Abstract
Background
Higher heart rate has been associated with an adverse prognosis, but most prior studies focused on individuals with known cardiovascular disease or examined a limited number of outcomes. We sought to examine the association of baseline heart rate with both fatal and nonfatal outcomes during 2 decades of follow‐up.
Methods and Results
Our study included 4058 Framingham Heart Study participants (mean age 55 years, 56% women). Cox models were performed with multivariable adjustment for clinical risk factors and physical activity. A total of 708 participants developed incident cardiovascular disease (303 heart failure, 343 coronary heart disease, and 216 stroke events), 48 received a permanent pacemaker, and 1186 died. Baseline heart rate was associated with incident cardiovascular disease (hazard ratio [HR] 1.15 per 1 SD [11 bpm] increase in heart rate, 95% CI 1.07 to 1.24, P=0.0002), particularly heart failure (HR 1.32, 95% CI 1.18 to 1.48, P<0.0001). Higher heart rate was also associated with higher all‐cause (HR 1.17, 95% CI 1.11 to 1.24, P<0.0001) and cardiovascular mortality (HR 1.18, 95% CI 1.04 to 1.33, P=0.01). Spline analyses did not suggest a lower threshold beyond which the benefit of a lower heart rate abated or increased. In contrast, individuals with a higher heart rate had a lower risk of requiring permanent pacemaker placement (HR 0.55, 95% CI 0.38 to 0.79, P=0.001).
Conclusions
Individuals with a higher heart rate are at elevated long‐term risk for cardiovascular events, in particular, heart failure, and all‐cause death. On the other hand, a higher heart rate is associated with a lower risk of future permanent pacemaker implantation.
Keywords: cardiovascular disease, epidemiology, heart failure, risk factor
Introduction
A higher heart rate has been associated with worse clinical outcomes,1–2 particularly in individuals with existing cardiovascular disease.3–4 The underlying mechanism of this association is not well understood: higher heart rates may reflect underlying autonomic dysfunction and sympathetic overactivity,5–6 although direct effects of heart rate on atherosclerosis7 and myocardial energetics may also contribute.8 Specific pharmacologic lowering of heart rate using the If channel inhibitor Ivabradine on background β‐blocker therapy has been associated with decreased cardiovascular events in patients with chronic systolic heart failure.9
Prior studies have focused largely on fatal outcomes1–2,1–11 and have had relatively short durations of follow‐up. In previous studies, the association of heart rate with events is often attenuated by adjustment for clinical characteristics.12–14 Further, analyses based on individuals with existing cardiovascular disease can be confounded by the use of β‐blockers or other heart rate–slowing medications.10,15 Thus, we examined the association of heart rate with a full range of cardiovascular outcomes, in a large community‐based cohort that has been followed for 2 decades. Specifically, we sought to examine the following outcomes: incident cardiovascular disease, including heart failure, coronary heart disease, and stroke, in addition to all‐cause mortality and future need for pacemaker implantation. We restricted our analyses to prospectively adjudicated events and accounted for numerous potential confounders including physical activity.
Methods
Study Sample
The Framingham Heart Study (FHS) original and offspring cohorts were recruited in 1948 and 1971, respectively, and have since been followed with serial examinations.16–17 A total of 5420 participants attended the original cohort 20th examination (1986–1990, n=1401) or the offspring fourth examination (1987–1991, n=4019). Of this group, a total of 1361 participants were excluded from this analysis due to the following reasons: missing heart rate (n=4), prevalent myocardial infarction (n=215), prevalent heart failure (n=58), use of medications affecting heart rate, including β‐blockade, calcium channel blockade, digoxin, or other antiarrhythmic medications (n=780), prevalent atrial fibrillation (n=22), previous permanent pacemaker insertion (n=9), atrioventricular dissociation on ECG (n=2), or missing covariates (n=271). After these exclusions, 4058 participants remained eligible for the present analysis. Participants provided informed consent, and the study was approved by the Institutional Review Board at Boston University Medical Center.
Clinical Assessment
All participants underwent routine history, physical examination, anthropometry, and laboratory assessment. Heart rate was measured using standard supine 12‐lead electrocardiography at a paper speed of 25 mm/s, which was performed after approximately 5 minutes of resting quietly. Seated blood pressure was measured after at least 5 minutes of rest using mercury sphygmomanometry and reported as the average of 2 physician‐obtained readings. Diabetes mellitus was defined as a fasting glucose level ≥126 mg/dL, nonfasting blood glucose level ≥200 mg/dL, or the use of insulin or oral hypoglycemic medications. Participants who regularly smoked cigarettes during the prior year were considered current smokers. Electrocardiographic left ventricular hypertrophy was defined using standard ECG criteria.18 Valvular heart disease was defined as a systolic murmur grade ≥3/6 or any diastolic murmur auscultated on physician examination. Baseline physical activity was assessed by using an administered questionnaire. A physical activity index composite score was calculated based on the sum of usual reported activity at a certain level within a 24‐hour period, weighted based on estimated oxygen consumption required for a given activity level.19–20 Fasting total and high density lipoprotein cholesterol levels were obtained. The presence of atrial fibrillation was determined after examining all available ECGs from FHS clinic visits and medical records.
Definition of Cardiovascular Outcome Events
FHS participants were monitored regularly for the occurrence of cardiovascular events and death. Cardiovascular events were adjudicated by a panel of 3 physicians after review of medical records. Heart failure was defined based on FHS criteria.21 A major cardiovascular disease event was defined as myocardial infarction, coronary insufficiency (prolonged ischemic symptoms with new ECG abnormalities in the absence of biomarker elevations indicative of infarction), heart failure, stroke, or cardiovascular death.22 For analyses examining cardiovascular disease, participants with prevalent disease were excluded (n=39).
Statistical Analysis
Cross‐sectional correlates of baseline heart rate were determined using multivariable linear regression. The associations of baseline resting heart rate and incident cardiovascular events were examined using multivariable proportional hazards regression (Cox) models. Individuals who died were censored for analyses of nonfatal events. Separate analyses were conducted for incident cardiovascular disease, heart failure, stroke, coronary heart disease, pacemaker implantation, all‐cause death, and cardiovascular death. Models were first adjusted for age and sex and further adjusted for systolic blood pressure (BP), use of antihypertensive medications, body mass index (BMI), diabetes mellitus, smoking status, physical activity index, valvular heart disease, ECG left ventricular hypertrophy, total–to–HDL cholesterol ratio, minor cardiovascular disease (angina, transient ischemic attack, and intermittent claudication), PR interval, and QRS duration.23–24 To account for multiple hypothesis testing (7 outcomes), a Bonferroni‐corrected P‐value of <0.007 was considered significant for primary analyses. We tested for proportionality of hazards for each outcome by fitting an interaction term of follow‐up time and heart rate and by visually checking Martingale‐based residuals. Proportionality of hazards was confirmed for all outcomes. The cumulative incidence of each of the clinical outcomes across heart rate quartiles was examined using a Kaplan–Meier‐like method while accounting for competing risk of death.25 A sensitivity analysis was conducted after exclusion of individuals with minor cardiovascular disease at baseline, including angina, transient ischemic attack, intermittent claudication, and valvular heart disease.
In secondary analyses, we tested for differential risk associated with heart rate for heart failure versus non–heart failure cardiovascular disease using the Lunn–McNeil method.26 The association of sex‐specific heart rate quartiles and primary events was examined using Cox models. To evaluate possible nonlinear relationships in secondary analyses, we fitted restricted cubic splines with 3 knots at 25th, 50th, and 75th percentiles. We formally tested for age×heart rate and sex×heart rate interaction terms. Given recent data showing a differential effect of heart rate on heart failure in men and women,27 we performed secondary analyses stratified by sex. We conducted analyses using mean heart rate across the preceding 3 exam cycles spanning approximately 8 years prior to the baseline examination (original cohort exams 16, 18, and 20 and offspring cohort exams 2, 3, and 4). We examined the effect of heart rate as a time‐dependent variable over 8 years following the baseline exam as a predictor of outcomes. In secondary analyses, we examined the association of heart rate and clinical outcomes in participants taking heart rate–modifying agents at the baseline examination (β‐blockers, calcium channel blockers). All analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, NC).
Results
The baseline characteristics of 4058 participants (mean age 55 years, 56% women) are displayed in Table 1. Baseline resting heart rate was 64±11 bpm in men, and 67±11 bpm in women. Older age, female sex, higher diastolic BP, higher BMI, diabetes, smoking, and lower HDL cholesterol were all associated with higher resting heart rate (P<0.05 for all, Table 2). Higher physical activity as measured by the physical activity index was associated with a small but significant decrease in resting heart rate in age‐ and sex‐adjusted analyses (0.56 bpm decrease per 1‐standard deviation [SD] increase in physical activity index, P=0.001) and remained a correlate of baseline heart rate after multivariable adjustment (0.56‐bpm decrease, P=0.001). In comparison, each 1‐SD increase in diastolic BP was associated with a 2‐bpm increase in heart rate (P<0.0001).
Table 1.
Baseline Characteristics of FHS Participants by Heart Rate Quartile
| Heart Rate Quartile | ||||
|---|---|---|---|---|
| Q1 (n=918) | Q2 (n=1077) | Q3 (n=998) | Q4 (n=1065) | |
| Age, y | 52 (12) | 54 (13) | 56 (13) | 59 (15) |
| Women, n (%) | 491 (53) | 626 (58) | 586 (59) | 586 (55) |
| Systolic blood pressure, mm Hg | 124 (20) | 128 (19) | 129 (20) | 135 (21) |
| Diastolic blood pressure, mm Hg | 76 (10) | 78 (10) | 79 (10) | 81 (11) |
| Heart rate, beats per minute | 52 (4) | 61 (2) | 68 (3) | 80 (8) |
| Body mass index, kg/m2 | 25.6 (3.9) | 26.2 (4.4) | 26.9 (5.0) | 27.4 (5.3) |
| Antihypertensive treatment, n (%) | 81 (9) | 120 (11) | 140 (14) | 229 (22) |
| Diabetes mellitus, n (%) | 13 (1) | 33 (3) | 44 (4) | 96 (9) |
| Current smoker, n (%) | 146 (16) | 240 (22) | 248 (25) | 304 (29) |
| Left ventricular hypertrophy, n (%) | 9 (1) | 9 (1) | 22 (2) | 19 (2) |
| Valvular heart disease, n (%) | 7 (1) | 8 (1) | 14 (1) | 43 (4) |
| Physical activity index | 37 (7) | 37 (7) | 36 (7) | 36 (7) |
| Total cholesterol, mg/dL | 200 (38) | 206 (37) | 208 (38) | 212 (41) |
| HDL cholesterol, mg/dL | 53 (14) | 52 (15) | 50 (15) | 48 (16) |
Values are means (SD) unless otherwise specified. Heart rate cut‐offs for men are: 25th percentile=55 bpm, 50th percentile=61 bpm, 75th percentile=69 bpm; for women: 25th percentile=59 bpm, 50th percentile=65 bpm, 75th percentile=74 bpm. FHS indicates Framingham Heart Study; HDL, high‐density lipoprotein.
Table 2.
Cross‐sectional Correlates of Baseline Resting Heart Rate
| Multivariable‐Adjusted Model* | ||
|---|---|---|
| Estimate (SE) | P Value | |
| Age, per 14 y | 0.16 (0.02) | <0.0001 |
| Female sex | 5.16 (0.38) | <0.0001 |
| Systolic blood pressure, per 20 mm Hg | −0.54 (0.27) | 0.046 |
| Diastolic blood pressure, per 10 mm Hg | 2.12 (0.24) | <0.0001 |
| Body mass index, per 5 kg/m2 | 0.65 (0.20) | 0.001 |
| Antihypertensive treatment | 1.56 (0.52) | 0.003 |
| Diabetes mellitus | 6.59 (0.82) | <0.0001 |
| Current smoker | 3.38 (0.40) | <0.0001 |
| Left ventricular hypertrophy | −0.33 (1.43) | 0.82 |
| Valvular heart disease | 5.80 (1.28) | <0.0001 |
| Physical activity index, per 7 units | −0.56 (0.17) | 0.001 |
| Total cholesterol, per 39 mg/dL | 0.27 (0.17) | 0.12 |
| HDL cholesterol, per 15 mg/dL | −0.75 (0.19) | <0.0001 |
HDL indicates high‐density lipoprotein.
Multivariable model includes all covariates listed in table. Estimate represents change in baseline heart rate per 1‐SD change in continuous variables (as noted) or the presence vs absence of dichotomous variables.
During a median follow‐up of 19 years, 708 participants developed incident cardiovascular disease. Specific cardiovascular events included 303 participants with incident heart failure, 343 participants with coronary heart disease, and 216 participants with stroke. An additional 48 participants underwent permanent pacemaker insertion. There were 1186 deaths, of which 252 were classified as cardiovascular death.
Baseline Heart Rate Predicts Cardiovascular Events
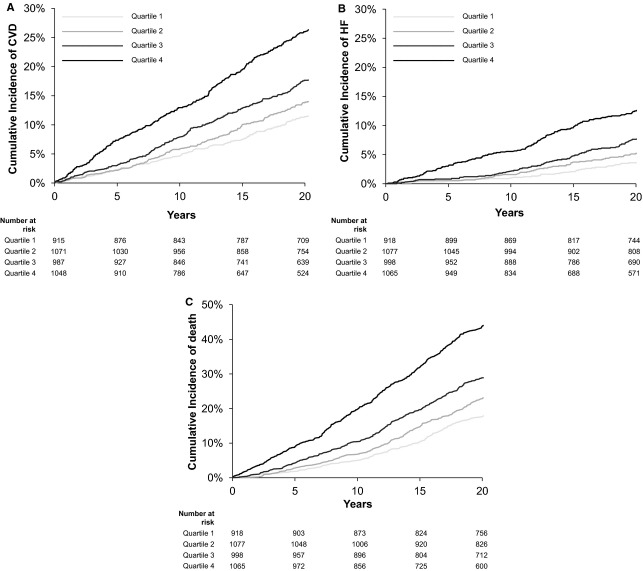
The cumulative incidence of cardiovascular disease, heart failure, and death increased across sex‐specific heart rate quartiles (Figure 1). Baseline resting heart rate predicted incident cardiovascular disease (Table 3). In multivariable‐adjusted analyses (including adjustment for physical activity), each 1‐SD (11 bpm) increase in baseline heart rate was associated with a 15% increased risk of cardiovascular disease (adjusted hazard ratio [HR] 1.15, 95% CI 1.07 to 1.24, P=0.0002). When individual components of cardiovascular disease were examined, this association appeared most pronounced for incident heart failure events, with each 1‐SD increase in baseline heart rate associated with a 32% increased risk of future heart failure (adjusted HR 1.32, 95% CI 1.18 to 1.48, P<0.0001). Specifically, there was a differential effect of heart rate on heart failure compared with non–heart failure cardiovascular disease (P=0.004 for difference).
Figure 1.
Heart rate quartiles and risk of long‐term cardiovascular events. Cumulative incidence of clinical events increases across sex‐specific heart rate quartiles for cardiovascular events (A), heart failure (B), and all‐cause mortality (C). Analyses for nonfatal events were adjusted for competing risk of death. Heart rate cut‐offs for men are 25th percentile=55 bpm, 50th percentile=61 bpm, 75th percentile=69 bpm; for women: 25th percentile=59 bpm, 50th percentile=65 bpm, and 75th percentile=74 bpm. CVD indicates cardiovascular disease; HF, heart failure.
Table 3.
Association of Baseline Resting Heart Rate and Cardiovascular Outcomes
| Age/Sex‐Adjusted Model | Multivariable‐Adjusted Model* | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Primary outcomes | ||||
| Cardiovascular disease (708 events) | 1.29 (1.20 to 1.39) | <0.0001 | 1.15 (1.07 to 1.24) | 0.0002 |
| Heart failure (303 events) | 1.46 (1.31 to 1.62) | <0.0001 | 1.32 (1.18 to 1.48) | <0.0001 |
| Coronary heart disease (343 events) | 1.26 (1.14 to 1.40) | <0.0001 | 1.08 (0.96 to 1.20) | 0.20 |
| Stroke (216 events) | 1.22 (1.07 to 1.39) | 0.003 | 1.10 (0.96 to 1.26) | 0.19 |
| Secondary outcomes | ||||
| Pacemaker (48 events) | 0.53 (0.37 to 0.75) | 0.0003 | 0.55 (0.38 to 0.79) | 0.001 |
| Death (1186 events) | 1.26 (1.19 to 1.33) | <0.0001 | 1.17 (1.11 to 1.24) | <0.0001 |
| Cardiovascular death (252 events) | 1.34 (1.19 to 1.51) | <0.0001 | 1.18 (1.04 to 1.33) | 0.01 |
HDL indicates high‐density lipoprotein; HR, hazard ratio per 1‐SD increase in heart rate.
Multivariable analyses adjusted for age, sex, systolic blood pressure, use of antihypertensive treatment, body mass index, diabetes, smoking status, physical activity index, valvular heart disease, electrocardiographic left ventricular hypertrophy, total/HDL cholesterol ratio, minor cardiovascular disease, and PR and QRS duration.
When examined across sex‐specific quartiles of heart rate, individuals in the top quartile had a 2‐fold increased risk of heart failure (adjusted HR 2.04, 95% CI 1.38 to 3.01, P for trend across quartiles <0.0001). Resting heart rate was associated with higher risk of coronary heart disease and stroke in age‐ and sex‐adjusted analyses, but that association was attenuated after multivariable adjustment (Table 3). Similar results were obtained when examining heart rate by sex‐specific quartiles.
In contrast to the increased risk of conventional cardiovascular events, higher heart rate was associated with a 40% decreased risk of requiring permanent pacemaker placement (per 1‐SD increase in heart rate, adjusted HR 0.55, 95% CI 0.38 to 0.79, P=0.001).
Baseline Heart Rate Predicts All‐Cause Mortality
Resting heart rate predicted increased risk of all‐cause mortality, with each 1‐SD increase in heart rate associated with a 17% increased risk of all‐cause death (multivariable‐adjusted hazard ratio 1.17, 95% CI 1.11 to 1.24, P<0.0001, Table 3). Resting heart rate predicted cardiovascular death, with each 1‐SD increase in heart rate associated with a 18% increased risk (adjusted HR 1.18, 95% CI 1.04 to 1.33, P=0.01), although this association did not meet the Bonferroni‐corrected P‐value threshold of P<0.007.
Effect Across Heart Rate Range
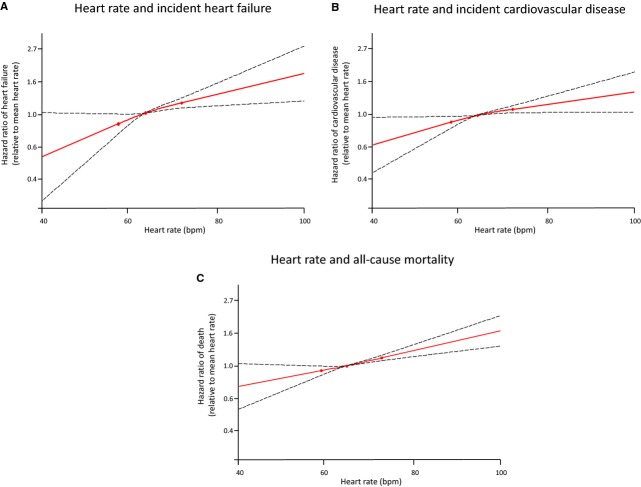
Restricted cubic splines were used to model the effect of heart rate on clinical outcomes. A lower heart rate appeared to be associated with lower risk of cardiovascular disease, heart failure, and death, even at very low heart rates <60 bpm. There were no significant nonlinear terms, and fit statistics of the spline analyses did not suggest a lower threshold beyond which the benefit of a lower heart rate abated or increased (Figure 2).
Figure 2.
Restricted cubic spline plots, showing the association of heart rate and clinical outcomes for cardiovascular disease (A), heart failure (B), and all‐cause mortality (C). Three knots were placed at the 25th, 50th, and 75th percentiles of heart rate, and dashed lines represent 95% CIs.
Secondary Analyses
After additionally excluding individuals with prevalent minor cardiovascular disease and valvular disease (n=281), results were not materially different from primary results (Table 4). We formally tested for interactions of age and sex with heart rate. There was no effect modification by sex (P>0.05 for all outcomes), and when examined separately in men and women, heart rate remained associated with incident cardiovascular disease, heart failure, and all‐cause and cardiovascular death. In contrast, age appeared to modify the association of resting heart rate and heart failure outcomes (P=0.03). Specifically, the association of resting heart rate and incident heart failure events was less pronounced at older ages. There was no effect modification by age for cardiovascular disease, death, or pacemaker events.
Table 4.
Association of Baseline Resting Heart Rate and Cardiovascular Outcomes After Exclusion of Prevalent Minor Cardiovascular Disease and Valvular Disease
| Age/Sex‐Adjusted Model | Multivariable‐Adjusted Model* | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Primary outcomes | ||||
| Cardiovascular disease (593 events) | 1.29 (1.19 to 1.39) | <0.0001 | 1.15 (1.06 to 1.25) | 0.0007 |
| Heart failure (233 events) | 1.45 (1.29 to 1.64) | <0.0001 | 1.34 (1.18 to 1.52) | <0.0001 |
| Coronary heart disease (285 events) | 1.26 (1.12 to 1.40) | <0.0001 | 1.07 (0.95 to 1.21) | 0.26 |
| Stroke (188 events) | 1.24 (1.08 to 1.42) | 0.003 | 1.11 (0.96 to 1.28) | 0.17 |
| Secondary outcomes | ||||
| Pacemaker (37 events) | 0.47 (0.31 to 0.72) | 0.0004 | 0.50 (0.33 to 0.77) | 0.002 |
| Death (987 events) | 1.26 (1.19 to 1.34) | <0.0001 | 1.20 (1.12 to 1.28) | <0.0001 |
| Cardiovascular death (188 events) | 1.32 (1.15 to 1.51) | <0.0001 | 1.17 (1.01 to 1.35) | 0.03 |
HDL indicates high‐density lipoprotein; HR, hazard ratio per 1 standard deviation increase in heart rate.
Multivariable analyses adjusted for age, sex, systolic blood pressure, use of antihypertensive treatment, body mass index, diabetes, smoking status, physical activity index, electrocardiographic left ventricular hypertrophy, total/HDL cholesterol ratio, and PR and QRS duration.
Average heart rate over approximately 8 years preceding the baseline examination remained a significant predictor of cardiovascular disease, heart failure, death, and need for permanent pacemaker implantation. When examined as a time‐dependent variable over the course of 8 years, the association with pacemaker implantation was no longer significant, whereas the association with coronary heart disease remained significant even after multivariable adjustment (Table 5).
Table 5.
Heart Rate as a Time‐Dependent Variable as a Predictor of Clinical Outcomes
| Age/Sex‐Adjusted Model | Multivariable‐Adjusted Model* | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Primary outcomes | ||||
| Cardiovascular disease (708 events) | 1.31 (1.22 to 1.40) | <0.0001 | 1.22 (1.13 to 1.30) | <0.0001 |
| Heart failure (303 events) | 1.51 (1.37 to 1.67) | <0.0001 | 1.41 (1.27 to 1.56) | <0.0001 |
| Coronary heart disease (343 events) | 1.31 (1.19 to 1.44) | <0.0001 | 1.19 (1.08 to 1.32) | 0.0004 |
| Stroke (216 events) | 1.13 (0.99 to 1.28) | 0.06 | 1.06 (0.93 to 1.20) | 0.40 |
| Secondary outcomes | ||||
| Pacemaker (48 events) | 0.90 (0.67 to 1.20) | 0.46 | 0.99 (0.75 to 1.33) | 0.97 |
| Death (1186 events) | 1.23 (1.16 to 1.29) | <0.0001 | 1.18 (1.12 to 1.25) | <0.0001 |
| Cardiovascular death (252 events) | 1.27 (1.13 to 1.42) | <0.0001 | 1.18 (1.05 to 1.32) | 0.005 |
HDL indicates high‐density lipoprotein; HR, hazard ratio per 1 standard deviation increase in heart rate.
Multivariable analyses adjusted for age, sex, systolic blood pressure, use of antihypertensive treatment, body mass index, diabetes, smoking status, physical activity index, valvular heart disease, electrocardiographic left ventricular hypertrophy, total/HDL cholesterol ratio, minor cardiovascular disease, and PR and QRS duration.
In secondary analyses examining the association of heart rate and clinical outcomes in participants taking heart rate–modifying agents at the baseline examination (n=575), we found similar trends as in the primary analysis for all outcomes except pacemaker insertion. After multivariable adjustment, only all‐cause mortality and cardiovascular death remained significantly associated with heart rate (P<0.0001, and P=0.02, respectively).
Discussion
Our study demonstrates that individuals with a higher heart rate have an excess risk of cardiovascular events and mortality, even after accounting for clinical risk factors and physical activity. The association with future events is particularly strong for heart failure, with individuals in the highest quartile of heart rate having a 2‐fold increased risk of incident heart failure. In contrast, we found that lower heart rate was associated with a greater risk of future permanent pacemaker implantation.
Existing community‐based studies have focused largely on fatal events of on individuals with existing cardiovascular disease.1–2,10,12,28 We now extend these data to a comprehensive assessment of fatal and nonfatal cardiovascular outcomes in a contemporary sample with extensive assessment of cardiovascular risk factors. In contrast to prior community‐based studies,29 we were able to exclude participants with prevalent cardiovascular disease and those taking heart rate–modifying medications at baseline.
Our findings suggest that the association of heart rate with cardiovascular disease is driven largely by incident heart failure. Two recent population‐based studies suggested an association of heart rate with heart failure in men but not women after exclusion of individuals taking heart rate–modifying medications,27,30 though our data suggest that the finding is equally prominent in both sexes. Our cohort was on average more than 10 years younger than individuals in the Rotterdam Study; this difference may have given us greater statistical power, because we found that the association weakened with advancing age.
Interestingly, we found that resting heart rate captured at a single examination was as strong a predictor of cardiovascular outcomes as repeated measurements of heart rate averaged over the course of 8 years. This highlights the potential role of resting heart rate as an easily obtainable measure of cardiovascular prognosis.
We also present novel population‐based data regarding the higher risk of future pacemaker implantation with lower heart rates, an association that was independent of other risk factors, including PR interval and QRS duration.23–24 Asymptomatic bradycardia has been associated with higher rates of pacemaker implantation in a clinical cohort of older patients.31 As such, sinus bradycardia may precede advanced conduction system and cardiac disease, which may be associated with an adverse prognosis. Indeed, some studies have demonstrated increased mortality with lower heart rates and a J‐shaped relationship of heart rate and outcomes in elderly community‐dwelling adults and in individuals with existing cardiovascular disease.28,32–34 Despite the increase in risk of permanent pacemaker implantation at lower heart rates, we found no evidence of concomitant increase in mortality or cardiovascular disease with bradycardia; to the contrary, our spline curves suggest continued benefit with regard to cardiovascular disease at lower heart rates. It may be that bradycardia portends increased overall risk in the elderly or in those with existing cardiovascular disease, whereas bradycardia in ostensibly healthy individuals is protective with regard to overall prognosis despite an increased risk of pacemaker implantation.
The mechanism by which increased heart rate predicts adverse cardiovascular outcomes is likely multifactorial. On one hand, heart rate may directly affect cardiovascular risk: previous studies have shown that higher heart rate may have proatherogenic effects by increasing shear stress,7 and higher heart rate has been associated with progression of atherosclerosis.35 Higher heart rate also has negative effects on myocardial energetics,8 which supports the particularly pronounced effect on heart failure outcomes. On the other hand, heart rate may reflect other underlying processes leading to adverse outcomes. For instance, elevated heart rate may reflect autonomic dysfunction and increased sympathetic activity.6 Higher heart rate has also been shown to precede the development of diabetes mellitus.5 Finally, higher heart rate could reflect poorer physical fitness.36 However, associations in our study persisted despite adjustment for physical activity. We found a strong association with cardiovascular‐specific outcomes and all‐cause mortality, suggesting that heart rate may be both mediator of cardiovascular risk, as well as a marker of overall poor prognosis. Further studies are needed to evaluate underlying mechanisms by which heart rate may lead to adverse outcomes.
Several limitations deserve mention. Ours is an observational study, and thus the ability to make causal inferences is limited. Generalizability of our results must also be interpreted with caution given our predominantly white sample. Given the modest number of permanent pacemaker implants during follow‐up, inferences about potential interactions of heart rate and age are not conclusive. Finally, the clinical implications our findings will need to be explored further. Heart rate could be a highly reproducible and routinely obtained measure that might serve in risk‐stratification of patients.
In summary, we show that increased heart rate is associated with elevated cardiovascular risk and increased mortality in a community‐based sample. Conversely, lower heart rate is associated with a greater risk of future permanent pacemaker implantation. These associations appeared to be independent of physical activity and other potential confounding factors. Whether treatment aimed at lowering heart rate in individuals without existing cardiovascular disease might reduce cardiovascular risk, as has been shown in patients with chronic heart failure,37 remains to be seen.
Sources of Funding
This work was partially supported by the Framingham Heart Study of the National Heart, Lung, and Blood Institute (contract N01‐HC‐25195). Dr Ho is supported by National Institutes of Health grant K23‐HL116780 and a Boston University School of Medicine Department of Medicine Career Investment Award. Dr Cheng is supported by an award from the Ellison Foundation.
Disclosures
None.
References
- 1.Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987; 113:1489-1494. [DOI] [PubMed] [Google Scholar]
- 2.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow‐Up Study. Am Heart J. 1991; 121:172-177. [DOI] [PubMed] [Google Scholar]
- 3.Zuanetti G, Mantini L, Hernández‐Bernal F, Barlera S, di Gregorio D, Latini R, Maggioni AP. Relevance of heart rate as a prognostic factor in patients with acute myocardial infarction: insights from the GISSI‐2 study. Eur Heart J. 1998; 19suppl F:F19-F26. [PubMed] [Google Scholar]
- 4.Ho JE, Bittner V, Demicco DA, Breazna A, Deedwania PC, Waters DD. Usefulness of heart rate at rest as a predictor of mortality, hospitalization for heart failure, myocardial infarction, and stroke in patients with stable coronary heart disease (Data from the Treating to New Targets [TNT] trial). Am J Cardiol. 2010; 105:905-911. [DOI] [PubMed] [Google Scholar]
- 5.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987‐1998. Circulation. 2003; 107:2190-2195. [DOI] [PubMed] [Google Scholar]
- 6.Grassi G, Vailati S, Bertinieri G, Seravalle G, Stella ML, Dell'Oro R. Heart rate as marker of sympathetic activity. J Hypertens. 1998; 16:1635-1639. [DOI] [PubMed] [Google Scholar]
- 7.Giannoglou GD, Chatzizisis YS, Zamboulis C, Parcharidis GE, Mikhailidis DP, Louridas GE. Elevated heart rate and atherosclerosis: an overview of the pathogenetic mechanisms. Int J Cardiol. 2008; 126:302-312. [DOI] [PubMed] [Google Scholar]
- 8.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004; 95:135-145. [DOI] [PubMed] [Google Scholar]
- 9.Böhm M, Swedberg K, Komajda M, Boerer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet. 2010; 376:886-894. [DOI] [PubMed] [Google Scholar]
- 10.Benetos A, Rudnichi A, Thomas F, Safar M, Guize L. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension. 1999; 33:44-52. [DOI] [PubMed] [Google Scholar]
- 11.Hansen TW, Thijs L, Boggia J, Li Y, Kikuya M, Bjorklund‐Bodegard K, Richart T, Ohkubo T, Jeppesen J, Torp‐Pedersen C, Lind L, Sandoya E, Imai Y, Wang J, Ibsen H, O'Brien E, Staessen JA. Prognostic value of ambulatory heart rate revisited in 6928 subjects from 6 populations. Hypertension. 2008; 52:229-235. [DOI] [PubMed] [Google Scholar]
- 12.Tverdal A, Hjellvik V, Selmer R. Heart rate and mortality from cardiovascular causes: a 12 year follow‐up study of 379,843 men and women aged 40‐45 years. Eur Heart J. 2008; 29:2772-2781. [DOI] [PubMed] [Google Scholar]
- 13.Shaper AG, Wannamethee G, Macfarlane PW, Walker M. Heart rate, ischaemic heart disease, and sudden cardiac death in middle‐aged British men. Br Heart J. 1993; 70:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jouven X, Empana JP, Escolano S, Buyck JF, Tafflet M, Desnos M, Ducimetiere P. Relation of heart rate at rest and long‐term (>=20 years) death rate in initially healthy middle‐aged men. Am J Cardiol. 2009; 103:279-283. [DOI] [PubMed] [Google Scholar]
- 15.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PW, Kritchevsky SB. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008; 1:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963; 107:539-556. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979; 110:281-290. [DOI] [PubMed] [Google Scholar]
- 18.Cupples LA, D'Agostino RB, Kannel WB, Wolf P, Garrison RJ. The Framingham Study, Section 35: An Epidemiological Investigation of Cardiovascular Disease: Survival Following Initial Cardiovascular Events: 30 Year Follow‐Up. 1988Bethesda, MD: National Heart Lung and Blood Institute; ‐ [Google Scholar]
- 19.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979; 139:857-861. [PubMed] [Google Scholar]
- 20.Wilson PW, Paffenbarger RS, Jr, Morris JN, Havlik RJ. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J. 1986; 111:1177-1192. [DOI] [PubMed] [Google Scholar]
- 21.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971; 285:1441-1446. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Wolf PA, Garrison RJ. The Framingham Heart Study, Section 34: An Epidemiological Investigation of Cardiovascular Disease: Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements: 30‐Year Follow‐Up. 1988Bethesda, MD: National Heart, Lung, and Blood Institute; ‐ [Google Scholar]
- 23.Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton‐Cheh C, Levy D, Benjamin EJ, Vasan RS, Wang TJ. Long‐term outcomes in individuals with prolonged PR interval or first‐degree atrioventricular block. JAMA. 2009; 301:2571-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng S, Larson MG, Keyes MJ, McCabe EL, Newton‐Cheh C, Levy D, Benjamin EJ, Vasan RS, Wang TJ. Relation of QRS width in healthy persons to risk of future permanent pacemaker implantation. Am J Cardiol. 2010; 106:668-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaynor JJ, Feuer EJ, Tan CC, Wu DH, Little CR, Straus DJ, Clarkson BD, Brennan MF. On the use of cause‐specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc. 1993; 88:400-409. [Google Scholar]
- 26.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995; 51:524-532. [PubMed] [Google Scholar]
- 27.Nanchen D, Leening MJ, Locatelli I, Cornuz J, Kors JA, Heeringa J, Deckers JW, Hofman A, Franco OH, Stricker BH, Witteman JC, Dehghan A. Resting heart rate and the risk of heart failure in healthy adults: the Rotterdam Study. Circ Heart Fail. 2013; 6:402-410. [DOI] [PubMed] [Google Scholar]
- 28.Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, Berkson DM, Lepper M, Schoenberger JA, Lindberg HA. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980; 112:736-749. [DOI] [PubMed] [Google Scholar]
- 29.Nanchen D, Stott DJ, Gussekloo J, Mooijaart SP, Westendorp RG, Jukema JW, Macfarlane PW, Cornuz J, Rodondi N, Buckley BM, Ford I, Satar N, de Craen AJ. Resting heart rate and incident heart failure and cardiovascular mortality in older adults: role of inflammation and endothelial dysfunction: the PROSPER study. Eur J Heart Fail. 2013; 15:581-588. [DOI] [PubMed] [Google Scholar]
- 30.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Resting heart rate and incident heart failure in apparently healthy men and women in the EPIC‐Norfolk study. Eur J Heart Fail. 2012; 14:1163-1170. [DOI] [PubMed] [Google Scholar]
- 31.Goldberger JJ, Johnson NP, Gidea C. Significance of asymptomatic bradycardia for subsequent pacemaker implantation and mortality in patients >60 years of age. Am J Cardiol. 2011; 108:857-861. [DOI] [PubMed] [Google Scholar]
- 32.Kuzuya M, Enoki H, Iwata M, Hasegawa J, Hirakawa Y. J‐shaped relationship between resting pulse rate and all‐cause mortality in community‐dwelling older people with disabilities. J Am Geriatr Soc. 2008; 56:367-368. [DOI] [PubMed] [Google Scholar]
- 33.Bangalore S, Messerli FH, Ou FS, Tamis‐Holland J, Palazzo A, Roe MT, Hong MK, Peterson ED. The association of admission heart rate and in‐hospital cardiovascular events in patients with non‐ST‐segment elevation acute coronary syndromes: results from 135 164 patients in the CRUSADE quality improvement initiative. Eur Heart J. 2010; 31:552-560. [DOI] [PubMed] [Google Scholar]
- 34.Kolloch R, Legler UF, Champion A, Cooper‐Dehoff RM, Handberg E, Zhou Q, Pepine CJ. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: findings from the INternational VErapamil‐SR/trandolapril STudy (INVEST). Eur Heart J. 2008; 29:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin J, Blaha MJ, Budoff MJ, Rivera JJ, Shaw LJ, Blankstein R, Mallah M‐A, Carr JJ, Jones D‐L, Blumenthal RS, Nasir K. The relationship between resting heart rate and incidence and progression of coronary artery calcification: the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2012; 220:194-200. [DOI] [PubMed] [Google Scholar]
- 36.Hodgson JL, Buskirk ER. Physical fitness and age, with emphasis on cardiovascular function in the elderly. J Am Geriatr Soc. 1977; 25:385-392. [DOI] [PubMed] [Google Scholar]
- 37.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet. 2010; 376:875-885. [DOI] [PubMed] [Google Scholar]