Abstract
Background
Therapeutic hypothermia (TH) is recommended to reduce ischemic brain injury after cardiac arrest. The variables that predict heat generation by patients receiving TH are uncertain, as is how this heat generation relates to neurologic outcome. We hypothesized that patient characteristics, medication use, inflammation, and organ injury would be associated with heat generation. We further hypothesized that neurologic outcome would be most strongly associated with heat generation.
Methods and Results
Surface and intravascular cooling devices were used to provide TH in 57 consecutive cardiac arrest patients. Device water temperatures during the maintenance (33°C) phase were collected. Patient heat generation was quantified as the “heat index” (HI), which was the inverse average water temperature over a minimum of 2 hours of maintenance hypothermia. Variables measuring reduced ischemic injury and improved baseline health were significantly associated with HI. After controlling for presenting rhythm, a higher HI was independently associated with favorable disposition (OR=2.2; 95% CI 1.2 to 4.1; P=0.014) and favorable Cerebral Performance Category (OR=1.8; 95% CI 1.0 to 3.1; P=0.035). Higher HI predicted favorable disposition (receiver‐operator area under the curve 0.71, P=0.029). HI was linearly correlated with arteriovenous CO2 (r=0.69; P=0.041) but not O2 (r=0.13; P=0.741) gradients.
Conclusions
In cardiac arrest patients receiving TH, greater heat generation is associated with better baseline health, reduced ischemic injury, and improved neurologic function, which results in higher metabolism. HI can control for confounding effects of patient heat generation in future clinical trials of rapid TH and offers early prognostic information.
Keywords: cardiac arrest, cardiopulmonary resuscitation, ischemia, prognosis
Introduction
Sudden cardiac arrest (CA) affects >500 000 people in the United States annually.1–2 Fewer than 1 in every 10 of these patients will survive to hospital discharge3 with the majority of deaths occurring as a result of neurologic injury.4 Since 2002, therapeutic hypothermia (TH) has emerged as the only effective post‐resuscitation therapy for neurologic injury.5–7 TH has a class I indication after ventricular fibrillation/ventricular tachycardia (VF/VT) out‐of‐hospital CA7 and neonatal hypoxic‐ischemic injury.8 At present, hypothermia is being actively investigated as an adjunct in treatment of other forms of acquired brain injury9 and myocardial infarction.10 Thus, TH offers benefit for a number of illnesses carrying significant morbidity and mortality, and gaining an understanding to its clinical application is crucial in future clinical trials.
Several animal studies11–14 and 2 clinical studies15–16 have shown that faster time to target temperature results in better outcomes. However, this result has not been confirmed in 2 other clinical studies prospectively comparing early versus late initiation of TH.17–18 Indeed some clinical studies have shown that several indirect measures of reduced patient heat generation (lower presenting body temperatures, shorter time to goal temperature, longer passive rewarming times) predict worsened outcomes.19–21 In designing studies aimed at rapid achievement of TH, it is therefore crucial to quantify the contribution of patient‐level variables in heat generation.
We hypothesized that differences in patient heat generation during TH could explain the inconsistencies between preclinical and clinical results. Furthermore, we hypothesized that patient heat generation, quantified as a “Heat Index” (HI), could be associated with patient demographics, body habitus, pre‐existing comorbidities, and the presence of infection, inflammation, or more severe ischemic injury, and could also be influenced by delivery of adjunctive medications commonly used to suppress shivering. Based on the existing literature,19–22 we hypothesized that greater heat generation would be associated with better neurologic outcomes and survival to hospital discharge. Therefore, the aim of this study was to determine which factors contribute to heat generation in patients receiving TH and to provide insight to clinicians for neuroprognostication and in designing future clinical trials of rapidly induced TH.
Methods
Study Population
This study used data prospectively gathered at 2 hospitals (UPMC Presbyterian and Mercy) as part of ongoing quality improvement (QI) efforts in post‐resuscitation care and TH implementation. It is our institutional standard to provide therapeutic hypothermia to all comatose survivors of CA except in the setting of active hemorrhage or severe antecedent bradycardia or heart block. Data was obtained from consecutive out‐of‐hospital and in‐hospital CA survivors who received TH and were maintained within the target temperature (33°C) for a minimum of 2 consecutive hours using either an intravascular device (ThermoGard XP; Zoll Medical) or adhesive surface cooling pads (Arctic Sun 5000; Bard Medical). As part of our QI efforts patient and water temperature data were downloaded from each device to analyze how units were performing at reaching and maintaining target cooling and rewarming temperatures. These data were linked to patient‐level data on a list of variables (Table 1) with intentions to understand variations from goals. The Institutional Review Board deemed this analysis of de‐identified QI data to be exempt from review.
Table 1.
Variables Included Within the Quality Improvement (QI) Database
| Variable | Explanation |
|---|---|
| Demographics | |
| Hospital | University of Pittsburg Medical Center (UPMC) Presbyterian, Mercy, Shadyside or Montefiore |
| Hospital unit | Intensive care unit name |
| Sex | |
| Age | |
| Living situation prior to arrest | Independent, dependent at home, nursing home, in hospital, psychiatric institute, or other |
| Weight | |
| Body mass index | |
| Body surface area | |
| Cardiac arrest | |
| Time of arrest | Time of day |
| Location of arrest | Out of hospital, in emergency department, or in hospital |
| Arrest witnessed or monitored | |
| Cause of arrest | Respiratory, myocardial infarction, arrhythmia, trauma, pulmonary embolism, or hypothermia |
| Cardiopulmonary resuscitation (CPR) | No CPR, untrained CPR, or trained CPR |
| Time from cardiac arrest (CA) to CPR | |
| Duration of CPR | |
| Presenting rhythm | |
| Number of defibrillations | |
| Time to defibrillation | |
| Type of resuscitative drugs used | No drugs, epinephrine, norepinephrine, vasopressin, atropine, sodium bicarbonate, amiodarone, dopamine, dobutamine, lidocaine, or other |
| Location of return of spontaneous circulation (ROSC) | OHCA‐ on scene, OHCA‐off scene, IHCA |
| Time to ROSC | |
| Time to hospital | |
| Intrafacility transport | |
| First Glasgow Coma Scale (GCS) | |
| First FOUR Score | |
| Pittsburgh CA Category (PCAC) | Category 1 to 4 within 6 h of ROSC (Ref 22) |
| Hypothermia | |
| Primary cooling device | Device used during all phases of cooling |
| Secondary cooling device | Optional devices used at initiation |
| Emergency department cooling device | Optional devices used at initiation |
| Pre‐hospital cooling | Was iced saline given? |
| Ice packs | No used, before cooling device, during cooling device, or used but unknown when |
| Cold fluid | No used, before cooling device, during cooling device, or used but unknown when |
| Time from CA to cold fluids | |
| Volume of cold fluids | |
| Pressure bag or infusion | |
| Pump infusion rate | min/L |
| Temperature measurement type | Axillary, bladder, brain, ear, esophageal, oral, or rectal |
| Time from CA to first cooling device | |
| Patient temperature at start of cooling | |
| Mean therapeutic hypothermia (TH) target temperature of device | |
| Patient temperature difference from start of TH and 34°C | |
| Time from start of TH to 34°C | |
| Time from CA to 34°C | |
| Time to reach target temperature | Never reached, ≤12 h, >12 h |
| Time from beginning of TH to target temperature | |
| Mean cooling rate (°C/h) | |
| Mean warming rate with a rise in target temperature (°C/h) | |
| Duration of TH (<34°C) | |
| Time from target temperature to rewarming | |
| Day 1: percent of time patient temperature is >0.5°C below target temperature | |
| Day 1: percent of time patient temperature is >1.0°C below target temperature | |
| Day 1: percent of time patient temperature is >0.5°C above target temperature | |
| Day 1: percent of time patient temperature is >1.0°C above target temperature | |
| Day 1: total percent of time patient temperature is within target temperature range | |
| Day 2: percent of time patient temperature is >0.5°C below target temperature | |
| Day 2: percent of time patient temperature is >1.0°C below target temperature | |
| Day 2: percent of time patient temperature is >0.5°C above target temperature | |
| Day 2: percent of time patient temperature is >1.0°C above target temperature | |
| Day 2: total percent of time patient temperature is within target temperature range | |
| Day 3: percent of time patient temperature is >0.5°C below target temperature | |
| Day 3: percent of time patient temperature is >1.0°C below target temperature | |
| Day 3: percent of time patient temperature is >0.5°C above target temperature | |
| Day 3: percent of time patient temperature is >1.0°C above target temperature | |
| Day 3: total percent of time patient temperature is within target temperature range | |
| All days: percent of time patient temperature is >0.5°C below target temperature | |
| All days: percent of time patient temperature is >1.0°C below target temperature | |
| All days: percent of time patient temperature is >0.5°C above target temperature | |
| All days: percent of time patient temperature is >1.0°C above target temperature | |
| All days: total percent of time patient temperature is within target temperature range | |
| Duration of time patient temperature below 30°C | |
| Lowest temperature reached | |
| Rewarming | |
| Initial patient temperature | Measures at start of rewarming |
| Mean rewarming target temperature of device | |
| Time from start temperature to target temperature | |
| Mean rewarming rate (°C/h) | |
| Time above 1.0°C of target temperature in first 24 h | |
| Duration of fever (>37.8°C) or fever management | |
| Fever management | Were measures used to prevent fever? |
| Hypothermia stopped early | Yes, due to complications; Yes, limitation of care; No |
| Interventions and labs | |
| Coronary angiography | |
| Stenting | |
| Lowest heart rate | Following ROSC |
| Highest heart rate | Following ROSC |
| Lowest mean arterial pressure (MAP) | Following ROSC |
| Highest MAP | Following ROSC |
| Lowest troponin | Following ROSC |
| Highest creatine kinase MB | Following ROSC |
| Highest creatine phosphokinase | Following ROSC |
| Lowest hemoglobin | Following ROSC |
| Lowest white blood count | Following ROSC |
| Highest white blood count | Following ROSC |
| Initial creatinine | Following ROSC |
| Highest creatinine | Following ROSC |
| Initial blood urea nitrogen | Following ROSC |
| Initial lactate | Following ROSC |
| Highest lactate | Following ROSC |
| Lowest magnesium | Following ROSC |
| Lowest glucose | Following ROSC |
| Highest glucose | Following ROSC |
| Lowest potassium | Following ROSC |
| Lowest phosphorus | Following ROSC |
| Day 1: total volume fluids in | |
| Day 1: total volume fluids out | |
| Day 2: total volume fluids in | |
| Day 2: total volume fluids out | |
| Day 3: total volume fluids in | |
| Day 3: total volume fluids out | |
| Blood gas and respiratory | |
| Initial pH | Following ROSC |
| Lowest pH | Following ROSC |
| Highest pH | Following ROSC |
| Initial pO2 | Following ROSC |
| Lowest pO2 | Following ROSC |
| Arteriovenous pO2 gradient | Following ROSC |
| Initial pCO2 | Following ROSC |
| Arteriovenous pCO2 gradient | Following ROSC |
| Initial HCO3 | Following ROSC |
| Initial pSaO2 | Following ROSC |
| Initial base deficit | Following ROSC |
| Initial base excess | Following ROSC |
| Highest FiO2 | Following ROSC |
| Days on ventilator | |
| Color of secretions | Within 24 h of rewarming |
| Time of secretions | No secretions, during cooling, or during rewarming |
| Infection suspected | |
| Infection | Documented infection based on positive culture or imaging plus clinical diagnosis/treatment noted in medical record |
| Positive culture results | Negative, bacterial, viral, fungal, or parasite |
| Time of positive culture results | Negative, during cooling, or during rewarming |
| Medication | |
| Day started on antibiotics | |
| Days given antibiotics | |
| Days magnesium >8 mg | |
| Days given skin counter warmer | |
| Fentanyl | Over initial 24 h following CA |
| Propofol | Over initial 24 h following CA |
| Aspirin | Over initial 24 h following CA |
| Acetaminophen | Over initial 24 h following CA |
| Midazolam | Over initial 24 h following CA |
| Lorazepam | Over initial 24 h following CA |
| Alprazolam | Over initial 24 h following CA |
| Rocuronium | Over initial 24 h following CA |
| Vercuronium | Over initial 24 h following CA |
| Morphine | Over initial 24 h following CA |
| Hydromorphine | Over initial 24 h following CA |
| Ketamine | Over initial 24 h following CA |
| Pressors | |
| Dobutamine | mg/h over induction phase |
| mg/h over maintenance phase | |
| mg/h over rewarming phase | |
| Epinephrine | mg/h over induction phase |
| mg/h over maintenance phase | |
| mg/h over rewarming phase | |
| Norepinephrine | mg/h over induction phase |
| mg/h over maintenance phase | |
| mg/h over rewarming phase | |
| Vasopressin | units/h over induction phase |
| units/h over maintenance phase | |
| units/h over rewarming phase | |
| Milrinone | mg/h over induction phase |
| mg/h over maintenance phase | |
| mg/h over rewarming phase | |
| Phenylephrine | mg/h over induction phase |
| mg/h over maintenance phase | |
| mg/h over rewarming phase | |
| Dopamine | mg/h over induction phase |
| mg/h over maintenance phase | |
| mg/h over rewarming phase | |
| Outcomes at discharge | |
| Pittsburgh cerebral performance category (CPC) | CPC 1 to 5 |
| Discharge location | Died, home, acute rehabilitation, nursing home, hospice, other |
| Mini‐mental state examination (MMSE) | Maximum score 30 |
| GCS | 3 to 15 |
| Days in hospital | |
| Survival | Yes/no |
IHCA indicates in hospital cardiac arrest; OHCA, out of hospital cardiac arrest.
Therapeutic Hypothermia
Standard protocols with accompanying computerized entry order sets were used in both hospitals following CA. Similar to prior work,23 these protocols included inducing TH to 33°C as quickly as possible using maximum cooling on the devices, along with most patients receiving 2 L iced saline and ice packs applied to the neck and groin before and during induction of TH. These adjuncts were not used in any of the included patients during maintenance TH. Patients were to be maintained at 33°C for 24 hours and rewarmed to 36.5 to 37°C at a goal rate of 0.25°C/h, (maximum rate 0.5°C/h). The patients included in this report were either treated using external gel‐coated pads (Arctic Sun), 2 placed on the torso and 2 on the legs, or a 3 or 4 balloon femoral venous intravascular cooling catheter (Zoll Icy or Quattro catheters). Our standard anti‐shivering regimen consists of use of sedation (propofol or midazolam), occasional opioid analgesia (fentanyl or morphine infusion), and occasional neuromuscular blockade (vecuronium or rocuronium, generally used only at induction of hypothermia). Continuous neuromuscular blockade is infrequently employed in our facilities for these patients.
Measuring Heat Generation
Patients' heat generation during the maintenance phase was quantified using a “Heat Index” (HI) developed for this study. The HI was calculated as ×100 the inverse average machine water temperature over the period in which patient temperature reached the target temperature of the maintenance phase (33°C) of TH for and was maintained for a minimum of 2 consecutive hours. Device water temperature is automatically recorded every minute. When TH was paused for patient transport, we did not resume this calculation until the patient was back at goal temperature. In all cases we averaged as much time as possible until rewarming began, which was indicated by a change in the goal temperature on the machine. HI as calculated directly quantifies patient heat generation since a higher HI indicates a lower water temperature was required to maintain the patient at 33°C.
Outcome Assessment
Our primary outcome endpoint was discharge disposition, which strongly correlates with other measures of neurologic outcome24 and is ultimately based on the patient's functional status at discharge. Other outcomes assessed were discharge Pittsburgh Cerebral Performance Category (CPC) and survival. CPC24 was defined using strict criteria such that patients with CPC 1 or 2 must be able to perform all activities of daily living and be able to return to work independently or in a sheltered environment. CPC and disposition were dichotomized as favorable or unfavorable. Favorable disposition was defined as discharge to home or acute rehabilitation, while unfavorable disposition was discharge to an advanced care or skilled nursing facility, hospice or death. A CPC of 1 or 2 was considered favorable whereas a CPC of 3 to 5 was unfavorable.
Additional Data Collection
Clinical, laboratory, and pharmacological data (Table 1) from the QI database was abstracted if it related to 1 of 6 predetermined patient characteristics: (1) demographics, (2) body habitus, (3) patient health at time of CA, (4) presence of infection or inflammation, (5) measures of ischemic injury severity, and (6) medication use to prevent shivering. A full list of the variables included in this study and how they were defined is stated in Table 2. CA data routinely collected include the location of arrest (out‐of‐ or in‐hospital), gender, the presenting rhythm (dichotomized as VF/VT or non‐VF/VT), and Pittsburgh CA category, a post‐arrest injury severity score.22 This score is based on the neurological examination, hemodynamics, and ventilator settings within 6 hours of arrest and is described elsewhere.22 Using this simple 4‐point scale, patients with the lowest CA category (I) had expected survival to discharge of ≈80% (60% with good neurologic outcome) as opposed to category IV patients who had survival <10% (<5% with good neurologic outcome). In patients who had paired arterial and superior vena cava central venous blood gases available within 30 minutes of one another, arteriovenous oxygen (A‐VO2) and carbon dioxide (A‐VCO2) gradients were calculated as they have been shown to correlate with cardiac output and metabolic energy expenditure.25–26 These were obtained as we hypothesized that higher heat generation was the result of higher metabolic rate and thus carbon dioxide generation presumably due to a higher hypothalamic/pituitary mediated set point. Both arteriovenous oxygen and carbon dioxide gradients have been demonstrated to correlate with cardiac output26 but only carbon dioxide differences correlate with metabolism and heat generation.25
Table 2.
Clinical, Laboratory, and Pharmacological Data Hypothesized to be Associated With Patient Heat Generation
| Variable | Explanation |
|---|---|
| Demographics | |
| Sex | |
| Age | |
| Location of arrest | In hospital cardiac arrest (IHCA) or out of hospital cardiac arrest (OHCA) |
| Presenting rhythm | Dichotomized to ventricular fibrillation (VF)/ventricular tachycardia (VT) or non‐VF/VT |
| Body habitus | |
| Weight | Weight |
| Body mass index | Body mass index |
| Body surface area | Body surface area |
| Patient health at time of cardiac arrest | |
| Comorbidities | Dichotomized to none/few, or moderate/many |
| Lowest white blood count | Following return of spontaneous circulation (ROSC) |
| Measures of ischemic injury severity | |
| Time to ROSC | |
| Patient temperature at start of cooling | |
| Pittsburgh cerebral performance category (CPC) | Favorable (1 to 2) or unfavorable (3 to 5) |
| Disposition | Favorable (home/acute rehabilitation) or unfavorable (other) |
| Pittsburgh CA category (PCAC) | Category 1 to 4 within 6 h of ROSC |
| Days in hospital | |
| Survival | Yes/no |
| Lowest heart rate | Following ROSC |
| Highest heart rate | Following ROSC |
| Lowest mean arterial pressure (MAP) | Following ROSC |
| Highest MAP | Following ROSC |
| Lowest troponin | Following ROSC |
| Highest creatine kinase MB | Following ROSC |
| Highest creatine phosphokinase | Following ROSC |
| Lowest hemoglobin | Following ROSC |
| Initial creatinine | Following ROSC |
| Highest creatinine | Following ROSC |
| Initial blood urea nitrogen | Following ROSC |
| Initial lactate | Following ROSC |
| Highest lactate | Following ROSC |
| Lowest glucose | Following ROSC |
| Highest glucose | Following ROSC |
| Lowest potassium | Following ROSC |
| Initial pH | Following ROSC |
| Lowest pH | Following ROSC |
| Arteriovenous pO2 gradient | Following ROSC |
| Arteriovenous pCO2 gradient | Following ROSC |
| Initial base deficit | Following ROSC |
| Medication use to prevent shivering (cumulative dose in mg) | |
| Fentanyl | Over initial 24 h following CA |
| Propofol | Over initial 24 h following CA |
| Aspirin | Over initial 24 h following CA |
| Acetaminophen | Over initial 24 h following CA |
| Midazolam | Over initial 24 h following CA |
| Lorazepam | Over initial 24 h following CA |
| Vercuronium | Over initial 24 h following CA |
| Presence of infection or inflammation | |
| Infection present | Documented infection based on positive culture or imaging plus clinical diagnosis/treatment noted in medical record |
| Highest white blood count | Following ROSC |
| Initial patient temperature | Measured at the start of cooling |
These variables were included in the regressions analysis for heat index.
Statistical Analysis
Univariate linear regressions determined the association of each of the grouped variables (Table 2) with HI. Variables associated with HI in the univariate analyses (P<0.05) were included in a multivariable backward stepwise linear regression to determine independent association with HI and are reported as coefficients with 95% confidence intervals (95% CI). Univariate binary logistic regressions determined the association of HI with each outcome endpoint expressed as an odds ratio (OR) with 95% CI. A series of univariate analyses determined the association of known predictors of outcome of CA with the primary endpoints (Table 3). To avoid over fitting the model, the strongest associated predictor was included in a bivariate binary logistic regression as a control to determine the independent association of HI with each endpoint, also expressed as an odds ratio (OR) with 95% CI. The area under the receiver‐operator curve (ROC) determined the predictive strength of HI for each outcome. Mann‐Whitney U test determined differences in median HI between favorable versus unfavorable outcome cohorts for each endpoint. Chi square analysis compared the proportion of patients with favorable and unfavorable discharge disposition based on Pittsburgh CA category.22 Correlation between HI and A‐VO2 and A‐VCO2 was determined based on Pearson's regression. In all cases 2‐tailed P<0.05 was considered significant. Regressions were performed using SPSS v19 (IBM Inc) and other analyses using Prism v6.01 (GraphPad Software, Inc).
Table 3.
Binary Logistic Regressions Examining the Association Between Known Predictors of Outcome Following Cardiac Arrest and Each Endpoint
| Disposition | Survival | Cerebral Performance Category | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | Odds Ratio (95% Confidence Interval) | P Value | |
| Age | 0.98 (0.94, 1.02) | 0.359 | 1.01 (0.98, 1.05) | 0.492 | 1.00 (0.96, 1.04) | 0.894 |
| Sex | 0.99 (0.27, 3.72) | 0.991 | 1.54 (0.52, 4.55) | 0.434 | 1.03 (0.30, 3.56) | 0.965 |
| Rhythm (VF/VT, non‐VF/VT) | 9.60 (2.14, 43.05) | 0.003 | 4.56 (1.39, 14.97) | 0.012 | 5.44 (1.45, 20.40) | 0.012 |
| Location of arrest (IHCA, OHCA) | 0.77 (0.19, 3.04) | 0.704 | 0.22 (0.07, 0.72) | 0.012 | 0.44 (0.12, 1.57) | 0.204 |
| PCAC | 0.42 (0.20, 0.86) | 0.018 | 0.14 (0.04, 0.43) | 0.001 | 0.45 (0.22, 0.90) | 0.024 |
IHCA indicates in hospital cardiac arrest; OHCA, out of hospital cardiac arrest; PCAC, Pittsburgh CA category; VF, ventricular fibrillation; VT, ventricular tachycardia.
Results
A total of 76 consecutive, comatose post‐CA patients were treated with TH using one of the designated devices between October 2011 and September 2012. Of these, 19 patients were excluded from the study due to the target temperature of the machine being set to a temperature other than 33°C (n=5), inadequate time at 33°C due to truncated cooling (n=3), or frequent (>10% of total time) interruptions of cooling (n=2), improper programming of the cooling device (n=7), or the patient not maintaining 33°C despite full protocol execution (n=2). To avoid bias we examined the 2 patients excluded due to inadequate time at goal temperature despite appropriate protocol use by using the available maintenance data. One patient maintained at goal for only 106 minutes had an HI of 6.93 and survived with favorable CPC. He was discharged back to the nursing home from which he came. The other patient was maintained at goal for 67 minutes with HI of 2.93. This patient died.
Thus, 57 patients remained for the final analysis. A total of 36 (63%) patients died before hospital discharge. Patient demographics are shown in Table 4. Patients were cooled using the Arctic Sun 5000® (n=20), and ThermoGard® Icy (n=18) and Quattro (n=19) catheters. We found minimal variation in the mean HI (Figure 1) or ranges for each device indicating similar cooling efficiencies (P=0.371), therefore the data were pooled for analysis.
Table 4.
Patient Demographics and Characteristics
| Overall Population (n=57) | Favorable Disposition (n=11) | Unfavorable Disposition (n=46) | |
|---|---|---|---|
| Age (SD) | 59 (17) | 55 (12) | 60 (18) |
| Male | 31 (54%) | 6 (55%) | 25 (54%) |
| OHCA | 39 (68%) | 7 (64%) | 32 (70%) |
| Presenting rhythm | |||
| VF/VT | 19 (33%) | 9 (82%) | 10 (22%) |
| PEA | 17 (30%) | 1 (9%) | 16 (35%) |
| Asystole | 18 (32%) | 1 (9%) | 17 (37%) |
| Unknown | 3 (5%) | — | 3 (6%) |
| Favorable CPC | 13 (23%) | 9 (82%) | 4 (9%) |
| Survival | 21 (37%) | 11 (100%) | 10 (22%) |
CPC indicates cerebral performance category; OHCA, out of hospital cardiac arrest; PEA, pulseless electrical activity; VF, ventricular fibrillation; VT, ventricular tachycardia.
Figure 1.
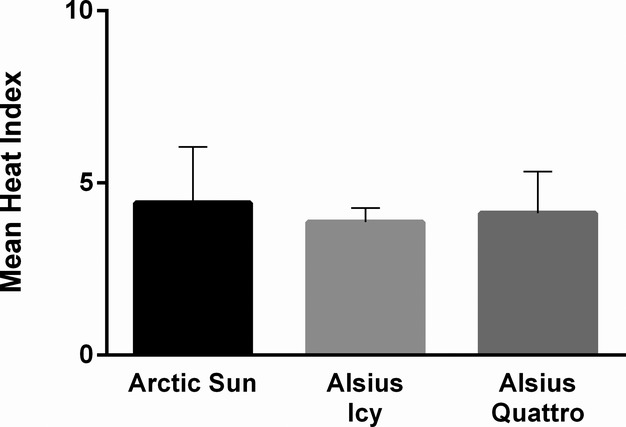
Mean heat generation by cooling device. Mean±SD of HI for each device during the maintenance phase of TH with target temperature set at 33°C. The HI did not differ significantly between groups (P=0.371). HI indicates heat index; TH, therapeutic hypothermia.
Patient Level Variables Associated With Heat Generation
To test our hypothesis that various patient‐level variables (Table 2) could account for fluctuations in heat generation, we performed univariate linear regressions to determine the association of these variables with the HI. Six of the analyzed variables had some association with HI (Table 5). Of these 6, only 3 were independently associated with HI (P<0.05) in a multivariable step‐wise backward linear regression. Increased heat generation was associated with higher minimum WBC count (ie, less leukopenia), a marker of poor health or immunosuppression, higher initial lactate, a marker of ischemia severity, and higher initial 24‐hour cumulative propofol dosage (Table 6). We were unable to analyze several medications (alprazolam, rocuronium, morphine, hydromorphine, and ketamine), which may potentially alter heat generation due to their underutilization (<10 patients having received the medication). Surprisingly, patient size did not associate with HI (BMI: P=0.440; BSA: P=0.417; weight: P=0.858).
Table 5.
Univariate Predictors of Heat Generation
| Unstandardized Coefficient (95% Confidence Interval) | P Value | |
|---|---|---|
| Lowest hemoglobin, mg/dL | 0.186 (0.060, 0.312) | 0.005 |
| Lowest WBC, ×109 | 0.068 (0.000, 0.135) | 0.049 |
| Initial lactate, mmol/L | −0.119 (−0.188, −0.051) | 0.001 |
| Maximum glucose, mg/dL | −0.028 (−0.050, −0.006) | 0.014 |
| Propofol, mg | 0.011 (0.002, 0.020) | 0.019 |
| Acetylsalicylic acid, mg | 0.028 (0.001, 0.056) | 0.041 |
Variables associated with heat index in univariate linear analyses. Minimum hemoglobin and white blood count (WBC) are the lowest values observed following return of spontaneous circulation, while maximum glucose is the highest value observed. Initial lactate is the earliest recorded value after return of spontaneous circulation. Propofol and acetylsalicylic acid are recorded as the cumulative dosage over the initial 24 hours following cardiac arrest.
Table 6.
Independent Predictors of Heat Generation
| Unstandardized Coefficient (95% Confidence Interval) | P Value | |
|---|---|---|
| Minimum WBC, ×109 | 0.072 (0.013, 0.130) | 0.018 |
| Initial lactate, mmol/L | −0.099 (−0.165, −0.033) | 0.004 |
| Propofol, mg | 0.009 (0.001, 0.017) | 0.025 |
Variables independently associated with heat index in a backward multivariable model. WBC indicates white blood count.
Association Between Heat Generation and Outcomes
To test the hypothesis that greater heat generation predicts better neurologic outcome, we performed a univariate binary logistic regression between HI and our endpoints. HI was associated (Figure 2A) with favorable disposition (OR=2.26; 95% CI: 1.19, 4.29; P=0.013) and CPC (OR=1.91; 95% CI: 1.08, 3.37; P=0.026), while a trend was observed with survival (OR=1.67; 95% CI: 0.97, 2.88; P=0.067). Our analysis of the known predictors of outcome after CA showed presenting rhythm, dichotomized as presentation with or without VF/VT, had the strongest association with the endpoints (Table 3). After adjustment for presenting rhythm, HI remained independently associated with favorable disposition and CPC (Figure 2B) in bivariate logistic regression. A sensitivity analysis performed defining “good outcome” to include patients who were admitted from a nursing home and discharged to a similar nursing home (n=2) did not alter these associations. A separate sensitivity analysis excluding patients with a clinical diagnosis of sepsis (n=2) or fever (n=4) antecedent to hypothermia induction also did not alter these results.
Figure 2.
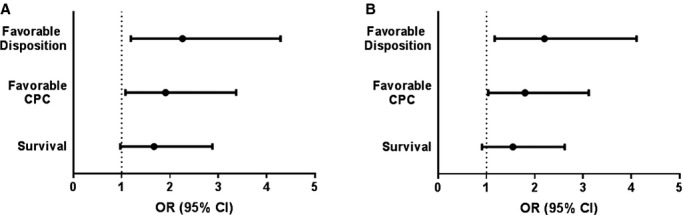
Association between heat index (HI) and outcomes. A, OR and 95% CI for each endpoint with HI, with favorable disposition and CPC demonstrating a significant association. B, OR and 95% CI for each endpoint with HI after adjusting for presenting rhythm, the major factor associated with CA outcome (Table 3). This adjustment minimally altered associations. CA indicates cardiac arrest; CPC, cerebral performance category; OR, odds ratio.
HI distribution differed significantly (P=0.029) between patients with favorable (median HI=4.63) and unfavorable (median HI=3.80) disposition (Figure 3A). HI predicted (Figure 3B) favorable disposition (AUC=0.71, P=0.029) with optimal discrimination at a cut‐off of HI=4.45, which had a sensitivity of 0.636 and specificity of 0.826 for predicting favorable disposition (likelihood ratio=3.66). Analyses for the endpoints of CPC (favorable CPC median HI=4.45, unfavorable CPC median HI=3.80, AUC=0.677, P=0.055) and survival (survivors median HI=4.19, nonsurvivors median HI=3.73, AUC=0.626, P=0.116) yielded similar trends.
Figure 3.
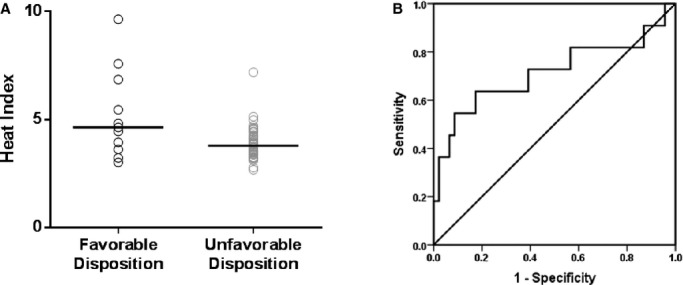
Association between heat index (HI) and favorable disposition. A, Distribution of HI within the primary endpoint demonstrates a higher median HI in patients with favorable compared to unfavorable disposition (P=0.029). B, HI significantly predicts favorable disposition with an area under the receiver operator curve of 0.71 (P=0.029).
Heat Index Measures Metabolic Rate
In order to corroborate the theory that HI was indeed measuring metabolic rate, we examined its correlation with arteriovenous carbon dioxide (A‐VCO2) and oxygen (A‐VO2) gradients. Others have demonstrated that A‐VCO2 is correlated with both metabolic rate (CO2 generation) and cardiac output whereas A‐VO2 tends to be primarily a measure of cardiac output.26 Consistent with the hypothesis that HI is measuring metabolic rate, HI correlated well with A‐VCO2 (Spearman's rho=0.68; P=0.043) but not with A‐VO2 (Spearman's rho=0.32, P=0.406) (Figure 4).
Figure 4.
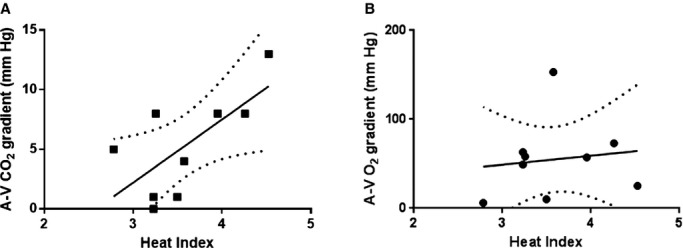
Correlation between heat index and arteriovenous gradients. Heat index was significantly correlated with the (A) arteriovenous carbon dioxide gradient (A‐VCO2; Spearman's rho=0.68; P=0.043) but not (B) arteriovenous oxygen gradient (A‐VO2; Spearman's rho=0.32, P=0.406). The linear regression line and 95% confidence intervals are also plotted for each correlation.
Differences in Heat Generation After Adjustment for Injury Severity
Based on the results above, it is clear that clinical trials of more rapid cooling would need to adjust for baseline neurologic injury or be seriously confounded by this interaction. We therefore looked to see how strongly the association between heat generation was mitigated after adjustment for injury severity after CA. Our recently reported Pittsburgh CA Category (PCAC) is a simple 4‐point injury severity score,22 which categorizes CA survivors based in large part on their early neurologic exam and cardiopulmonary injury. Higher PCAC corresponds to worsened injury with increasing mortality and worsened neurologic function. Adding PCAC as a continuous variable in a bivariate logistic regression removed the association between HI and favorable disposition.
A univariate linear regression between HI and PCAC demonstrated a strong negative association (B=−0.561; 95% CI: −0.920, −0.202; P=0.003) with higher HI tracking with lower PCAC (ie, less injury). When we plotted patient disposition by PCAC, there was a trend to more favorable disposition in patients with HI above the regression line (8/27) compared with those below it (3/29; OR=3.65; 95% CI 0.085, 15.6; P=0.081) (Figure 5). Thus, the diminishing effect that early categorization of injury severity has on the association of HI with outcomes is likely a result of the 2 variables both measuring injury severity.
Figure 5.
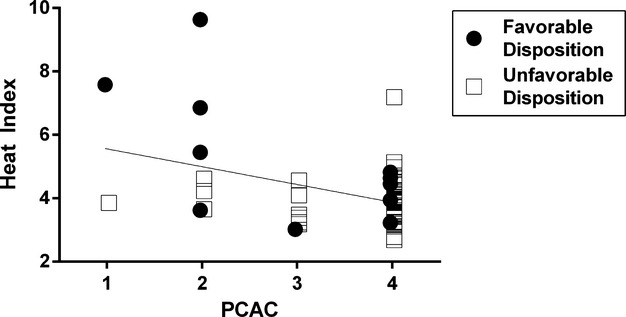
Association between heat generation and post‐arrest injury severity score. Plotted is the regression line that correlates heat index (HI) to the Pittsburgh cardiac arrest category (PCAC). Points lying above the line represent patients with a higher than expected HI for that category and those below the line represent a lower than expected HI. The solid points represent good outcomes and the open points bad outcomes. HI was strongly associated with the PCAC, P=0.003. A HI greater than that predicted based on PCAC trended towards higher likelihood of favorable disposition (P=0.081).
Discussion
There are several important findings of this study, which is the first to quantify heat generation in cardiac arrest survivors receiving therapeutic hypothermia. First, of an extensive list of variables analyzed, only 3 had independent associations with greater heat generation: a lower initial lactate level, a higher minimum WBC count, and a higher 24 hour cumulative propofol dose. Second, greater heat generation was associated with neurologically intact survival. Finally, these data suggest that greater heat generation is the result of higher metabolic rates in less injured patients. Heat index is therefore a quantitative prognostic factor that can be obtained early in treatment of cardiac arrest patients even when sedation and neuromuscular blockade used adjunctively with TH clouds the neurological exam making prognostication difficult.27–28
Several studies have shown an association between elevated initial lactate levels and both mortality and worsened neurologic outcomes in cardiac arrest patients.29–31 Therefore, it is not surprising that a higher HI is also associated with lower initial lactate level. This finding and the association between HI with post‐arrest injury severity (PCAC) may be useful in alerting clinicians to the fact that the patient being treated with therapeutic hypothermia will likely be easier or harder to cool. The higher propofol requirement in patients with higher HI is likely a reflection of this as propofol is commonly used in shivering prevention.32 Although we do not know of any papers documenting leukopenia as a risk factor for worsened post‐CA outcome, this plus the univariate association between HI and anemia likely speak to patients with lower baseline health having worsened outcomes. Because other measures such as albumin and pre‐arrest functional status were not available to us, this is only speculative and requires future validation.
At least 2 other groups19,21 have reported that poikilothermia on presentation after ROSC is associated with worsened survival. Benz‐Woerner and colleagues19 reported that longer time to rewarming was associated with non‐survival. Haugk and colleagues21 reported rapid time to target temperature (34°C) was associated with unfavorable neurologic outcomes after CA. These results suggest patients with more severe neurologic injury are generating less heat and are therefore easier to cool and harder to rewarm.
The correlation between HI and arteriovenous carbon dioxide gradients corroborate the hypothesis that the measured HI reflects metabolic rate with lower heat generation present in more severely injured CA patients. We can only speculate on the physiologic mechanism of this effect. One potential etiology is that more severe global brain injury impacts the hypothalamus, which impairs thermoregulation.33 The hypothalamus is not one of the well‐characterized, selectively vulnerable (to ischemia) regions of the brain34 and thus may only be affected in more severe cases. Alternatively, mitochondrial dysfunction after cardiac arrest35–37 and hence systemic ability to generate heat may limit heat generation and predicts worsened survival.36 Given the differential sensitivities of various tissue types to ischemia, and the relative resistance to ischemia by muscle38 where most heat generation occurs, compared with the brain, where it is regulated, we favor the former hypothesis.
An important finding of our study is the independent and quantitative prognostic value of HI. This effect persisted even after controlling for the most important known association with CA outcomes, namely the presenting rhythm. Admittedly the predictive value of comparing median HI between groups is modest with a likelihood ratio of 3.6. What adds to its value in clinical practice is the ability to use it at a time when neuroprognosis is nearly impossible, namely during hypothermia when most patients are sedated and perhaps paralyzed. The PCAC is limited to these constraints and requires a fair degree of expertise to assign effectively; therefore, it may be difficult to implement at most institutions. The objective nature of HI will allow it to be calculated with minimal expertise on all patients, regardless of their level of sedation, rendering it a valuable early predictor of injury severity. Additionally, the quantitative nature of HI makes it more powerful in more extreme cases than the group medians. In this report, the median difference in HI between those with favorable versus unfavorable neurologic outcome translated to a nearly 5°C difference in water temperature throughout maintenance phase. Though not linear, mean water temperature increases from 16.6 to 20°C and 25 to 33°C (corresponding to HI decrease from 6 to 3) are each associated with a 2.2‐fold increase in odds of poor neurologic outcome. Thus in cases where low (<3) or high (>6) HI is present the value is substantial. Given the increasing use of hypothermia in post‐resuscitation care frequently mediated by devices where water temperature is available,39 this provides clinicians an additional early marker of prognosis. When using HI in conjunction with PCAC, another early prognostic marker, we saw greatest discernment in the lower injury severities where outcomes are often hardest to predict. In more severe cases, the outcome is often manifest.
Our results are also important in the planning of randomized clinical trials aimed at achieving more rapid initiation of hypothermia and achievement of goal temperature. Several trials of this sort have been performed with hopes of reducing reperfusion injury to the brain. Although this approach has yielded good results in animal models,11–12,14 these results have not been consistently replicated clinically.17–18 Animal models deliver similar degrees of ischemia, resulting in a more homogenous injury severity such that the rapidity of cooling depends only on the therapeutic cooling methods used. In the clinical setting where injury is heterogeneous, patients' heat generation will vary substantially so discerning the benefit of external hypothermic therapies is challenging without an adjustment for this patient level variable. The use of HI is the only quantitative method to perform such a post‐hoc adjustment. Our findings regarding the correlation between HI and arteriovenous CO2 gradients, which can be obtained even in the field, permit a means to estimate heat generation permitting stratification prior to randomization in trials testing rapid hypothermia.
There are limitations to this study. The study population is small, though it is comprised of patients from 2 centers, which increases generalizability. The sample size may explain the limited number of associations we observed and larger studies may detect weaker associations. Though the data collection on HI and many peri‐arrest variables were collected prospectively, other data elements (such as drug dosing and blood gases) were gathered retrospectively. Thus, there is a reliance on accurate record keeping and data entry, which is not always present. As much as possible, we used objective lab measures and for the calculation of HI all data were directly downloaded from the devices, which should mitigate inaccuracies. We also note that continuous neuromuscular blockade is rarely employed in these facilities. Facilities using continuous neuromuscular blockade may yield differing results and potentially a significant association with decreased heat generation. CPC assessment at discharge is limited by the evaluations provided in physician's reports, which are inherently subjective in nature.40 Hence our use of a slightly more objective endpoint for neurologic outcome, the discharge location, which is more clearly dictated by the patient's functional status.
Conclusion
Heat generation is independently associated with neurologic outcome in patients successfully resuscitated from cardiac arrest, and shows a strong association with initial illness severity. Markers of better baseline health and reduced ischemic injury were associated with greater heat generation. Our findings demonstrate one potential reason for the inability to translate more rapid cooling to improved clinical outcomes and provide a means to control for this important confounding variable in future clinical trials.
Sources of Funding
Dr Dezfulian is supported by K08NS069817. P. Sonder and G. Janssens are supported by NSH. Dr E. Dekker Fellowship, Dutch Heart Association.
Disclosures
Disclosures are correct.
References
- 1.Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, Carr BG, Mitra N, Bradley SM, Abella BS, Groeneveld PWInvestigators tAHAGWTG‐R. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011; 39:2401-2406.2410.1097/CCM.2400b2013e3182257459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out‐of‐hospital cardiac arrest incidence and outcome. JAMA. 2008; 300:1423-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MBAmerican Heart Association Statistics C, Stroke Statistics S. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012; 125:188-197. [DOI] [PubMed] [Google Scholar]
- 4.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004; 30:2126-2128. [DOI] [PubMed] [Google Scholar]
- 5.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002; 346:557-563. [DOI] [PubMed] [Google Scholar]
- 6.The Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002; 346:549-556. [DOI] [PubMed] [Google Scholar]
- 7.Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL. Part 9: post‐cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122:S768-S786. [DOI] [PubMed] [Google Scholar]
- 8.Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, Guinsburg R, Hazinski MF, Morley C, Richmond S, Simon WM, Singhal N, Szyld E, Tamura M, Velaphi S. Part 11: neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010; 122:S516-S538. [DOI] [PubMed] [Google Scholar]
- 9.Choi HA, Badjatia N, Mayer SA. Hypothermia for acute brain injury–mechanisms and practical aspects. Nat Rev Neurol. 2012; 8:214-222. [DOI] [PubMed] [Google Scholar]
- 10.Tissier R, Ghaleh B, Cohen MV, Downey JM, Berdeaux A. Myocardial protection with mild hypothermia. Cardiovasc Res. 2012; 94:217-225. [DOI] [PubMed] [Google Scholar]
- 11.Nozari A, Safar P, Stezoski SW, Wu X, Kostelnik S, Radovsky A, Tisherman S, Kochanek PM. Critical time window for intra‐arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006; 113:2690-2696. [DOI] [PubMed] [Google Scholar]
- 12.Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra‐arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004; 109:2786-2791. [DOI] [PubMed] [Google Scholar]
- 13.Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011; 39:1423-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993; 21:1348-1358. [DOI] [PubMed] [Google Scholar]
- 15.Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009; 133:223-228. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama H, Nagao K, Hase M, Tahara Y, Hazui H, Arimoto H, Kashiwase K, Sawano H, Yasuga Y, Kuroda Y, Kasaoka S, Shirai S, Yonemoto N, Nonogi HInvestigators JP‐H. Impact of therapeutic hypothermia in the treatment of patients with out‐of‐hospital cardiac arrest from the J‐PULSE‐HYPO study registry. Circ J. 2011; 75:1063-1070. [DOI] [PubMed] [Google Scholar]
- 17.Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, Kelly A‐M, Silvester WInvestigators ftRIoCH. Induction of therapeutic hypothermia by paramedics after resuscitation from out‐of‐hospital ventricular fibrillation cardiac arrest. Circulation. 2010; 122:737-742. [DOI] [PubMed] [Google Scholar]
- 18.Group TICEIS. Early‐ versus late‐initiation of therapeutic hypothermia after cardiac arrest: preliminary observations from the experience of 17 Italian intensive care units. Resuscitation. 2012; 83:823-828. [DOI] [PubMed] [Google Scholar]
- 19.Benz‐Woerner J, Delodder F, Benz R, Cueni‐Villoz N, Feihl F, Rossetti AO, Liaudet L, Oddo M. Body temperature regulation and outcome after cardiac arrest and therapeutic hypothermia. Resuscitation. 2012; 83:338-342. [DOI] [PubMed] [Google Scholar]
- 20.Lyon RM, Richardson SE, Hay AW, Andrews PJ, Robertson CE, Clegg GR. Esophageal temperature after out‐of‐hospital cardiac arrest: an observational study. Resuscitation. 2010; 81:867-871. [DOI] [PubMed] [Google Scholar]
- 21.Haugk M, Testori C, Sterz F, Uranitsch M, Holzer M, Behringer W, Herkner HGroup tTtTTS. Relationship between time to target temperature and outcome in patients treated with therapeutic hypothermia after cardiac arrest. Crit Care. 2011; 15:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011; 82:1399-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rittenberger JC, Guyette FX, Tisherman SA, DeVita MA, Alvarez RJ, Callaway CW. Outcomes of a hospital‐wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation. 2008; 79:198-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between cerebral performance category, modified rankin scale, and discharge disposition after cardiac arrest. Resuscitation. 2011; 82:1036-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanhela R, Mustonen A, Korhonen I, Salomäki T. The effects of two rewarming strategies on heat balance and metabolism after coronary artery bypass surgery with moderate hypothermia. Acta Anaesthesiol Scand. 1999; 43:979-988. [DOI] [PubMed] [Google Scholar]
- 26.Teboul J‐L, Mercat A, Lenique F, Berton C, Richard C. Value of the venous‐arterial pCO2 gradient to reflect the oxygen supply to demand in humans: effects of dobutamine. Crit Care Med. 1998; 26:1007-1010. [DOI] [PubMed] [Google Scholar]
- 27.Perman SM, Kirkpatrick JN, Reitsma AM, Gaieski DF, Lau B, Smith TM, Leary M, Fuchs BD, Levine JM, Abella BS, Becker LB, Merchant RM. Timing of neuroprognostication in postcardiac arrest therapeutic hypothermia. Crit Care Med. 2012; 40:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010; 67:301-307. [DOI] [PubMed] [Google Scholar]
- 29.Mullner M, Sterz F, Domanovits H, Behringer W, Binder M, Laggner AN. The association between blood lactate concentration on admission, duration of cardiac arrest, and functional neurological recovery in patients resuscitated from ventricular fibrillation. Intensive Care Med. 1997; 23:1138-1143. [DOI] [PubMed] [Google Scholar]
- 30.Kliegel A, Losert H, Sterz F, Holzer M, Zeiner A, Havel C, Laggner AN. Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine. 2004; 83:274-279. [DOI] [PubMed] [Google Scholar]
- 31.Cocchi MN, Miller J, Hunziker S, Carney E, Salciccioli J, Farris S, Joyce N, Zimetbaum P, Howell MD, Donnino MW. The association of lactate and vasopressor need for mortality prediction in survivors of cardiac arrest. Minerva Anestesiol. 2011; 77:1063-1071. [PubMed] [Google Scholar]
- 32.Cheong KF, Chen FG, Yau GH. Postanaesthetic shivering–a comparison of thiopentone and propofol. Ann Acad Med Singapore. 1998; 27:729-732. [PubMed] [Google Scholar]
- 33.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007; 292:R37-R46. [DOI] [PubMed] [Google Scholar]
- 34.White BC, Grossman LI, O'Neil BJ, DeGracia DJ, Neumar RW, Rafols JA, Krause GS. Global brain ischemia and reperfusion. Ann Emerg Med. 1996; 27:588-594. [DOI] [PubMed] [Google Scholar]
- 35.Han F, Da T, Riobo NA, Becker LB. Early mitochondrial dysfunction in electron transfer activity and reactive oxygen species generation after cardiac arrest. Crit Care Med. 2008; 36:S447-S453.410.1097/CCM.1090b1013e31818a31818a31851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radhakrishnan J, Wang S, Ayoub IM, Kolarova JD, Levine RF, Gazmuri RJ. Circulating levels of cytochrome c after resuscitation from cardiac arrest: a marker of mitochondrial injury and predictor of survival. Am J Physiol Heart Circ Physiol. 2007; 292:H767-H775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh ST, Lee H‐L, Aune SE, Chen C‐L, Chen Y‐R, Angelos MG. Preservation of mitochondrial function with cardiopulmonary resuscitation in prolonged cardiac arrest in rats. J Mol Cell Cardiol. 2009; 47:789-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002; 10:620-630. [DOI] [PubMed] [Google Scholar]
- 39.Gillies MA, Pratt R, Whiteley C, Borg J, Beale RJ, Tibby SM. Therapeutic hypothermia after cardiac arrest: a retrospective comparison of surface and endovascular cooling techniques. Resuscitation. 2010; 81:1117-1122. [DOI] [PubMed] [Google Scholar]
- 40.Ajam K, Gold LS, Beck SS, Damon S, Phelps R, Rea TD. Reliability of the cerebral performance category to classify neurological status among survivors of ventricular fibrillation arrest: a cohort study. Scand J Trauma Resusc Emerg Med. 2011; 19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]


