Abstract
Background
Percutaneous coronary intervention (PCI) is sometimes performed with the intent to lower cardiovascular risk before high‐risk noncardiac surgery (HRNCS). There are limited data on the frequency and outcome of PCIs performed in this setting.
Methods and Results
We assessed the frequency, characteristics, and in‐hospital outcomes of patients undergoing PCI as part of the preoperative workup for HRNCS among all 61 145 elective PCIs performed between 2002 and 2009 at 14 hospitals in the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Propensity matching was performed to compare outcomes of patients undergoing PCI before HRNCS with all other elective PCI patients. The frequency of PCI before HRNCS was low (4.2%). Patients undergoing PCI before HRNCS were older (67.3 versus 64.9 years, P<0.0001) and had a greater burden of comorbidity. Patients undergoing PCI before HRNCS had an increase in unadjusted major adverse cardiovascular events, postprocedure transfusion, contrast‐induced nephropathy, nephropathy requiring dialysis, and same‐admission coronary artery bypass graft surgery, but there was no difference in mortality (0.27% versus 0.14%, P=0.11). However, in propensity score–matched samples, there was a significant difference only in nephropathy requiring dialysis.
Conclusions
The incidence of PCI performed in preparation for high‐risk noncardiac surgery is low, and these procedures are currently being performed on a highly selected high‐risk patient population.
Keywords: angioplasty, epidemiology, preoperative revascularization, stents
Introduction
Coronary artery disease is a source of major complications after surgery, and the role of preoperative coronary revascularization to reduce the risk of adverse cardiovascular events before high‐risk noncardiac surgery (HRNCS) remains controversial. Since the early part of the past decade, several important studies have shown that prophylactic coronary revascularization and optimal medical management produce similar outcomes after major noncardiac surgery.1–4
Accordingly, the American College of Cardiology/American Heart Association guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery state that “the role of prophylactic perioperative percutaneous coronary intervention in reducing untoward perioperative complications appears limited to patients with unstable active coronary artery disease who would be appropriate candidates for emergent or urgent revascularization.”5 The 2011 American College of Cardiology Foundation/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines echo this by issuing a Class III recommendation: “Routine prophylactic coronary revascularization should not be performed in patients with stable coronary artery disease before noncardiac surgery.”6
Given the recommendations of these guidelines and the publication of these studies suggesting a lack of benefit for prophylactic percutaneous coronary interventions (PCIs) before HRNCS, one would expect the number of PCIs performed as part of the preparation for HRNCS to be few and to be performed only on highly selected patients who are at high risk for postoperative cardiovascular complications. However, it is unclear how commonly PCI is performed in preparation for HRNCS in contemporary practice. Furthermore, the characteristics of patients undergoing PCI before HRNCS and how they might differ from the larger population of patients receiving PCI are unknown. Using data from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) registry, we assessed for trends in and outcome of PCIs performed before HRNCS.
Methods
Our study cohort was composed of 61 145 consecutive elective PCIs performed at the 14 hospitals in Michigan that participated in the BMC2 registry continuously between January 2002 and December 2009.
Standardized data collection forms were used by trained personnel to collect demographic, procedural, and outcomes information. Medical records of all patients who underwent coronary artery bypass graft surgery (CABG) or who died during their hospital stay were reviewed to ensure accuracy. An additional 2% of cases were randomly selected for audit. All data collection processes were prospectively defined, and the collection of data was approved by the University of Michigan's Institutional Review Board, as well as the local review board from each participating center. Further details have been previously published.7–10
The study population for this analysis included only patients who were classified as undergoing elective PCI. PCIs were classified as elective if the patients on whom the PCI was performed met all of the following conditions: they had not had a myocardial infarction (MI) within the previous 7 days; were not in shock; had not had a cardiac arrest during or immediately before the current admission; were not receiving intravenous heparin, nitroglycerin, glycoprotein IIb‐IIIa inhibitor, or vasopressors before the procedure; and did not have an intra‐aortic balloon pump before the start of the procedure. Patients were classified as undergoing PCI before HRNCS if the operator performing the PCI deemed HRNCS to be the primary indication for the PCI procedure. Of note, there were 332 patients for whom the HRNCS status was not defined; these patients were included in the elective PCI cohort but were excluded from comparisons between pre‐HRNCS PCIs and non‐HRNCS PCIs. Clinical outcomes evaluated included in‐hospital death, vascular complications, in‐hospital MI, transfusion rates, gastrointestinal bleed, nephropathy requiring dialysis, contrast‐induced nephropathy (defined as a rise in the serum creatinine level of 0.5 mg/dL), stroke or transient ischemic attack, emergency CABG, any CABG, and overall major adverse cardiovascular events, defined as any in‐hospital stroke or transient ischemic attack, MI, CABG, revascularization, or death.
Statistical Analysis
Continuous variables are expressed as mean±SD, and discrete variables are expressed as frequency counts and percentages. Differences in discrete variables between groups were evaluated by the χ2 test and, when needed, Fisher's exact test. Continuous variables were analyzed using the t test and Wilcoxon rank sum test. Trends over time were analyzed using the Cochran‐Armitage test. Because of multiple baseline differences in the population of patients who were undergoing PCI before HRNCS, propensity matching was used before comparing between‐group outcomes. The probability or propensity score of being a pre‐HRNCS patient if all other variables were known was calculated using a nonparsimonious logistic regression model. The variables included in the propensity model were patient age, patient body mass index, renal function and dialysis status, World Health Organization defined anemia (<13 g/dL for men and <12 g/dL for women), current tobacco use, presence of extra‐cardiac vascular disease, hypertension, diabetes, chronic obstructive pulmonary disease, congestive heart failure, significant valve disease, atrial fibrillation, history of cardiac arrest, recent gastrointestinal bleeding, previous PCI, previous CABG, and previous MI. Greedy matching was then used to match pre‐HRNCS patients with elective non‐HRNCS patients with similar characteristics. In‐hospital outcomes for the PCI‐related hospitalizations were then compared within the propensity‐matched cohort. SAS Version 9.2 software (SAS Institute) was used for analysis.
Results
There were 61 145 elective PCIs performed at the 14 hospitals in the BMC2 registry that participated between January 2002 and December 2009. Of these, 2592 (4.23% of patients with HRNCS status defined) PCIs were performed as part of the preoperative preparation for HRNCS. The incidence of preoperative PCIs significantly declined during the study period (Figure 1). The majority of this decline was from 2002 to 2003. In a post‐hoc analysis, we reexamined the trend from 2003 to 2009 and found that the decline was not significant if 2002 data were excluded.
Figure 1.
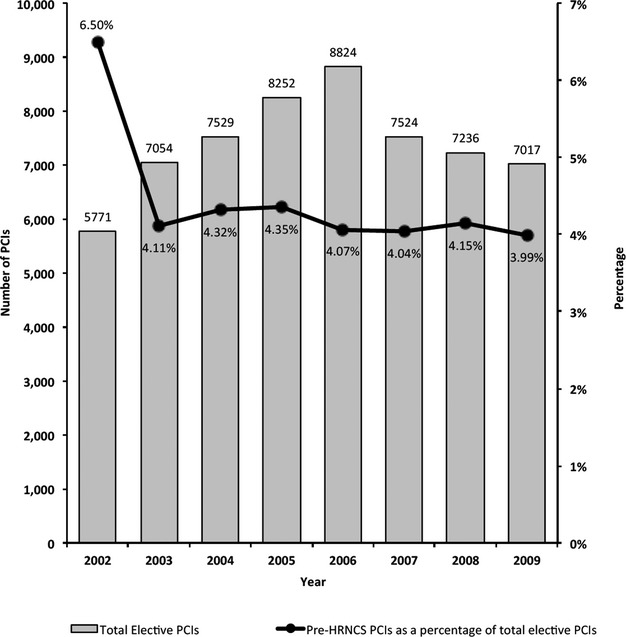
PCIs done in preparation for high‐risk noncardiac surgery (HRNCS) as a percentage of total elective PCIs in the BMC2 cohort, 2002–2009. BMC2 indicates Blue Cross Blue Shield of Michigan Cardiovascular Consortium; PCI, percutaneous coronary intervention.
There was significant variability in the proportion of pre‐HRNCS PCIs performed at the participating hospitals (Figure 2); this variability was not correlated with the total number of PCIs performed at each hospital.
Figure 2.
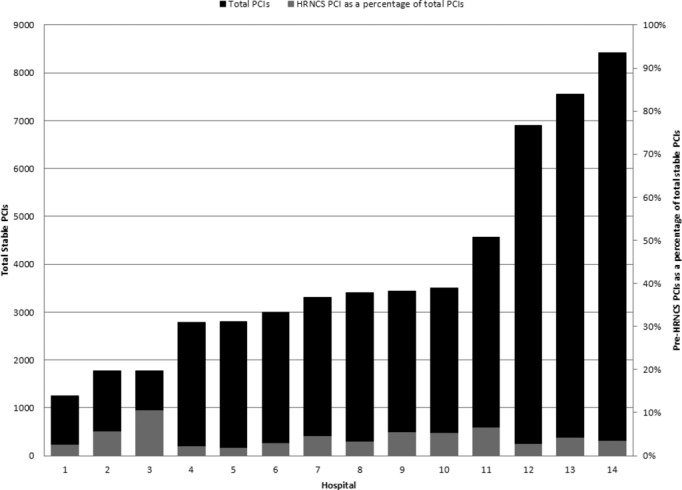
Hospital variation in pre‐HRNCS PCIs as a percentage of total elective PCIs. BMC2 indicates Blue Cross Blue Shield of Michigan Cardiovascular Consortium; HRNCS, high‐risk noncardiac surgery; PCI, percutaneous coronary intervention.
The baseline clinical characteristics of the patient populations are shown in Table 1. The pre‐HRNCS population was older, and the patients were more likely to be female and to be white. The pre‐HRNCS population also had a higher burden of comorbidity; there were higher rates of current tobacco use, hypertension, diabetes, extracardiac vascular disease, valve disease, renal failure, gastrointestinal bleeding, and chronic obstructive pulmonary disease. In addition, the pre‐HRNCS patients had higher baseline creatinine and lower baseline hemoglobin levels. The nonpreoperative, elective PCI population had a higher incidence of prior MI, prior PCI, and prior CABG. PCIs performed in preparation for HRNCS required more contrast and more fluoroscopy time. The patients undergoing PCI in preparation for HRNCS had fewer restenotic lesions, although the lesions were more likely to be calcified. They were significantly more likely to have PCI for more than one vessel and significantly more likely to receive bare metal rather than drug‐eluting stents. There were no significant differences in the ejection fraction or number of patients with 3‐vessel disease.
Table 1.
Characteristics of All Elective PCIs With Defined HRNCS Status in the BMC2 Cohort, 2002–2009
| Pre‐HRNCS PCI (n=2570) | Nonpreoperative PCI (n=58 243) | P Value | |
|---|---|---|---|
| Age, y, SD | 67.3 (10.8) | 64.9 (11.3) | <0.0001 |
| Female, % | 39.0 | 33.2 | <0.0001 |
| White, % | 77.0 | 79.4 | 0.004 |
| African American, % | 6.9 | 8.1 | 0.03 |
| Current tobacco use, % | 21.8 | 19.4 | 0.002 |
| History of hypertension, % | 86.4 | 84.6 | 0.01 |
| Diabetes mellitus, % | 42.0 | 35.6 | <0.0001 |
| History of atrial fibrillation, % | 7.5 | 7.3 | 0.64 |
| Prior myocardial infarction, % | 29.9 | 36.5 | <0.0001 |
| History of congestive heart failure, % | 12.2 | 12.5 | 0.64 |
| Extracardiac vascular disease, % | 43.2 | 24.6 | <0.0001 |
| Significant valve disease, % | 5.9 | 3.9 | <0.0001 |
| History of cardiac arrest, % | 0.7 | 1.0 | 0.1 |
| History of percutaneous coronary intervention, % | 33.4 | 51.2 | <0.0001 |
| History of coronary artery bypass graft surgery, % | 18.3 | 21.2 | 0.0004 |
| Chronic obstructive pulmonary disease, % | 19.1 | 15.4 | <0.0001 |
| History of gastrointestinal bleeding, % | 1.4 | 0.9 | 0.006 |
| Baseline creatinine, mg/dL, SD | 1.62 (1.9) | 1.15 (0.9) | <0.0001 |
| Renal failure and dialysis, % | 8.3 | 1.3 | <0.0001 |
| Baseline hemoglobin, g/dL, SD | 13.4 (1.8) | 13.7 (1.7) | <0.0001 |
| Ejection fraction, SD | 54.5 (10.9) | 54.2 (10.6) | 0.16 |
| Total contrast, mL, SD | 218 (99) | 209 (91) | <0.0001 |
| Total fluoroscopy time, min, SD | 16.1 (12.8) | 15.1 (11.9) | <0.0001 |
| Left main stenosis >70% | 3.0 | 3.7 | 0.05 |
| Restenotic lesion, % | 6.0 | 9.2 | <0.0001 |
| Thrombus, % | 2.5 | 3.1 | 0.01 |
| Calcification, % | 24.4 | 20.3 | <0.0001 |
| 3‐Vessel disease, % | 19.1 | 19.0 | 0.88 |
| 2 Lesions, % | 23.5 | 22.9 | 0.48 |
| ≥3 Lesions, % | 6.0 | 5.9 | 0.83 |
| PCI for 1 vessel, % | 86.3 | 88.0 | 0.01 |
| PCI for 2 vessels, % | 12.8 | 11.3 | 0.02 |
| PCI for ≥3 vessels, % | 0.8 | 0.7 | 0.62 |
BMC2 indicates Blue Cross Blue Shield of Michigan Cardiovascular Consortium; HRNCS, high‐risk noncardiac surgery; PCI, percutaneous coronary intervention.
The post‐PCI outcomes of interventions performed on patients before HRNCS compared with all elective non‐HRNCS patients are shown in Table 2. In an unadjusted analysis, patients undergoing PCI in preparation for HRNCS had a higher risk of major adverse cardiovascular events, postprocedure transfusion, contrast‐induced nephropathy, in‐hospital nephropathy requiring dialysis, and need for CABG. There were no significant differences with regard to in‐hospital mortality, vascular complications, stroke and transient ischemic attack, MI, gastrointestinal bleeding, or need for emergency CABG. However, when outcomes were adjusted via propensity matching, patients undergoing PCI before HRNCS had higher rates only for the event of nephropathy requiring dialysis (Table 3). In a supplementary analysis with patients exact‐matched within institutions (total number of matched patients 1875), results were similar (data not shown).
Table 2.
Unadjusted Outcomes in Patients Undergoing PCI Before HRNCS Compared With Non‐HRNCS Elective Patients
| PCI Before HRNCS (n=2570), % | Nonpreoperative PCI (n=58 243), % | P Value | |
|---|---|---|---|
| Emergency CABG | 0.39 | 0.21 | 0.06 |
| All CABG | 0.89 | 0.48 | 0.004 |
| Contrast nephropathy* | 2.87 | 1.73 | 0.001 |
| Nephropathy requiring dialysis | 0.34 | 0.03 | <0.0001 |
| Postprocedure transfusion | 2.98 | 1.88 | <0.0001 |
| Stroke/TIA | 0.12 | 0.15 | >0.999 |
| Myocardial infarction | 1.95 | 1.50 | 0.07 |
| Vascular complication | 1.71 | 1.47 | 0.32 |
| Gastrointestinal bleeding | 0.31 | 0.39 | 0.53 |
| Death | 0.27 | 0.14 | 0.10 |
| MACE | 3.35 | 2.31 | 0.0007 |
PCI indicates percutaneous coronary intervention; HRNCS, high‐risk noncardiac surgery; CABG, coronary artery bypass graft surgery; TIA, transient ischemic attack; MACE, major adverse cardiovascular events.
Patients with history of renal failure requiring dialysis are excluded.
Table 3.
Propensity‐Matched Outcomes in Patients Undergoing PCI Before HRNCS Compared With Non‐HRNCS Elective Patients
| PCI Before HRNCS (n=2358), % | Nonpreoperative PCI (n=2358), % | P Value | |
|---|---|---|---|
| Emergency CABG | 0.38 | 0.34 | 0.81 |
| All CABG | 0.81 | 0.81 | >0.999 |
| Contrast nephropathy* | 2.87 | 2.61 | 0.66 |
| Nephropathy requiring dialysis | 0.32 | 0.05 | 0.04 |
| Postprocedure transfusion | 2.88 | 3.10 | 0.67 |
| Stroke/TIA | 0.13 | 0.17 | >0.999 |
| Myocardial infarction | 2.04 | 1.61 | 0.28 |
| Vascular complication | 1.65 | 1.99 | 0.38 |
| Gastrointestinal bleeding | 0.30 | 0.34 | 0.80 |
| Death | 0.25 | 0.47 | 0.22 |
| MACE | 3.31 | 2.80 | 0.31 |
PCI indicates percutaneous coronary intervention; HRNCS, high‐risk noncardiac surgery; CABG, coronary artery bypass graft surgery; TIA, transient ischemic attack; MACE, major adverse cardiovascular events.
Patients with history of renal failure requiring dialysis are excluded.
Discussion
In this study, we analyzed the incidence, characteristics, and outcomes of patients with stable coronary artery disease undergoing PCI as part of the preoperative preparation for HRNCS. We found that the incidence of PCIs performed in preparation for HRNCS was relatively low and declined during the study period. Furthermore, PCI before HRNCS is performed mainly on a select group of patients with a large burden of perioperative comorbidity.
Our data suggest that only a small percentage of highly selected patients are undergoing PCI as part of the preoperative preparation for HRNCS. The patients in the pre‐HRNCS PCI population were older and had higher rates of hypertension, diabetes, prior stroke, extracardiac vascular disease, valve disease, gastrointestinal bleeding, chronic obstructive pulmonary disease, and renal failure than did the elective, non‐HRNCS PCI population. These comorbidities are likely to be associated with a high risk of perioperative complications. Similarly, these factors mean that the patients who undergo PCI in preparation for HRNCS are at a higher risk for complications from the PCI itself. In an unadjusted analysis, we found that preoperative PCI led to higher rates of major adverse cardiovascular events, postprocedure MI, postprocedure transfusion, and in‐hospital nephropathy requiring dialysis. However, when a propensity‐adjusted analysis was performed, the only difference found was a higher rate of nephropathy requiring dialysis in patients undergoing PCI in preparation for HRNCS. This may be related to the higher volume of contrast used in these patients and their greater degree of comorbidity. It is also possible that this was simply a chance finding, as no adjustment for multiple comparisons was made.
The small number of patients combined with the high‐risk characteristics suggests that physicians are highly selective in performing PCI with the intent of reducing operative risk. However, it is not clear if the high risk of complications with PCI seen in this population negates any expected reduction in perioperative complications. This question merits assessment in a randomized controlled trial.
Our data show a decline in the proportion of PCIs during the study period, although the largest decline occurred during the first 2 years of the study. The reasons for this sharp decline versus the smaller degree of decline during the remainder of the study period remain unclear; of note, there were no differences in the data collection practices or definitions during this time.
We observed a considerable variation between hospitals in the proportion of PCIs performed as part of the preparation for HRNCS (1.71% to 10.48%). There was no correlation between the total number of PCIs performed at a hospital and the proportion of PCIs performed in preparation for HRNCS. It is possible that this difference may relate to differences in the type and number of surgical procedures being performed at these institutions; some of the institutions with a high proportion of PCI before HRNCS have a busy organ transplant program or are referral centers for patients with advanced aortic disease, and the practice of regularly performing pre‐HRNCS PCI in these patients may explain part of the variation. Data on the specific surgery that followed PCI are not collected, and we were thus unable to explore this possibility. It is also possible that these differences may reflect practice variation at these institutions, and further research is warranted to explore this hypothesis.
It is also interesting to note that there were several landmark trials published during the study period. The CARP (Coronary Artery Revascularization Prophylaxis) trial, with results published in December 2004, showed that preoperative PCI did not improve outcomes in 510 patients randomized to revascularization or medical therapy before major vascular surgery; the DECREASE‐V (fifth Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography) Pilot Study, with results published in May 2007, showed that preoperative PCI did not improve outcomes in 101 high‐risk patients randomized to revascularization or medical therapy before major vascular surgery.3–4 We did not see a decline in the number of pre‐HRNCS PCIs performed in our cohort in association with publication of these study results. It is possible that practitioners were already being selective in choosing patients for preoperative PCI and that further declines were not possible. It is also possible that the publication of these studies, and the subsequent publication in September 2007 of the American College of Cardiology/American Heart Association guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery, did not produce a significant impact on practice to be discerned within the study period.5
Study Limitations
This study has several limitations. First, we analyzed data from 14 hospitals in one state, and, accordingly, our results may not be generalizable to other settings. However, these hospitals represent a wide range of practice patterns, from small hospitals to quaternary referral centers. Second, our data are observational and were entered into a quality improvement registry; even though the data were abstracted by trained professionals and randomly audited for accuracy, the results may be susceptible to ascertainment bias. Third, our data relied on the physician performing the PCI included in the registry to decide whether the PCI was being performed in preparation for HRNCS. Fourth, our data are limited to in‐hospital outcomes, and we are not able to assess the outcomes of the patients after the index hospitalization or the specific types of HRNCS or the outcomes of the surgery for which patients underwent preparatory PCI. Finally, we do not have data on the total number and type of surgical procedures that are performed at these institutions. However, it is likely that these patients make up a very small proportion of the total number of patients undergoing noncardiac procedures at these hospitals.
Conclusion
The incidence of PCI performed in preparation for HRNCS is low. These procedures are currently being performed on a highly selected high‐risk patient population.
Sources of Funding
The BMC2 registry is funded by Blue Cross Blue Shield of Michigan. The sponsor had no role in analysis, study design, or decision to publish these results.
Disclosures
Dr Gurm receives research funding from Blue Cross Blue Shield of Michigan, the National Institutes of Health, and the Agency for Healthcare Research and Quality. Dr Share is employed part‐time by Blue Cross Blue Shield of Michigan. None of the authors have any conflicts directly relevant to this study.
Acknowledgments
We are indebted to all the study coordinators, investigators, and patients who participated in BMC2.
References
- 1.Godet G, Riou B, Bertrand M, Fleron MH, Goarin JP, Montalescot G, Coriat P. Does preoperative coronary angioplasty improve perioperative cardiac outcome? Anesthesiology. 2005; 102:739-746. [DOI] [PubMed] [Google Scholar]
- 2.Illuminati G, Ricco JB, Greco C, Mangieri E, Calio' F, Ceccanei G, Pacile MA, Schiariti M, Tanzilli G, Barilla F, Paravati V, Mazzesi G, Miraldi F, Tritapepe L. Systematic preoperative coronary angiography and stenting improves postoperative results of carotid endarterectomy in patients with asymptomatic coronary artery disease: a randomised controlled trial. Eur J Vasc Endovasc Surg. 2010; 39:139-145. [DOI] [PubMed] [Google Scholar]
- 3.McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, Pierpont G, Santilli S, Rapp J, Hattler B, Shunk K, Jaenicke C, Thottapurathu L, Ellis N, Reda DJ, Henderson WG. Coronary‐artery revascularization before elective major vascular surgery. N Engl J Med. 2004; 351:2795-2804. [DOI] [PubMed] [Google Scholar]
- 4.Poldermans D, Schouten O, Vidakovic R, Bax JJ, Thomson IR, Hoeks SE, Feringa HH, Dunkelgrun M, de Jaegere P, Maat A, van Sambeek MR, Kertai MD, Boersma EDECREASE Study Group. A clinical randomized trial to evaluate the safety of a noninvasive approach in high‐risk patients undergoing major vascular surgery: the DECREASE‐V pilot study. J Am Coll Cardiol. 2007; 49:1763-1769. [DOI] [PubMed] [Google Scholar]
- 5.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Tarkington LG, Yancy CWAmerican College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery), American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society for Vascular Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007; 116:e418-e499. [DOI] [PubMed] [Google Scholar]
- 6.Kline‐Rogers E, Share D, Bondie D, Rogers B, Karavite D, Kanten S, Wren P, Bodurka C, Fisk C, McGinnity J, Wright S, Fox S, Eagle KA, Moscucci MBlue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Development of a multicenter interventional cardiology database: the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) experience. J Interv Cardiol. 2002; 15:387-392. [DOI] [PubMed] [Google Scholar]
- 7.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. ACCF/AHA/SCAI guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2011; 2012:453-495. [DOI] [PubMed] [Google Scholar]
- 8.Moscucci M, Share D, Kline‐Rogers E, O'Donnell M, Maxwell‐Eward A, Meengs WL, Clark VL, Kraft P, De FrancoAC, Chambers JL, Patel K, McGinnity JG, Eagle KABlue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). The Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) collaborative quality improvement initiative in percutaneous coronary interventions. J Interv Cardiol. 2002; 15:381-386. [DOI] [PubMed] [Google Scholar]
- 9.Jackson EA, Moscucci M, Smith DE, Share D, Dixon S, Greenbaum A, Grossman PM, Gurm HS. The association of sex with outcomes among patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction in the contemporary era: Insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am Heart J. 2011; 161:e1. [DOI] [PubMed] [Google Scholar]
- 10.Duvernoy CS, Smith DE, Manohar P, Schaefer A, Kline‐Rogers E, Share D, McNamara R, Gurm HS, Moscucci M. Gender differences in adverse outcomes after contemporary percutaneous coronary intervention: an analysis from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) percutaneous coronary intervention registry. Am Heart J. 2010; 159:e1. [DOI] [PubMed] [Google Scholar]


