Abstract
Unprecedented access to the biology of single cells is now feasible, enabled by recent technological advancements that allow us to manipulate and measure sparse samples and achieve a new level of resolution in space and time. This review focuses on advances in tools to study single cells for specific areas of biology. We examine both mature and nascent techniques to study single cells at the genomics, transcriptomics, and proteomics level. In addition, we provide an overview of tools that are well suited for following biological responses to defined perturbations with single-cell resolution. Techniques to analyze and manipulate single cells through soluble and chemical ligands, the microenvironment, and cell-cell interactions are provided. For each of these topics, we highlight the biological motivation, applications, methods, recent advances, and opportunities for improvement. The toolbox presented in this review can function as a starting point for the design of single-cell experiments.
Keywords: single-cell analysis, genomics, transcriptomics, proteomics, soluble factors, microenvironment, cell-cell interaction
INTRODUCTION
Unprecedented access to the biology of single cells is now feasible, enabled by recent technological advancements that allow us to manipulate and measure sparse samples and achieve a new level of resolution in space and time. Variations at the single-cell level manifest in many forms, from the genome, to the transcriptome, to how the cell integrates signals and distributes cues. Bulk measurements on populations of cells mask single-cell responses and therefore regularly fail to accurately quantify biological processes or identify rare events. For example, in the case of tumorigenesis or immunological responses to pathogens, a few cells may drive the overall processes. To decipher the underlying mechanisms, it is useful to enhance the resolution of the underlying biology through single-cell analysis (SCA). Many new technologies are currently coming online to enable characterization of an organism at both the molecular and single-cell level. To understand how complex biological systems function, we must assemble our models from the single-cell building block using these tools.
Studying single cells across multiple biological dimensions (see Figure 1) has already opened new avenues in basic research (1), changed how we approach diagnosis of diseases (2), and provided novel tools for biotechnology (3). For example, in basic research, unique cellular biological responses occur on many levels and can be attributed to epigenetic variation (4), transcript stochastic noise (5–8), and cell cycle or circadian clock mechanisms (9), and the impact of cellular microenvironment (10, 11) on functional responses is often masked by the aggregate signal from many cells (1). In addition, SCA can reveal allelic expression differences (12, 13). The tools provided to deconvolute the cellular heterogeneity allow us to gain insight into the unique processes occurring on multiple functional levels of the single cell.
Figure 1.
An overview of approaches for the analysis and perturbation of single cells. Both conventional and novel methods to perform single-cell intracellular analysis at the genomic, transcriptomic, and proteomic level are provided, along with methods to perturb and analyze single cells at the level of secretory responses, microenvironments, and cell-cell interactions. Abbreviations: ESI MS, electrospray ionization mass spectrometry; FISH, fluorescence in situ hybridization; LOC, lab-on-a-chip; MALBAC, multiple annealing and looping-based amplification cycle; MALDI-TOF, matrix-assisted laser desorption ionization/time-of-flight; MDA, multiple displacement amplification; MSI, mass spectrometry imaging; SLB, supported lipid bilayer; STRT, single-cell tagged reverse transcription; WGA, whole-genome amplification.
Access to information about single cells on multiple functional levels is enabled by the recent development of novel tools. There are both mature and emerging technologies for SCA. This review emphasizes accessible tools to conduct experiments at the single-cell level and highlights technologies that overcome current limitations. For instance, traditional methods, such as ELISpot (14), commonly determine only a single functional parameter (cytokine secretion) and therefore yield a limited view of the functional diversity. Flow cytometry (15) can record multifunctional data (cytokine secretion and cell-surface markers) but often requires fixing and permeabilizing the cells. This requirement precludes further analysis of gene expression or other functions like proliferation, senescence, and cytolytic activity. Technologies that enable the simultaneous determination of multiple phenotypic and functional aspects of these small numbers of cells would improve basic clinical research on human biology and the pathogenesis of diseases.
One class of tools with the potential to provide new opportunities by integrating (16) multiple functions is based on microsystems such as lab-on-a-chip (LOC) devices (17). Lindström et al. (17) provide an overview of microdevice-based single-cell tools, such as LOC microfluidics and microwell-based technologies, as well as applications of these technologies. Several reviews focus on the topic of SCA, covering aspects of basic (18, 19), clinical (2), and biotechnological research (3, 16, 20). Furthermore, comprehensive reviews covering different aspects of single-cell “omics” (1, 20) have been published. Chemical and biological single-cell perturbations and analysis methods are presented in References 18 and 21.
This review focuses on advances in tools to study single cells for specific areas of biology (Figure 1). We cover mature and nascent techniques to study single cells at the genomics, transcriptomics, and proteomics level. In addition, we provide an overview of tools that are well suited for following the biological responses to defined perturbations with single-cell resolution. Techniques to analyze and manipulate single cells through soluble and chemical ligands, the microenvironment, and cell-cell interactions are provided. For each of these topics, we highlight the biological motivation, applications, methods, recent advances, and opportunities for improvement. The toolbox presented in this review can function as a starting point for the design of single-cell experiments.
INTRACELLULAR SINGLE-CELL ANALYSIS
The capacity to study intracellular mechanisms of single cells has enabled new approaches to basic biology and diagnosis of disease. For example, access to the single-cell genome and transcriptome allows screening for rare events in clinical samples. We can screen for circulating tumor cells for diagnostic purposes (22, 23), investigate tumor development by tracing cell lineages (24), or perform in situ RNA hybridization analysis (25). SCA can immediately impact studies in clinical medicine where the measurement and detailed profiling of clinical samples of limited size are often required. The low yields of cells present, and the inefficiencies of existing analytical systems, often constrain the breadth of analysis possible for cells isolated from tissue biopsies (26). For example, a pinch biopsy from a tissue or a needle aspirate of a tumor may yield at most ~105 lymphocytes. Samples from pediatrics, particularly infants, are similarly difficult to study because the volume of the sample available is small. Standard characterization procedures for such samples include either ELISpot (14) or flow cytometry (15), but each one requires ~105–106 cells per condition tested. Therefore, the entire sample must be used in a single assay, and the scope of knowledge gained about the biological system is limited.
Single-Cell DNA and Genomics
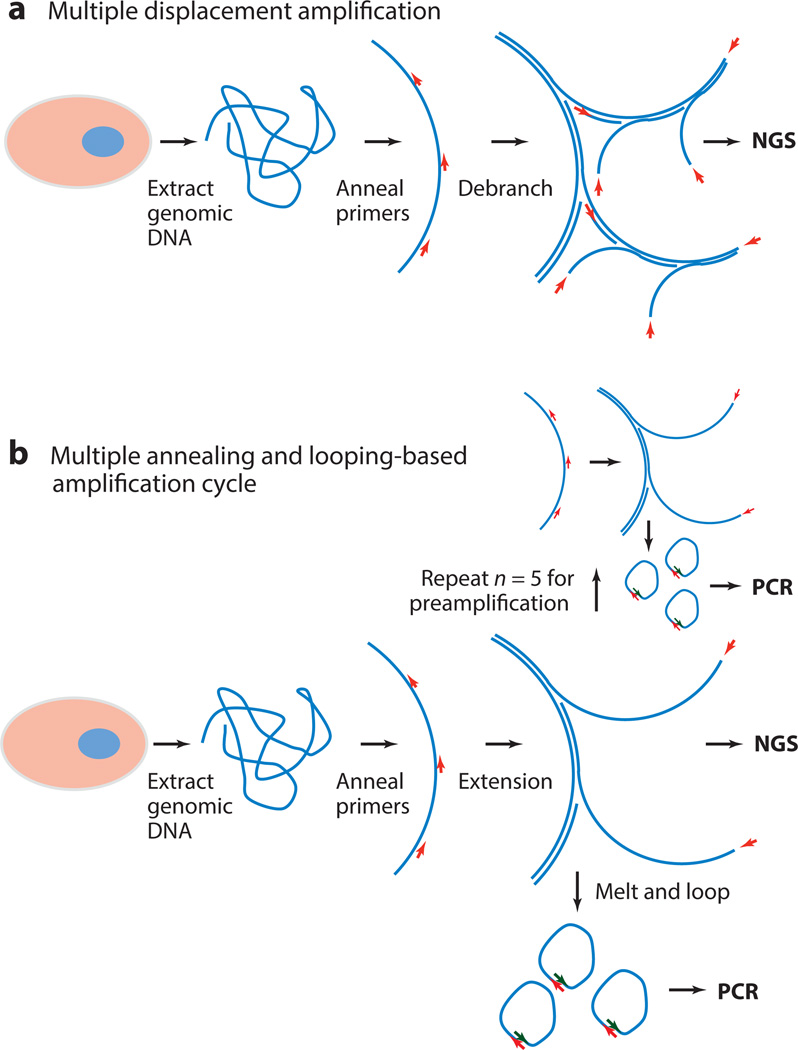
Recent advances in next-generation sequencing (NGS) (24, 27) enable whole-genome sequencing of single cells (1, 28–32). In this section, we review methods to amplify minute amounts of genomic DNA, a prerequisite for single-cell genomic analysis with current sequencing technologies. We provide an overview of multiple displacement amplification (MDA), the current gold-standard method for whole-genome amplification (WGA), as well as a discussion on multiple annealing and looping-based amplification cycles (MALBAC) as an emerging method that can reduce amplification bias (see Figure 2). An overview of other WGA methods can be found in Reference 28.
Figure 2.
Two methods for whole-genome amplification. (a) Multiple displacement amplification is a commercially available and broadly used method for single-cell whole-genome amplification. The method amplifies the genomic starting material exponentially, potentially introducing amplification biases. (b) Multiple annealing and looping-based amplification cycle uses a quasilinear preamplification step to reduce biases followed by polymerase chain reaction (PCR). This method has the potential to transform single-cell next-generation sequencing (NGS) but requires further validation and benchmarking.
Single-cell genome sequencing can be broken down into three basic steps, of which WGA is specific to SCA. The three steps are (a) identification and isolation of cells of interest using cytometry, micromanipulators, or optical tools; (b) cell lysis, WGA, and sequencing library preparation (28, 30); and (c) NGS with the prepared genomic material. We review WGA specifically in this section.
The current standard for single-cell WGA is MDA (33, 34) owing to accessibility (a kit is commercially available through Qiagen) and ease of implementation.MDA is a non–polymerase chain reaction (PCR)-based method to obtain nano- to microgram quantities of DNA for NGS from high–molecular weight genomic single-cell material. Random hexamer primers are annealed to the DNA template, and DNA synthesis is carried out under isothermal conditions by the high-fidelity DNA polymerase Φ29. The strong strand displacement activity of Φ29 creates single-stranded displacement products from the template DNA (35). In contrast to PCR, the displaced single strands function as new templates for further exponential amplification of the genomic starting material. The product from MDA can exceed that of PCR-based methods with a tenfold increase in size, higher accuracy, and reduced bias. Owing to the exponential amplification principle used in MDA, certain regions of the genome are still preferentially amplified. To bypass the issue of amplification bias, other methods for WGA are under development.
One emerging method for WGA is MALBAC, which was developed to improve single-cell WGA by reducing biases associated with nonlinear amplification (36, 37). MALBAC introduces a quasilinear preamplification step to amplify picograms of DNA. Amplicons form loops and are therefore prevented from functioning as a template for further amplification, allowing only the original genomic DNA to function as a template for amplification. In contrast to MDA, MALBAC requires cycling of multiple temperatures to hybridize, extend, and melt. A pool of random primers is hybridized to the DNA template to initiate the amplification. Subsequently, DNA polymerases with strand-displacement activity are used to produce semi amplicons (amplicons with only one primer site on the 5′ end), followed by a melting step. Full amplicons are looped with the complementary 3′ and 5′ ends and therefore cannot be used as a template for further amplification. Preamplification is composed of five thermocycles followed by multiple additional rounds of PCR to generate microgram levels of DNA for library preparation and sequencing. The inventors of this method describe MALBAC as superior to MDA with respect to bias and therefore uniformity of coverage. MALBAC is a promising method for single-cell WGA but requires validation in other studies before it can be adopted as the new gold standard for WGA.
WGA methods have been implemented successfully in the study of tumors (24, 38), which comprise many cell types with specific spatial and temporal distributions and functions. Analysis of single cells is crucial in these studies, as only small subpopulations of these cells might be responsible for tumor growth (39) and metastasis. Some examples of successful clinical applications of single-cell genomic analysis include the use of circulating and disseminated tumor cells for diagnostic tests (40–42) and SCA for the investigation of tumor heterogeneity (2, 24,38,39,43, 44).
In one study, Navin et al. (24) quantified genomic copy number within individual tumor cells to investigate the structure and evolution in two human breast cancer cases. Analysis of 100 flow-sorted polygenomic cancer single-cell nuclei indicated sequential clonal expansion into three distinct clonal subpopulations. Analysis of monogenomic cancer single-cell nuclei revealed that tumors and metastases formed from a single clonal expansion. Using single-nuclei sequencing techniques, the authors concluded that tumors form from punctuated clonal expansion and not granular starting sites, as common cancer models suggest.
In another application of single-cell sequencing, Xu et al. (39) used single-cell exon sequencing to investigate the intratumoral genetic landscape on the single-cell level. This study analyzed 25 clear cell renal cell carcinomas and adjacent healthy kidney tissue. No common known mutations were found among the carcinomas sequenced. The authors concluded that frequent mutations identified in the general patient population might not be relevant for individual patients or tumors. Interestingly, the study did not discover subpopulations within the tumor, and somatic mutations were discovered in only a small fraction of the carcinoma cells.
These studies demonstrate the utility of single-cell genomic analysis in clinical research. Extending these techniques into the fields of developmental biology and immunology will greatly aid our understanding of spatial and temporal development of embryos, as well as identify single-cell responses to immunogens. The parallel development of novel WGA methods will expedite discovery.
Single-Cell RNA and Transcriptomics
In contrast to genomic analysis, analyzing the transcriptome at the single-cell level can provide a snapshot into events occurring at specific time points. Analyzing single-cell expression levels at a given time point enables us to perform perturbation experiments offering insight into the dynamics of cellular events (45, 46). Bulk transcript analysis has been performed for decades by quantitative PCR (47) and has been expanded for high throughput by microarray (48) and NanoString (49). Some of these technologies, such as quantitative PCR (50) and microarray (51, 52), are achieving single-cell or near-single-cell resolution, but the limited throughput of these techniques constrains their efficacy in the discovery of novel transcripts. More recently, bulk RNA sequencing (RNA-seq) (53) has enabled insight into cellular functions by providing NGS-based high-throughput capability to identify transcript start sites (54, 55), novel transcripts (56, 57), and splicing variants (58). Various techniques are under development, including digital RNA-seq (59) and methods to directly sequence RNA (60) without the need for cDNA conversion and amplification, thereby reducing biases. Furthermore, RNA-seq provides a straightforward method to link phenotype and genotype (61). More recently, methods to analyze the transcriptomes of single cells (61, 62) have been developed. Single-cell RNA-seq has provided the means to identify alternative transcript isoforms, candidate biomarkers for melanoma circulation tumor cells (63), and low–copy number transcripts (64). It was demonstrated that observed average expression levels in cell populations can often be contributed to only a few cells with transiently high expression levels (65). In addition, single-cell methods to screen for siRNAs are also in development (66).
A comprehensive overview of current single-cell RNA-seq methods is provided elsewhere (61, 62, 67). In this section, we focus on two methods that are versatile, representative, and easily integrated into single-cell RNA-seq, namely, single-cell tagged reverse transcription (STRT) (68) and single-cell RNA by multiplexed linear amplification (Cel-seq) (21). Recently, other methods have also been reported (65), but these methods, in principle, can be reduced to the basics of STRT (exponential amplification by PCR) and linear amplification by in vitro transcription (IVT). Furthermore, we briefly evaluate the utility of fluorescence in situ hybridization (FISH) (69) in the context of single-cell RNA-seq as a useful tool for experimental validation and quantification of relative expression levels in live single cells. These two single-cell RNA-seq methods were selected owing to the integration of barcoded multiplexing to measure expression levels in multiple individual cells simultaneously by taking advantage of high-throughput NGS methods. We first recapitulate the technical foundation of these methods and follow with specific examples (see Figure 3).
Figure 3.
Overview of two major single-cell RNA-seq library preparation techniques. (a) Single-cell tagged reverse transcription (STRT), a polymerase chain reaction (PCR)-based method to obtain indexed libraries for next-generation sequencing. The STRT kit is commercially available through Clontech, enabling straightforward implementation of this method. STRT also reduces bias during library preparation by exclusively using full-length transcripts. (b) Cel-seq provides indexing capabilities through in vitro transcription (IVT) linear amplification. This method provides higher sensitivity than STRT at lower cost but requires larger efforts to initially implement.
In the STRT method, single cells are lysed, and poly(A)RNAis captured with an anchored oligo d(T) primer containing an NGS sequencing adaptor. Using oligo d(T) primers for first-strand synthesis avoids selecting structural RNAs such as tRNA and rRNA, which would otherwise occupy a majority of the reads in subsequent sequencing runs. The template-switching activity of Moloney murine leukemia virus reverse transcriptase is used to add cytosines to the 3′ end of the first-strand cDNA. Subsequently, the template RNA is digested with RNase and a second oligonucleotide containing a poly(G)3, a barcode, and a sequencing adapter binds to the 3′end of the first strand, effectively switching from an RNA to a cDNA template. The barcode strategy allows pooling of the individual samples at this point. The second strand is synthesized, and subsequent PCR is performed to amplify the template, using the NGS sequencing adaptors as priming sites. Note that template switching occurs only on full-length RNA templates, and therefore, only full-length transcripts (63) are amplified and sequenced; this reduces bias against shorter, incomplete transcript fragments. Details on the experimental procedures can be found in Reference 70.
STRT has been implemented to distinguish and map various cell types based on transcripts, comparing subtypes of mouse embryonic stem cells and embryonic fibroblasts (68). Single-cell RNA-seq could therefore be used to differentiate cell types beyond cell-surface markers, potentially allowing discovery of novel cell types that could not be identified through traditional cytometry methods.
In addition, Shalek et al. (71) implemented STRT in perturbation experiments to investigate effects on gene-expression levels and found bimodal distribution of mRNA expression and splicing patterns in immune cell populations previously thought to be homogeneous. Mouse bone marrow–derived dendritic cell heterogeneity was investigated in response to lipopolysaccharide. The bimodal distribution of immune gene expression could be explained by various closely related stages of cell maturation. Therefore, single-cell RNA-seq can provide the resolution to distinguish various stages of cell development. From hundreds of immune genes identified, FISH validated the bimodal distribution of a few selected transcripts.
FISH is a useful tool for noninvasive single-cell transcript analysis for validation of RNA-seq experiments. In FISH, labeled complementary cDNA or RNA strands are used to localize and identify specific transcript sections in individual cells by microscopy. This technique is versatile for validation experiments with RNA-seq, as it is implemented as multiplexed probes (FISH and CHIPS) (69) or can be used in time-resolved perturbation experiments to perform dynamic transcript analysis through RNA-binding fusion proteins (72). The major limitation of FISH is the small number of genes that can be covered in one experiment. Various flavors are being developed; for example, flow FISH (73) and FISH and CHIPS (72) can be used for validation of single-cell RNA-seq experiments.
The Cel-seq approach (74) uses IVT instead of PCR to amplify the final product for sequencing. Similar to in STRT, mRNA is reverse transcribed to cDNA using anchored oligo d(T) primers. In addition to the poly(T) tail, the oligo also contains a barcode sequence, NGS sequencing adaptor, and T7 promoter. The barcode sequence allows pooling of transcripts from multiple single-cell products. Subsequently, IVT is initiated with T7 polymerase to amplify the RNA product. In contrast to PCR, IVT amplification occurs in a linear fashion, therefore avoiding bias from exponential amplification of dominant transcripts. The RNA is fragmented, and a sequencing library is prepared. In a final step, the RNA library is converted to cDNA. The study introducing Cel-seq also performed a comparison between STRT and Cel-seq and demonstrated the superior accuracy and sensitivity of Cel-seq.
Cel-seq has been used to distinguish expressional differences in early-stage embryonic development. The resolution provided with this method allowed identification of transcriptional differences in sister cells at the embryo two-cell stage in Caenorhabditis elegans. This resolution enables us to distinguish between different cell types that are developmentally similar, allowing for potential identification of novel cell types.
Both STRT and CEL-seq provide multiplexing capabilities from indexed sequencing adapters. The STRT kit can be commercially obtained through Clontech and therefore is easily implemented. This technique reduces amplification bias by amplifying exclusively full-length transcripts. CEL-seq provides higher sensitivity (according to the authors) and is more cost efficient to implement, but it requires additional time to initially set up owing to custom components that must be obtained independently.
Single-cell RNA-seq is a powerful tool for obtaining gene-expression information with inhomogeneous cellular populations at unprecedented resolution. This information will allow identification of novel cellular functions and potentially enable us to distinguish if diseases are caused by individual intrinsic cellular dysfunction or if issues are systemic in nature, thus providing potential novel paths for treatment.
Single-Cell Proteomics
Genomic and transcriptional information provide details about the origins and the current state of single cells but limited functional information. The posttranslational protein level must be taken into account to paint a sufficient image of cellular mechanisms. Molecular functions in the cell are controlled by proteins. The high complexity of the proteome poses challenges to analyzing single cells at the protein level. In addition, proteins undergo posttranslational modification, adding another layer of complexity. The state of proteins in a single cell changes constantly over time. These factors make single-cell protein analysis difficult (1, 20,75, 76, 77).
Single-cell protein analysis can be performed by employing a variety of methods. To study the dynamic changes of proteins in live cells, methods based on genetically introduced and expressed fluorescent markers [fluorescent proteins, Förster resonance energy transfer (FRET), and luciferase reporters] can provide insight into static and dynamic processes in the single cell by microscopy (78). Furthermore, methods of flow cytometry (79), LOC devices (80), capillary electrophoresis (81), nano-liquid chromatography (82), and mass spectrometry (76) have been implemented. No single method covers all aspects of single-cell proteomics analysis; therefore, an integrated approach becomes important.
We review single-cell mass spectrometry, as this emerging method has the potential to provide detailed information about the entire proteome of a single cell, an advantage over current techniques. This method provides femtomolar resolution without the need for labeling. Current methods include secondary ion mass spectrometry and matrix-assisted laser desorption ionization mass spectrometry (MALDI MS) (83). This technique is still in the beginning stages, and improvement of a low signal-to-noise ratio is necessitated to fully utilize this technique for SCA.
Various forms of mass spectrometry have been developed with applications in SCA, including secondary ion mass spectrometry with application in cancer research (84–86), electrospray ionization (ESI) mass spectrometry, and MALDI MS enabling proteomic and metabolomic analysis of various cell types (82, 83). These methods provide the resolution to perform functional imaging as well, as they are capable of direct spatial mapping via in situ peptide sequencing. Of the approaches for mass spectrometry, MALDI is of particular interest because it is well suited for biological samples, as it can be used under atmospheric conditions.
In MALDI, a sample is embedded in a matrix, and the encapsulated target undergoes desorption from a UV laser. This causes ablation of the top layer of the sample, and the evaporated species becomes ionized and analyzed by time-of-flight mass spectrometry. Because the sample is embedded in a matrix, MALDI does not require sample separation, which enables spatial information to be preserved (87).
In conclusion, various single-cell proteomics tools are currently available; however, several tools must be used in concert to gain a full image of proteins at the single-cell level. The emerging use of MALDI for single-cell proteomic analysis could overcome this challenge, but sensitivity remains an issue. Moving forward, changes to the sample preparation, tool setup, and ablation method could enable greater sensitivity when using MALDI and may make single-cell proteomics a standard tool in the single-cell toolbox (87).
SINGLE-CELL RESPONSES TO DEFINED EXTRACELLULAR STIMULI
The study and manipulation of cellular systems are central to all areas of biology and biotechnology. In many cases, it is important to understand the relationships between external cues (media, stimuli, proteins) and functional outputs at the scale of the cell or a system of cells (tissues, bioreactors). Most of these relationships are defined by correlating data from independent, single-cell, or bulk measures, but these correlations often are not simple and may exclude critical, but rare, subsets of interesting cells. Understanding of multicomponent systems usually requires more information to resolve complex relationships or to improve the efficiency of a process than is possible to collect with standard bioanalytical tools (88).
Cell Responses to Stimulations by Chemical or Soluble Ligands
Investigations of cellular responses are typically performed using assays that depend on ensemble measurements of cell populations. Biological samples, however, can comprise heterogeneous populations presenting different phenotypes and abilities to respond to defined stimuli. So far, a diverse set of tools, such as flow cytometry (15), ELISpot assays (14), and droplet microfluidic devices (89, 90), have been developed to investigate cellular phenotypes at the single-cell level. Nonetheless, these technologies can be destructive and do not allow for time-resolve measurements that are important to investigate changes in the functional stage of cells that occur after activation. Highly integrated measures that score three or more classes of information (e.g., gene expression, secretion profiles, lineages, growth rates) for single cells using microsystems remain largely underdeveloped. In lieu of the necessity to address the complexities of cellular heterogeneity, various investigators have developed approaches that take advantage of micro- and nanofabrication techniques to provide spatial and chemical control at the level of cell operation.
Micro- and nanofabrication tools emerged in large part from the realms of microcircuits and materials science. Together with fabrication methods, materials can be designed with chemical features that resemble spatial cues sensed by cells. In particular, the use of arrays of microwells has proven to be an effective method to spatially isolate cells and control cues that cells receive to initiate a response. Dense arrays of wells with nominal (sub) nanoliter volumes are well suited to isolate individual cells for characterization by imaging (91–93).The defined addresses of individual cells, and the ability to immerse the array in a bulk reservoir of media, make measurements of temporal responses to stimuli straightforward on many live cells in parallel (88). One example of this method is to monitor drug-induced responses among individual cells (10, 94). The entire surface of the array, or just the basal surfaces of the well, can also be functionalized with biologic reagents to detect secreted proteins such as antibodies or cytokines from single cells (95–97), allowing the subsequent recovery of the cell of interest. Alternatively, a second substrate can be applied to the planar surface of the array to confine the cells temporarily in subnanoliter volumes in a process called microengraving (98, 99). This confinement enhances the sensitivity of assays designed to capture secreted proteins by 10 to 100 times relative to conventional surface-based capture assays (99) and allows repeated multiplex sampling of the secretions from the same cells (Figure 4a) (100).
Figure 4.
(a) Schematic of a process (microengraving) using a microwell-based system yielding multidimensional data from single cells that provide information about protein secretion, surface-expressed markers, viability, and gene expression. (b) Characterization of polyfunctional T cell responses with single-cell temporal resolution. Cells were isolated in a microwell array, and the dynamic patterns of cytokine secretion were investigated by following operations similar to those described in a. The viability (calcein label) and lineage of the cells were characterized by image cytometry, and cytokine secretion was probed at different time points by microengraving (left). The data collected from thousands of single cells were clustered based on the pattern of cytokine secretion and displayed in a heat map, in which each row represents time-resolved data on secretions from a single cell. The color code represents different combinations of cytokine secretion. Abbreviations: IFN, interferon; PDMS, polydimethylsiloxane; TNF, tumor necrosis factor. Adapted from Reference 100 with permission from Proceedings of the National Academy of Sciences.
One primary advantage of microwell-based systems is that they are simple. They have no external connections and can integrate into standard research practices for in vitro assays. A second advantage is that minimal manipulation is required to isolate cells. Typically, they are dispersed onto an array by pipette and allowed to settle by gravity (101). It is also straightforward to recover specific cells by micromanipulation to rescue particular genes or generate clonal lines of interest (102). The integration of microfluidic systems with these types of arrays can further expand their utility by enabling the modulation of microenvironments or application of stimuli (103). Like most examples of droplet microfluidics (104), these systems are also limited by the efficiency with which cells are distributed into individual wells. Reducing the size of the wells, or recycling cells during deposition, can improve loading of the arrays with single cells to achieve loading efficiencies of up to 70% (101, 105).
We highlight here a recent example that demonstrates bioanalytical processes using SCA yielding multidimensional datasets. A set of data, including surface-expressed markers, viability, and relative rates of secretion for cytokines, was collected for thousands of individual primary T cells (Figure 4b) (100). This single bioanalytical process incorporated modular operations, yielding data equivalent to at least three independent assays on both the lineages of the T cells and the polyfunctionality of the time-dependent responses to specific soluble stimuli. This approach provides a comprehensive means to assess cells involved in an adaptive immune response and suggests one strategy for monitoring responses to a soluble stimuli with single-cell resolution.
Artificial Niches to Regulate the Cellular Microenvironment: Cell-Surface Receptor Activation and Functional Responses
The evolving behaviors of a cell are often directed by local environmental cues, such as soluble and surface-bound biochemical signals, cell-cell junctions, or the biophysical properties of the extracellular matrix (106). In recent years, the tumor microenvironment has risen to prominence as a key factor in malignant progression (107). Study of a cell’s microenvironment is also important in stem cell biology and regenerative medicine, given that stem cell expansion, differentiation, and capacity to self-renew are regulated through finely tuned signals in the stem cell microenvironmental niche (108) (see Figure 5).
Figure 5.
Methods to perturb the single-cell microenvironment. Cell-patterning techniques can be used for targeted drug delivery by embedding the drug in spots of polymer. Seeding density on a flat substrate can be precisely modulated while avoiding initial cell-cell contact by loading a single-occupancy microwell chip with cells and inverting onto the substrate. The influence of cell morphology can be studied using patterned adhesive proteins organized in different geometries, distinguishing the area of adhesive contact area per se from cell spreading. Substrate stiffness can be modulated by altering the concentration of polyethylene glycol (PEG) precursor when forming a PEG hydrogel. The effect of 2D versus 3D microenvironments can be determined by comparing growth in microwells to growth on flat substrates. Cells may be exposed to tethered protein coatings on prespecified surfaces of microwells: Subtractive microcontact printing can limit proteins to the inner surface of the well. Alternatively, proteins may be tethered to the basal surface of the well by spotting proteins onto the pins of the microwell mold, with the added benefit of allowing different protein concentrations and combinations to be tethered in different wells.
SCA is well situated for elucidating the effects of biophysical characteristics of the microenvironment, including adhesive contact area, cell geometry, and substrate rigidity. Chen et al. (109) probed the importance of environmental influence on cell geometry, in this case as a determinant of apoptosis in capillary cells. This subject is pertinent to medicine given the role of angiogenesis in tumor growth. The authors found that cells in suspension or adhered to 10-µm fibronectin-coated beads experienced a high rate of apoptosis not seen with beads with a diameter ≥25 µm. To clarify that the critical variable was cell spreading rather than extracellular matrix curvature or bead internalization, Chen et al. (109) showed a similar correlation using patterned cells on planar fibronectin-coated adhesive islands of different sizes. Finally, to distinguish geometric effects from those of fibronectin binding, they compared capillary cell behavior on larger single, round islands with collections of smaller islands with a similar total adhesive area that would allow greater cell spreading, and they confirmed that cell-spreading area was the key variable in apoptosis (109).
Two- versus three-dimensional environments are known to change how cells respond to drugs. This is an important consideration in preclinical drug development, where drug candidates tend to be screened in a two-dimensional environment. A three-dimensional environment may be provided in vitro, for instance by using a basement membrane–like matrix (Matrigel™). The use of Matrigel, however, brings confounding variables, such as extensive extracellular matrix exposure and large cluster sizes, that may interfere with our understanding of the contribution of three-dimensional environments per se. By comparing cells grown in microwells versus those grown on flat surfaces, Håkanson et al. (10) confirmed the protective effect of a three-dimensional environment onMCF-7breast cancer cells exposed to Taxol, an effect independent of proliferation rate. By coating the inner surfaces of the wells with either fibronectin or collagen I through subtractive microcontact printing, they identified an interaction between dimensionality and type of adhesive protein presented to cells in determining the magnitude of this protective effect.
SCA is useful in the context of stem cell biology for understanding how elements of the microenvironment influence the pattern of stem cell differentiation and self-renewal. Different genealogical trees may result in the same numbers of stem cells and differentiated daughter cells through contrasting paths of self-renewal, differentiation, death, and reversion. The puzzle of deciphering which possible path a cell’s progeny actually followed may be solved by following the fate of single stem cells seeded to microwells using monitoring with time-lapse microscopy (110).
Substrate rigidity has been shown to critically influence stem cell fates (106). Gilbert et al. (111) used time-lapse microscopy to explore the influence of substrate rigidity on the propagation of functional adult stem cells in vitro. This observation is especially important for regenerative medicine given that the propagation of adult stem cells has proved challenging. Using a microwell platform made of polyethylene glycol hydrogel with tunable stiffness, crosslinked with laminin as an adhesion ligand, they monitored the fate of single muscle stem cells and their progeny, comparing outcomes from a range of substrate rigidities. Although the substrate elastic modulus had no effect on cell-division rate, soft substrates improved viability and prevented differentiation. Cells cultured on a substrate with stiffness similar to skeletal muscle also showed superior capacity for tissue renewal when engrafted into mice (111).
Precise dynamic control over soluble factors in the microenvironment of single cells can be achieved using microfluidic devices. See Toh et al. (112) for a discussion of these technologies in the context of the stem cell microenvironment. Alternatively, Collins et al. (11) used tip-based lithography to develop a method for controlled delivery of soluble factors to single cells patterned on a flat surface. They showcased their method by delivering several live cell indicators to single cells (or cell clusters) using Eudragit® polymer composites or gelatin embedded with viability dyes. Nearby cells were selectively targeted with differing concentrations of the indicators. The same group used tip-based protein patterning to coculture different cell types in predefined patterns. They demonstrated this technique using 3T3 fibroblasts and C2C12 myoblasts, exploiting the differing binding dynamics of these cell types with fibronectin and laminin (113).
Gobaa et al. (114) devised a method to expose cells to immobilized protein on the basal surface of the microwell by robotically spotting the protein layer onto the pins of the stamp used to mold their hydrogels. This approach enables researchers to vary the composition of the tethered biomolecule coating from well to well. Their artificial niche platform exemplifies the capacity of SCA to modulate multiple parameters of the microenvironment in tandem, by combining targeted protein deposition with hydrogels of tunable stiffness and allowing precise control and monitoring of local cell density. The investigators demonstrated the utility of their platform by studying the seeding-density dependence of the differentiation of adipocytes from human mesenchymal stem cells. They found that this relationship persisted in the absence of permanent cell-cell contact and could be recapitulated by functionalizing the wells with N-cadherin, which promoted differentiation in a concentration-dependent manner (114).
When studying the impact of cell density, variation of seeding density alone may not allow investigators to distinguish between the contact-dependent effects of cluster size and the influence of diluting secreted paracrine signaling factors. Doh et al. (115) studied the effect of cluster size on activating murine CD4+ T cells using microwells of varying sizes in a single device. Whereas any degree of contact-dependent interaction between the T cells was sufficient to minimize apoptosis, larger clusters correlated with greater proliferation.
Arrays of wells or adhesive proteins impose limitations on cell growth, movement, and substrate composition, which may interfere with studying phenomena such as growth and motility. Rosenthal et al. (116) developed a patterning technique that allows single cells to be deposited on any substrate while allowing the experimenter to determine cell spacing, giving incremental control over contact-mediated signaling without restricting cell growth and migration. Cells fall into single-occupancy wells patterned on their chips, which can then be inverted onto the growth substrate and removed after the deposited cells attach. Using this method, the investigators produced the first quantitative evidence that single-cell seeding of mouse embryonic stem cells maximizes colony formation (116).
The flexibility of approaches to modify the microenvironment to investigate cellular responses has been highlighted by the use of supported membranes to present either adhesion proteins to investigate mechanisms in cell adhesion or peptide ligands attached to major histocompatibility complex (MHC) molecules to activate antigen-specific T cells (117, 118). The influence of lateral mobility and dimensionality in cell adhesion and stress fiber formation has been investigated by creating an in vitro platform that mimics the three-dimensional environment of neighboring cells by modifying microwells to present a functionalized supported lipid bilayer (SLB) displaying the extracellular domain of E-cadherin (117). Using either laterally mobile or immobile ligands, the authors demonstrated that enhanced cadherin lateral mobility significantly decreased the formation of actin bundles while constraining the cell shape to that of the microwell.
SLB-presenting tethered ligands on planar substrates are also an important tool for characterizing spatiotemporal and structural aspects of T cell receptor reorganization during the formation of immunological synapses (118). This receptor reorganization occurs upon recognition of peptide antigens bound to MHC molecules. Structural aspects of the immunological synapse have been thoroughly investigated via fluorescence microscopy, but more detailed analysis into the functional aspect of the functional synapse (specifically, downstream cellular responses after activation) has been limited by approaches that rely only on imaging. Our group has developed a new method that uses microwell arrays modified with SLB-presenting MHC/peptides and ICAM-1 to activate cells in a way that allows each microwell to function as an artificial antigen-presenting cell. This construct allows investigation of both structural aspects of the synapse (by imaging) and functional aspects of cell activation by analyzing secretion of cytokines and other mediators (by microengraving) (Figure 6a).
Figure 6.
Single-cell analysis of cellular responses using microwell-based systems. (a) An artificial microenvironment inside microwells was created to activate specific receptors on the surface of T helper (Th) cells by using supported lipid bilayers presenting ligands to mimic antigen-presenting cells. (b) The study of cell-cell interactions can be achieved by spatially isolating the cells to track behavior by microscopy and functional responses (i.e., cytokine secretion) by microengraving. In these examples, cytotoxic killing of target cells by natural killer cells was followed by labeling of dead cells with a fluorescent probe that binds to DNA when the plasma membrane has been compromised by apoptosis. Adapted from References 118 and 132 with permission from the Royal Society of Chemistry.
Investigation of Cell-Cell Interactions at the Single-Cell Level
SCA can provide valuable information in the field of cell biology. In any given biological system (e.g., immune system, tissue, tumor microenvironment), cells not only respond to soluble factors released by other cells (i.e., secretion of chemical mediators) but also mediate interactions by binding to cell-surface receptors. These cell-cell interactions can initiate target cell death [as in the case of cytotoxic killing (119, 120)], cell proliferation (121), and other functional responses.
Assays to monitor cell-cell interactions depend on indirect reporters of some functional response. For example, cytotoxic killing, cell proliferation, and changes in gene expression can be monitored in bulk by following fluorescent reporters (122) or gene profiling (5) in a 96-well plate format. Such a bulk assay can be informative when a homogeneous cell population is assumed. However, information on cell-cell interactions that occur in complex samples can be obscured by ensemble measurements. More robust approaches are needed to allow monitoring of individual cells by imaging and to probe functional responses, including morphological changes, cell secretion, and apoptosis. Advances in microscopy and fluorescent reporters, including genetically engineered fluorescent proteins, make it possible to investigate many aspects of cell-cell interactions in real time. Miniaturized systems (such as microwell arrays and microfluidics) are amenable for these investigations owing to the ability to isolate groups of cells in spatially defined regions (92, 123–125). By spatially isolating cells into single wells, it is possible to monitor dynamic behaviors for defined numbers of cells in the wells over time. These approaches to isolate cells have been used to monitor dynamics of single-cell growth and proliferation and the effects of cell crowding, including epigenetics changes that occur over multiple generations (126–131). Specifically, the development of a plethora of LOC devices has been pushing forward the integrated study of cancer cytomics, allowing investigation into the functional heterogeneities of cancer cells (123).
The study of cell-cell interactions spans many complex aspects of cell biology, with important implications in cancer biology and immunology. In particular, the interactions between immune cell cytotoxicity by CD8+T cell or natural killer (NK) cells is an important biological problem that is readily accessible for investigation using SCA (132–134). NK cells are important players in the innate immune system that identify potential target cells and respond by secreting inflammatory immunomodulators that cause apoptosis of target cells, such as tumor cells or virally infected cells (135). The relationship between the characteristics of these cell-cell interactions, cytolysis, and secretory activity had not been investigated until recently, in part owing to the limitation of traditional approaches that relied on bulk measurements of indirect reports of cell function. By employing an integrated approach to SCA, we recently showed that NK cells operate independently when lysing a single target cell and that lysis occurs during the first encounter with a target (132). Furthermore, we demonstrated that the secretion of interferon-γ occurs mainly among NK cells that become the least motile upon contact with a target cell but is largely uncorrelated to cytolysis (Figure 6b). Our findings showed that integrated analysis of the cell-cell interaction can reveal new insights into complex mechanisms in cell biology.
CHALLENGES AND OPPORTUNITIES FOR INTEGRATING SINGLE-CELL TECHNOLOGIES
To truly understand the detailed workings of biological systems, we must capture the unique properties of all levels of the single cell. By integrating data from SCA of the genome, transcriptome, and proteome, we can curate a deep understanding of the properties of each unique cell, allowing us to develop accurate models of biological systems. In addition, we can begin to understand how biological systems interact with one another and the environment by probing effects of soluble factors, the microenvironment, and cell-cell interactions at single-cell resolution. This provides a road map for understanding unique properties of cells, cell-cell interactions, and cell-environment interactions. Ultimately, the goal is to move toward LOC devices that can simultaneously capture, measure, and integrate all these aspects on the single-cell level.
Despite significant efforts, major hurdles in SCA still exist and must be addressed. The throughput in SCA depends on the number of parameters measured in an experiment. For example, we are limited to a few hundred cells in one experiment when we sequence single-cell genomes or transcripts together with functional parameters interrogation, such as cytokine secretion or surface receptor specificities. To draw accurate conclusions on a systems level, these numbers must be extended from hundreds to thousands of cells. Furthermore, as the number of single cells and parameters measured steadily increases, the complexity of the data generated increases exponentially. This provides an opportunity to develop new analysis pipelines and visualization tools to identify correlations in these large, multiparameter data sets. The insights from large-scale single-cell data will allow us to formulate and test hypotheses to gain new understandings in basic biological processes and to address clinical issues in diagnostics and diseases.
SUMMARY POINTS.
The latest next-generation sequencing methods have enabled sequencing of a large number of single cells on the genomic and transcriptomic level at high throughput.
Methods to perform SCA at the genomic, transcriptomic, and proteomic level have become available commercially and have enabled us to distinguish cellular subtypes and stages of embryo and cancer development.
SCA has reached clinical relevance as it is implemented to identify circulating and disseminated tumor cells. Exon sequencing enables us to focus sequencing efforts and reduce costs for potential clinical applications.
Mass spectrometry has the potential to provide detailed information about single-cell proteomics.
Microwell technology has enabled multiple single-cell studies.
Controlling the microenvironment allows perturbation of single cells.
Studying cell-cell interactions at the single-cell level reveals insight into complex biological mechanisms.
ACKNOWLEDGMENTS
This work was supported in part by the Bill & Melinda Gates Foundation and by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) (5P01AI045757, 1DP3DK097681). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH. J.C.L. is a Camille Dreyfus Teacher-Scholar. The authors dedicate this paper to the memory of Officer Sean Collier, for his caring service to the MIT community and for his sacrifice.
Glossary
- Single-cell analysis (SCA)
a collection of methods and techniques to investigate single cells across multiple biological dimensions
- Lab-on-a-chip (LOC)
miniaturized, integrated devices that implement principles from microfabrication, material sciences, and microfluidics to efficiently manipulate and measure sparse input material from single cells
- Next-generation sequencing (NGS)
a collection of methods for high-throughput sequencing at high depth and coverage, enabling detailed genomic and transcriptomic nucleotide sequence information from large numbers of single cells
- Multiple displacement amplification (MDA)
the gold standard for whole-genome amplification using strong strand-displacement activity DNA polymerase Φ29
- Whole-genome amplification (WGA)
a collection of methods used to amplify minute amounts of single-cell genomic DNA for next-generation sequencing
- Multiple annealing and looping-based amplification cycle (MALBAC)
a novel method for whole-genome amplification that reduces amplification bias
- Single-cell tagged reverse transcription (STRT)
a highly multiplexed method for single-cell RNA-seq that uses template switching to obtain full-length transcripts
- Cel-seq
single-cell RNA-seq by multiplexed linear amplification
- Fluorescence in situ hybridization (FISH)
a noninvasive method to quantify transcripts in live cells by hybridization of a fluorescent probe to the complementary RNA strand of interest; used in combination to validate single-cell RNA-seq data
- Supported lipid bilayer (SLB)
presents tethered ligands on planar substrates as an important tool for characterizing spatiotemporal and structural aspects of cell receptors
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Konstantinos Tsioris, Email: kosta@mit.edu.
Alexis J. Torres, Email: ajtorres@mit.edu.
Thomas B. Douce, Email: tbdouce@gmail.com.
J. Christopher Love, Email: clove@mit.edu.
LITERATURE CITED
- 1.Wang D, Bodovitz S. Single cell analysis: the new frontier in “omics”. Trends Biotechnol. 2010;28(6):281–290. doi: 10.1016/j.tibtech.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navin N, Hicks J. Future medical applications of single-cell sequencing in cancer. Genome Med. 2011;3(5):1–12. doi: 10.1186/gm247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Love KR, Bagh S, Choi J, Love JC. Microtools for single-cell analysis in biopharmaceutical development and manufacturing. Trends Biotechnol. 2013;31:280–286. doi: 10.1016/j.tibtech.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 5.Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, et al. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150(6):1209–1222. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai L, Friedman N, Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006;440(7082):358–362. doi: 10.1038/nature04599. [DOI] [PubMed] [Google Scholar]
- 7.Paulsson J. Models of stochastic gene expression. Phys. Life Rev. 2005;2(2):157–175. [Google Scholar]
- 8.Munsky B, Neuert G, van Oudenaarden A. Using gene expression noise to understand gene regulation. Science. 2012;336(6078):183–187. doi: 10.1126/science.1216379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Q, Pando BF, Dong G, Golden SS, van Oudenaarden A. Circadian gating of the cell cycle revealed in single cyanobacterial cells. Science. 2010;327(5972):1522–1526. doi: 10.1126/science.1181759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Håkanson M, Textor M, Charnley M. Engineered 3D environments to elucidate the effect of environmental parameters on drug response in cancer. Integr. Biol. 2011;3(1):31–38. doi: 10.1039/c0ib00074d. [DOI] [PubMed] [Google Scholar]
- 11.Collins JM, Lam RTS, Yang Z, Semsarieh B, Smetana AB, Nettikadan S. Targeted delivery to single cells in precisely controlled microenvironments. Lab Chip. 2012;12(15):2643–2648. doi: 10.1039/c2lc40216e. [DOI] [PubMed] [Google Scholar]
- 12.White AK, VanInsberghe M, Petriv I, Hamidi M, Sikorski D, et al. High-throughput microfluidic single-cell RT-qPCR. Proc. Natl. Acad. Sci. USA. 2011;108(34):13999–14004. doi: 10.1073/pnas.1019446108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang F, Barbacioru C, Nordman E, Bao S, Lee C, et al. Deterministic and stochastic allele specific gene expression in single mouse blastomeres. PLoS ONE. 2011;6(6):e21208. doi: 10.1371/journal.pone.0021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slota M, Lim J-B, Dang Y, Disis ML. ELISpot for measuring human immune responses to vaccines. Expert Rev. Vaccines. 2011;10(3):299–306. doi: 10.1586/erv.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freer G, Rindi L. Intracellular cytokine detection by fluorescence-activated flow cytometry: basic principles and recent advances. Methods. 2013;61(1):30–38. doi: 10.1016/j.ymeth.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Love JC. Integrated process design for single-cell analytical technologies. AIChE J. 2010;56(10):2496–2502. [Google Scholar]
- 17.Lindström S, Andersson-Svahn H. Overview of single-cell analyses: microdevices and applications. Lab Chip. 2010;10(24):3363–3372. doi: 10.1039/c0lc00150c. [DOI] [PubMed] [Google Scholar]
- 18.Schmid A, Kortmann H, Dittrich PS, Blank LM. Chemical and biological single cell analysis. Curr. Opin. Biotechnol. 2010;21(1):12–20. doi: 10.1016/j.copbio.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka YJ, Gierahn TM, Love JC. The dynamic lives of T cells: new approaches and themes. Trends Immunol. 2012;34:59–66. doi: 10.1016/j.it.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritzsch FSO, Dusny C, Frick O, Schmid A. Single-cell analysis in biotechnology, systems biology, and biocatalysis. Annu. Rev. Chem. Biomol. Eng. 2012;3:129–155. doi: 10.1146/annurev-chembioeng-062011-081056. [DOI] [PubMed] [Google Scholar]
- 21.Borland LM, Kottegoda S, Phillips KS, Allbritton NL. Chemical analysis of single cells. Annu. Rev. Anal. Chem. 2008;1:191–227. doi: 10.1146/annurev.anchem.1.031207.113100. [DOI] [PubMed] [Google Scholar]
- 22.Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE. 2012;7(5):e33788. doi: 10.1371/journal.pone.0033788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol. Med. 2010;16(9):398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Navin N, Kendall J, Troge J, Andrews P, Rodgers L, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472(7341):90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leversha MA, Han J, Asgari Z, Danila DC, Lin O, et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin. Cancer Res. 2009;15(6):2091–2097. doi: 10.1158/1078-0432.CCR-08-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson JB, Webb AJ. Fine-needle aspiration biopsy and the diagnosis of thyroid cancer. Br. J. Surg. 1987;74(4):292–296. doi: 10.1002/bjs.1800740422. [DOI] [PubMed] [Google Scholar]
- 27.Mardis ER. Next-generation sequencing platforms. Annu. Rev. Anal. Chem. 2013;6:287–303. doi: 10.1146/annurev-anchem-062012-092628. [DOI] [PubMed] [Google Scholar]
- 28.Blainey PC. The future is now: single-cell genomics of bacteria and archaea. FEMS Microbiol. Rev. 2013;37:407–427. doi: 10.1111/1574-6976.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovett M. The applications of single-cell genomics. Hum. Mol. Genet. 2013;22:R22–R26. doi: 10.1093/hmg/ddt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalisky T, Blainey P, Quake SR. Genomic analysis at the single-cell level. Annu. Rev. Genet. 2011;45:431–445. doi: 10.1146/annurev-genet-102209-163607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 2013;14:618–630. doi: 10.1038/nrg3542. [DOI] [PubMed] [Google Scholar]
- 32.Lasken RS, Egholm M. Whole genome amplification: abundant supplies of DNA from precious samples or clinical specimens. Trends Biotechnol. 2003;21(12):531–535. doi: 10.1016/j.tibtech.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Dean FB, Nelson JR, Giesler TL, Lasken RS. Rapid amplification of plasmid and phage DNA using Phi29 DNA polymerase and multiply-primed rolling circle amplification. Genome Res. 2001;11(6):1095–1099. doi: 10.1101/gr.180501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spits C, Le Caignec C, De Rycke M, Van Haute L, Van Steirteghem A, et al. Whole-genome multiple displacement amplification from single cells. Nat. Protoc. 2006;1(4):1965–1970. doi: 10.1038/nprot.2006.326. [DOI] [PubMed] [Google Scholar]
- 35.Paez JG, Lin M, Beroukhim R, Lee JC, Zhao X, et al. Genome coverage and sequence fidelity of Ф29 polymerase-based multiple strand displacement whole genome amplification. Nucleic Acids Res. 2004;32(9):e71. doi: 10.1093/nar/gnh069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zong C, Lu S, Chapman AR, Xie XS. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science. 2012;338(6114):1622–1626. doi: 10.1126/science.1229164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasken RS. Single-cell sequencing in its prime. Nat. Biotechnol. 2013;31(3):211–212. doi: 10.1038/nbt.2523. [DOI] [PubMed] [Google Scholar]
- 38.Russnes HG, Navin N, Hicks J, Borresen-Dale A-L. Insight into the heterogeneity of breast cancer through next-generation sequencing. J. Clin. Investig. 2011;121(10):3810. doi: 10.1172/JCI57088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Hou Y, Yin X, Bao L, Tang A, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148(5):886–895. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heitzer E, Auer M, Gasch C, Pichler M, Ulz P, et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 2013;73(10):2965–2975. doi: 10.1158/0008-5472.CAN-12-4140. [DOI] [PubMed] [Google Scholar]
- 41.Auer M, Heitzer E, Ulz P, Geigl JB, Speicher MR. Single circulating tumor cell sequencing for monitoring. Oncotarget. 2013;4(6):812. doi: 10.18632/oncotarget.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathiesen RR, Fjelldal R, Liestøl K, Due EU, Geigl JB, et al. High-resolution analyses of copy number changes in disseminated tumor cells of patients with breast cancer. Int. J. Cancer. 2012;131(4):E405–E415. doi: 10.1002/ijc.26444. [DOI] [PubMed] [Google Scholar]
- 43.Navin N, Krasnitz A, Rodgers L, Cook K, Meth J, et al. Inferring tumor progression from genomic heterogeneity. Genome Res. 2010;20(1):68–80. doi: 10.1101/gr.099622.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou Y, Song L, Zhu P, Zhang B, Tao Y, et al. Single-cell exome sequencing and monoclonal evolution of a jak 2-negative myeloproliferative neoplasm. Cell. 2012;148(5):873–885. doi: 10.1016/j.cell.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 45.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2012;31(1):46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Licatalosi DD, Darnell RB. RNA processing and its regulation: global insights into biological networks. Nat. Rev. Genet. 2010;11(1):75–87. doi: 10.1038/nrg2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44(5):619. doi: 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- 48.Hoheisel JD. Microarray technology: beyond transcript profiling and genotype analysis. Nat. Rev. Genet. 2006;7(3):200–210. doi: 10.1038/nrg1809. [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni MM. Digital multiplexed gene expression analysis using the NanoString nCounter system. Curr. Protoc. Mol. Biol. 2011;94:25B.10.1–25B.10.17. doi: 10.1002/0471142727.mb25b10s94. [DOI] [PubMed] [Google Scholar]
- 50.Guo G, Huss M, Tong GQ, Wang C, Sun LL, et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell. 2010;18(4):675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Esumi S, Wu S-X, Yanagawa Y, Obata K, Sugimoto Y, Tamamaki N. Method for single-cell microarray analysis and application to gene-expression profiling of GABAergic neuron progenitors. Neurosci. Res. 2008;60(4):439–451. doi: 10.1016/j.neures.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Kurimoto K, Yabuta Y, Ohinata Y, Ono Y, Uno KD, et al. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic Acids Res. 2006;34(5):e42. doi: 10.1093/nar/gkl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat. Rev. Genet. 2010;12(2):87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Gerstein M, Snyder M. RNA-seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28(5):511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 57.Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-seq. Bioinformatics. 2011;27(17):2325–2329. doi: 10.1093/bioinformatics/btr355. [DOI] [PubMed] [Google Scholar]
- 58.Trapnell C, Pachter L, Salzberg SL. Tophat: discovering splice junctions with RNA-seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang F, Barbacioru C, Nordman E, Li B, Xu N, et al. RNA-seq analysis to capture the transcriptome landscape of a single cell. Nat. Protoc. 2010;5(3):516–535. doi: 10.1038/nprot.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozsolak F, Platt AR, Jones DR, Reifenberger JG, Sass LE, et al. Direct RNA sequencing. Nature. 2009;461(7265):814–818. doi: 10.1038/nature08390. [DOI] [PubMed] [Google Scholar]
- 61.Tang F, Lao K, Surani MA. Development and applications of single-cell transcriptome analysis. Nat. Methods. 2011;8:S6–S11. doi: 10.1038/nmeth.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu YY, Machleder EM, Chenchik A, Li R, Siebert PD. Reverse transcriptase template switching: a SMART™ approach for full-length cDNA library construction. Biotechniques. 2001;30(4):892–897. doi: 10.2144/01304pf02. [DOI] [PubMed] [Google Scholar]
- 63.Ramsköld D, Luo S, Wang Y-C, Li R, Deng Q, et al. Full-length mRNA-seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 2012;30(8):777–782. doi: 10.1038/nbt.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinkle D, Glanzer J, Sarabi A, Pajunen T, Zielinski J, et al. Single neurons as experimental systems in molecular biology. Prog. Neurobiol. 2004;72(2):129–142. doi: 10.1016/j.pneurobio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Pan X, Durrett RE, Zhu H, Tanaka Y, Li Y, et al. Two methods for full-length RNA sequencing for low quantities of cells and single cells. Proc. Natl. Acad. Sci. USA. 2013;110(2):594–599. doi: 10.1073/pnas.1217322109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snijder B, Sacher R, Rämö P, Liberali P, Mench K, et al. Single-cell analysis of population context advances RNAi screening at multiple levels. Mol. Syst. Biol. 2012;8(1):579. doi: 10.1038/msb.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hebenstreit D. Methods, challenges and potentials of single cell RNA-seq. Biology. 2012;1(3):658–667. doi: 10.3390/biology1030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Islam S, Kjällquist U, Moliner A, Zajac P, Fan JB, et al. Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Res. 2011;21(7):1160–1167. doi: 10.1101/gr.110882.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levsky JM, Shenoy SM, Pezo RC, Singer RH. Single-cell gene expression profiling. Science. 2002;297(5582):836–840. doi: 10.1126/science.1072241. [DOI] [PubMed] [Google Scholar]
- 70.Islam S, Kjällquist U, Moliner A, Zajac P, Fan JB, et al. Highly multiplexed and strand-specific single-cell RNA 5′ end sequencing. Nat. Protoc. 2012;7(5):813–828. doi: 10.1038/nprot.2012.022. [DOI] [PubMed] [Google Scholar]
- 71.Shalek AK, Satija R, Adiconis X, Gertner RS, Gaublomme JT, et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature. 2013;498:236–240. doi: 10.1038/nature12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le TT, Harlepp S, Guet CC, Dittmar K, Emonet T, et al. Real-time RNA profiling within a single bacterium. Proc. Natl. Acad. Sci. USA. 2005;102(26):9160–9164. doi: 10.1073/pnas.0503311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nat. Protoc. 2006;1(5):2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 74.Hashimshony T, Wagner F, Sher N, Yanai I. CEL-seq: single-cell RNA-seq by multiplexed linear amplification. Cell Rep. 2012;2:666–673. doi: 10.1016/j.celrep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Amantonico A, Urban PL, Zenobi R. Analytical techniques for single-cell metabolomics: state of the art and trends. Anal. Bioanal. Chem. 2010;398(6):2493–2504. doi: 10.1007/s00216-010-3850-1. [DOI] [PubMed] [Google Scholar]
- 76.Klepárník K, Foret F. Recent advances in the development of single cell analysis—a review. Anal. Chim. Acta. 2013;800:12–21. doi: 10.1016/j.aca.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 77.Wu M, Singh AK. Single-cell protein analysis. Curr. Opin. Biotechnol. 2012;23(1):83–88. doi: 10.1016/j.copbio.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ni Q, Titov DV, Zhang J. Analyzing protein kinase dynamics in living cells with FRET reporters. Methods. 2006;40(3):279–286. doi: 10.1016/j.ymeth.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 79.Bendall SC, Simonds EF, Qiu P, Amir ED, Krutzik PO, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332(6030):687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Urban PL, Amantonico A, Zenobi R. Lab-on-a-plate: extending the functionality of MALDI-MS and LDI-MS targets. Mass Spectrom. Rev. 2011;30(3):435–478. doi: 10.1002/mas.20288. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H, Jin W, et al. Single-cell analysis by intracellular immuno-reaction and capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. A. 2006;1104(1):346–351. doi: 10.1016/j.chroma.2005.11.083. [DOI] [PubMed] [Google Scholar]
- 82.Rubakhin SS, Romanova EV, Nemes P, Sweedler JV. Profiling metabolites and peptides in single cells. Nat. Methods. 2011;8(4s):S20–S29. doi: 10.1038/nmeth.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rubakhin SS, Sweedler JV. Quantitative measurements of cell-cell signaling peptides with single-cell MALDI MS. Anal. Chem. 2008;80(18):7128–7136. doi: 10.1021/ac8010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lorey DR, Morrison GH, Chandra S. Dynamic secondary ion mass spectrometry analysis of boron from boron neutron capture therapy drugs in co-cultures: single-cell imaging of two different cell types within the same ion microscopy field of imaging. Anal. Chem. 2001;73(16):3947–3953. doi: 10.1021/ac0103266. [DOI] [PubMed] [Google Scholar]
- 85.Chandra S, Lorey DR., 2nd SIMS ion microscopy in cancer research: single cell isotopic imaging for chemical composition, cytotoxicity and cell cycle recognition. Cell. Mol. Biol. 2001;47(3):503–518. [PubMed] [Google Scholar]
- 86.Kulp KS, Berman ES, Knize MG, Shattuck DL, Nelson EJ, et al. Chemical and biological differentiation of three human breast cancer cell types using time-of-flight secondary ion mass spectrometry. Anal. Chem. 2006;78(11):3651–3658. doi: 10.1021/ac060054c. [DOI] [PubMed] [Google Scholar]
- 87.Passarelli MK, Ewing AG. Single-cell imaging mass spectrometry. Curr. Opin. Chem. Biol. 2013;17:854–859. doi: 10.1016/j.cbpa.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El-Ali J, Sorger PK, Jensen KF. Cells on chips. Nature. 2006;442(7101):403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 89.Joensson HN, Andersson Svahn H. Droplet microfluidics—a tool for single-cell analysis. Angew. Chem. Int. Ed. 2012;51(49):12176–1292. doi: 10.1002/anie.201200460. [DOI] [PubMed] [Google Scholar]
- 90.Guo MT, Rotem A, Heyman JA, Weitz DA. Droplet microfluidics for high-throughput biological assays. Lab Chip. 2012;12(12):2146–2155. doi: 10.1039/c2lc21147e. [DOI] [PubMed] [Google Scholar]
- 91.Tajiri K, Kishi H, Ozawa T, Sugiyama T, Muraguchi A. SFMAC: a novel method for analyzing multiple parameters on lymphocytes with a single fluorophore in cell-microarray system. Cytometry. 2009;75A(4):282–288. doi: 10.1002/cyto.a.20675. [DOI] [PubMed] [Google Scholar]
- 92.Roach KL, King KR, Uygun BE, Kohane IS, Yarmush ML, Toner M. High throughput single cell bioinformatics. Biotechnol. Prog. 2009;25(6):1772–1779. doi: 10.1002/btpr.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindström S, Andersson-Svahn H. Miniaturization of biological assays—overview on microwell devices for single-cell analyses. Biochim. Biophys. Acta. 2011;1810(3):308–316. doi: 10.1016/j.bbagen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Asphahani F, Wang K, Thein M, Veiseh O, Yung S, et al. Single-cell bioelectrical impedance platform for monitoring cellular response to drug treatment. Phys. Biol. 2011;8(1):015006. doi: 10.1088/1478-3975/8/1/015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kinoshita K, Ozawa T, Tajiri K, Kadowaki S, Kishi H, Muraguchi A. Identification of antigen-specific B cells by concurrent monitoring of intracellular Ca2+ mobilization and antigen binding with microwell array chip system equipped with a CCD imager. Cytometry. 2009;75A(8):682–687. doi: 10.1002/cyto.a.20758. [DOI] [PubMed] [Google Scholar]
- 96.Jin A, Ozawa T, Tajiri K, Obata T, Kondo S, et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody–secreting cells from human peripheral blood. Nat. Med. 2009;15(9):1088–1092. doi: 10.1038/nm.1966. [DOI] [PubMed] [Google Scholar]
- 97.Zhu H, Stybayeva G, Silangcruz J, Yan J, Ramanculov E, et al. Detecting cytokine release from single T-cells. Anal. Chem. 2009;81(19):8150–8156. doi: 10.1021/ac901390j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Love JC, Ronan JL, Grotenbreg GM, van der Veen AG, Ploegh HL. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat. Biotechnol. 2006;24(6):703–707. doi: 10.1038/nbt1210. [DOI] [PubMed] [Google Scholar]
- 99.Han Q, Bradshaw EM, Nilsson B, Hafler DA, Love JC. Multidimensional analysis of the frequencies and rates of cytokine secretion from single cells by quantitative microengraving. Lab Chip. 2010;10(11):1391. doi: 10.1039/b926849a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han Q, Bagheri N, Bradshaw EM, Hafler DA, Lauffenburger DA, Love JC. From the cover: Polyfunctional responses by human T cells result from sequential release of cytokines. Proc. Natl. Acad. Sci. USA. 2012;109(5):1607–1612. doi: 10.1073/pnas.1117194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rettig JR, Folch A. Large-scale single-cell trapping and imaging using microwell arrays. Anal. Chem. 2005;77(17):5628–5634. doi: 10.1021/ac0505977. [DOI] [PubMed] [Google Scholar]
- 102.Choi JH, Ogunniyi AO, Du M, Du M, Kretschmann M, et al. Development and optimization of a process for automated recovery of single cells identified by microengraving. Biotechnol. Prog. 2010;26(3):888–895. doi: 10.1002/btpr.374. [DOI] [PubMed] [Google Scholar]
- 103.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA. 2006;103(8):2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.El Debs B, Utharala R, Balyasnikova IV, Griffiths AD, Merten CA. Functional single-cell hybridoma screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. USA. 2012;109(29):11570–11575. doi: 10.1073/pnas.1204514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park JY, Morgan M, Sachs AN, Samorezov J, Teller R, et al. Single cell trapping in larger microwells capable of supporting cell spreading and proliferation. Microfluid. Nanofluidics. 2010;8(2):263–268. doi: 10.1007/s10404-009-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 107.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 108.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 109.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 110.Lutolf MP, Doyonnas R, Havenstrite K, Koleckar K, Blau HM. Perturbation of single hematopoietic stem cell fates in artificial niches. Integr. Biol. 2009;1(1):59–69. doi: 10.1039/b815718a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gilbert P, Havenstrite K, Magnusson K, Sacco A, Leonardi N, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Toh Y-C, Blagović K, Voldman J. Advancing stem cell research with microtechnologies: opportunities and challenges. Integr. Biol. 2010;2(7–8):305–325. doi: 10.1039/c0ib00004c. [DOI] [PubMed] [Google Scholar]
- 113.Collins JM, Nettikadan S. Subcellular scaled multiplexed protein patterns for single cell cocultures. Anal. Biochem. 2011;419(2):339–341. doi: 10.1016/j.ab.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 114.Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods. 2011;8(11):949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 115.Doh J, Kim M, Krummel MF. Cell-laden microwells for the study of multicellularity in lymphocyte fate decisions. Biomaterials. 2010;31(12):3422–3428. doi: 10.1016/j.biomaterials.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 116.Rosenthal A, Macdonald A, Voldman J. Cell patterning chip for controlling the stem cell microenvironment. Biomaterials. 2007;28(21):3208–3216. doi: 10.1016/j.biomaterials.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andreasson-Ochsner M, Romano G, Håkanson M, Smith ML, Leckband DE, et al. Single cell 3-D platform to study ligand mobility in cell-cell contact. Lab Chip. 2011;11(17):2876–2883. doi: 10.1039/c1lc20067d. [DOI] [PubMed] [Google Scholar]
- 118.Torres AJ, Contento RL, Gordo S, Wucherpfennig KW, Love JC. Functional single-cell analysis of T-cell activation by supported lipid bilayer-tethered ligands on arrays of nanowells. Lab Chip. 2013;13(1):90–99. doi: 10.1039/c2lc40869d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Front Immunol. 2012;3:335. doi: 10.3389/fimmu.2012.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lopez JA, Jenkins MR, Rudd-Schmidt JA, Brennan AJ, Danne JC, et al. Rapid and unidirectional perforin pore delivery at the cytotoxic immune synapse. J. Immunol. 2013;191(5):2328–2334. doi: 10.4049/jimmunol.1301205. [DOI] [PubMed] [Google Scholar]
- 121.Néron S, Suck G, Ma X-Z, Sakac D, Roy A, et al. B cell proliferation following CD40 stimulation results in the expression and activation of Src protein tyrosine kinase. Int. Immunol. 2006;18(2):375–387. doi: 10.1093/intimm/dxh377. [DOI] [PubMed] [Google Scholar]
- 122.Selimkhanov J, Hasty J, Tsimring LS. Recent advances in single-cell studies of gene regulation. Curr. Opin. Biotechnol. 2012;23(1):34–40. doi: 10.1016/j.copbio.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wlodkowic D, Cooper JM. Tumors on chips: oncology meets microfluidics. Curr. Opin. Chem. Biol. 2010;14(5):556–567. doi: 10.1016/j.cbpa.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 124.Lindström S, Andersson-Svahn H. Overview of single-cell analyses: microdevices and applications. Lab Chip. 2010;10(24):3363–3372. doi: 10.1039/c0lc00150c. [DOI] [PubMed] [Google Scholar]
- 125.Charnley M, Textor M, Khademhosseini A, Lutolf MP. Integration column: microwell arrays for mammalian cell culture. Integr. Biol. 2009;1(11–12):625. doi: 10.1039/b918172p. [DOI] [PubMed] [Google Scholar]
- 126.Lindström S, Larsson R, Andersson Svahn H. Towards high-throughput single cell/clone cultivation and analysis. Electrophoresis. 2008;29(6):1219–1227. doi: 10.1002/elps.200700536. [DOI] [PubMed] [Google Scholar]
- 127.Lindström S, Hammond M, Brismar H, Andersson-Svahn H, Ahmadian A. PCR amplification and genetic analysis in a microwell cell culturing chip. Lab Chip. 2009;9(24):3465–3471. doi: 10.1039/b912596e. [DOI] [PubMed] [Google Scholar]
- 128.Wakamoto Y, Ramsden J, Yasuda K. Single-cell growth and division dynamics showing epigenetic correlations. Analyst. 2005;130(3):311–317. doi: 10.1039/b409860a. [DOI] [PubMed] [Google Scholar]
- 129.Yamamura S, Yatsushiro S, Yamaguchi Y, Abe K, Shinohara Y, et al. Accurate detection of carcinoma cells by use of a cell microarray chip. PLoS ONE. 2012;7(3):e32370. doi: 10.1371/journal.pone.0032370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yasuda K. On-chip single-cell-based microcultivation method for analysis of genetic information and epigenetic correlation of cells. J. Mol. Recognit. 2004;17(3):186–193. doi: 10.1002/jmr.672. [DOI] [PubMed] [Google Scholar]
- 131.Zaretsky I, Polonsky M, Shifrut E, Reich-Zeliger S, Antebi Y, et al. Monitoring the dynamics of primary T cell activation and differentiation using long term live cell imaging in microwell arrays. Lab Chip. 2012;12(23):5007–5015. doi: 10.1039/c2lc40808b. [DOI] [PubMed] [Google Scholar]
- 132.Yamanaka YJ, Berger CT, Sips M, Cheney PC, Alter G, Love JC. Single-cell analysis of the dynamics and functional outcomes of interactions between human natural killer cells and target cells. Integr. Biol. 2012;4(10):1175–1184. doi: 10.1039/c2ib20167d. [DOI] [PubMed] [Google Scholar]
- 133.Varadarajan N, Julg B, Yamanaka YJ, Chen H, Ogunniyi AO, et al. A high-throughput single-cell analysis of human CD8+ T cell functions reveals discordance for cytokine secretion and cytolysis. J. Clin. Investig. 2011;121(11):4322–4331. doi: 10.1172/JCI58653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guldevall K, Vanherberghen B, Frisk T, Hurtig J, Christakou AE, et al. Imaging immune surveillance of individual natural killer cells confined in microwell arrays. PLoS ONE. 2010;5(11):e15453. doi: 10.1371/journal.pone.0015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J. Allergy Clin. Immunol. 2013;132(3):536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]