Abstract
Background
We sought to perform a study assessing the association between electrocardiographic ST‐segment deviations and cardiovascular death (CVD), in relation to sex and age (≥ and <65 years), in a large primary care population without overt ischemic heart disease.
Methods and Results
Using computerized analysis of ECGs from 285 194 persons, we evaluated the association between precordial ST‐segment deviations and the risk of CVD. All data on medication, comorbidity, and outcomes were retrieved from Danish registries. After a median follow‐up period of 5.8 years, there were 6679 cardiovascular deaths. Increasing ST‐depression was associated with an increased risk of CVD in almost all of the precordial leads, with the most robust association seen in lead V5 to V6. ST‐elevations in lead V2 to V6 were associated with increased risk of CVD in young women, but not in men. However, ST‐elevations in V1 increased the risk for both genders and age groups, exemplified by a HR of 1.80 (95% CI [1.19 to 2.74], P=0.005) for men <65 years with ST‐elevations ≥150 μV versus a nondeviating ST‐segment (−50 μV to +50 μV). In contrast, for men <65 years, ST‐elevations in lead V2 to V3 conferred a decreased risk of CVD with a HR of 0.77 (95% CI [0.62 to 0.96], P<0.001) for ST‐elevations ≥150 μV in V2.
Conclusion
We found that ST‐depressions were associated with a dose‐responsive increased risk of CVD in nearly all the precordial leads. ST‐elevations conferred an increased risk of CVD in women and with regard to lead V1 also in men. However, ST‐elevations in V2 to V3 were associated with a decreased risk of CVD in young men.
Keywords: Brugada, ECG, gender differences, general population, Marquette 12SL validation, ST‐segment
Introduction
ST‐segment deviations in the standard surface electrocardiogram (ECG) are a common finding. The electrocardiographic ST‐segment reflects the depolarized state and initial repolarization of the ventricles and several factors are known to affect the ST‐segment including acute ischemic disease, ventricular hypertrophy, electrolytes, various medications, gender, and age.
ST‐depression in the lateral precordial leads (V5 to V6) has previously been associated with increased mortality in a wide variety of populations.1–5 Such ST‐depressions often appear as a strain pattern with inverted T‐waves thought to result from subendocardial ischemia or as a consequence of an increased ventricular workload.6
The case of ST‐elevation in the precordial leads is more complex in its origin and implications. Right and antero‐septal precordial ST‐elevations has been described as a normal variant in young males7–8 but is also associated with potentially arrythmogenic conditions such as Brugada and early repolarization syndromes.9
We sought to investigate the spectrum and prevalence of precordial ST‐segment deviations and their relation to age and gender in a large contemporary primary care population. Additionally, we aimed to investigate the association between various degrees of precordial ST‐deviations and the risk of cardiovascular death (CVD). We believe that such knowledge could lead to a better pathophysiological understanding of the nature of ST‐deviations and potentially improve risk stratification of patients undergoing standard cardiovascular evaluation.
Methods
Study Population
In the greater region of Copenhagen, Denmark, the vast majority of general practitioners refer their patients to one core facility (CGPL; Copenhagen General Practitioners' Laboratory) for clinical tests, such as biochemistry and electrocardiogram (ECG) recordings. The present study population is part of the Copenhagen ECG study and consists of all individuals who had an ECG recorded at CGPL from 2001 to 2011.10–11 We excluded individuals <15 and >90 years of age, individuals who were in treatment with digoxin on the day of ECG recording, individuals with a history of ischemic heart disease at baseline, or with ECG abnormalities inconsistent with ST‐segment assessment (see Electrocardiography). Further details on the study population have been described previously.10–12
In addition, in order to compare the ECG study population with the general population with respect to incidence rate of CVD, we randomly sampled individuals from the entire Danish population (5.6 million), matched 1:2 based on gender, age, and without ischemic heart disease at the time of inclusion.
Because our study was registry based with no active participation from study subjects, no approval from an ethics committee was required according to Danish law. The use of registry data was approved by the Danish Data Protection Agency.
Electrocardiography
All ECGs were digitally recorded and stored in the MUSE® Cardiology Information System (GE Healthcare) and were later processed using version 21 of the Marquette 12SL algorithm. With the use of 12SL statements and intervals, we excluded ECGs with the following findings that were not suitable for measurement of the ST‐segment: rhythms different from sinus‐ or ectopic atrial rhythms, bradyarrhythmias (heart rate <40 beats per minute [bpm]), tachyarrhythmias (heart rate >110 bpm), ventricular rhythms, delta waves, second‐ and third‐degree AV‐blocks, bundle branch blocks, multiple premature ventricular complexes, multiple premature atrial complexes, junctional rhythms, pace spikes, and ST‐segment deviations from the isoelectric line below the 0.005th percentile and above the 99.995th percentile.
The 12SL algorithm measures ST‐segment deviation by constructing a representative median beat from all PQRST complexes of the 10‐second ECG tracing in the particular lead. The median complex is shifted so that the voltage at the QRS onset is 0 by definition (isoelectric line). The point measured on the ST‐segment is defined as the ST‐level at the QRS offset plus 1/16 of the average RR interval (denoted as “STM” in the 12SL algorithm). This point is in most cases equivalent to 80 ms after the J‐point, which by convention is the point used for measuring ST‐depression. We chose this point of measurement for ST‐elevation, as our interest was not the notched or slurred appearance of the terminal part of the QRS complex, which some denote early repolarization and which makes definition of a J‐point difficult. Additionally, we only categorized deviations as elevations if the end of the ST‐segment was higher or equal to the mid‐section, and for ST‐depressions only if the end was lower or equal to the mid‐section. This was done to avoid descending ST‐elevations and ascending ST‐depressions, as these specific types of electrocardiographic ST‐deviations were not the focus of the study.
To evaluate the agreement between the 12SL algorithm and manual ST‐segment measurement, a number of ECGs (N=200) were sampled for manual evaluation with 100 ECG's for evaluation in lead V3 and 100 ECG's for evaluation in lead V6. To be able to explore the validity of the automated measurements also at the extremes of ST‐segment deviations, ECGs were randomly sampled from each category of ST‐deviation (see Statistical Analysis). For all manually assessed ECGs, ST‐segments were measured manually in lead V3 for ST‐elevations and lead V6 for ST‐depressions at ×10 magnification and with the use of a digital caliper (MUSE® Cardiology Information System, GE Healthcare). The manual rater (P.V.R.) was blinded to results from the 12SL algorithm. To evaluate agreement between manual and 12SL measured ST‐segments, results were summarized in a Bland‐Altman plot. Mean differences between manual and 12SL algorithm measurements were calculated together with the limits of agreement (±2 standard deviations).
Baseline Variables and Follow‐Up
Using Danish administrative registries and a unique personal identification number assigned to all persons with permanent residence in Denmark, it is possible to follow individuals with respect to death, emigration, the use of prescription drugs, and any hospital, ambulatory, or emergency room discharge diagnoses. With the use of such registry data, we identified baseline variables concerning comorbidity and medication status as described previously.12
We defined a modified version of the Charlson Comorbidity Index in which we excluded the major cardiovascular ICD‐10 codes, as these covariates were adjusted for separately. Using the modified Charlson co‐morbidity index, we were able to take several comorbidities including diabetes, various cancer diseases, liver diseases, and vascular diseases into account.13
Death from cardiovascular causes (ICD‐10: I00‐I99) was the endpoint of interest with supplementary analyses of all‐cause mortality.
Statistical Analysis
Age was used as time‐scale in all survival analyses. Follow‐up started at the day of first ECG recording (index ECG) and ended in case of death, emigration, or on December 31, 2011 which was the end of follow‐up. Cox regression was used to assess the association of ST‐segment deviation, measured on the day of inclusion (index ECG), with the instantaneous risk (hazard) of CVD and all‐cause mortality. All Cox models were adjusted for the following covariates obtained at baseline: prior treatment with non‐loop diuretics, calcium antagonists, angiotensin‐converting enzyme inhibitors, beta blockers, history of heart failure, history of valvular heart disease, a modified Charlson comorbidity index, R‐wave amplitude, S‐wave amplitude, QRS duration, and heart rate.
We performed the analysis based on categorization of the ST‐segment deviation in groups of 50 μV with reference to a nondeviating ST‐segment (−50 μV to +50 μV). We chose this range of ST‐deviation for our reference group, as we believe 50 μV, corresponding to half of one small square on the standard ECG, is a credible threshold for what amplitude of deviations from the isoelectric line is actually possible to measure on an ECG. Furthermore, this range provided a reference group of sufficient size.
In addition to the categorical analysis, the relationship between ST‐segment deviations and the risk of CVD was assessed with the use of restricted cubic regression splines based on Cox regression models adjusted for the same covariates as in the categorical analyses.
A subgroup analysis was performed in which we excluded individuals being hospitalized with myocardial infarction within 14 days after inclusion.
Poisson regression was used to compare rates of CVD per 1000 person‐years in the ECG study population with the sample from the general population.
We considered a 2‐tailed P‐value below 0.05 as statistically significant. Proportional hazard assumptions were checked using log‐log survival plots and intersecting curves were observed for ST‐deviations corresponding to ≈65 years of age, in particular with respect to lead V6. Accordingly, to avoid violation of the proportional hazard assumption, analyses were done separately for persons younger and older than 65 years of age. Tests for interaction between gender and ST‐deviations, within the 2 age groups, were performed by introducing interaction terms to Cox models and by comparing models applying likelihood ratio tests. These tests indicated statistical significant interactions for all leads (P<0.0001). Consequently, we made separate analyses for women and men older and younger than 65 years of age, respectively. In order to test whether ST‐changes in lead V1 and V5/V6 were reflections of each other, we analyzed the degree of correlation between ST‐deviations in the precordial leads.
All analyses were conducted using the Stata 12.0 software package (StataCorp LP).
Results
A total of 343 639 individuals had one or more ECGs recorded at CGPL during the period from 2001 to 2011. Of these, 285 194 were eligible for inclusion. Baseline characteristics of the study population are shown in Table 1.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | Total (n=285 194) | Women | Men | ||||
|---|---|---|---|---|---|---|---|
| All (n=160 879) | Age <65 (n=115 651) | Age ≥65 (n=45 097) | All (n=124 315) | Age <65 (n=100 616) | Age ≥65 (n=23 699) | ||
| Age—y, median (IQR) | 53 (41 to 65) | 54 (40 to 67) | 47 (36 to 56) | 75 (70 to 81) | 54 (40 to 62) | 47 (37 to 56) | 72 (68 to 78) |
| Medical history—no. (%) | |||||||
| Congestive heart failure | 1113 (0.4) | 742 (0.5) | 131 (0.1) | 611 (1) | 371 (0.3) | 112 (0.1) | 259 (1) |
| Valvular heart disease | 702 (0.2) | 426 (0.3) | 166 (0.1) | 260 (0.6) | 276 (0.2) | 150 (0.2) | 126 (1) |
| Modified Charlson co‐morbidity index | |||||||
| 0 points | 236 699 (83) | 131 413 (80) | 100 603 (87) | 30 810 (68) | 105 286 (85) | 88 962 (88) | 16 324 (69) |
| 1 point | 27 705 (10) | 16 408 (10) | 9314 (8) | 7094 (16) | 11 297 (9) | 7682 (8) | 3615 (15) |
| ≥2 points | 20 790 (7) | 13 058 (8) | 5865 (5) | 7193 (16) | 7732 (6) | 3972 (4) | 3760 (16) |
| Medication history—no. (%) | |||||||
| ACE inhibitors | 34 610 (12) | 20 168 (13) | 9465 (8) | 10 703 (24) | 14 442 (12) | 9122 (9) | 5320 (23) |
| Beta‐blockers | 34 489 (12) | 23 571 (15) | 14 526 (13) | 9045 (20) | 10 918 (9) | 7490 (7) | 3428 (14) |
| Calcium‐antagonists | 27 946 (10) | 16 951 (11) | 6999 (6) | 9952 (22) | 10 995 (9) | 6435 (6) | 4542 (19) |
| Non‐loop diuretics | 59 466 (21) | 42 967 (27) | 21 029 (18) | 21 938 (48) | 16 499 (13) | 9067 (9) | 7432 (31) |
| ECG variables | |||||||
| Heart rate—bpm, median (IQR) | 69 (62 to 78) | 71 (63 to 79) | 70 (62 to 78) | 73 (65 to 82) | 67 (60 to 77) | 67 (59 to 76) | 69 (61 to 80) |
| Left ventricular hypertrophy—no. (%) | 12 286 (4) | 3509 (2) | 1737 (2) | 1772 (4) | 8777 (7) | 7371 (7) | 1406 (6) |
| QRS duration—ms, median (IQR) | 92 (86 to 100) | 88 (82 to 94) | 88 (84 to 94) | 88 (82 to 94) | 98 (90 to 104) | 98 (92 to 104) | 96 (88 to 102) |
| Subjects in ECG reference range (−50 to +50 μV)—no. (%) | |||||||
| V1 | 196 654 (69) | 122 242 (76) | 90 909 (79) | 31 333 (70) | 74 412 (60) | 59 524 (59) | 14 888 (63) |
| V2 | 68 332 (24) | 52 482 (33) | 32 994 (29) | 19 488 (43) | 15 850 (13) | 9433 (9) | 6417 (28) |
| V3 | 105 227 (37) | 83 130 (52) | 57 143 (49) | 25 987 (58) | 22 097 (18) | 13 614 (14) | 8483 (36) |
| V4 | 178 139 (63) | 130 603 (81) | 93 539 (81) | 37 064 (83) | 47 536 (38) | 32 780 (33) | 14 756 (67) |
| V5 | 224 851 (79) | 146 434 (91) | 106 387 (92) | 40 047 (89) | 78 417 (63) | 59 442 (59) | 18 975 (81) |
| V6 | 254 266 (89) | 152 797 (95) | 111 296 (96) | 41 501 (92) | 101 469 (82) | 80 563 (80) | 20 906 (89) |
bpm indicates beats per minute; IQR, inter‐quartile range; ms, milliseconds.
Spectrum and Prevalences of ST‐Segment Deviations
We observed distinct differences between the genders and age groups in the prevalences of ST‐segment deviations (Figure 1). ST‐depressions were generally prevalent in the lateral and mid‐precordial leads and almost non‐existent in the right precordial leads. Additionally, the prevalences of ST‐depression were much higher in the population ≥65 years of age compared with those ≤65 years of age and almost equally distributed between the genders.
Figure 1.
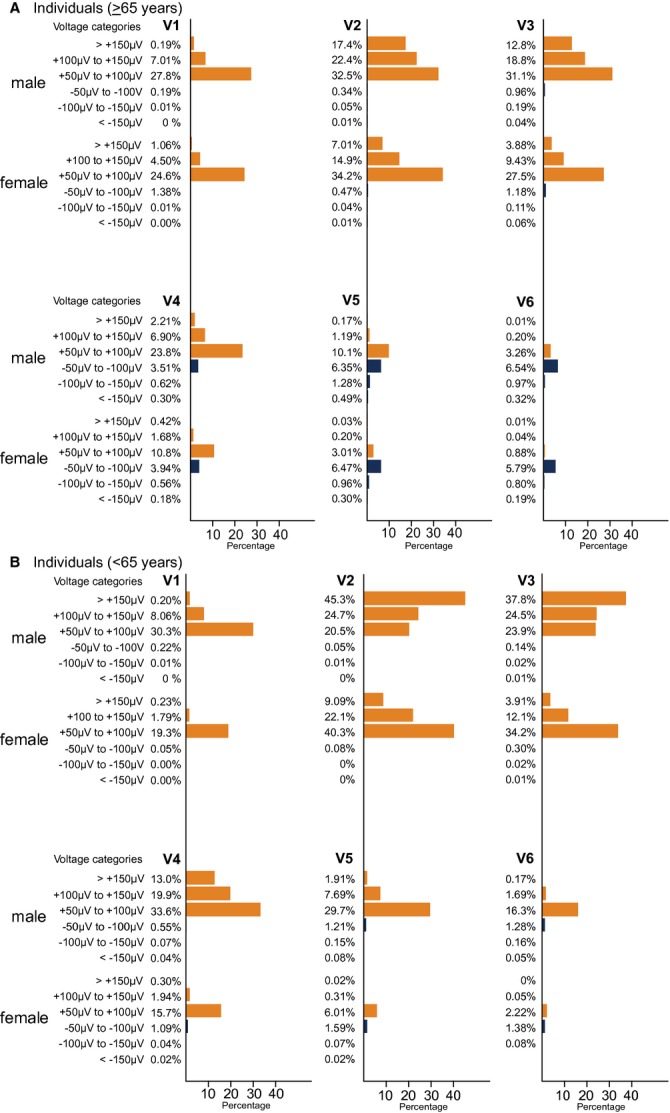
Bar charts displaying the prevalences and distribution of precordial ST‐segment deviations in the different voltage categories of (≥50 to <100 μV, ≥100 to <150 μV and ≥150 μV) in the subgroup of men and women younger and older than 65 years of age. Orange bars represent ST‐elevations and blue bars represent ST‐depressions. It is supposed to be A, Old (≥65 years), B, Young (<65 years).
ST‐segment elevations were generally more prevalent among men compared with women, especially in the subgroup <65 years of age. As such, we observed that almost 91% of young men had some degree of ST‐elevation in lead V2 and 45% had ST‐elevations ≥150 μV.
In male subjects ≥65 years of age, 17% had ST‐elevations ≥150 μV in lead V2, whereas this proportion was 7% for females.
In lead V3, 38% of the young men and 13% of the older men had elevations ≥150 μV. The same types of elevations were observed with a prevalence of 4% in both age categories of women.
Minor lateral ST‐elevation was present, especially among the young men, but these were not nearly as prevalent as in V2 through V4 (Figure 1).
Risk of CVD Associated With ST‐Segment Deviations
The median follow‐up period for the ECG study population was 5.8 years (interquartile range [IQR] 3.1 to 8.6), corresponding to 1651 219 person‐years. During follow‐up, we identified 6679 cardiovascular deaths and 22 578 deaths from noncardiovascular causes.
ST‐depressions and the risk of CVD
In general, increasing ST‐segment depression was associated with a dose‐response increase in the risk of CVD for all 4 gender and age determined subgroups (Figure 2).
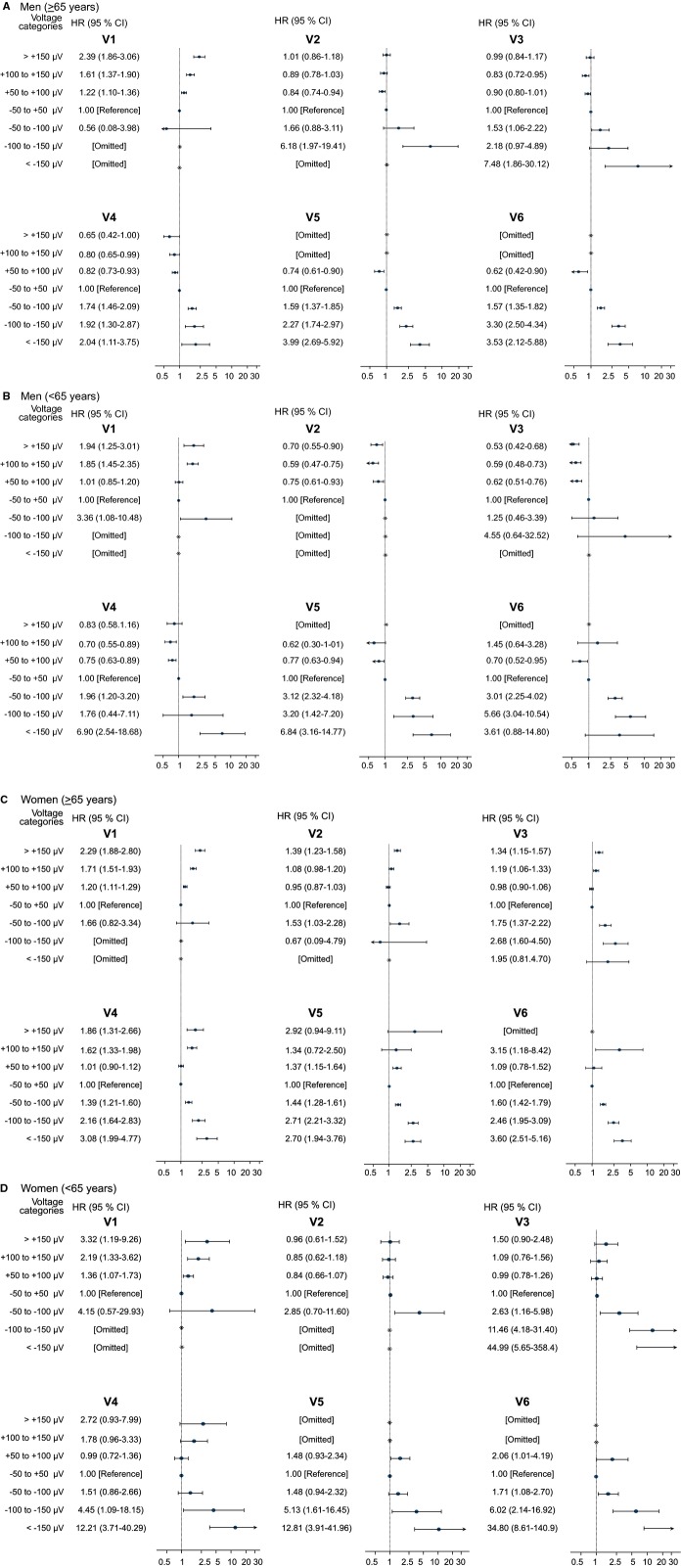
Figure 2.
Associations of ST‐segment deviation and cardiovascular death. Forest plots depicting multivariable‐adjusted hazard ratios for cardiovascular death by voltage categories (≥50 to 100> μV, ≥100 to 150> μV and ≥150 μV) with reference to an isoelectric ST‐segment. All models were adjusted for heart failure, valvular heart disease, Charlson comorbidity index (0 points, 1 point, or ≥2 points), treatment with angiotensin converting enzyme (ACE)‐inhibitors, beta‐blockers, or calcium‐antagonists prior to inclusion, digoxin at the day of ECG recording, R‐wave and S‐wave amplitude on the index ECG, and age was the timescale. The horizontal solid lines represent 95% confidence intervals and vertical lines represent a HR of 1. A, Men, old (≥65 years); (B) Men, young (<65 years); (C) Women, old (≥65 years); (D) Women, young (<65 years).
This association existed for all precordial leads, where ST‐depressions were prevalent (ie, mid‐precordial and lateral leads). This is illustrated by the group of men ≥65 years of age, where increasing depression in lead V5 was associated with a HR of 1.59 (95% confidence interval [CI] 1.37 to 1.85, P<0.0001), 2.27 (95% CI 1.74 to 2.97, P<0.0001), and 3.99 (95% CI 2.69 to 5.92, P<0.0001) for depressions in the range of ≥50 to <100 μV, ≥100 to <150 μV, and ≥150 μV, respectively, when compared with a nondeviating ST‐segment (−50 μV to +50 μV).
ST‐elevations and the risk of CVD
The association between ST‐segment elevations and the risk of CVD was generally much more heterogenic compared with ST‐segment depressions. We found that elevations in lead V1 conferred an increased risk of CVD for both genders and age groups (Figure 2) and thus, within our subgroup of young men, we found elevations in V1 to be associated with a highly significant increased risk of CVD as evidenced by an HR of 1.85 (95% CI 1.45 to 2.35, P<0.001) for elevations between 100 to 150 μV and an HR of 1.94 (95% CI 1.25 to 3.01, P=0.005) for elevations ≥150 μV compared with a nondeviating ST‐segment (−50 μV to +50 μV). This association was the least strong in the group of young women.
We found ST‐elevations in V2 to be associated with a modest increased risk of CVD only in older women. This was in contrast to young men where we observed a decreased risk for the same elevations with an HR of 0.59 (95% CI 0.47 to 0.75, P<0.0001) for having elevations between 100 to 150 μV.
The same pattern of association was found for ST‐elevations in V3. In the subgroup of young men, we observed a decreased risk associated with ST‐elevations, similar to those observed for lead V2, with HRs of 0.59 (95% CI 0.48 to 0.73, P=0.002) and 0.53 (95% CI 0.42 to 0.68, P=0.008) for CVD with respect to elevations in the range 100 to 150 μV and ≥150 μV, respectively. This is in contrast to young women, where the same elevations conferred HRs of 1.09 (95% CI 0.76 to 1.56, P=0.109) and 1.50 (95% CI 0.90 to 2.48, P=0.003), respectively.
ST‐elevations in lead V4 were associated with an increased risk of CVD in both young and old women, with the strongest effect seen in young women where elevations ≥150 μV were associated with an HR of 2.72 (95% CI 0.93 to 7.99, P=0.069) for CVD compared with a nondeviating ST‐segment (Figure 2). There was no significant association among men, albeit with a slight indication of a decreased risk of CVD for both age groups in lead V4.
Lateral ST‐elevations in V5 to V6 seemed to be associated with an increased risk of CVD, but the relatively few endpoints made any conclusions impossible.
Representative examples of the described ST‐segment deviations taken from the population are provided in Figure 3.
Figure 3.
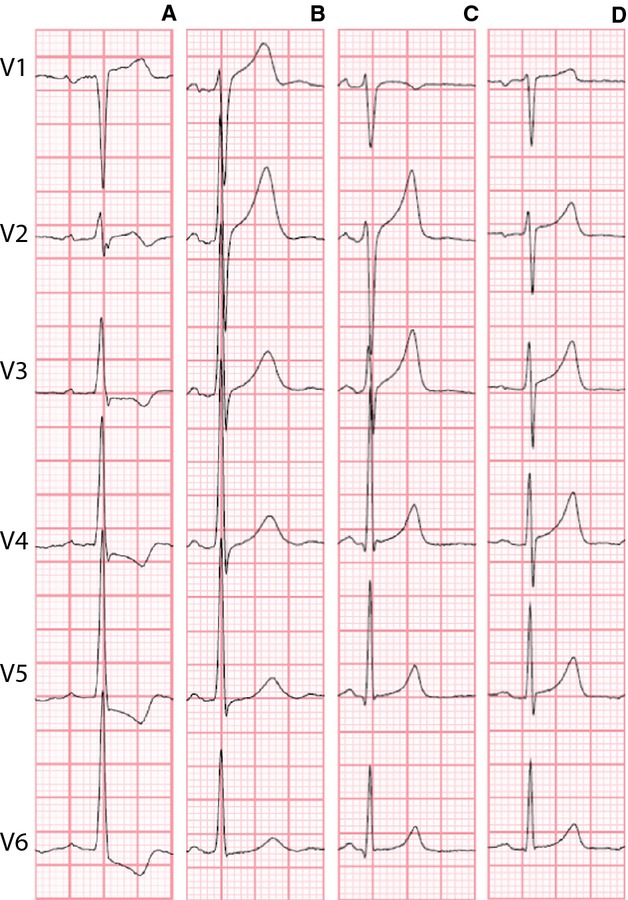
Representative examples of the precordial leads of ECG's emphasized in the text. A, Older man with lateral ST‐depression in V5/V6; (B) Younger man with ST‐elevation in V1; (C) Younger man with ST‐elevation in V2/V3; (D) Younger woman with ST‐elevation in V4.
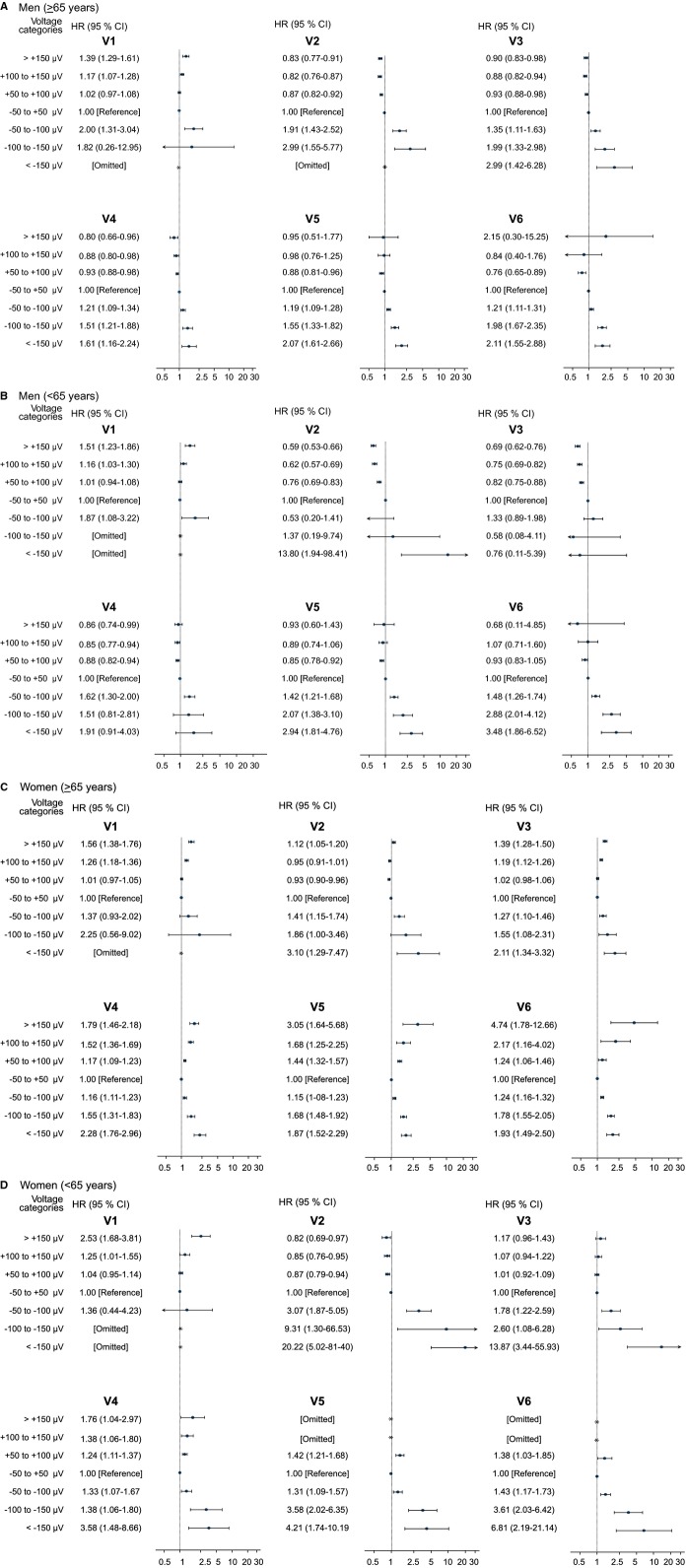
ST‐deviations and the risk of all‐cause mortality
Results for all‐cause mortality in general revealed results similar to those for CVD but with attenuated effects and narrower confidence limits due to the larger number of endpoints (see Figure 4).
Figure 4.
Forest plots showing the association between ST‐segment deviations and all‐cause mortality demonstrating the same associations as the main analyses with regard to cardiovascular death (CVD) but with attenuated effect. A, Men, old (≥65 years); (B) Men, young (<65 years); (C) Women, old (≥65 years); (D) Women, young (<65 years).
Additional Results
Electrocardiographic validations
We performed a validation analysis and found good agreement between manual and 12SL automated ST‐segment measurement (see Figure 5). For comparison of automated ST‐segment measuring of ST‐elevation in lead V3 with manual measurement we found a mean difference of −16.66 μV (95% CI −22.41 to −10.91 μV) with limits of agreement (±2 standard deviations) between −74.6 to 41.3 μV.
Figure 5.
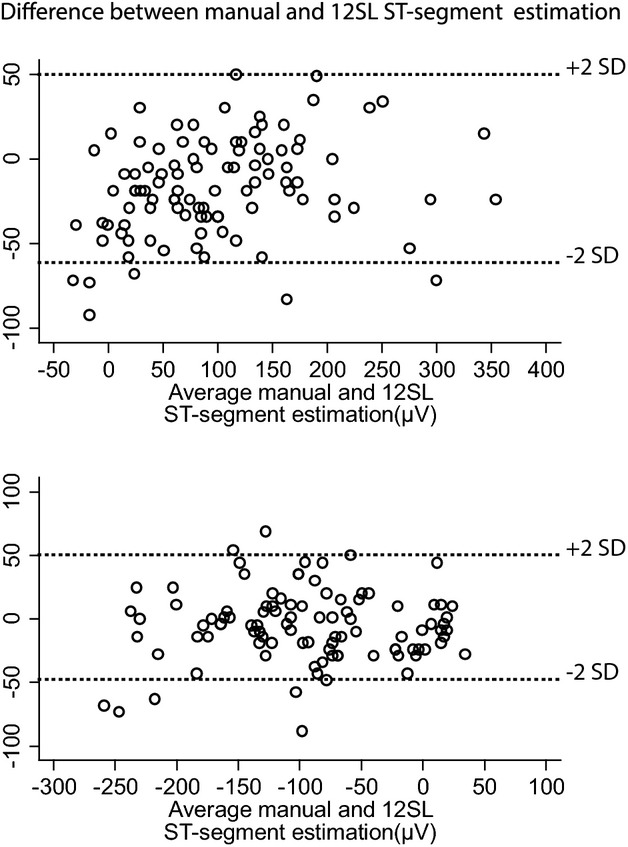
Agreement between automated ST‐segment measurements (Marquett 12SL algorithm) and manual measurements. Bland‐Altman plot for ST‐elevation (top) and ST‐depression (bottom). SD, standard deviation.
Automated ST‐segment depression in lead V6 compared with manual measurement showed a mean difference of −5.545 μV (95% CI −11.07 to 0.02) with limits of agreement between −74.64 to 41.320 μV.
Comparison to controls sampled from the general population
The incidence rate of CVD was 4.05 (95% CI 3.96 to 4.16) per 1000 person‐years in the ECG study population compared with 3.78 (95% CI 3.71 to 3.84) in the sampled control population (matched 1:2 based on gender and age at the time of inclusion, P<0.001).
Sensitivity analysis
We analyzed a subgroup in which we excluded individuals being hospitalized with ischemic heart disease within 14 days after inclusion (N=1264 or 0.4%) and found that the patterns of association did not change (data not shown).
Correlation analysis
We found a certain degree of correlation between adjacent leads with the strongest correlation seen between lead V5 and V6 (R2=0.909). We found no noteworthy inverse correlation between ST‐elevations in V1 and ST‐depressions in lead V5 and lead V6 (R2=−0.03 and R2=−0.15, respectively). See Table 2.
Table 2.
Correlation Coefficients (R2) Illustrating the Degree of Correlation Between the Different Electrocardiographic Leads Based on the Voltage Categories
| V1 | V2 | V3 | V4 | V5 | V6 | |
|---|---|---|---|---|---|---|
| V1 | 1.0000 | |||||
| V2 | 0.6220 | 1.0000 | ||||
| V3 | 0.3782 | 0.7792 | 1.0000 | |||
| V4 | 0.1601 | 0.6391 | 0.8702 | 1.0000 | ||
| V5 | −0.0304 | 0.4866 | 0.6731 | 0.8823 | 1.0000 | |
| V6 | −0.1513 | 0.3568 | 0.5087 | 0.7312 | 0.9090 | 1.0000 |
Median voltages
The median voltages in each precordial lead for both age and gender groups are summarized in Table 3.
Table 3.
Median (IQR) ST‐Segment Voltages (μV) for Both Age‐ and Gender Groups
| Men <65 Years | Men ≥65 Years | Women <65 Years | Women ≥65 Years | |
|---|---|---|---|---|
| V1 | 43 (24 to 73) | 39 (19 to 68) | 34 (14 to 48) | 34 (19 to 58) |
| V2 | 141 (87 to 200) | 83 (48 to 126) | 78 (48 to 107) | 58 (29 to 92) |
| V3 | 122 (78 to 180) | 68 (34 to 112) | 53 (24 to 83) | 39 (14 to 73) |
| V4 | 73 (39 to 117) | 29 (0 to 63) | 19 (0 to 43) | 9 (−10 to 34) |
| V5 | 39 (14 to 68) | 4 (−20 to 29) | 9 (−10 to 24) | −5 (−25 to 14) |
| V6 | 24 (4 to 43) | 0 (−25 to 14) | 4 (−10 to 19) | −10 (−25 to 9) |
IQR indicates inter‐quartile range.
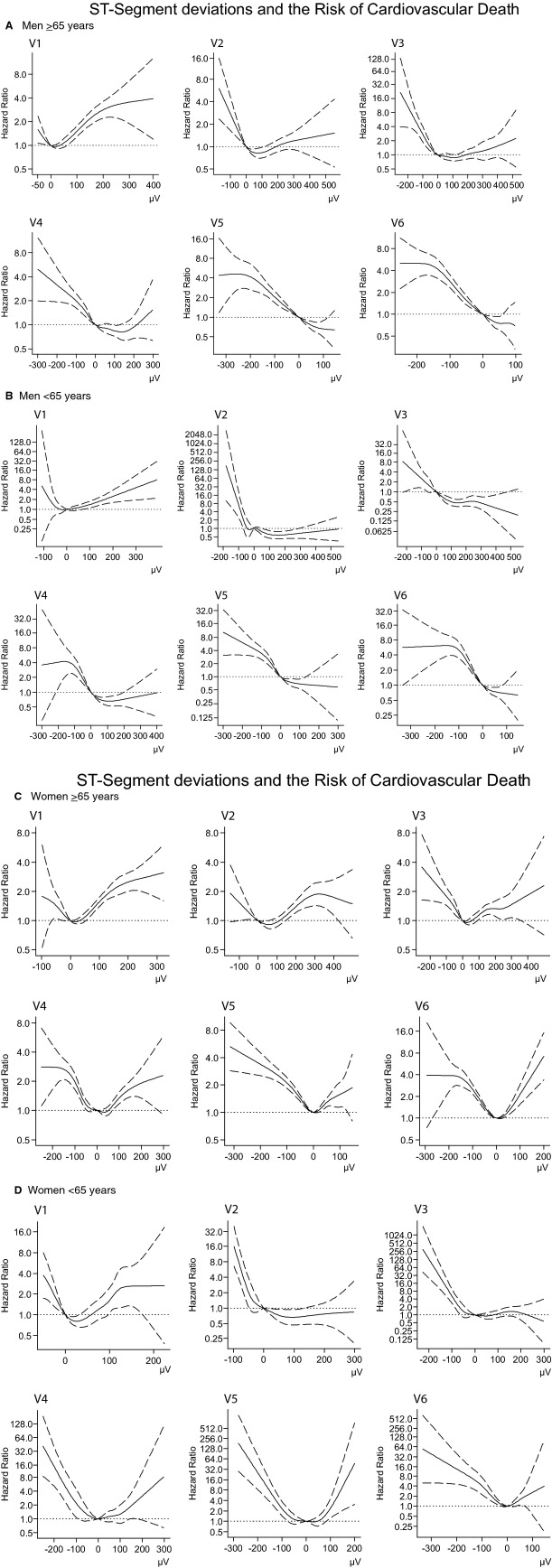
Restricted cubic spline analyses
The restricted cubic regression spline analyses were in concordance with the categorical analysis regarding the relationship between ST‐segment deviations and the risk of CVD (see Figure 6).
Figure 6.
Restricted cubic regression splines depicting the association between the ST‐segment amplitude and the risk of CVD based on cox regression models adjusted for the same covariates as the categorical analysis (see Methods). Note the different axes due to variation between leads. A, Men, old (≥65 years); (B) Men, young (<65 years); (C) Women, old (≥65 years); (D) Women, young (age <65 years).
Discussion
In a large primary care population without a history ischemic heart disease, we examined the spectrum and prevalence of precordial ST‐segment deviations and investigated the association with CVD. This revealed significant differences across age and gender determined subgroups.
We observed the highest prevalence of ST‐depressions in the lateral leads and found that the population ≥65 years had a much higher prevalence of depressions compared with the younger population. This is in accordance with the primary etiology for ST‐depression probably being ischemia or strain due to increased left ventricular workload. We found no notable differences in the occurrence of ST‐depression between genders.
The largest relative risk of CVD associated with ST‐depression was seen among the women and the young, although, as a result of low prevalences of ST‐depressions and outcomes in these groups, confidence interval limits were wide. However, when focusing on leads with higher prevalences of ST‐depression we found that ST‐segment depressions in leads V4 to V6 were associated with an increased risk of CVD in a dose‐response manner as seen in Figure 2. Our sample size and detailed measurement data allowed us to obtain information of prevalences and prognosis associated with various degrees of ST‐depression in individual precordial leads. As a result of this, we could identify leads V5 and V6 as those leads where the most robust association, with narrowest confidence limits, between ST‐depression and CVD is found.
A widely used method of categorizing ECG abnormalities is by Minnesota Coding, which contains codes for varying degrees of ST‐depression in groups of leads. However, these categories are often grouped into minor or major ST‐T abnormalities in order to obtain sufficient statistical power.14 Hence, previous studies have not been able to show convincing dose response associations or have not been able to report in what electrocardiographic lead that the statistical association was most robust.
Our findings regarding ST‐depressions are generally in line with other publications concerning ST‐depression being a marker of severe cardiovascular morbidity.1–3 The prognostic value of this was emphasized by De Bacquer et al, who showed that ST‐depression on the baseline ECG was the electrocardiographic abnormality, among several ECG findings, that showed the strongest predictive power of CVD.15
Data on prevalence and associated outcomes of ST‐segment elevations is a more complex matter, probably because of mixed etiologies and definitions. Our findings show that 70% among our subgroup of men <65 years of age had more than 100 μV of ST‐elevation in lead V2. In men ≥65 years of age, this proportion was almost halved to a prevalence of 40%. It is highly probable that the proportion of subjects with this “male‐type” ST‐elevation would have been even higher with a lower age cut‐off which is concordant with previous findings: In a study of 6014 healthy men aged 16 to 58 years from the United States Air Force, 91% had more than 100 μV of ST‐elevation in one or more of the precordial leads with V2 as the lead where ST‐elevations were most commonly seen.7 In a more recent study of 529 men between 17 to 24 years of age, 93% had ST‐elevations in leads V1 through V4. This prevalence declined with age and only 30% of the men who were ≥76 years had right precordial ST‐elevations on their resting ECG.8
Our findings in men are in contrast with the prevalence of ST‐elevation we found among women. We observed a much lower prevalence of major (>100 μV) ST‐elevations in lead V2 to V3 among women, and correspondingly a higher prevalence of minor elevations. This prevalence was not age dependent with 40% of the young women and 34% of the older women having the minor ST‐elevation. This “female‐type” of ST‐elevation with ST‐elevations <100 μV and with the same prevalence throughout age groups has previously been described by Surawicz et al.8
The fact that the majority of the young men had >100 μV ST‐elevation in lead V2 and V3 emphasizes a point previously proposed; that this is not only a normal variant but effectively the normal pattern of a young male subject, possibly associated with physical fitness.9
This point is further underlined by our association analyses in which we observed a decreased risk of cardiovascular death associated with V2 to V3 ST‐elevations in young men. We believe we are the first to show this. However, previous studies have hinted at this. Accordingly, the electrocardiographic pattern of early repolarization also with an upsloping ST‐segment in inferolateral leads has, in contrast to other types of early repolarization, been shown not to be associated with sudden arrhythmic death in a general population.16 In addition, a recent study of healthy middle‐aged males showed that the same form of ST‐elevation during exercise test was associated with a 30% reduced risk of death from coronary heart disease.17 Accordingly, having a nondeviating ST‐segment in these leads actually seems to represent a difference from the benign standard in young males, something that may signal poor fitness. Unfortunately, we do not possess data regarding physical fitness on our study population.
Also worth noting is that we observed no such decreased mortality associated with ST‐elevations in V2 to V3 among young women.
We observed a significantly increased risk of CVD for women and men with ST‐elevations in V1. However, major ST‐elevations (>100 μV) in lead V1 are generally very uncommon compared with lead V2 and lead V3 (Figure 1). The arrythmogenic condition known as Brugada Syndrome is characterized, among other things, by a characteristic ST‐elevation in V1 and a high risk of sudden cardiac death.18 Whether the causal mechanism underlying our observed increased risk of CVD for persons with ST‐elevations in lead V1, is caused by pathophysiological mechanisms similar to those underlying Brugada syndrome is not known. Nevertheless, major ST‐elevations in V1 remain a relatively rare but ominous harbinger of cardiovascular mortality in our study population. Furthermore, it is worth noting that the association, by far, is the least robust among young women.
We examined if ST‐elevations in lead V1 could simply be a mirror image of lateral ST‐depressions and hence explain the increased risk of CVD seen for lead V1 but this did not seem to be the case (see Table 2).
J‐point elevations in the left precordial leads, as a part of the early repolarization syndrome, has been associated with sudden cardiac death and cardiovascular mortality, but is still subject to some controversy.19–22 Interestingly, a recent study looking a J‐point elevation in any lead showed a significant increase in the risk of sudden cardiac death and coronary heart disease for women, but not for men.23 This finding could be explained by the association in men being cancelled out because of the presence of both protective and harmful ST‐elevation in this group and are thus in line with our findings.
All ECGs were analyzed digitally with the use of clinically validated software, thus avoiding any intra‐ or interobserver variability. We evaluated the computer‐based Marquette 12SL algorithm with respect to ST‐segment measurement against manual measurement and found a high degree of agreement (see Results and Figure 5). This is in accordance with previous findings that computer‐based algorithms are equal, or in fact superior, to visual measurement in terms of ST‐deviation measurement for the purpose of risk prediction.3,24
We performed restricted cubic regression splines, which showed similar associations between ST‐segment deviations and CVD as the categorical analyses. This underlines the fact that our findings are not affected whether a categorical or continuous approach is used. It should be noted that the spline analyses include ascending ST‐depressions and descending ST‐elevations contrary to the categorical analyses, indicating that these specific types of deviations do not distort our results.
Limitations
Our study cohort accounts for one‐third of the population in the greater region of Copenhagen, the capital of Denmark. Hence, we believe that our study sample is a fair representation of the background population. Nevertheless, it is appropriate to address that the individuals were referred to ECG recording by their general practitioner for a reason. To address this issue, we performed supplementary analyses in which we compared the rate of cardiovascular mortality in the study population with individuals from the general Danish population matched on age, gender, and without individuals with ischemic heart disease at the time of inclusion. We found a slightly increased CVD rate of 4.05 (95% CI 3.96 to 4.16) per 1000 person‐years in the study population compared with 3.78 (95% CI 3.71 to 3.84) in background population (P<0.001). Although this difference was statistically significant it seems negligible, indicating that our study population is fairly representative of the general population. Additionally most non‐opportunistic cohort studies suffer from the opposite issue with an often‐pronounced, healthy responder bias.25 Accordingly, we believe that our study population is a fair representation of the general population.
We investigated whether selection bias due to acute coronary syndrome could be an issue by performing a subgroup analysis in which we excluded individuals being hospitalized with ischemic heart disease within 14 days after inclusion. This did not significantly alter our results. Even though this approach represents some methodological problems with conditioning on the future, we believe it is justified in order to exclude a potential causal link to acute ischemia in our results.
Finally, we lack important anthropometric data on our study population such as body mass index (BMI), blood pressure, and smoking status and therefore we cannot exclude some residual confounding. However, we were able to adjust for several cardiovascular risk factors that are likely intermediate phenotypes for the possible confounding effect of BMI, blood pressure, and smoking status on the association between ST‐segment deviations and the risk of CVD. Instead of controlling for blood pressure, we were able to adjust for treatment with antihypertensive medication at inclusion. We believe that this approach might be as good as controlling for a single ambulatory blood pressure measurement, as such a blood pressure is not always a good estimate of an individual's true resting blood pressure.26–27
Conclusions
In this study of more than 280 000 primary care patients without a history of ischemic heart disease, we examined in detail the association between ST‐segment deviations and risk of CVD and found significant age and gender differences in some of the electrocardiographic leads.
We found ST‐depressions to confer an increased risk of CVD in a dose‐response manner in nearly all leads for both genders and age groups, with the most robust association seen in leads V5 and V6. ST‐elevations in women, in general, were associated with increased risk of CVD as were elevations in V1 in men. Among young men, however, we observed a decreased risk of CVD associated with ST‐elevations in V2 and V3. We believe we are the first to show a decreased risk of CVD with certain ST‐elevations. Prognostic information regarding these ST‐segment deviations could contribute towards improving the risk stratification of patients.
Sources of Funding
This study was supported by the University of Copenhagen, the Danish National Research Foundation, The Danish Council for Independent Research (Grant No 11‐107 456), The John and Birthe Meyer Foundation, The Villadsen Family Foundation, The Beckett Foundation, and the Copenhagen Medical Society (DMSK).
Disclosures
A.G. Holst is an employee of Novo Nordisk A/S, Denmark.
References
- 1.Auer R, Bauer DC, Marques‐Vidal P, Butler J, Min LJ, Cornuz J, Satterfield S, Newman AB, Vittinghoff E, Rodondi N. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012; 307:1497-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daviglus ML, Liao Y, Greenland P, Dyer AR, Liu K, Xie X, Huang CF, Prineas RJ, Stamler J. Association of nonspecific minor ST‐T abnormalities with cardiovascular mortality: the Chicago Western Electric Study. JAMA. 1999; 281:530-536. [DOI] [PubMed] [Google Scholar]
- 3.Okin PM, Devereux RB, Kors JA, van Herpen G, Crow RS, Fabsitz RR, Howard BV. Computerized ST depression analysis improves prediction of all‐cause and cardiovascular mortality: the strong heart study. Ann Noninvasive Electrocardiol. 2001; 6:107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen CT, Dahlin J, Blackburn H, Scharling H, Appleyard M, Sigurd B, Schnohr P. Prevalence and prognosis of electrocardiographic left ventricular hypertrophy, ST segment depression and negative T‐wave; the Copenhagen City Heart Study. Eur Heart J. 2002; 23:315-324. [DOI] [PubMed] [Google Scholar]
- 5.Sigurdsson E, Sigfusson N, Sigvaldason H, Thorgeirsson G. Silent ST‐T changes in an epidemiologic cohort study–a marker of hypertension or coronary heart disease, or both: the Reykjavik study. J Am Coll Cardiol. 1996; 27:1140-1147. [DOI] [PubMed] [Google Scholar]
- 6.Gorgels APM. Explanation for the electrocardiogram in subendocardial ischemia of the anterior wall of the left ventricle. J Electrocardiol. 2009; 42:248-249. [DOI] [PubMed] [Google Scholar]
- 7.Hiss RG, Lamb LE, Allen MF. Electrocardiographic findings in 67,375 asymptomatic subjects. X. Normal values. Am J Cardiol. 1960; 6:200-231. [DOI] [PubMed] [Google Scholar]
- 8.Surawicz B, Parikh SR. Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J Am Coll Cardiol. 2002; 40:1870-1876. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, Asinger RW, Marriott HJL. ST‐segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003; 349:2128-2135. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen JB, Graff C, Pietersen A, Lind B, Struijk JJ, Olesen MS, Haunsø S, Gerds TA, Svendsen JH, Køber L, Holst AG. J‐shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol. 2013; 25:2557-2564. [DOI] [PubMed] [Google Scholar]
- 11.Bille Nielsen J, Pietersen A, Graff C, Lind B, Jan Struijk J, Salling Olesen M, Haunsø S, Aalexander Gerds T, Thomas Ellinor P, Køber L, Hastrup Svendsen J, Gaarsdal Holst A. Risk of atrial fibrillation as a function of the electrocardiographic PR interval: results from the Copenhagen ECG Study. Heart Rhythm. 2013; 9:1249-1256. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen JB, Graff C, Rasmussen PV, Pietersen A, Lind B, Olesen MS, Struijk JJ, Haunsø S, Svendsen JH, Køber L, Gerds TA, Holst AG. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur Heart J. 2014. 10.1093/eurheartj/ehu081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD‐10 version of the Charlson comorbidity index predicted in‐hospital mortality. J Clin Epidemiol. 2004; 57:1288-1294. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn H. Classification of the electrocardiogram for population studies: Minnesota Code. J Electrocardiol. 1969; 2:305-310. [DOI] [PubMed] [Google Scholar]
- 15.De Bacquer D, De Backer G, Kornitzer M, Myny K, Doyen Z, Blackburn H. Prognostic value of ischemic electrocardiographic findings for cardiovascular mortality in men and women. J Am Coll Cardiol. 1998; 32:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, Sager SJ, Rissanen HA, Myerburg RJ, Reunanen A, Huikuri HV. Early repolarization: electrocardiographic phenotypes associated with favorable long‐term outcome. Circulation. 2011; 123:2666-2673. [DOI] [PubMed] [Google Scholar]
- 17.Hodnesdal C, Prestgaard E, Erikssen G, Gjesdal K, Kjeldsen SE, Liestol K, Skretteberg PT, Erikssen J, Bodegard J. Rapidly upsloping ST‐segment on exercise ECG: a marker of reduced coronary heart disease mortality risk. Eur J Prev Cardiol. 2012; 4:541-548. [DOI] [PubMed] [Google Scholar]
- 18.Mizusawa Y, Wilde AAM. Brugada syndrome. Circ Arrhythm Electrophysiol. 2012; 5:606-616. [DOI] [PubMed] [Google Scholar]
- 19.Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquié J‐L, Nogami A, Babuty D, Yli‐Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O'Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clémenty J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008; 358:2016-2023. [DOI] [PubMed] [Google Scholar]
- 20.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long‐term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009; 361:2529-2537. [DOI] [PubMed] [Google Scholar]
- 21.Haruta D, Matsuo K, Tsuneto A, Ichimaru S, Hida A, Sera N, Imaizumi M, Nakashima E, Maemura K, Akahoshi M. Incidence and prognostic value of early repolarization pattern in the 12‐lead electrocardiogram. Circulation. 2011; 123:2931-2937. [DOI] [PubMed] [Google Scholar]
- 22.Hisamatsu T, Miura K, Ohkubo T, Yamamoto T, Fujiyoshi A, Miyagawa N, Kadota A, Takashima N, Okuda N, Matsumura Y, Yoshita K, Kita Y, Murakami Y, Nakamura Y, Okamura T, Horie M, Okayama A, Ueshima HNIPPON DATA80 Research Group. Interaction between dietary marine‐derived n‐3 fatty acids intake and J‐point elevation on the risk of cardiac death: a 24‐year follow‐up of Japanese men. Heart. 2013; 99:1024-1029. [DOI] [PubMed] [Google Scholar]
- 23.Olson KA, Viera AJ, Soliman EZ, Crow RS, Rosamond WD. Long‐term prognosis associated with J‐point elevation in a large middle‐aged biracial cohort: the ARIC study. Eur Heart J. 2011; 32:3098-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ammar KA, Kors JA, Yawn BP, Rodeheffer RJ. Defining unrecognized myocardial infarction: a call for standardized electrocardiographic diagnostic criteria. Am Heart J. 2004; 148:277-284. [DOI] [PubMed] [Google Scholar]
- 25.Drivsholm T, Eplov LF, Davidsen M, Jørgensen T, Ibsen H, Hollnagel H, Borch‐Johnsen K. Representativeness in population‐based studies: a detailed description of non‐response in a Danish cohort study. Scand J Public Health. 2006; 34:623-631. [DOI] [PubMed] [Google Scholar]
- 26.Stergiou GS, Baibas NM, Gantzarou AP, Skeva II, Kalkana CB, Roussias LG, Mountokalakis TD. Reproducibility of home, ambulatory, and clinic blood pressure: implications for the design of trials for the assessment of antihypertensive drug efficacy. Am J Hypertens. 2002; 15:101-104. [DOI] [PubMed] [Google Scholar]
- 27.Niiranen TJ, Jula AM, Kantola IM, Reunanen A. Comparison of agreement between clinic and home‐measured blood pressure in the Finnish population: the Finn‐HOME Study. J Hypertens. 2006; 24:1549-1555. [DOI] [PubMed] [Google Scholar]