on the tree (figure 2b) and tree diameter (figure 2c). In contrast, the proportion of leaf-ants re-sighted on leaves did not correlate with the three estimators of colony size (figure 2d–f). We also analysed the dataset excluding the largest colony, because there was a gap in the size range of sampled trees (between 60 and 113 spines; figure 2a,d); in that case, foraging specialization positively correlated with number of spines (r = 0.62, p = 0.01) and workers (r = 0.49, p = 0.051), but not with tree diameter (r = 0.40, p = 0.11); defence specialization still correlated with number of spines (r = 0.69, p = 0.003), workers (r = 0.58, p = 0.01) and with tree diameter (r = 0.57, p = 0.02). The proportion of trunk-ants re-sighted on the trunk did not correlate with the proportion of leaf-ants re-sighted on the leaves; defence specialization in a colony therefore did not correlate with foraging specialization (electronic supplementary material, figure S2; r = 0.38, p = 0.12), but the correlation is marginally significant when excluding the largest colony (r = 0.43, p = 0.08).
Figure 2.
Proportion of trunk-ants or leaf-ants that were re-sighted working on the same tasks as on the previous day, in relation to colony-size parameters (n = 17 colonies). Re-sighting of an ant performing the same task is a measure of behavioural specialization. The specialization of trunk-ants increased with number of (a) tree spines, (b) workers and (c) tree diameter. Leaf-ants were not more specialized (d) in trees with more spines, nor (e) in colonies with more workers, nor (f) in trees with greater trunk diameter. Correlations of foraging specialization with number of spines and workers are statistically significant when excluding the largest colony (see text).
(b). Behavioural assays
We did not find evidence that, as colony size increased, differences between trunk- and leaf-ants increased in the reactions towards food or intruders, as predicted by the TSH. Specifically, the likelihood of workers to discard experimental Beltian bodies decreased as foraging specialization increased, but this decrease depended on the type of ant. For trunk-ants, a 1% increase in foraging specialization decreased the odds of discarding by 4% (exponentiated β2 in table 1; electronic supplementary material, figure S3b), whereas for leaf-ants, the odds of discarding increased by 0.03% (β2 + β3 in table 1; electronic supplementary material, figure S2b). Defence specialization did not have an effect on the probability of discarding food (electronic supplementary material, table S3 and figure S3a). In the intruder test (presenting an Atta worker), the odds of attacking were 2% lower for leaf-ants than for trunk-ants, but these odds were not affected by foraging specialization or defence specialization (electronic supplementary material, tables S4 and S5, and figure S3b,c).
Table 1.
Effect of foraging specialization on the log odds of discarding food (log odds of discarding = β0 intercept + β1 ant type (leaf-ants = 1) + β2 foraging specialization + β3 ant type × foraging specialization), from a generalized estimating equation. For trunk-ants, the odds ratio of discarding food with a unit increase in foraging specialization is exp(β2) = 0.96; for leaf-ants, the odds ratio is exp(β2 + β3) = 1.0003. Significant values are in bold and marginally significant values are in italics.
| factor | estimate (log-odds) | s.e. | Wald | p-value |
|---|---|---|---|---|
| β0 intercept | 2.97 | 0.43 | 45.87 | <0.0001 |
| β1 ant type (trunk-ants are reference, i.e. when leaf-ants = 0) | −3.04 | 1.66 | 3.33 | 0.07 |
| β2 foraging specialization | −0.0425 | 0.007 | 31.8 | <0.0001 |
| β3 ant type × foraging specialization | 0.0428 | 0.02 | 3.53 | 0.060 |
(c). Brain anatomy
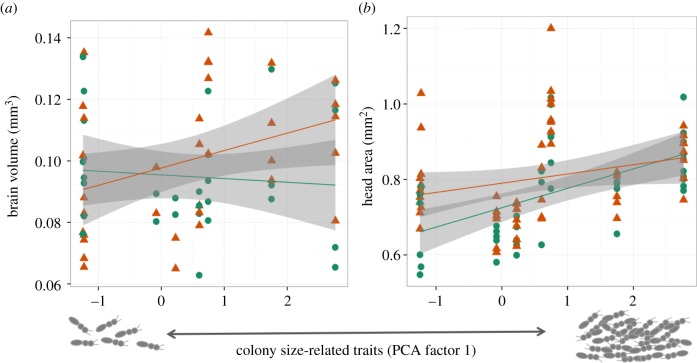
Colony-size-related traits had a marginally significant effect on total brain volume that was different between leaf- and trunk-ants (figure 3a; interaction term, F1,59 = 3.3, p = 0.07): total brain volume of trunk-ants increased with colony size-related traits (r2 = 0.35, CI: 0.018–0.61, p = 0.04), whereas total brain volume of leaf-ants was not affected by size-related traits (r2 = −0.08, CI: −0.44 to 0.28, p = 0.65). Descriptive statistics for mean relative volume of different neuropiles are shown in the electronic supplementary material, table S6. Head area showed a positive correlation with colony size (figure 3b; F1,93 = 16.05, p < 0.0001, 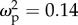 , CI: 0.04–0.25), regardless of the type of ant (interaction of colony size and ant type, F1,93 = 2.23, p = 0.14,
, CI: 0.04–0.25), regardless of the type of ant (interaction of colony size and ant type, F1,93 = 2.23, p = 0.14, 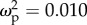 , CI: 0–0.064).
, CI: 0–0.064).
Figure 3.
Absolute (a) brain volume (n = 63 brains, eight colonies) and (b) head area (n = 98, eight colonies) of acacia ants performing tasks on the trunk (triangles) or foraging on leaves (circles) of the host tree, as a function of colony-size-related traits (see text). The shaded area represents 95% CI for the linear fit. As colony size and task specialization increased, absolute brain volume tended to increase more for trunk- than for leaf-ants, while head size increased equally for both types of ants. (Online version in colour.)
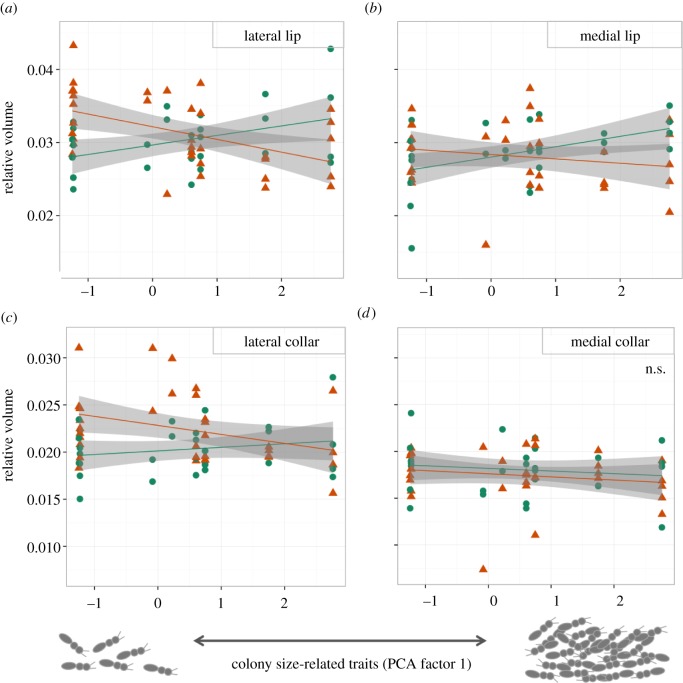
The correlation of three brain regions within the MB's calyces and colony size-related traits was task-dependent (figure 4), which supports the TSH but not the SBH. The lip of the medial and lateral calyces (figure 4a,b) and the collar of the lateral calyx (figure 4c) were relatively smaller in trunk-ants living in larger colonies with more specialized workers than in trunk-ants defending smaller colonies. Conversely, for leaf-ants, those same regions increased in relative size as colony size increased (figure 4 and table 2). The interaction between colony-size-related traits and type of ant explained about 18% of the variation in lateral lip volume, and 8.5% and 5.6% of the variation in medial and lateral collar, respectively.
Figure 4.
Relative volume of regions within the mushroom body's calyces, for trunk (triangles) and leaf-ants (circles), as a function of colony-size-related traits (see text). Trunk-ants in smaller colonies have relatively larger volume of (a) lateral lip, (b) medial lip and (c) lateral collar than when living in larger societies. Conversely, those same brain regions are relatively larger in leaf-ants living in larger societies. (d) The medial collar did not change with colony-size-related traits for either of the two types of ants. (Online version in colour.)
Table 2.
F-statistics, p-values and effect sizes (partial omega-squared, ω2p) of a general linear model interaction term between type of ant (leaf- or trunk-ant) and colony-size-related traits (see text). Significant p-values after correction for false discovery rate are in bold. Confidence intervals for estimated effect sizes are in parentheses.
| brain region | F1,59 | p-value | effect size 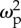
|
|---|---|---|---|
| optic lobes lamina | 0.23 | 0.63 | |
| medulla | 0.008 | 0.92 | |
| lobula | 1.20 | 0.28 | |
| olfactory lobes | 0.02 | 0.89 | |
| mushroom bodies | |||
| calyces medial lip | 6.76 | 0.01 | 0.085 (0–0.25) |
| medial collar | 0.01 | 0.92 | |
| lateral lip | 15.4 | 0.0002 | 0.19 (0.04–0.37) |
| lateral collar | 5.15 | 0.027 | 0.056 (0–0.24) |
| lobes vertical (alpha) | 0.68 | 0.41 | |
| medial (beta) | 0.47 | 0.49 |
Correlations of sensory neuropiles in the brain with colony-size-related traits were not affected by task specialization, which supports the SBH and not the TSH. The relative volume of some regions within the optic lobes slightly increased with colony size: the lamina and medulla tended to be larger for workers living in larger colonies, regardless of task, although colony size explained, respectively, only 4% and 3% of the variation, and the confidence intervals for effect size include zero (lamina: F1,59 = 3.56, p = 0.06, 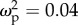 , CI: 0–0.19; medulla: F1,59 = 2.90, p = 0.09,
, CI: 0–0.19; medulla: F1,59 = 2.90, p = 0.09, 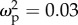 , CI: 0–0.17; electronic supplementary material, figure S4). MB lobes (electronic supplementary material, figure S5) and olfactory lobes were not statistically correlated with colony-size-related traits, and they did not differ between leaf-ants and trunk-ants (table 2).
, CI: 0–0.17; electronic supplementary material, figure S4). MB lobes (electronic supplementary material, figure S5) and olfactory lobes were not statistically correlated with colony-size-related traits, and they did not differ between leaf-ants and trunk-ants (table 2).
4. Discussion
The observed effects of colony size on worker behaviour and brain anatomy in the acacia ant P. spinicola support the TSH (increased behavioural specialization in larger colonies may generate anatomical specialization of worker brains), rather than the SBH (increased levels of social stimuli in larger colonies lead to enlarged brain regions in all workers, regardless of their task specialization). Larger colonies exhibited more specialized workers engaged in defence (figure 2) and to a lesser degree in foraging (when excluding the outlier large colony). This increased specialization in defence and foraging in larger colonies was correlated with task-dependent anatomical changes in the relative volume of brain regions, specifically in subregions within the integration centres (MBs), as predicted by the TSH.
(a). Behavioural tests of task specialization hypothesis
Behavioural observations confirmed one of the two assumptions of the TSH: that task specialization increases with colony size. Our measurement of task specialization depends on site fidelity (re-sighting an ant on leaves or trunk to perform site-related tasks), and fidelity to a site in the colony therefore may facilitate task specialization [36,37]. Specifically, when acacia ants return to the same site to work after spending the night inside the spines, they are exposed to the same stimuli and less frequently to other stimuli, which presumably induced the observed behavioural and neuroanatomical differences. In large colonies, a higher fidelity to a particular tree site and exposure to task-related stimuli correlated with the behaviour of workers: trunk-ants are more likely to discard food and are more prone to attack intruders than leaf-ants [29]. These observations support the first assumption of the TSH.
A second assumption of the TSH is that colony size enhances behavioural differences among workers. Trunk-ants were more likely to discard food (consistent with the TSH), but contrary to the expectation of the TSH, the likelihood of discarding food was affected not by the degree of defence specialization but by the degree of foraging specialization. In other words, trunk-ants were less likely to discard food when there were fewer leaf-ants on the trunk. The growth of ant colonies engaged in obligatory mutualism with plants is largely limited by food provided by the host tree [30], and large colonies of acacia ants are often seen collecting food bodies from nearby acacia saplings [28]. Hence, a possible explanation for the decrease in the likelihood of discarding food could be that large colonies preserve (rather than discard) food more effectively because of their high food demands; this explanation remains to be tested. Likewise, in the intruder assay, leaf-ants were less prone to attack than trunk-ants, but contrary to the TSH prediction, this behavioural difference was not affected by colony size. Colonies may be able to tolerate leaf-ants that ignore some intruders, but trunk-ants must be less tolerant, because the fitness of the entire colony largely depends on how trunk-ants guard and defend the acacia tree [26]. Therefore, although we tested only two behaviours that could be affected by colony size, observations did not support the assumption of the TSH of increased behavioural differentiation between workers in larger colonies.
(b). Brain anatomical tests of task specialization and social brain hypotheses
Worker subcaste differences in brain anatomy agree with predictions of the TSH, and are inconsistent with the SBH. The MBs are crucial for testing the SBH, as they are involved in multisensory integration, memory and learning, which would be of larger size for processing the increased social stimuli in larger colonies in all society members, regardless of task specialization [19,38,39]. In contrast to this prediction of the SBH, but consistent with the TSH, we found a task-dependent effect of colony size and specialization on the relative size of regions within the MB's calyces (figure 4).
A decrease in the relative size of MB with defence specialization was previously unknown for monomorphic workers. Specifically, we observed a decrease in the regions for integration of olfactory and visual input. Only one other study on wasps documented the effects of aggressive behaviour on the volume of brain regions, but this study reported an increase, rather than a decrease, in the size of the MB's calyces and in the ratio of lips to Kenyon cell bodies [40]. Studies of other ant species with workers specialized in defence (e.g. Dinoponera [41]) may be useful in understanding whether this is a general trend among social insects. In contrast, the observed increase in those same MB regions for foragers is congruent with findings in other social insects, such as Camponotus ants and honeybees [17,42].
What are the behavioural implications of relatively larger or smaller lips or collars in the MB? MBs, in general, are involved in multimodal sensory processing [38,43], context generalization [44], problem solving and decision making [45]. Specifically, the MB's calyces receive sensory input, whereas the lobes are mostly output areas (although they also receive some input). Within the calyces, the lips are the regions where axons from projection neurons of the olfactory lobe synapse with dendrites of Kenyon cells, whereas the collars have direct visual input from the medulla and the lobula of the optic lobe [46]. The observed patterns therefore suggest for P. spinicola that workers specialized in defence in large colonies may show reduced olfaction-related processing (e.g. learning, decision making). Future behavioural studies should compare learning abilities between workers specialized in different tasks.
The observed correlation between colony size and brain anatomy also has important implications for the symmetry and function of the MB's calyces: the observed changes in relative size of lip and collar in foragers and workers involved in defence were stronger on the lateral than on the medial calyx. Previous studies on axon projections have documented mirrored projections to both calyces, and therefore they are expected (and often assumed) to be symmetrical [17,47–49]. However, foraging experience had stronger effects on the medial than on the lateral calyx of bumblebees [50]; and asymmetry between calyces has been argued to increase with colony size and worker polymorphism [51]. These results underline the need in future studies to distinguish between calyces when studying brain anatomy, and to explore the respective functional differences.
Although previous studies have shown that workers from some species with larger colonies have larger brains [18,52], we did not find evidence in P. spinicola of an increase in absolute brain size with colony size. Instead, we found a small task-dependent effect of colony size on absolute brain volume (interaction between colony size and type of ant explained 3.4% of the variation). Head size increased with colony size for both leaf- and trunk-ants. Hence, in larger colonies, workers were overall larger, but foragers had relatively smaller brains, which agrees with predictions of the TSH, but also with the known general trend that larger animals have proportionally smaller brains [12]. This result implies that other structures inside the head capsule (e.g. glands, muscles, infrabuccal pocket) may be relatively larger in foragers from larger colonies, a pattern observed also in larger castes of polymorphic ants [17,18].
One potential shortcoming of our study is that we did not know the workers' age. Working with colonies in the field makes it difficult to track the activity and age of each individual, which prevented us from directly assessing the age effects on neuroanatomy. If worker age is responsible for the observed behavioural plasticity, then age differences between task-specialized workers should also be greater in larger colonies, which is currently unknown. However, even if ants specialized in different tasks differed in age in our experiments, the conclusion is still valid that colony size has a task-dependent effect on the neuroanatomical differentiation among workers (where the effect could be mediated by age). Understanding the role of worker age in field colonies is one of the challenges in future studies testing effects of task specialization and colony size on worker behaviour and brain anatomy.
5. Conclusion
The SBH and the TSH propose contrasting effects of social life and group size on brain regions [19,38]. We tested both hypotheses in the same species of acacia ants, without the confounding effects of the worker external morphology or natural history differences that complicate comparative studies using other species. Our study shows that workers exhibit greater task specialization as group size increases, especially in defence tasks, which confirms the main assumption of the TSH. In addition, the task-dependent effect of colony size on brain anatomy agrees with predictions of the TSH. Processes underlying learning and memory should be the focus of future studies testing effects of society size on behavioural specialization and neuroanatomy.
Supplementary Material
Acknowledgements
We thank Natalia Ramírez, Israel Carrión and Norma Mujica for their patience and dedication during fieldwork; Amelia Muñoz and Rafael Gómez at Parque Natural Metropolitano; Gloria Vargas, Lissette Jiménez, Simon Tierney, Hermógenes Fernández, Franziska Beran, Jorge Ceballos, Edwin Domínguez, James Coronado, Andre Riveros, Paola Galgani and Adriana Bilgray for support at the Smithsonian Tropical Research Institute (STRI); Theresa Jones, Nicole Donlan and Dwight Romanovicz (Microscopy and Imaging Facility, University of Texas Austin) for technical support; Joshua T. Lackey, George Cao, Kevin Clauss, Sadia Karani, Giancarlo Mignucci, Mackenzie Mueller, Danny Nguyen and Taylor Smith for assistance with brain reconstruction; Chad Smith, Emma Dietrich, Chi-Chun Fang, Hannah Marti, and Rong Ma for comments on the manuscript; Alejandro Farji-Brener for helpful suggestions; and Raif and Suzanne Ishak for supporting local scientists. S.A.-V. conceived the idea, collected field and laboratory data, and analysed the results; S.A.-V. and U.M. drafted the manuscript; all authors collaborated in the design of the study and gave final approval for publication.
Funding statement
This research was supported by an STRI short-term fellowship, NSF-DDIG and EEB grant to S.A.-V.; NSF award IOS-0920138 to U.M.; and the Wheeler Lost Pines Endowment from the University of Texas at Austin.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Szathmáry E, Maynard Smith J. 1995. The major evolutionary transitions. Nature 374, 227–232. ( 10.1038/374227a0) [DOI] [PubMed] [Google Scholar]
- 2.Bourke AFG. 2011. Principles of social evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Herron MD, Michod RE. 2008. Evolution of complexity in the Volvocine algae: transitions in individuality through Darwin's eye. Evolution 62, 436–451. ( 10.1111/j.1558-5646.2007.00304.x) [DOI] [PubMed] [Google Scholar]
- 4.Bonner JT. 2004. Perspective: the size-complexity rule. Evolution 58, 1883–1890. ( 10.1554/04-146) [DOI] [PubMed] [Google Scholar]
- 5.Anderson C, McShea DW. 2001. Individual versus social complexity, with particular reference to ant colonies. Biol. Rev. 76, 211–237. ( 10.1017/S1464793101005656) [DOI] [PubMed] [Google Scholar]
- 6.Bourke AFG, Franks NR. 1995. Social evolution in ants. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Pacala SW, Gordon DM, Godfray HCJ. 1996. Effects of social group size on information transfer and task allocation. Evol. Ecol. 10, 127–165. ( 10.1007/BF01241782) [DOI] [Google Scholar]
- 8.Gautrais J, Theraulaz G, Deneubourg J-L, Anderson C. 2002. Emergent polyethism as a consequence of increased colony size in insect societies. J. Theor. Biol. 215, 363–373. ( 10.1006/jtbi.2001.2506) [DOI] [PubMed] [Google Scholar]
- 9.Thomas ML, Elgar MA. 2003. Colony size affects division of labour in the ponerine ant Rhytidoponera metallica. Naturwissenschaften 90, 88–92. ( 10.1007/s00114-002-0396-x) [DOI] [PubMed] [Google Scholar]
- 10.Holbrook CT, Barden PM, Fewell JH. 2011. Division of labor increases with colony size in the harvester ant Pogonomyrmex californicus. Behav. Ecol. 22, 960–966. ( 10.1093/beheco/arr075) [DOI] [Google Scholar]
- 11.Dornhaus A, Holley J-A, Franks NR. 2009. Larger colonies do not have more specialized workers in the ant Temnothorax albipennis. Behav. Ecol. 20, 922–929. ( 10.1093/beheco/arp070) [DOI] [Google Scholar]
- 12.Eberhard WG, Wcislo WT. 2011. Grade changes in brain–body allometry: morphological and behavioral correlates of brain size in miniature spiders, insects and other invertebrates. Adv. Physiol. 40, 155–214. ( 10.1016/B978-0-12-387668-3.00004-0) [DOI] [Google Scholar]
- 13.Laughlin SB, van Steveninck RRD, Anderson JC. 1998. The metabolic cost of neural information. Nat. Neurosci. 1, 36–41. ( 10.1038/236) [DOI] [PubMed] [Google Scholar]
- 14.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 15.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, Frith CD. 2000. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl Acad. Sci. USA 97, 4398–4403. ( 10.1073/pnas.070039597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Withers GS, Fahrbach SE, Robinson GE. 1993. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364, 238–240. ( 10.1038/364238a0) [DOI] [PubMed] [Google Scholar]
- 17.Gronenberg W, Heeren S, Holldöbler B. 1996. Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol. 199, 2011–2019. [DOI] [PubMed] [Google Scholar]
- 18.Muscedere ML, Traniello JFA. 2012. Division of labor in the hyperdiverse ant genus Pheidole is associated with distinct subcaste- and age-related patterns of worker brain organization. PLoS ONE 7, e31618 ( 10.1371/journal.pone.0031618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronenberg W, Riveros A. 2009. Social brains and behavior: past and present. In Organization of insect societies: from genome to sociocomplexity (eds Gadau J, Fewell J.), pp. 377–401. Cambridge, MA: Harvard University Press. [Google Scholar]
- 20.Dunbar RIM. 1998. The social brain hypothesis. Evol. Anthropol. Issues News Rev. 6, 178–190. () [DOI] [Google Scholar]
- 21.Byrne RW, Bates LA. 2007. Sociality, evolution and cognition. Curr. Biol. 17, R714–R723. ( 10.1016/j.cub.2007.05.069) [DOI] [PubMed] [Google Scholar]
- 22.Dunbar RIM, Shultz S. 2007. Evolution in the social brain. Science 317, 1344–1347. ( 10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- 23.Riveros AJ, Seid MA, Wcislo WT. 2012. Evolution of brain size in class-based societies of fungus-growing ants (Attini). Anim. Behav. 83, 1043–1049. ( 10.1016/j.anbehav.2012.01.032) [DOI] [Google Scholar]
- 24.Farris SM, Schulmeister S. 2011. Parasitoidism, not sociality, is associated with the evolution of elaborate mushroom bodies in the brains of hymenopteran insects. Proc. R. Soc. B 1707, 940–951. ( 10.1098/rspb.2010.2161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith AR, Seid MA, Jiménez LC, Wcislo WT. 2010. Socially induced brain development in a facultatively eusocial sweat bee Megalopta genalis (Halictidae). Proc. R. Soc. B 277, 2157–2163. ( 10.1098/rspb.2010.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janzen DH. 1966. Coevolution of mutualism between ants and acacias in Central America. Evolution 20, 249–275. ( 10.2307/2406628 [DOI] [PubMed] [Google Scholar]
- 27.Amador-Vargas S. 2008. Spartan defense in the Thermopylae pass: strategic defense by aggregations of Pseudomyrmex spinicola (Hymenoptera, Formicidae) on the trunk of Acacia collinsii (Mimosaceae). Insect. Soc. 55, 241–245. ( 10.1007/s00040-008-1000-y) [DOI] [Google Scholar]
- 28.Amador-Vargas S. 2012. Plant killing by mutualistic ants increases the density of host species seedlings in the dry forest of Costa Rica. Psyche (Stuttg.) 2012, 28–33. ( 10.1155/2012/491592) [DOI] [Google Scholar]
- 29.Amador-Vargas S. 2012. Behavioral responses of acacia ants correlate with age and location on the host plant. Insect. Soc. 59, 341–350. ( 10.1007/s00040-012-0226-x) [DOI] [Google Scholar]
- 30.Fonseca CR. 1993. Nesting space limits colony size of the plant-ant Pseudomyrmex concolor. Oikos 67, 473–482. ( 10.2307/3545359) [DOI] [Google Scholar]
- 31.Krebs CJ. 1999. Ecological methodology. Menlo Park, CA: Addison-Welsey. [Google Scholar]
- 32.Fiala JC. 2005. Reconstruct: a free editor for serial section microscopy. J. Microsc. 218, 52–61. ( 10.1111/j.1365-2818.2005.01466.x) [DOI] [PubMed] [Google Scholar]
- 33.Storey JD, Taylor JE, Siegmund D. 2004. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J. Roy. Stat. Soc. B, Stat. Methodol. 66, 187–205. ( 10.1111/j.1467-9868.2004.00439.x) [DOI] [Google Scholar]
- 34.Dabney A, Storey JD. 2004. qvalue: Q-value estimation for false discovery rate control. R package. See http://www.bioconductor.org/packages/release/bioc/html/qvalue.html
- 35.Canty A, Ripley B. 2014. boot: bootstrap R (S-Plus). R package. See http://cran.r-project.org/package=boot
- 36.Wilson EO. 1971. The insects societies. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 37.Sendova-Franks A, Franks NR. 1993. Task allocation in ant colonies within variable environments (a study of temporal polyethism: experimental). Bull. Math. Biol. 55, 75–96. ( 10.1016/S0092-8240(05)80062-X) [DOI] [Google Scholar]
- 38.Gronenberg W. 2008. Structure and function of ant (Hymenoptera: Formicidae) brains: strength in numbers. Myrmecol. News 11, 25–36. [Google Scholar]
- 39.Lihoreau M, Latty T, Chittka L. 2012. An exploration of the social brain hypothesis in insects. Front. Physiol. 3 ( 10.3389/fphys.2012.00442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina Y, O'Donnell S. 2007. Mushroom body volume is related to social aggression and ovary development in the paperwasp Polistes instabilis. Brain Behav. Evol. 70, 137–144. ( 10.1159/000102975) [DOI] [PubMed] [Google Scholar]
- 41.Asher CL, Nascimento FS, Sumner S, Hughes WO. 2013. Division of labour and risk taking in the dinosaur ant, Dinoponera quadriceps (Hymenoptera: Formicidae). Myrmecol. News 18, 121–129. [Google Scholar]
- 42.Farris SM, Robinson GE, Fahrbach SE. 2001. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci. 21, 6395–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heisenberg M. 1998. What do the mushroom bodies do for the insect brain? An introduction. Learn. Mem. 5, 1–10. ( 10.1101/lm.5.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L, Wolf R, Ernst R, Heisenberg M. 1999. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature 400, 753–756. ( 10.1038/23456) [DOI] [PubMed] [Google Scholar]
- 45.Tang S, Guo A. 2001. Choice behavior of Drosophila facing contradictory visual cues. Science 294, 1543–1547. ( 10.1126/science.1058237) [DOI] [PubMed] [Google Scholar]
- 46.Gronenberg W. 2001. Subdivisions of hymenopteran mushroom body calyces by their afferent supply. J. Comp. Neurol. 435, 474–489. ( 10.1002/cne.1045) [DOI] [PubMed] [Google Scholar]
- 47.Giraldo YM, Patel E, Gronenberg W, Traniello JFA. 2013. Division of labor and structural plasticity in an extrinsic serotonergic mushroom body neuron in the ant Pheidole dentata. Neurosci. Lett. 534, 107–111. ( 10.1016/j.neulet.2012.11.057) [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa M, Watanabe H, Yokohari F. 2012. Higher brain centers for social tasks in worker ants, Camponotus japonicus. J. Comp. Neurol. 520, 1584–1598. ( 10.1002/cne.23001) [DOI] [PubMed] [Google Scholar]
- 49.Strausfeld NJ. 2012. Arthropod brains: evolution, functional elegance, and historical significance. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 50.Riveros AJ, Gronenberg W. 2010. Brain allometry and neural plasticity in the bumblebee Bombus occidentalis. Brain Behav. Evol. 75, 138–148. ( 10.1159/000306506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaffe K, Perez E. 1989. Comparative study of brain morphology in ants. Brain Behav. Evol. 33, 25–33. ( 10.1159/000115895) [DOI] [PubMed] [Google Scholar]
- 52.Wehner R, Fukushi T, Isler K. 2007. On being small: brain allometry in ants. Brain Behav. Evol. 69, 220–228. ( 10.1159/000097057) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.