Abstract
Background
The European Carotid Surgery Trial (ECST) risk model is a validated tool for predicting cerebrovascular risk in patients with symptomatic carotid disease. Carotid plaque hemorrhage as detected by MRI (MRIPH) and microembolic signals (MES) detected by transcranial Doppler (TCD) are 2 emerging modalities in assessing instability of the carotid plaque. The aim of this study was to assess the strength of association of MES and MRIPH with cerebrovascular recurrence in patients with symptomatic carotid artery disease in comparison with the ECST risk prediction model.
Methods and Results
One hundred and thirty‐four prospectively recruited patients (mean [SD]: age 72 [9.8] years, 33% female) with symptomatic severe (50% to 99%) carotid stenosis underwent preoperative TCD, MRI of the carotid arteries to assess MES, PH, and the ECST risk model. Patients were followed up until carotid endarterectomy, recurrent cerebral event, death, or study end. Event‐free survival analysis was done using backward conditional Cox regression analysis.
Of the 123 patients who had both TCD and MRI, 82 (66.7%) demonstrated PH and 46 (37.4%) had MES. 37 (30.1%) cerebrovascular events (21 transient ischemic attacks, 6 amaurosis fugax, and 10 strokes) were observed. Both carotid PH (HR=8.68; 95% CI 2.66 to 28.40, P<0.001) as well as MES (HR=3.28; 95% CI 1.68 to 6.42, P=0.001) were associated with cerebrovascular event recurrence. Combining MES and MRIPH improved the strength of association (HR=0.74, 95% CI 0.65 to 0.83; P<0.001). The ECST risk model was not associated with recurrence (HR=0.86; 95% CI 0.45 to 1.65; P=0.65).
Conclusions
The presence of carotid plaque hemorrhage is better associated with recurrent cerebrovascular events in patients with symptomatic severe carotid stenosis than the presence of microembolic signals; combining MES and MRIPH, further improves the association while the ECST risk score was insignificant.
Keywords: carotid arteries, cerebrovascular embolism, magnetic resonance imaging, relative risk, stroke
Introduction
Randomized controlled trials have demonstrated the efficacy of performing early carotid endarterectomy in patients with symptomatic carotid disease.1 However, a significant proportion of patients with symptomatic moderate and high‐grade carotid disease will not have a recurrent cerebrovascular event if treated medically.1 The determination of individual patient risk remains challenging2 but would be a prerequisite for rational selection of best therapy (medical or surgical) to prevent stroke.
Over the past decade multiple biomarkers and subsequent models have been developed to assess the risk of stroke in patients with carotid disease. The European Carotid Surgery Trial (ECST) risk model is a validated model that predicts the absolute risk of ipsilateral stroke in recently symptomatic patients with 50% to 99% carotid stenosis who were treated in the medical arm of the ECST study.2 The ECST risk model is derived from a Cox model comprised of clinical information (sex, age, type, and timing of event) as well as a measure of carotid plaque morphology (irregularity) and degree of stenosis.
An emerging noninvasive technique to assess carotid plaque activity is the detection of circulating microembolic signals (MES) by the use of transcranial Doppler (TCD).3–4 In observational studies, detection of spontaneous microembolic signals (MES) has been shown to be associated with stroke recurrence in symptomatic carotid disease as well as stroke in asymptomatic carotid disease.4 Carotid plaque hemorrhage detected by magnetic resonance imaging (MRIPH) is a simple noninvasive MRI technique that can detect the complicated carotid plaque5 and has been shown to be associated with embolization6–7 and recurrent cerebrovascular events in patients with carotid disease.8–11 However, it is unclear whether the relationship between MES and MRIPH and recurrence is additive.
The aims of this study were to determine whether MES and PH are associated with recurrent cerebrovascular events in symptomatic carotid artery disease.
Methods
Patients
The study population is comprised of patients recruited in 2 prospective single‐center studies that were undertaken between 2003 and 2009 in Nottingham University Hospitals. Previous published data includes the first 47 patients in this population that described the association between MRIPH and MES7 as well 63 patients who underwent preliminary follow‐up studies.9 Two recent publications, including one that assessed for the mediator effect of sex differences of MRIPH on recurrent events12 and another that assessed the relationship between MRIPH and recurrent cerebrovascular events,13 used the follow‐up and events from this database.
The study was approved by an institutional review committee and all subjects gave informed consent. All recruited patients were symptomatic in the preceding 6 months with amaurosis fugax, hemispheric TIA, or stroke clinically localized to the carotid artery territory and confirmed by a neurologist or a stroke physician. Patients had ipsilateral internal carotid artery stenosis >60% by the North American Symptomatic Carotid Endarterectomy Trial criteria using carotid duplex ultrasound. Best medical treatment and appropriate surgical intervention were offered to all patients for secondary prevention according to national and local standard guidelines at the time, and none of the treatments were altered or delayed for the purpose of the studies.
Patients were excluded if they were claustrophobic, or had any other contraindications to MRI and if there was an inadequate temporal bone window for TCD.
Two hundred and twenty‐five symptomatic patients were assessed for carotid endarterectomy with >60% stenosis between December 2003 and September 2009 (170 in one study between December 2003 and January 2004; and 55 in the other study between November 2007 and September 2009). There were 17 patients who declined to participate in the studies; 25 claustrophobic to MRI; 35 patients who presented to the surgeon 6 months after the presenting event; and in 14 patients we were unable to perform a MRI in time before carotid endarterectomy. Therefore, 134 patients were recruited into the studies.
MRI
Carotid MRI was performed on one of the three 1.5 T scanners: Vision (Siemens Medical), Intera (Philips), or Signa (General Electric) using standard receive‐only quadrature neck array coils, as previously described. All patients underwent a coronal T1‐weighted 3‐dimensional gradient echo sequence with effective blood nulling and fat suppression due to selective water‐excitation (TR 10.3 ms, TE 4.0 ms, FA 15, TI 20 ms, FOV 350×300 mm, matrix 256×140, 140 partitions, volume thickness 120 to 150 mm). The acquisition took <5 minutes.
Image Analysis
As previously described, image analysis was performed using standard image reconstruction techniques as provided by JAVA Imaging (JIM) software.7 Presence of carotid PH was determined by 2 trained researchers (N.A.; N.K.) and adjudicated by an experienced Neuroradiologist (DPA), blinded to the clinical data. Using previously published criteria, plaque hemorrhage was present if the intensity of the signal in the carotid plaque was greater than 50% of the intensity in the adjacent sternocleidomastoid muscle. We have previously demonstrated a high inter‐ and intra‐rater correlation using this method.7 Plaques with plaque hemorrhage are denoted as MRIPH+ and without plaque hemorrhage as MRIPH−.
TCD
Using a pulsed Doppler device (EmboDop; Compumedics DWL, Singen, Germany/Doppler box; DWL, Germany), the middle cerebral artery, ipsilateral to the symptomatic carotid artery, was insonated by 2 authors (N.A., N.K.). MES were monitored for 1 hour after MR imaging. A sample volume of 8 mm and a low gain provided a setting optimal from discriminating emboli from the background settings.14
The recordings were continuously analyzed both online and offline for the presence of MES, by investigators blinded to the status of the MRIPH signal. MES were identified as emboli using established criteria. MES were identified as high‐intensity signals (HITS) that were unidirectional within the velocity spectrum, had intensity >7 dB above the background spectrum, lasted <300 ms, and were associated with a characteristic chirping sound.14
Plaques were considered to be MES+ if 1 or more MES were detected in the ipsilateral middle cerebral artery during the hour‐long recording.
Follow‐Up
The clinical assessments for any cerebrovascular ischemic event (stroke, TIA, or AmF) were recorded at the entry to the study. All patients were followed‐up until the primary endpoint (ipsilateral ischaemic symptom), carotid endarterectomy, and death until October 2011. All recurrent cerebrovascular ischemic events were verified by review of clinical details, and all strokes were confirmed as ischemic by neuroimaging.
ECST Risk Model
This is a validated model that has been derived from patients randomized from the ECST.2 It is comprised of 4 clinical variables (age, sex, presentation type, time since last event) and 2 imaging characteristics (degree of stenosis and type of plaque—smooth/irregular).2 As all patients did not have any formal angiograms, as in the ECST study, the surface of the carotid plaque was commented upon by ultrasound. The ECST model results were further categorized in 2 groups: (1) <30% risk at 5 years and (2) >30% recurrence risk at 5 years.
Statistical Analysis
Analysis was performed using SPSS 18 and STATA 11. A χ2 test was used to assess the association between MRIPH and MES presence and to assess the categorical risk factors differences between patients with MRIPH and MES presence and absence, respectively.
Kaplan–Meier curves for the time from symptom to recurrent event were generated for the 4 groups classified by MRIPH and MES: (1) MES−, MRIPH+; (2) MES+, MRIPH−; (3) MES−, MRIPH+; (4) MES+, MRIPH+). The log‐rank test was used to test the differences between the groups.
A univariate Cox regression analysis was performed to explore the association between factors and cerebrovascular event recurrence. Furthermore, adjusted Cox regression analysis was undertaken to account for age and sex as potential confounders. A backward conditional Cox regression analysis was performed to assess the factors associated with recurrence. Factors included were known factors that have been previously shown to be risk factors for recurrence (grade of stenosis, age, sex, time to event) and significant factors derived from the univariate analysis in this study and for the factor to remain in the model when the associated P value <0.1 (MRIPH+, MES+, smoking; Table 2).
Table 2.
Table Demonstrating the Number of Patients at Risk in the Study Period
| Days | Numbers at Risk |
|---|---|
| 0 | 123 |
| 30 | 71 |
| 60 | 44 |
| 90 | 29 |
| 500 | 12 |
| 1000 | 6 |
| 1500 | 4 |
| 1831 | 1 |
To assess whether adding MES to MRIPH improved the model fit we compared the −2 log likelihood values for Cox regression models derived from the backward conditional analysis (MES+, MRIPH+, sex, smoking) with MES removed from the model using a χ2 test. Similarly, to test whether adding MRIPH improved the model fit, the derived model was compared with MRIPH removed from the model.
To compare the models with different predictor variables, the goodness of fit was assessed using the Akaike Information Criterion (AIC). The most appropriate model is the one that minimizes AIC.
Discrimination and calibration methods were used to demonstrate the performance of the 4 models based on (1) ECST scores, (2) presence of MRIPH, (3) MES, and (4) combined MRIPH/MES/sex/smoking. Discrimination is the ability to estimate the probability of assigning a higher risk to those who develop cerebrovascular events compared with those who do not. This is quantified with the calculation of the area under the receiver operating characteristic curve (ROC); where the value 1 represents perfect discrimination. Calibration measures how closely the predicted cerebrovascular risk agrees with our observed outcome (actual cerebrovascular event). This was assessed for each 10th predicted risk score and visually presented by plotting the predicted probability versus observed proportions.
Results
Of the 134 patients recruited, 11 (8.2%) patients did not have an adequate temporal window for TCD. One hundred twenty‐three patients (mean [SD]: age 72[10] years) with moderate or high‐grade stenosis were followed up for a median of 36 days (IQR 15 to 87) after the index cerebrovascular event. The basic demographics are shown in Table 1.
Table 1.
Demographics of the Patient Population
| ALL | MR Status | MES Status | |||||
|---|---|---|---|---|---|---|---|
| MRIPH+ 82 (67%) | MRIPH−41 (33%) | P Value | MES+ 46 (37%) | MES− 77 (63%) | P Value | ||
| Age, mean years±SD | 72±10 | 72.2 (8.8) | 70.3 (9.4) | 0.31 | 73.0 (7.4) | 70.8 (10.7) | 0.19 |
| Sex—female, % | 38 (31) | 21 (26) | 17 (34) | 0.09 | 11 (24) | 27 (35) | 0.23 |
| Hypertension, n (%) | 95 (77) | 65 (79) | 30 (73) | 0.45 | 37 (80) | 58 (75) | 0.60 |
| Diabetes mellitus, n (%) | 16 (8) | 7 (9) | 3 (7) | 0.81 | 5 (12) | 5 (7) | 0.51 |
| Smoker, n (%) | 69 (56) | 45 (55) | 24 (57) | 0.70 | 24 (52) | 45 (58) | 0.53 |
| Presenting complaint | |||||||
| AmF | 30 (24) | 17 (21) | 13 (32) | 0.39 | 7 (15) | 23 (30) | 0.09 |
| TIA | 51 (42) | 35 (43) | 16 (39) | 16 (35) | 35 (45) | ||
| Stroke | 42 (34) | 30 (37) | 12 (29) | 23 (50) | 19 (25) | ||
| Degree of carotid stenosis | |||||||
| 50% to 69% | 26 (21) | 17 (21) | 9 (22) | 0.87 | 11 (24) | 15 (20) | 0.73 |
| 70% to 99% | 97 (79) | 65 (79) | 32 (79) | 35 (76) | 62 (80) | ||
| Time from presenting symptom and MRI—median (IQR) | 33 (16 to 97) | 31.5 (15 to 78) | 41.0 (22 to 81) | 0.19 | 28 (15 to 72) | 36 (17 to 82) | 0.17 |
Amf indicates amaurosis fugax; IQR, inerquartile range; MES, microembolic signals; MR, magnetic resonance; MRI, magnetic resonance imaging; MRIPH, plaque hemorrhage detected by magnetic resonance imaging; Tia, transient ischemic attack.
Twenty (16.3%) patients did not have a carotid endarterectomy for the following reasons: 10 were unfit, 3 had large strokes, and 1 occluded while waiting for the carotid endarterectomy, 3 did not want an operation, 2 had atrial fibrillation in addition to carotid stenosis and because of significant co‐morbidities were treated medically with anticoagulation; and 1 presented 6 months after the initial event thus meeting an exclusion criteria of the study. Ninety‐five percent of the patients who underwent a carotid endarterectomy had one within 3 months of presentation.
There were no significant differences with respect to age and other risk factors in patients with and without MRIPH. Similarly, there were no differences in patients with and without MES (Table 1).
Of the 123 patients, 46 (37.4%) patients exhibited ipsilateral MES and 82 (66.7%) of the ipsilateral carotid plaques were MRIPH+. There was an association between MRIPH and MES (χ2=6.2, P=0.01).
There were 37 (30.1%) recurrent cerebrovascular events (21 TIA, 6 amaurosis fugax, and 10 strokes) in the follow up period. Of the patients who had strokes, 7 patients had mild strokes (Rankin scores <3) and 3 patients had severe strokes (Rankin scores 4 and 5). Twenty‐one (56.8%) and 34 (91.9%) of the patients who developed recurrent events had MES and MRIPH+ carotid plaques, respectively.
Among the 123 patients, the risk of recurrence was identified to be highest when patients had both MRIPH and MES, and lowest when patients had neither MRIPH nor MES (Kaplan‐Meier log rank statistic 33, P<0.001) (Figure 1, Table 2).
Figure 1.
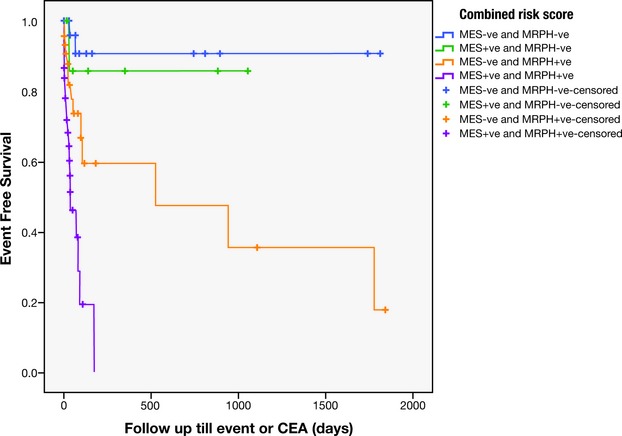
Kaplan‐Meier showing the recurrence rates in the 4 groups of patients: (1) MES −ve and MRIPH−ve; (2) MES+, and MRIPH−; (3) MES−ve and MRIPH +ve, and (4) MES+ve and MRIPH+ve. CEA indicates carotid endarterectomy; MES, microembolic signals; MRIPH, plaque hemorrhage detected by magnetic resonance imaging.
Using univariate Cox regression analysis, MRIPH was associated with recurrence (HR=8.7; 95% CI 2.7 to 28.4, P<0.001) as did MES, although to a lesser extent (HR=3.3; 95% CI 1.7 to 6.4, P=0.001). Table 3 shows the association between unadjusted as well as adjusted (age and sex) risk factors and recurrence using Cox regression analysis.
Table 3.
Unadjusted and Adjusted (for Sex and Age) Cox Regression Demonstrating Factors Effecting Recurrence
| Risk Factor | Unadjusted Hazard Ratios (95% CI) | P Value | Adjusted Hazard Ratios (95% CI)* | P Value* |
|---|---|---|---|---|
| Sex (female) | 0.74 (0.35 to 1.56) | 0.423 | ||
| Age, y | 0.99 (0.96 to 1.03) | 0.735 | ||
| Hypertension (present) | 1.34 (0.58 to 3.09) | 0.489 | 1.35 (0.58 to 3.10) | 0.485 |
| Ischemic heart disease (present) | 0.67 (0.32 to 1.40) | 0.291 | 0.66 (0.31 to 1.41) | 0.283 |
| Diabetes mellitus (present) | 0.29 (0.04 to 2.09) | 0.216 | 0.26 (0.04 to 1.94) | 0.191 |
| Smoking (present) | 2.11 (1.01 to 4.41) | 0.047 | 2.16 (0.97 to 4.80) | 0.058 |
| Degree of carotid stenosis | 1.06 (0.75 to 1.49) | 0.744 | 1.06 (0.76 to 1.49) | 0.726 |
| Time from symptom to MRI | 1.00 (0.99 to 1.01) | 0.901 | 1.00 (0.99 to 1.01) | 0.812 |
| MES presence | 3.28 (1.68 to 6.42) | 0.001 | 3.35 (1.69 to 6.67) | 0.001 |
| MRIPH presence | 8.68 (2.66 to 28.40) | <0.001 | 9.25 (2.78 to 30.83) | <0.001 |
CI indicates confidence interval; MES, microembolic signals; MRI, magnetic resonance imaging; MRIPH, plaque hemorrhage detected by magnetic resonance imaging.
Adjusted for age and sex.
On performing backward conditional analysis, 4 factors were retained in the model (MES, MRIPH, sex, and smoking) given that P value <0.1 which were associated with recurrence. As shown in Table 3, sensitivity analysis using −2 log likelihood values with Cox regression and AIC showed that removing MES (loss χ2=9.32, P=0.02; AIC=276.12) and removing MRIPH (loss χ2=23.8, P<0.0001; AIC=290.25) from the combined model worsened the model, indicating that MRIPH and MES were associated with an increased cerebrovascular ischemic event risk. Combining MES and MRIPH improved the model (AIC=268.8, Table 4).
Table 4.
Sensitivity Analysis of MES and MRIPH Presence/Absence—Goodness of Fit
| Risk Factor | Full Model: Hazard Ratios (95% CI) | Model Without MES: Hazard Ratios (95% CI) | Model Without MRIPH: Hazard Ratios (95% CI) |
|---|---|---|---|
| Sex (female) | 2.39 (0.95 to 6.01) | 2.25 (0.88 to 5.77) | 1.18 (0.52 to 2.70) |
| Smoking (present) | 3.17 (1.32 to 7.62)* | 3.37 (1.38 to 8.25)* | 2.21 (1.01 to 4.83)* |
| MES presence | 2.91 (1.45 to 5.84)* | — | 3.36 (1.69 to 6.67)* |
| MRIPH presence | 10.98 (3.12 to 38.64)* | 12.67 (3.55 to 45.28)* | — |
| Goodness of fit (AIC) | 268.80 | 276.12 | 290.25 |
AIC indicates Akaike information criterion; CI, confidence interval; MES, microembolic signals; MRIPH, plaque haemorrhage detected by magnetic resonance imaging.
P value<0.05.
Information to complete all the variables for the ECST 2 group model was present in 123 patients. The ECST risk model was not associated with the recurrence in this population of patients (HR=0.868; 95% CI 0.45 to 1.65; P=0.65).
The performance and discriminative ability was highest for the combined MRIPH and MES risk score associated with the risk of recurrence (area under the curve=0.743; 95% CI 0.652 to 0.833; P<0.001), followed by presence of MRIPH alone (area under the curve=0.680; 95% CI 0.585 to 0.766; P=0.002) and MES (area under the curve=0.638; 95% CI 0.529 to 0.748; P=0.015). On the other hand, the performance and discriminative ability associated with ECST was very low and nonsignificant (area under the curve=0.470; 95% CI 0.358 to 0.582; P=0.598) (Figure 2).
Figure 2.
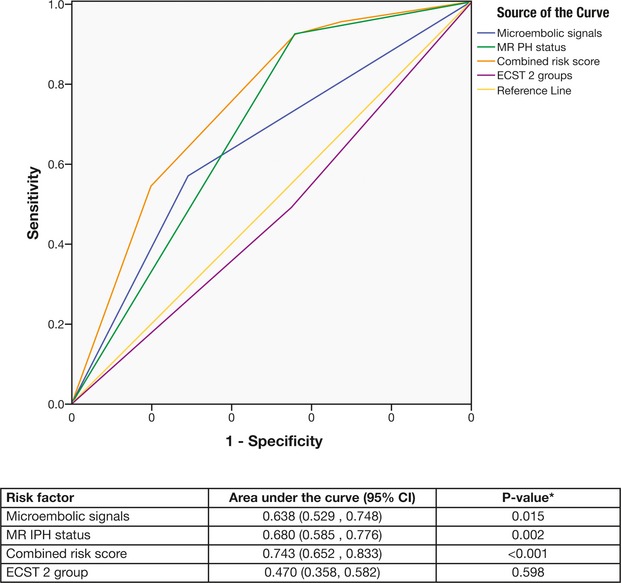
Receiver operating characteristic curves (ROC) for each risk factor. CI indicates confidence interval; ECST, European Carotid Surgery Trial; MRIPH, plaque haemorrhage detected by magnetic resonance imaging.
Figure 3 displays the agreement between mean predicted risk and mean observed risk by 10th of predicted risk for our full model and ECST 2 group model. Both models have a tendency to overestimate.
Figure 3.
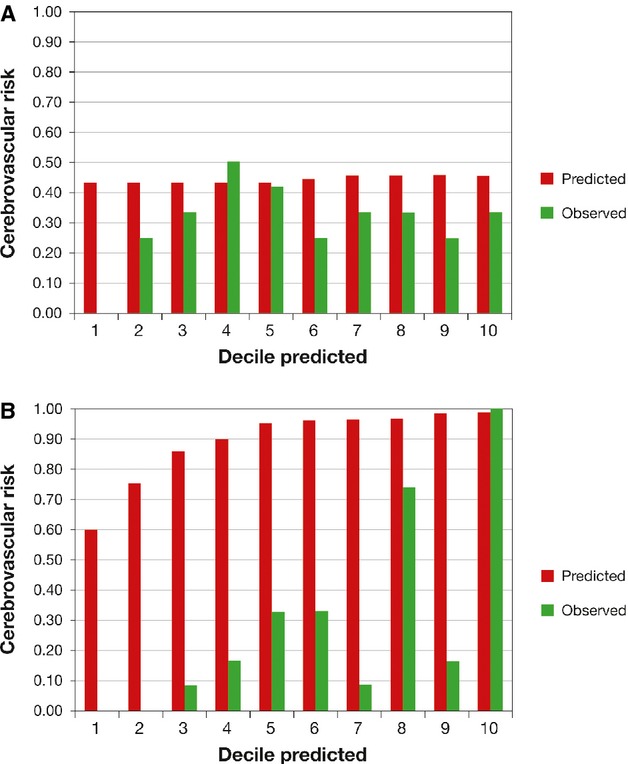
Calibration—predicted and actual number of cerebrovascular events by decile of predicted risk. ECST indicates European Carotid Surgery Trial; MES, microembolic signals; MRIPH, plaque hemorrhage detected by magnetic resonance imaging.
Discussion
This study assessed 2 new markers of carotid plaque instability, carotid plaque hemorrhage and microembolic signals, compared with the established ECST risk score. We demonstrated that MRIPH and MES, but not the ECST risk score were associated with recurrent cerebrovascular events in patients with symptomatic carotid stenosis.
Carotid plaque hemorrhage as detected by MRI has been previously shown to be associated with an increased risk of stroke/TIA in patients with asymptomatic and symptomatic carotid disease with similar hazard ratios seen in this study.8–11,15 Plaque hemorrhage has been increasingly identified to be an adverse pathological finding that is associated with embolization and carotid plaque instability.6–7 Intraplaque hemorrhage stimulates atherogenic activity by being a source of cholesterol to the necrotic core and inflammatory load.16 MRI studies have shown that plaque hemorrhage is associated with an increased mechanical load and repeated episodes of plaque hemorrhage increases the carotid plaque load.16–17 Due to the simple and noninvasive nature of this MRI sequence, this imaging technique has become incorporated as part of routine clinical assessment of stroke in some units.18–19
Microembolic signals have been previously demonstrated to be associated with carotid plaque instability and a recent meta‐analysis has shown that MES predict recurrent stroke in patients with symptomatic carotid disease.3–4 The association demonstrated between MRIPH presence and MES presence is similar to previous smaller publication, which used half of the patients from the current study.3 After adjusting for vascular risk factors, both of the individual tools demonstrated were associated with cerebrovascular event recurrence. However, the clinical implementation of both these modalities may be difficult. As MRI is being increasingly used in the assessment stroke and carotid disease, the addition of this noninvasive MRI technique can be used clinically without the use of significant resources or cost. There is no requirement of advanced carotid coils and both 1.5 and 3 T MRI scanners can be used.
However, the widespread use of TCD in clinical work is limited by the use of specialized TCD machines and necessary clinical expertise to apply the monitor and subsequently assess for MES. This will increase the upfront cost, but the use of automated microembolic detection software may be useful to further limit the cost in the long term; however, this has not been validated for routine clinical use.
To assess the potential clinical merit of these imaging modalities, we used the ECST risk model to compare the effectiveness of each of these tools in predicting cerebrovascular event recurrence. The ECST risk model performed poorly in predicting recurrence in our population showing no significant association or risk discrimination over the study period. However, the limited performance of ECST may be explained by the fact that the follow‐up period in this study was significantly shorter than what the ECST model2 was validated against. Through our study we were able to observe patients with symptomatic carotid disease in the highest risk period (4 weeks within the initial event) in which over 75% of recurrent events occur.1 Therefore, we were able to observe the majority of the events that would have normally occurred over the time period that the ECST model would have assessed.
We used an ultrasound measure of carotid surface irregularity, rather than angiographic appearances, as in the ECST study.2 Ultrasound assessment is subjective, but a previous study had demonstrated ultrasound‐determined ulceration to be more prevalent in symptomatic patients, with similar sensitivities and specificities in determining ulceration to angiography.20
Just under half of patients with plaque hemorrhage had recurrent cerebrovascular events. We did not discriminate between intraplaque hemorrhage and thrombus, nor did we assess other plaque features such as rupture using the more time‐consuming technique of multisequence MRI.11 In addition, a significant proportion of patients followed up had carotid endarterectomy, which limited the follow‐up of these high‐risk patients. This would decrease the strength of relationship between MRIPH and recurrence. In fact, our calibration analysis highlights that even the best model we derived from combining MRIPH and MES overestimates the risk of recurrence. This is unsurprising given the availability of CEA as an effective preventive treatment and current treatment recommendations for patients who in the majority would not have experienced a stroke over a 5‐year follow‐up.1
Surgical intervention for asymptomatic carotid disease remains controversial. Despite the small absolute benefit from surgery, asymptomatic carotid disease accounts for a significant stroke burden.19 The Asymptomatic Carotid Emboli Study (ACES) demonstrated that asymptomatic embolization identifies the high‐risk patients with asymptomatic carotid disease.4 Smaller MRI studies have also demonstrated that IPH is associated with future TIA/stroke in asymptomatic patients.10,15 However, to demonstrate whether MRIPH is able to predict stroke in this subgroup, a much larger study population would be required.
The prevalence of MES may have been underestimated in this study due to temporal variation of MES presence, with MES most prevalent after the initial event.21 In addition we only used one 1‐hour recording to assess for MES rather than multiple recordings that may have decreased the prevalence further.
The results in this study will need to be interpreted with caution because it was limited by the short follow‐up and the small number of events resulting in wide confidence intervals as well as by the delay in presentation. The marginal discrimination and poor calibration of the joint MES and MRIPH model, in conjunction with the wide confidence intervals in this study, suggests that this model is not an adequate predictive tool over the short observation period chosen. Nevertheless, in the initial recruitment period, there were significant delays between the presenting symptom and CEA, which allowed us to observe patients for longer periods than what would be possible in current practice. A larger similar observational study would thus be impossible in view of current recommendations for early intervention.
In summary, this study suggests that the combination of TCD and MRI may be useful tools in identifying patients with symptomatic carotid disease who are at high risk of stroke or TIA, and also identify patients at low risk in whom surgical intervention may not be beneficial Larger studies in asymptomatic patients and as part of randomized controlled trials in symptomatic patients are warranted to assess the clinical usefulness of this imaging‐based risk model in carotid artery disease.
Sources of Funding
We would like to acknowledge the funders of the study: Vascular Research Fund of Nottingham, Stroke Association, UK, Mason Medical Research Fund, the Special Trustees of Nottingham University Hospitals and the National Institute of Health Research (NIHR).
Disclosures
None.
Acknowledgments
We would like to acknowledge Dr Graham Warren (medical statistician) for statistical advice.
References
- 1.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004; 363:915-924. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. Treating individuals 3: from subgroups to individuals: general principles and the example of carotid endarterectomy. Lancet. 2005; 365:256-265. [DOI] [PubMed] [Google Scholar]
- 3.King A, Markus HS. Doppler embolic signals in cerebrovascular disease and prediction of stroke risk: a systematic review and meta‐analysis. Stroke. 2009; 40:3711-3717. [DOI] [PubMed] [Google Scholar]
- 4.Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S, Bornstein NM, Schaafsma A. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (aces): a prospective observational study. Lancet Neurol. 2010; 9:663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moody AR, Murphy RE, Morgan PS, Martel AL, Delay GS, Allder S, MacSweeney ST, Tennant WG, Gladman J, Lowe J, Hunt BJ. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation. 2003; 107:3047-3052. [DOI] [PubMed] [Google Scholar]
- 6.Altaf N, Beech A, Goode SD, Gladman JR, Moody AR, Auer DP, MacSweeney ST. Carotid intraplaque hemorrhage detected by magnetic resonance imaging predicts embolization during carotid endarterectomy. J Vasc Surg. 2007; 46:31-36. [DOI] [PubMed] [Google Scholar]
- 7.Altaf N, Goode SD, Beech A, Gladman JR, Morgan PS, MacSweeney ST, Auer DP. Plaque hemorrhage is a marker of thromboembolic activity in patients with symptomatic carotid disease. Radiology. 2011; 258:538-545. [DOI] [PubMed] [Google Scholar]
- 8.Altaf N, Daniels L, Morgan PS, Auer D, MacSweeney ST, Moody AR, Gladman JR. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008; 47:337-342. [DOI] [PubMed] [Google Scholar]
- 9.Altaf N, MacSweeney ST, Gladman J, Auer DP. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high‐grade carotid stenosis. Stroke. 2007; 38:1633-1635. [DOI] [PubMed] [Google Scholar]
- 10.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke. 2006; 37:818-823. [DOI] [PubMed] [Google Scholar]
- 11.Kwee RM, van Oostenbrugge RJ, Mess WH, Prins MH, van der Geest RJ, Ter Berg JW, Franke CL, Korten AG, Meems BJ, Van Engelshoven JM, Wildberger JE, Kooi ME. MRI of carotid atherosclerosis to identify tia and stroke patients who are at risk of a recurrence. J Magn Reson Imaging. 2012; 37:1189-1194. [DOI] [PubMed] [Google Scholar]
- 12.Kandiyil N, Altaf N, Hosseini AA, Macsweeney ST, Auer DP. Lower prevalence of carotid plaque hemorrhage in women, and its mediator effect on sex differences in recurrent cerebrovascular events. PLoS One. 2012; 7:e47319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseini AA, Kandiyil N, MacSweeney S, Altaf N, Auer D. Carotid plaque hemorrhage on MRI strongly predicts recurrent ischemia and stroke. Ann Neurol. 2013; 73:774-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consensus committee of the ninth international cerebral hemodynamic symposium. Basic identification criteria of Doppler microembolic signals. Stroke. 1996; 26:1123. [PubMed] [Google Scholar]
- 15.Singh N, Moody AR, Gladstone DJ, Leung G, Ravikumar R, Zhan J, Maggisano R. Moderate carotid artery stenosis: MR imaging‐depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology. 2009; 252:502-508. [DOI] [PubMed] [Google Scholar]
- 16.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003; 349:2316-2325. [DOI] [PubMed] [Google Scholar]
- 17.Kockx MM, Cromheeke KM, Knaapen MW, Bosmans JM, De Meyer GR, Herman AG, Bult H. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2003; 23:440-446. [DOI] [PubMed] [Google Scholar]
- 18.Cheung HM, Moody AR, Singh N, Bitar R, Zhan J, Leung G. Late stage complicated atheroma in low‐grade stenotic carotid disease: MR imaging depiction–prevalence and risk factors. Radiology. 2011; 260:841-847. [DOI] [PubMed] [Google Scholar]
- 19.Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, Pan H, Peto R, Potter J, Rahimi K, Rau A, Robertson S, Streifler J, Thomas D. 10‐year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (acst‐1): a multicentre randomised trial. Lancet. 2010; 376:1074-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furst H, Hartl WH, Jansen I, Liepsch D, Lauterjung L, Schildberg FW. Color‐flow doppler sonography in the identification of ulcerative plaques in patients with high‐grade carotid artery stenosis. AJNR Am J Neuroradiol. 1992; 13:1581-1587. [PMC free article] [PubMed] [Google Scholar]
- 21.Molloy J, Khan N, Markus HS. Temporal variability of asymptomatic embolization in carotid artery stenosis and optimal recording protocols. Stroke. 1998; 29:1129-1132. [DOI] [PubMed] [Google Scholar]


