Abstract
Background
Comorbidities are common in heart failure (HF), and the number of comorbidities has been associated with poor outcomes in HF patients. However, little is known about the effect of multiple comorbidities on cardiac mechanics, which could impact the pathogenesis of HF. We sought to determine the relationship between comorbidity burden and adverse cardiac mechanics.
Methods and Results
We performed speckle‐tracking analysis on echocardiograms from the HyperGEN study (n=2150). Global longitudinal, circumferential, and radial strain, and early diastolic (e') tissue velocities were measured. We evaluated the association between comorbidity number and cardiac mechanics using linear mixed effects models to account for relatedness among subjects. The mean age was 51±14 years, 58% were female, and 47% were African American. Dyslipidemia and hypertension were the most common comorbidities (61% and 58%, respectively). After adjusting for left ventricular (LV) mass index, ejection fraction, and several potential confounders, the number of comorbidities remained associated with all indices of cardiac mechanics except global circumferential strain (eg, β=−0.32 [95% CI −0.44, −0.20] per 1‐unit increase in number of comorbidities for global longitudinal strain; β=−0.16 [95% CI −0.20, −0.11] for e' velocity; P≤0.0001 for both comparisons). Results were similar after excluding participants with abnormal LV geometry (P<0.05 for all comparisons).
Conclusions
Higher comorbidity burden is associated with worse cardiac mechanics, even in the presence of normal LV geometry. The deleterious effect of multiple comorbidities on cardiac mechanics may explain both the high comorbidity burden and adverse outcomes in patients who ultimately develop HF.
Keywords: cardiac mechanics, comorbidities, echocardiography, risk factors, strain
Introduction
The progression from cardiac risk factors to heart failure is typically mediated by an asymptomatic stage in which structural and/or functional left ventricular (LV) abnormalities can be discerned, highlighting a prime opportunity for both prevention of further progression and intervention.1 Previous studies have emphasized, in particular, the effects of cardiac and non‐cardiac comorbidities on abnormal LV geometry, considered both in isolation and in concert with other comorbidities.2–8 The addition of comorbidities results in a progressive increase in LV mass, a major risk factor for future adverse cardiovascular events.9 However, prior to overt cardiac remodeling or reduced ejection fraction detected by conventional 2‐dimensional (2D) echocardiography, speckle‐tracking echocardiography can detect subclinical alterations in cardiac performance by examining intrinsic indices of cardiac mechanics (LV strain and tissue velocities). Understanding the link between comorbidity burden and cardiac mechanics may explain why an increasing number of comorbidities predicts hospitalization in patients with heart failure.10
The evaluation of echocardiograms from the Hypertension Genetic Epidemiology Network (HyperGEN) study permits comprehensive assessment of the relationship between comorbidities and cardiac mechanics. Strengths of the HyperGEN study for studying mechanical alterations include a large bi‐racial sample of >2000 participants, comprehensive clinical and laboratory data collection, and 2‐dimensional (2D) and Doppler echocardiographic data.11 Speckle‐tracking analysis has now allowed for the measurement of indices of cardiac mechanics in HyperGEN.
Therefore, we sought to determine whether comorbidity burden (ie, number of comorbidities) is associated with incremental worsening of cardiac mechanics. We hypothesized that accumulation of comorbidities is associated with worse cardiac mechanics (decreased LV strain and early diastolic [e'] tissue velocities), independent of its effects on LV mass and ejection fraction and even in those with normal LV geometry.
Methods
Study Population
HyperGEN, part of the National Institutes of Health Family Blood Pressure Program (FBPP), is a cross‐sectional study consisting of 5 US sites, with 4 participating in an ancillary echocardiographic study (Salt Lake City, UT; Forsyth County, NC; Minneapolis, MN; and Birmingham, AL). The goal of HyperGEN was to identify and characterize the genetic basis of familial hypertension; the complete details of the HyperGEN study design have been reported previously.11 Study eligibility required a diagnosis of hypertension prior to the age of 60 and at least 1 sibling willing to participate in the study. Hypertension was defined by an average systolic blood pressure ≥140 mm Hg or an average diastolic blood pressure ≥90 mm Hg (on at least 2 separate clinic visits) or by self‐reporting treatment for hypertension. A random sample of normotensive individuals who represented the source cohort from which the HyperGEN affected sibships were identified was also recruited. Individuals with a history of type 1 diabetes mellitus or severe chronic kidney disease were excluded due to the high risk of secondary forms of hypertension. None of the study participants had symptomatic heart failure. All HyperGEN study participants gave written informed consent, and the HyperGEN study was approved by each study site's local institutional review board.
Clinical Characteristics and Definition of Comorbidity Burden
Demographic, clinical, and laboratory data were collected during the initial HyperGEN visit. Height, weight, blood pressure, and waist circumference were measured by trained personnel, using a standardized protocol. Three sitting, upright blood pressure measurements were obtained per person and averaged; further details regarding blood pressure measurements have been previously reported.11 Histories of myocardial infarction, transient ischemic attack, or stroke were obtained by self‐report. Diabetes mellitus was defined by fasting glucose ≥126 mg/dL, use of hypoglycemic medication, or a self‐reported history. Dyslipidemia was defined by use of lipid‐lowering medication, low‐density lipoprotein cholesterol ≥160 mg/dL, triglycerides >150 mg/dL, or high‐density lipoprotein cholesterol <40 mg/dL (for men) or <50 mg/dL (for women). Obesity was defined by a body mass index ≥30 kg/m2. Chronic kidney disease was defined by an estimated glomerular filtration rate ≤60 mL/min per 1.73 m2.
Comorbidity burden was defined as the number of the following comorbidities: hypertension, dyslipidemia, obesity, diabetes mellitus, chronic kidney disease, coronary artery disease, and transient ischemic attack/stroke.
Echocardiography
Echocardiography (including 2D, M‐mode, and Doppler imaging) was acquired on all study participants using standardized acquisition protocols and stored in analog format (high grade, medical quality videocassette tapes) at the time of study visit.12–13 Cardiac structure and function were quantified as recommended by the American Society of Echocardiography (ASE).14–15 LV ejection fraction was calculated using the biplane method of discs. LV mass was calculated using the linear method recommended by the ASE and indexed to body surface area. LV hypertrophy was defined by a LV mass index >95 g/m2 in women or >115 g/m2 in men. Normal geometry was defined by the absence of LV hypertrophy. Diastolic function was quantitated using early diastolic (E) and late/atrial diastolic (A) transmitral velocities, E/A ratio, isovolumic relaxation time, and E deceleration time.
Digitization of Echocardiograms and Interpretation of Image Quality
Archived echocardiograms in analog format were converted to digital format using the TIMS 2000 DICOM System (Foresight Imaging). Cine loops of 2 to 4 cardiac cycles from the parasternal short axis (papillary muscle level) and apical 4‐chamber views were digitized at a high rate and stored offline in DICOM format. Each study was assessed for image quality by an experienced operator, blinded to all other clinical and echocardiographic data, using a 4‐point scale based on the degree of endocardial border visualized (1=0% to 25%; 2=25% to 50%; 3=50% to 75%; 4=75% to 100%), similar to scales used previously.16–17
Two‐Dimensional Speckle‐Tracking Analysis
Digitized cine loops were analyzed using 2D wall motion tracking software (2D Cardiac Performance Analysis [CPA], TomTec v4.5). After isolating the highest quality cardiac cycle, the endocardial and epicardial borders were traced at end‐systole in each view. Computerized speckle‐tracking analysis was performed and endocardial and epicardial border tracings were manually adjusted to optimize tracking. Indices of LV mechanics included peak global longitudinal strain (GLS), peak global radial strain (GRS), peak global circumferential strain (GCS), and early diastolic (e') tissue velocities. LV filling pressures were estimated using E/e' ratio. For ease of display, all strain values were converted to absolute values (ie, longitudinal and circumferential strain values were converted from negative to positive values). Lower absolute strain values, lower e' tissue velocities, and higher E/e' ratio were used to indicate worse cardiac function. A validation of the digitization and speckle‐tracking techniques employed here have been published elsewhere.18 Intra‐ and inter‐observer reliability data for speckle‐tracking measurements in HyperGEN were excellent, as detailed previously.19
Statistical Analysis
Clinical characteristics, laboratory data, and both conventional echocardiographic parameters and speckle‐tracking parameters are displayed for the total cohort. Continuous data are presented as mean±standard deviation. Categorical variables are presented as a count and percentage.
We performed multivariable analyses to determine whether number of comorbidities is associated with worse indices of cardiac mechanics and increased LV filling pressure (ie, E/e' ratio) after accounting for potential confounders. We used linear mixed effects models, thereby accounting for relatedness among HyperGEN participants. Analyses were repeated after excluding participants with a prior history of myocardial infarction to determine whether number of non‐cardiac comorbidities is associated with worse indices of cardiac mechanics and increased LV filling pressure. Further analyses were performed in participants with normal LV geometry.
Multivariable‐adjusted logistic mixed effects models were used to determine which comorbidities predicted abnormal GLS by the ASE definition (<12%).20 For the outcome of abnormal GLS, we performed additional analyses to explore whether a weighted comorbidity index would perform better than equal weighting of comorbidities. We performed a logistic regression analysis with abnormal GLS (<12%) as the dependent variable and individual comorbidities as the independent variables. Next, we created a weighted comorbidity index based on the model coefficients for each of the comorbidities. Finally, we compared the un‐weighted comorbidity index with the weighted comorbidity index with receiver operating characteristic (ROC) analyses.
For our multivariable analyses, Model 1 included speckle‐tracking analyst, image quality, and center (as well as familial relatedness, which was entered into the model as a random effect). Model 2 included all Model 1 covariates plus age, sex, race, LV mass index, ejection fraction, and wall motion score index. We also conducted subgroup analyses with participants stratified by race and sex. Multiplicative interaction terms were created and entered into regression models to determine whether there were interactions between comorbidity burden and race/sex.
A 2‐sided P value <0.05 was considered statistically significant. Analysis was performed using Stata v.12 (StataCorp) and SAS v. 9.0 (SAS Institute).
Results
Characteristics of Study Participants
Descriptive characteristics of the study sample from HyperGEN are displayed in Table 1. The study cohort consisted of 2150 participants, randomly sampled from all 4 participating sites, representing 1089 unique families. The mean age was 51±14 years, 58% were female, 47% were African American, and 53% were white. Recruitment was relatively split among the 4 centers. Comorbidities were common; only 14% of study participants were free of comorbidities, and most participants had 1 or 2 comorbidities. Figure 1 displays the histograms of number of comorbidities, stratified by sex and race. In participants with 1 or 2 comorbidities, dyslipidemia, obesity, and hypertension were the most common risk factors. Notably, 131 (6%) of the study participants had a history of myocardial infarction, and 134 (6%) had a history of coronary revascularization (coronary artery bypass surgery, angioplasty, or stent). Medication use reflected standard therapies used in the comorbidities detailed in Table 1. Calcium channel blockers (23%) and angiotensin‐converting enzyme inhibitors (21%) were the most commonly employed anti‐hypertensive therapies. Blood pressure was relatively well controlled (127±21/72±11 mm Hg), and obesity was common (mean body mass index 31±7 kg/m2, 47% obese [BMI>30 kg/m2]). Laboratory results revealed preserved kidney function (estimated glomerular filtration rate 85±20 mL/min per 1.73 m2) in the majority of study participants.
Table 1.
Clinical Characteristics of the Study Sample
| Characteristic | All Study Participants (n=2150) |
|---|---|
| Age, y | 51±14 |
| Female, n (%) | 1255 (58) |
| Race/ethnicity, n (%) | |
| White | 1144 (53) |
| African‐American | 998 (47) |
| Other | 8 (0.3) |
| Recruiting center, n (%) | |
| Birmingham, AL | 590 (27) |
| Minneapolis, MN | 426 (20) |
| Salt Lake City, UT | 589 (27) |
| Forsyth County, NC | 545 (25) |
| Comorbidities, n (%) | |
| Hypertension | 1251 (58) |
| Dyslipidemia | 1310 (61) |
| Obesity | 1021 (47) |
| Diabetes mellitus | 365 (17) |
| Chronic kidney disease | 197 (9) |
| Myocardial infarction | 131 (6) |
| Transient ischemic attack or stroke | 97 (5) |
| Number of comorbidities, n (%) | |
| 0 | 304 (14) |
| 1 | 517 (24) |
| 2 | 585 (27) |
| 3 | 452 (21) |
| 4 | 226 (11) |
| 5+ | 66 (3) |
| Medications, n (%) | |
| Anti‐hypertensive medication | 1095 (51) |
| Angiotensin‐converting enzyme inhibitor | 444 (21) |
| Alpha blocker | 168 (8) |
| Angiotensin receptor blocker | 54 (3) |
| Beta‐blocker | 275 (13) |
| Calcium channel blocker | 488 (23) |
| Loop diuretic | 141 (7) |
| Thiazide diuretic | 275 (13) |
| Hypoglycemic medication | 226 (11) |
| Insulin | 85 (4) |
| Lipid lowering medication | 197 (9) |
| Statin | 174 (8) |
| Physical examination | |
| Systolic blood pressure, mm Hg | 127±21 |
| Diastolic blood pressure, mm Hg | 72±11 |
| Body‐mass index, kg/m2 | 31±7 |
| Waist circumference, cm | 102±17 |
| Laboratory data | |
| Sodium, mEq/L | 142±3 |
| Creatinine, mg/dL | 0.98±0.32 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 85±20 |
| Fasting glucose, mg/dL | 106±43 |
| Total serum cholesterol, mg/dL | 196±39 |
| High density lipoprotein, mg/dL | 51±15 |
| Low density lipoprotein, mg/dL | 119±34 |
| Triglycerides, mg/dL | 140±98 |
Figure 1.
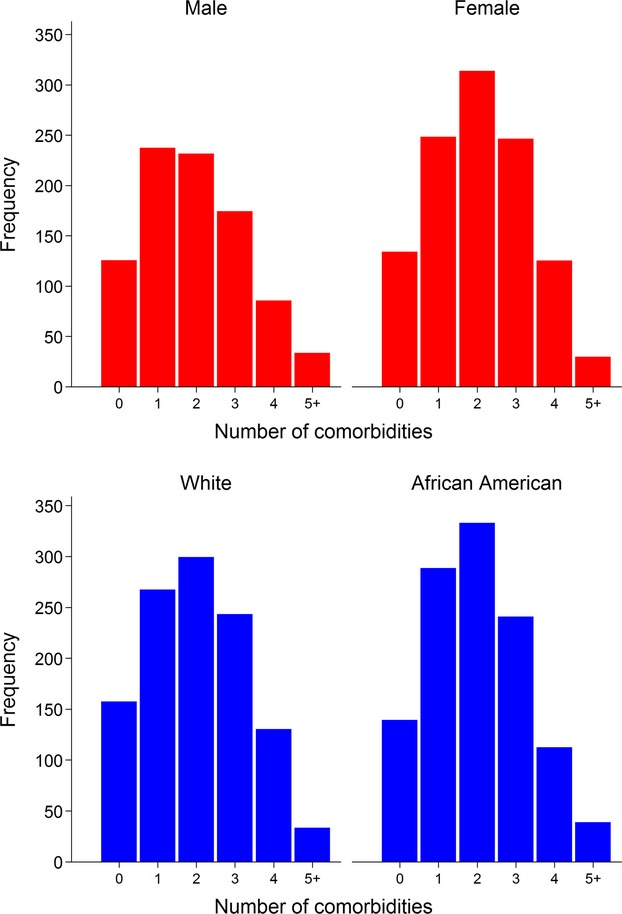
Histogram plots of number of comorbidities stratified by sex and race. Stratified by sex (top panel) and race (bottom panel). There were no significant differences in number of comorbidities by sex (P=0.07) or race (P=0.58).
Table 2 lists the 2D, Doppler, and speckle‐tracking echocardiographic parameters of the study participants. Average LV structure fell within normal limits (LV end‐systolic volume 51±23 mL; LV end‐diastolic volume 130±31 mL; LV mass index 85±22 kg/m2), though roughly one‐fifth had evidence of LV hypertrophy (22%). Global LV systolic function was preserved (ejection fraction 62±8%) in the majority of participants.
Table 2.
Two‐Dimensional, M‐Mode, Doppler, and Speckle‐tracking Echocardiographic Parameters of the Study Sample
| Echocardiographic Parameter | All Study Participants (n=2150) |
|---|---|
| Traditional echocardiographic parameters | |
| LV end‐diastolic volume, mL | 130±31 |
| LV end‐systolic volume, mL | 51±23 |
| LV mass index, g/m2 | 85±22 |
| LV hypertrophy, n (%) | 469 (22) |
| Left atrial diameter, cm | 3.5±0.5 |
| LV ejection fraction, % | 62±8 |
| Stroke volume, mL | 76±16 |
| Cardiac index, L/min per m2 | 2.6±0.6 |
| Early (E) transmitral velocity, cm/s | 73±20 |
| Late/atrial (A) transmitral velocity, cm/s | 66±19 |
| E/A ratio | 1.19±0.50 |
| E deceleration time, ms | 204±58 |
| Isovolumic relaxation time, ms | 80±18 |
| Speckle‐tracking echocardiographic parameters | |
| e' velocity, cm/s* | 3.5±1.3 |
| E/e' ratio* | 23.8±11.9 |
| Global radial strain, % | 26.6±11.9 |
| Global circumferential strain, % | 20.6±5.3 |
| Global longitudinal strain, % | 14.6±3.6 |
LV indicates left ventricular.
Tissue velocity values derived from speckle‐tracking software are lower than values derived from tissue Doppler imaging, resulting in lower e' velocities and higher E/e' velocities.
Images used for speckle‐tracking analysis were generally of high quality. In the parasternal short‐axis and apical 4‐chamber views, 85% and 97% of images had an image quality score of ≥2, respectively, indicating good image quality for the majority of myocardial segments. The values obtained for most strain parameters were lower (ie, worse) than values obtained in patients with mild hypertension.21 By ASE standards, 20% had abnormally low GLS.
Association of Number of Comorbidities With Worse Cardiac Mechanics and Higher LV Filling Pressures
Figure 2 displays bar graphs demonstrating the relationship between number of comorbidities and progressively worse GLS, GCS, and GRS (P<0.001 for trend). Figure 3 shows a similar relationship of comorbidity burden with diastolic parameters (e' velocity and E/e' ratio) (P<0.001 for trend). Table 3 demonstrates the strength these associations. After adjusting for factors that could have affected the measurement of speckle‐tracking parameters (Model 1; covariates=speckle‐tracking analyst, recruitment center, and image quality), comorbidity burden was still associated with GLS, tissue e' velocity, and E/e' ratio (P<0.0001 for all associations). For instance, for each 1‐unit increase in the number of comorbidities, GLS decreased by 0.41%‐units. These findings were consistent in both the entire cohort and in study participants with normal LV geometry. Table 4 shows that the findings were similar even after further adjustment for additional covariates (Model 2), including age, sex, race, wall motion score index, LV mass index, and ejection fraction. Further adjustment for smoking status, systolic blood pressure, and number of anti‐hypertensive medications did not attenuate the key associations between comorbidity burden and GLS (P=0.004), e' velocity (P<0.0001), and E/e' ratio (P<0.0001). Excluding participants with a prior history of myocardial infarction resulted in similar findings (Table 5).
Figure 2.
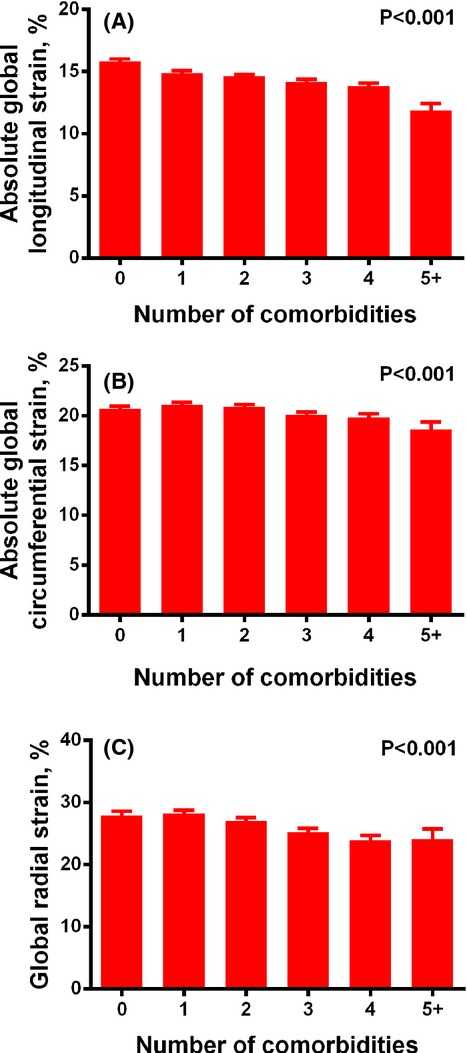
Bar graphs of number of comorbidities vs indices of cardiac mechanics. Increasing comorbidity burden is associated with worsening global longitudinal strain (A), global circumferential strain (B), and global radial strain (C). P values represent significance for the trend across number of comorbidities. Error bars represent standard errors.
Figure 3.
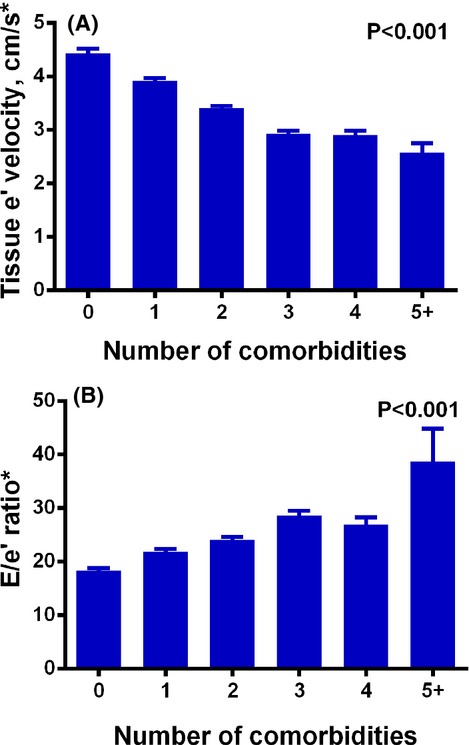
Bar graphs of number of comorbidities vs diastolic parameters. Increasing comorbidity burden is associated with worsening e' velocity (A) and progressively increased E/e' ratio (B), an index of increase left ventricular filling pressure. P values represent significance for the trend across number of comorbidities. Error bars represent standard errors. *Values for speckle tracking‐derived e' velocity are lower (and therefore E/e' are higher) than those obtained clinically (via tissue Doppler imaging).
Table 3.
Association of Number of Comorbidities With Cardiac Mechanics and Filling Pressures After Multivariable Adjustment (Model 1)*
| Dependent Variable | All Participants (n=2150) | Participants With Normal Left Ventricular Geometry (n=1620) | ||
|---|---|---|---|---|
| β‐Coefficient (95% CI) | P Value | β‐Coefficient (95% CI) | P Value | |
| Global radial strain, % | −0.19 (−0.59, −0.20) | 0.34 | −0.14 (−0.60, 0.32) | 0.54 |
| Global circumferential strain, % | 0.05 (−0.13, 0.22) | 0.58 | 0.18 (−0.01, 0.39) | 0.06 |
| Global longitudinal strain, % | −0.41 (−0.52, −0.30) | <0.0001 | −0.32 (−0.44, −0.19) | <0.0001 |
| e' velocity, cm/s | −0.36 (−0.41, −0.32) | <0.0001 | −0.36 (−0.41, −0.31) | <0.0001 |
| E/e' ratio | 2.69 (2.16, 3.22) | <0.0001 | 2.19 (1.64, 2.72) | <0.0001 |
Adjusted for speckle‐tracking analyst, recruiting center, image quality.
Table 4.
Association of Number of Comorbidities With Cardiac Mechanics and Filling Pressures After Multivariable Adjustment (Model 2)*
| Dependent Variable | All Participants (n=2150) | Participants With Normal Left Ventricular Geometry (n= 1620) | ||
|---|---|---|---|---|
| β‐Coefficient (95% CI) | P Value | β‐Coefficient (95% CI) | P Value | |
| Global radial strain, % | −0.49 (−0.93, −0.06) | 0.02 | −0.52 (−1.02, −0.03) | 0.04 |
| Global circumferential strain, % | 0.02 (−0.16, 0.20) | 0.83 | 0.02 (−0.19, 0.22) | 0.87 |
| Global longitudinal strain, % | −0.32 (−0.44, −0.20) | <0.0001 | −0.29 (−0.42, −0.16) | <0.0001 |
| e' velocity, cm/s | −0.16 (−0.20, −0.11) | <0.0001 | −0.15 (−0.20, −0.10) | <0.0001 |
| E/e' ratio | 1.53 (0.96, 2.11) | <0.0001 | 1.45 (0.86, 2.02) | <0.0001 |
Adjusted for speckle‐tracking analyst, recruiting center, image quality, age, sex, race, left ventricular mass index, wall motion score, and ejection fraction.
Table 5.
Association of Number of Comorbidities With Cardiac Mechanics and Filling Pressures After Multivariable Adjustment—Excluding Participants With Prior Myocardial Infarction (Model 2)*
| Dependent Variable | All Participants (n=2019) | Participants With Normal Left Ventricular Geometry (n=1551) | ||
|---|---|---|---|---|
| β‐Coefficient (95% CI) | P Value | β‐Coefficient (95% CI) | P Value | |
| Global radial strain, % | −0.46 (−0.91, −0.00) | 0.048 | −0.46 (−0.96, 0.5) | 0.08 |
| Global circumferential strain, % | 0.04 (−0.15, 0.23) | 0.68 | 0.04 (−0.17, 0.25) | 0.69 |
| Global longitudinal strain, % | −0.33 (−0.45, −0.21) | <0.0001 | −0.29 (−0.43, −0.16) | <0.0001 |
| e' velocity, cm/s | −0.16 (−0.21, −0.12) | <0.0001 | −0.16 (−0.21, −0.11) | <0.001 |
| E/e' ratio | 1.60 (1.01, 2.18) | <0.0001 | 1.54 (0.95, 2.13) | <0.0001 |
Adjusted for speckle‐tracking analyst, recruiting center, image quality, age, sex, left ventricular mass index, wall motion score, and ejection fraction.
Table 6 demonstrates the association of number of comorbidities with cardiac mechanics and LV‐filling pressure stratified by race/ethnicity. The effect sizes demonstrated by the beta‐coefficients in Table 6 demonstrate that for every 1‐unit increase in the number of comorbidities, African Americans (compared with whites) had disproportionately worse GLS and GRS, and higher LV‐filling pressure (E/e' ratio). In addition, the association of number of comorbidities with GRS was only significant in the African‐American subgroup. African‐American participants were more likely to be obese compared with whites (52% versus 43%, respectively). However, additional adjustment for body mass index in our multivariable models did not change the differential associations between comorbidity burden and cardiac mechanics by race. Race×comorbidities interaction testing demonstrated that there was a significant interaction (P<0.05) between race and comorbidity burden for GLS, GCS, and E/e' ratio (Table 6). Table 7 lists the demographic and comorbidity differences between whites and African Americans. We found no sex‐based differences in the association of number of comorbidities with cardiac mechanics (interaction P>0.10 for all indices of cardiac mechanics).
Table 6.
Association of Number of Comorbidities With Cardiac Mechanics and Filling Pressures After Multivariable Adjustment (Model 2)—Race‐Stratified Analyses*
| Dependent Variable | White Participants (n=1144) | African American Participants (n=998) | Race×Comorbidities Interaction P Value | ||
|---|---|---|---|---|---|
| β‐Coefficient (95% CI) | P Value | β‐Coefficient (95% CI) | P Value | ||
| Global radial strain, % | −0.23 (−0.86, 0.40) | 0.48 | −0.69 (−1.29, −0.09) | 0.025 | 0.44 |
| Global circumferential strain, % | 0.11 (−0.15, 0.36) | 0.43 | −0.12 (−0.38, 0.13) | 0.34 | 0.003 |
| Global longitudinal strain, % | −0.23 (−0.39, −0.07) | 0.005 | −0.39 (−0.57, −0.21) | <0.0001 | 0.026 |
| e' velocity, cm/s | −0.16 (−0.22, −0.10) | <0.0001 | −0.16 (−0.22, −0.09) | <0.0001 | 0.79 |
| E/e' ratio | 1.26 (0.61, 1.92) | <0.0001 | 1.95 (0.99, 2.91) | <0.0001 | <0.001 |
Adjusted for speckle‐tracking analyst, recruiting center, image quality, age, sex, left ventricular mass index, wall motion score, and ejection fraction.
Table 7.
Differences in Demographics and Comorbidities Between White and African American Participants
| Characteristic | White Participants (n=1144) | African American Participants (n=998) | P Value |
|---|---|---|---|
| Age, y | 54±13 | 48±13 | <0.001 |
| Female, n (%) | 588 (51) | 660 (66) | <0.001 |
| Recruiting center, n (%) | <0.001 | ||
| Birmingham, AL | 3 (<1) | 587 (59) | |
| Minneapolis, MN | 425 (37) | 0 (0) | |
| Forsyth County, NC | 584 (51) | 0 (0) | |
| Salt Lake City, UT | 132 (12) | 411 (41) | |
| Number of comorbidities, n (%) | 0.43 | ||
| 0 | 172 (15) | 131 (13) | |
| 1 | 267 (23) | 247 (25) | |
| 2 | 299 (26) | 285 (29) | |
| 3 | 243 (21) | 206 (21) | |
| 4 | 130 (11) | 96 (10) | |
| 5+ | 33 (3) | 33 (3) | |
| Average number of comorbidities | 2.0±1.3 | 2.0±1.3 | 0.58 |
| Individual comorbidities, n (%) | |||
| Hypertension | 624 (55) | 620 (63) | <0.001 |
| Dyslipidemia | 798 (70) | 507 (51) | <0.001 |
| Obesity | 494 (43) | 523 (52) | <0.001 |
| Diabetes mellitus | 160 (14) | 205 (21) | <0.001 |
| Chronic kidney disease | 141 (12) | 56 (6) | <0.001 |
| Myocardial infarction | 74 (6) | 57 (6) | 0.46 |
| Transient ischemic attack or stroke | 38 (3) | 58 (6) | 0.005 |
Independent Predictors of Abnormal Global Longitudinal Strain
Table 8 displays the odds ratios for each comorbidity, as well as number of comorbidities, in predicting abnormal GLS (<12%) on mixed‐effects logistic regression analyses (adjusted for all factors in Model 2). After multivariable adjustment, only obesity (odds ratio 1.31 per comorbidity, P=0.049) and number of comorbidities (odds ratio 1.22 per comorbidity, P=0.0009) were independently associated with abnormal GLS. We found that a weighted comorbidity index (more weight given to those comorbidities that were more closely associated with abnormal GLS) was only slightly better than the unweighted comorbidity index (c‐statistic=0.62 versus 0.61, respectively; P=0.006).
Table 8.
Predictors of Abnormal Global Longitudinal Strain by Comorbidity*
| Comorbidity | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Hypertension | 1.35 (0.98, 1.85) | 0.07 |
| Dyslipidemia | 1.21 (0.91, 1.59) | 0.18 |
| Obesity | 1.31 (1.00, 1.72) | 0.049 |
| Diabetes mellitus | 1.19 (0.85, 1.67) | 0.32 |
| Chronic kidney disease | 1.52 (0.95, 2.43) | 0.08 |
| Myocardial infarction | 1.42 (0.84, 2.39) | 0.19 |
| Transient ischemic attack or stroke | 1.63 (0.92, 2.84) | 0.08 |
| Number of comorbidities | 1.22 (1.08, 1.37) | 0.0009 |
Abnormal global longitudinal strain defined as <12% (see text for details); Multivariable mixed‐effects logistic regression models were adjusted for speckle‐tracking analyst, recruiting center, image quality, age, sex, race, left ventricular mass index, wall motion score, and ejection fraction.
Discussion
In a speckle‐tracking study of 2150 HyperGEN participants, we found that comorbidity burden was associated with several indices of abnormal cardiac mechanics and higher LV‐filling pressures in a dose‐response fashion. The findings were significant after controlling for LV ejection fraction and LV mass index and even in participants without evidence of LV remodeling by conventional echocardiography. To our knowledge, our study is one of the largest speckle‐tracking investigations to date and is the first to show the adverse effects of comorbidity burden on intrinsic measures of myocardial function.
Our findings are clinically relevant. Based on prior studies relating GLS to outcomes, the differences in GLS across the number of comorbidities is clinically significant. Studies have shown that for every 1% worsening in GLS, the risk of adverse outcomes increases by 18% to 28%.22–23 Furthermore, the 4% worsening in GLS detected between participants with the lowest (0) and highest (5+) number of comorbidities has been found to be associated with an adjusted hazard ratio of 1.45 for increased mortality.24
The association of comorbidity burden with worse GLS is likely explained by the vulnerability of the subendocardium. Longitudinal LV mechanics, which are largely determined by the subendocardium, are the most sensitive and vulnerable to myocardial disease processes.25 Diastolic indices of impaired relaxation and increased filling pressures are likewise sensitive indicators of cardiac function, which often precede overt diastolic dysfunction.25 Though abnormalities in GRS and GCS, which both evaluate transmural function, often occur later in disease processes, the reason why only one (GRS) correlated well with comorbidity burden remains unclear, though the lack of association between comorbidities and GCS may have been due to the decreased accuracy of post‐hoc speckle‐tracking GCS compared with prospective, native GCS.18
The finding in our subgroup analysis that African Americans are disproportionately affected by comorbidity burden is particularly notable. African Americans develop heart failure at the highest incidence rate in the United States and show symptoms at early ages.26–27 Increased comorbidity burden has been hypothesized as one etiology.26 We provide further evidence here for the role of comorbidities in disproportionate cardiac dysfunction in African‐American adults. In our subgroup analyses, we found that the magnitude of the association between comorbidity burden and worse cardiac mechanics (GLS and GCS) and higher E/e' ratio was greater in African Americans compared with whites, as demonstrated by differences in effect sizes and significant interaction P values. Though African Americans develop LV hypertrophy more commonly than whites,28 our analyses, which controlled for LV mass index, show that intrinsic inequalities in myocardial mechanics related to comorbidity burden may also play a role in the racial disparities in heart failure development and severity. The results shown in Table 7 suggest that race/ethnic differences in the pattern of comorbidities may also play a role in the greater role of comorbidities on cardiac mechanics in African Americans.
We found that obesity and the number of comorbidities significantly predicted abnormal GLS. Obesity has been previously implicated as a substantial risk factor for LV remodeling, and thus its relationship to GLS is not surprising.3,29 Previous studies have shown that individual comorbidities are associated with worse myocardial mechanics.30 However, to our knowledge, ours is the first to comprehensively quantitate the adverse effects of comorbidity burden. Understanding the relationship between comorbidity burden, in addition to individual comorbidities, and cardiac mechanics is particularly clinically relevant, given the high number of patients with multiple comorbidities. Determination of cardiac mechanics may be a more sensitive and useful assay for cardiovascular prevention. Further, our results demonstrate the potential utility of examining the total number of comorbidities in the cardiac assessment of patients, since the number of comorbidities was the only significant predictor of abnormal GLS apart from obesity.
Interest in the role of comorbidities in cardiovascular disease, specifically heart failure, is growing. The number of comorbidities has recently been shown to predict heart failure hospitalization, all‐cause hospitalization, and death, particularly in those with preserved ejection fraction.10,31 Of note, a substantial percentage of patients with heart failure, regardless of ejection fraction, do not have evidence of antecedent LV hypertrophy. The reduction in indices of cardiac mechanics in participants with normal LV geometry observed in our study may underlie this transition from multiple comorbidities to the heart failure syndrome in the presence and absence of overt LV remodeling.
The importance of worsening cardiac mechanics to cardiovascular disease cannot be overemphasized. Changes in tissue deformation often precede alterations observed in traditional 2‐dimensional echocardiography. Thus, a “normal” echocardiogram may not be as reassuring in the context of abnormal cardiac mechanics. In addition, GLS, in particular, is an independent predictor of all‐cause mortality and is superior to conventional echocardiographic indices such as the ejection fraction or wall motion score index.24 Though still an emerging tool in cardiovascular imaging, speckle‐tracking echocardiography may highlight the highest risk individuals, including those with high comorbidity burden.
Our results should be interpreted in the context of a few limitations. First, the cross‐sectional design of our study precludes our ability to determine whether the reductions in cardiac mechanics as a consequence of comorbidity burden result in adverse outcomes. Second, some of the comorbidities were obtained only by self‐reported history. Third, speckle‐tracking was performed on archival echocardiograms stored on videotapes that may have degraded image quality.25 However, the vast majority of images acquired were of good or high quality; in addition, image quality was entered into all regression analyses. Fourth, we were unable to adjust for preload in our analyses, which may influence cardiac mechanics, since this echocardiographic data is not available in HyperGEN. Finally, the definition of abnormal GLS was based off measurements using a different software package, EchoPAC. However, there is low inter‐platform variability when compared with TomTec speckle‐tracking software.32
In conclusion, comorbidity burden is associated with a worse cardiac mechanics and higher LV‐filling pressures in a dose‐response fashion, even in those with normal LV geometry and particularly in African Americans. Obesity and the number of comorbidities are significantly associated with the presence of abnormal strain. The deleterious effect of multiple comorbidities on cardiac mechanics may explain both the high comorbidity burden and adverse outcomes in patients who ultimately develop heart failure. Whether treatment of comorbidities improves cardiac mechanics and/or outcomes should be evaluated prospectively.
HyperGEN Participating Institutions and Principal Staff
Network Center/University of Utah Field Center: Steven C. Hunt, Roger R. Williams, Hilary Coon, Paul N. Hopkins, Janet Hood, Lily Wu, Jan Skuppin; University of Alabama at Birmingham Field Center: Albert Oberman, Cora E. Lewis, Michael T. Weaver, Phillip Johnson, Susan Walker, Christie Oden; Boston University/Framingham Field Center: R. Curtis Ellison, Richard H. Myers, Yuqing Zhang, Luc Djoussé, Jemma B. Wilk, Greta Lee Splansky; University of Minnesota Field Center: Donna Arnett, Aaron R. Folsom, Mike Miller, Jim Pankow, Gregory Feitl, Barb Lux; University of North Carolina Field Center: Gerardo Heiss, Barry I. Freedman, Kari North, Kathryn Rose, Amy Haire; Data Coordinating Center, Washington University: D.C. Rao, Michael A. Province, Ingrid B. Borecki, Avril Adelman, Derek Morgan, Karen Schwander, David Lehner, Aldi Kraja, Stephen Mandel; Central Biochemistry Laboratory, University of Minnesota: John H. Eckfeldt, Catherine Leiendecker‐Foster, Ronald C. McGlennen, Greg Rynders, Michael Y. Tsai, Jean Bucksa; Molecular Genetics Laboratory, University of Utah: Mark Leppert, Steven C. Hunt, Jean‐Marc Lalouel, Robert Weiss; National Heart, Lung, and Blood Institute: Susan E. Old, Millicent Higgins, Cashell Jaquish, Martha Lundberg, Mariana Gerschenson. Echocardiographic reading center, Weill Cornell Medical College: Richard B. Devereux, Giovanni de Simone, Jonathan N. Bella.
Sources of Funding
The HyperGEN cardiac mechanics ancillary study was funded by the National Institutes of Health (R01 HL 107577 to Shah). The HyperGEN parent study was funded by cooperative agreements (U10) with the National Heart, Lung, and Blood Institute: HL54471, HL54472, HL54473, HL54495, HL54496, HL54497, HL54509, HL54515.
Disclosures
None.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009; 119:e391-e479. [DOI] [PubMed] [Google Scholar]
- 2.Aijaz B, Ammar KA, Lopez‐Jimenez F, Redfield MM, Jacobsen SJ, Rodeheffer RJ. Abnormal cardiac structure and function in the metabolic syndrome: a population‐based study. Mayo Clin Proc. 2008; 83:1350-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002; 20:323-331. [DOI] [PubMed] [Google Scholar]
- 4.Burchfiel CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, Taylor HA. Metabolic syndrome and echocardiographic left ventricular mass in blacks—the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2005; 112:819-827. [DOI] [PubMed] [Google Scholar]
- 5.Halldin M, Fahlstadius P, de Faire U, Vikstrom M, Hellenius ML. The metabolic syndrome and left ventricular hypertrophy—the influence of gender and physical activity. Blood Press. 2012; 21:153-160. [DOI] [PubMed] [Google Scholar]
- 6.Iwashima Y, Horio T, Kamide K, Tokudome T, Yoshihara F, Nakamura S, Ogihara T, Rakugi H, Kawano Y. Additive interaction of metabolic syndrome and chronic kidney disease on cardiac hypertrophy, and risk of cardiovascular disease in hypertension. Am J Hypertens. 2010; 23:290-298. [DOI] [PubMed] [Google Scholar]
- 7.Patel DA, Srinivasan SR, Chen W, Berenson GS. Influence of the metabolic syndrome versus the sum of its individual components on left ventricular geometry in young adults (from the Bogalusa Heart Study). Am J Cardiol. 2009; 104:69-73. [DOI] [PubMed] [Google Scholar]
- 8.Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, Siepi D, Vaudo G, Mannarino E. Different impact of the metabolic syndrome on left ventricular structure and function in hypertensive men and women. Hypertension. 2006; 47:881-886. [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990; 322:1561-1566. [DOI] [PubMed] [Google Scholar]
- 10.Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012; 59:998-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams RR, Rao DC, Ellison RC, Arnett DK, Heiss G, Oberman A, Eckfeldt JH, Leppert MF, Province MA, Mockrin SC, Hunt SC. NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann Epidemiol. 2000; 10:389-400. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Roman MJ, de Simone G, O'Grady MJ, Paranicas M, Yeh JL, Fabsitz RR, Howard BV. Relations of left ventricular mass to demographic and hemodynamic variables in American Indians: the Strong Heart Study. Circulation. 1997; 96:1416-1423. [DOI] [PubMed] [Google Scholar]
- 13.Palmieri V, Dahlof B, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999; 34:1625-1632. [DOI] [PubMed] [Google Scholar]
- 14.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978; 58:1072-1083. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440-1463. [DOI] [PubMed] [Google Scholar]
- 16.Galema TW, Geleijnse ML, Yap SC, van Domburg RT, Biagini E, Vletter WB, Ten Cate FJ. Assessment of left ventricular ejection fraction after myocardial infarction using contrast echocardiography. Eur J Echocardiogr. 2008; 9:250-254. [DOI] [PubMed] [Google Scholar]
- 17.Peteiro J, Pinon P, Perez R, Monserrat L, Perez D, Castro‐Beiras A. Comparison of 2‐ and 3‐dimensional exercise echocardiography for the detection of coronary artery disease. J Am Soc Echocardiogr. 2007; 20:959-967. [DOI] [PubMed] [Google Scholar]
- 18.Aguilar FA, Selvaraj S, Martinez EE, Beussink L, Kim K‐Y, Ping J, Rasmussen‐Torvik L, Sha J, Irvin R, Arnett DK, Shah SJ. Archeological echocardiography: digitization and speckle‐tracking analysis of archival echocardiograms in the HyperGEN Study [abstract]. J Am Soc Echocardiogr. 2012; 25:B10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz DH, Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim KY, Peng J, Sha J, Irvin MR, Eckfeldt JH, Turner ST, Freedman BI, Arnett DK, Shah SJ. Association of low‐grade albuminuria with adverse cardiac mechanics: findings from the Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2014; 129:42-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mor‐Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011; 24:277-313. [DOI] [PubMed] [Google Scholar]
- 21.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle‐strain imaging study. Circ Cardiovasc Imaging. 2009; 2:382-390. [DOI] [PubMed] [Google Scholar]
- 22.Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, Burrell LM, Srivastava PM. Global longitudinal strain is a strong independent predictor of all‐cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2012; 13:827-833. [DOI] [PubMed] [Google Scholar]
- 23.Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging. 2012; 5:719-725. [DOI] [PubMed] [Google Scholar]
- 24.Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009; 2:356-364. [DOI] [PubMed] [Google Scholar]
- 25.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010; 23:351-369. [DOI] [PubMed] [Google Scholar]
- 26.Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009; 360:1179-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005; 96:3i-12i. [DOI] [PubMed] [Google Scholar]
- 28.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population—the Dallas Heart Study. Hypertension. 2005; 46:124-129. [DOI] [PubMed] [Google Scholar]
- 29.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000; 101:2981-2988. [DOI] [PubMed] [Google Scholar]
- 30.Dalen H, Thorstensen A, Romundstad PR, Aase SA, Stoylen A, Vatten LJ. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr. 2011; 24:322-332.e326. [DOI] [PubMed] [Google Scholar]
- 31.Marechaux S, Six‐Carpentier MM, Bouabdallaoui N, Montaigne D, Bauchart JJ, Mouquet F, Auffray JL, Le Tourneau T, Asseman P, LeJemtel TH, Ennezat PV. Prognostic importance of comorbidities in heart failure with preserved left ventricular ejection fraction. Heart Vessels. 2011; 26:313-320. [DOI] [PubMed] [Google Scholar]
- 32.Kraigher‐Krainer E, Querejeta G, Gupta DK, Dimaano VL, Luo H‐C, Abraham T, Shah AM, Solomon SD. Comparison of vendor‐independent platforms for speckle tracking echocardiography derived strain and strain rate with a vendor‐specific platform. J Am Soc Echocardiogr. 2012; 25:B82 [Google Scholar]


