Abstract
Background
Epidemiologic studies have yielded mixed findings on the association of psychosocial stressors with cardiovascular disease (CVD) risk. In this study, we examined associations of stressful life events (SLE) and social strain with incident coronary heart disease (CHD) and stroke (overall, and for hemorrhagic and ischemic strokes) independent of sociodemographic characteristics, and we evaluated whether these relationships were explained by traditional behavioral and biological risk factors.
Methods and Results
Data from approximately 82 000 Women's Health Initiative Observational Study participants were used for the SLE and social strain analyses, respectively. Participants were followed for events for up to 18.0 years (median, 14.0). Separate Cox proportional hazards models were generated to estimate associations of each stress measure with incident CVD. After adjusting for sociodemographic characteristics and depressive symptoms, higher SLE and social strain were associated with higher incident CHD and stroke (each P trend <0.05). Hazard ratios and 95% confidence intervals were 1.12 (1.01, 1.25) for incident CHD and 1.14 (1.01, 1.28) for incident stroke among participants reporting high versus low SLE. Findings were similar for social strain. Associations were attenuated with further adjustment for mediating behavioral and biological risk factors. Findings were similar for associations of SLE with ischemic stroke and hemorrhagic stroke, but social strain was only associated with ischemic stroke.
Conclusions
Higher SLE and social strain were associated with higher incident CVD independent of sociodemographic factors and depressive symptoms, but not behavioral and biological risk factors.
Keywords: cardiovascular diseases, epidemiology, stress
Introduction
Despite the long‐standing notion that chronic exposure to psychosocial stressors is associated with higher cardiovascular disease (CVD) risk, findings from epidemiologic studies are mixed. The few existing studies that have investigated the association between chronic stress and stroke risk suggest that stress is a significant predictor of stroke.1–3 There is also some evidence suggesting that stress is associated with ischemic, but not hemorrhagic, stroke.3 Findings for coronary heart disease (CHD) are less consistent. Although some earlier studies found significant associations between chronic stress and CHD risk,4–8 recent research has challenged these findings.2,9–12 CHD definitions used by previous studies have often included angina pectoris based on self‐report of symptoms, and some researchers contend that individuals who report higher stress may also report more symptoms, thus biasing studies of chronic stress and CHD.
Another potential explanation for the inconsistent findings is the use of differing measures of chronic stress across studies, making comparisons difficult. The majority of prospective studies on stress and CVD risk have focused on job strain7 and general, everyday stressors, such as feelings of irritability or anxiety.4–6,9,11 Though some studies have examined accumulation of major life events as a measure of chronic stress,2,5,12 they typically used a measure consisting of counts of events without any assessment of the perceived severity of the stressor. As such, it may not capture the extent to which a major life event is perceived as stressful, which may affect how it influences CHD risk. Few studies have examined adverse aspects of social relationships, but there is some evidence implicating social stressors as a risk factor for incident coronary events.8
In this study, we used data from the Women's Health Initiative (WHI) observational study, a large, multiethnic cohort of postmenopausal women, to investigate associations of baseline stressful life events (SLE) and social strain with incident CHD and stroke over 16 years of follow‐up independent of sociodemographic characteristics. We also evaluated whether these associations were explained by traditional behavioral and biological risk factors hypothesized to mediate these relationships, including cigarette smoking, alcohol use, poor diet, physical activity, hypertension, and abdominal obesity.13–18 This study builds on the existing literature by using well‐defined, largely adjudicated CVD outcome measures and by utilizing 2 different measures of psychosocial stress exposure.
Methods
Study Population
The WHI observational study is a multiethnic cohort of 93 676 postmenopausal women ages 50 to 79 years at baseline. Women were enrolled from 1993 to 1998 at 40 geographically diverse clinical centers throughout the United States. All participants provided informed consent using materials approved by institutional review boards at each center. Further details have been previously described.19–20
Measures
Stressful life events and social strain
The life events questionnaire completed by WHI participants at baseline contained a life events inventory adapted for the Beta‐Blocker Heart Attack Trial based on the measure used in the Alameda County Epidemiologic Study.21 This measure was further modified to ensure relevance to older women. Participants were asked whether any of the following 11 life changes had occurred over the past year: spouse died; spouse had serious illness; close friend died; had major problems with money; divorced or break up; close friend divorced; major conflict with children or grandchildren; lost job; physically abused; verbally abused; or pet died. In addition, women were asked to indicate the extent to which the event upset them based on a scale ranging from 1 (did not upset me) to 3 (upset me very much). The resulting SLE score ranged from 0 to 33, with a higher score indicating that a participant experienced a greater number of and/or more upsetting SLE. This score was divided into approximate quartiles (low: 0; medium‐low: 1 to 2; medium‐high: 3 to 4; and high: 5 or more) based on the observed distribution of the responses.
Social strain was evaluated at baseline with 4 items derived from a previously validated measure of negative aspects of social relationships.22 Participants were asked how many of the people who were important to them got on their nerves, asked too much of them, did not include them, and tried to get them to do things they do not want to do. Responses to each item could range from 1 (none) to 5 (all). These items were summed to yield a social strain score that could range from 4 to 20, with higher scores indicating greater social strain. This score was divided into approximate tertiles (low: 4; medium: 5 to 6; and high: 7 or more) based on the observed distribution of responses.
Covariates
Age, self‐reported race/ethnicity (white, black/African American, Hispanic, American Indian/Alaska Native, Asian/Pacific Islander, or other), education (less than high school completed, high school diploma/general equivalency diploma, some college, or college or more completed), annual family income (<$35 000, $35 000 to $49 999, $50 000 to $74 999, and ≥$75 000), and marital status (married or in a marriage‐like relationship versus not married) were included as potential confounders of associations of the stress measures with the cardiovascular (CV) outcomes. Depressive symptoms, assessed using the Burnam 8‐item depression screening instrument, were also adjusted for as a potential confounder.23 The Burnham instrument consists of 6 items from the Center for Epidemiologic Studies Depression Scale about the frequency of depressive symptoms in the past week and 2 items from the National Institute of Mental Health's Diagnostic Interview Schedule about the duration of symptoms. Possible scores range from 0 to 0.99, with higher scores indicating greater depressive symptomatology.
Baseline cigarette smoking, alcohol use, dietary quality, physical activity, hypertension, and waist circumference were included as potential mediators of the associations of SLE and social strain with incident CHD and stroke.13–14,18,24 Cigarette smoking was dichotomized as current versus not current. Alcohol use was categorized as heavy (>7 drinks per week), none, and moderate (≤7 alcoholic drinks per week; reference group). Physical activity was categorized as high (≥1000 metabolic equivalent [MET]‐minutes per week of energy expenditure from recreational activity), intermediate (<1000 MET‐minutes per week), or none based on the 2008 Physical Activity Guidelines for Americans.25
Dietary information was obtained from a validated food frequency questionnaire developed by the WHI to estimate mean daily nutrient intakes during the previous 3‐month period.26–27 The 2005 U.S. Department of Agriculture Healthy Eating Index (HEI) was used to evaluate dietary quality.28 The HEI score is based on consumption levels of total fruit, whole fruit, total vegetables, dark green vegetables, orange vegetables, and legumes, total grains, whole grains, milk, meat and beans, oils, saturated fat, sodium, and calories from solid fats, alcoholic beverages, and added sugars. HEI scores range from 0 to 100, with a higher score indicating better dietary quality.
Blood pressure was measured twice during the baseline clinic visit by certified staff using standardized procedures and instruments. Hypertension was defined as having mean systolic blood ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self‐reported use of antihypertensive medication. Waist circumference was measured to the nearest 0.1 cm at the natural waist or narrowest part of the body. High cholesterol and diabetes status were based on self‐report. Participants were classified as having high cholesterol if they were told by a doctor that they have high cholesterol requiring pills. Participants were considered diabetic if they reported being told by a doctor they had sugar diabetes or high blood sugar.
Fatal and nonfatal incident CVD
Outcome follow‐up data were available for events occurring on or before and adjudicated through September 17, 2012.29–31 Outcome data were obtained annually by mailed medical history update questionnaires, direct contact when subjects attended the clinical follow‐up visits in years 1 and 3 of the study, or by proxy respondents. National death index searches were performed to verify reports of participant fatalities. Participants were initially followed for outcomes as part of the original WHI study between 1993 and 2005. Between 2005 and 2010, consenting original study participants were enrolled in the WHI Extension Study I for an additional 5 years of follow‐up. Follow‐up of consenting participants from the first extension study has continued with the WHI Extension Study II, which will continue following participants for outcomes through 2015.
The adjudication process changed for the CV outcomes over these follow‐up periods.30–31 Incident CHD was defined as the first occurrence of clinical myocardial infarction (MI), definite silent MI, or death resulting from definite or possible CHD. These events were locally adjudicated during the original follow‐up and centrally adjudicated during the Extension Study I. Incident stroke events, defined as the first occurrence of stroke or death resulting from cerebrovascular disease, were centrally adjudicated during the original and Extension Study I follow‐up periods. In the Extension Study II, participants were divided into 2 subsamples: a medical records cohort and a self‐report cohort. Stroke and CHD events were only adjudicated in the medical records cohort; outcomes were self‐reported in the self‐report cohort. Sensitivity analyses were conducted to determine how robust our findings were to the inclusion of self‐reported stroke and CHD during the Extension Study II.
Statistical Analysis
The distribution of all study covariates as well as age‐adjusted CHD and stroke incidence rates were examined by SLE and social strain categories. Poisson regression was used to estimate age‐adjusted incidence rates per 10 000 person‐years.32
Cox proportional hazard models were used to estimate the hazard ratio (HR) and 95% confidence intervals (CIs) for incident CHD and stroke associated with SLE and social strain categories after adjusting for age, race/ethnicity, marital status, education, and income. Time in the proportional model was the follow‐up time from enrollment to the event (for cases) or to the last contact for outcome information (for noncases). The proportional hazards assumption was tested by evaluating interactions of analysis time with the full complement of covariates; there was no evidence of violation. Associations did not vary significantly by race or ethnicity (P for interaction ≥0.57), so pooled point estimates are presented. The mediating role of each behavioral and biological risk factor was calculated as the percentage reduction in the beta coefficient for high (versus low) SLE/social strain after inclusion of each factor, using the formula: 100×([βModel 1−βModel 1+risk factor]/βModel 1).
The primary analyses for this study were based on the 82 107 and 82 009 WHI observational study participants who were not missing data on SLE or social strain, respectively, and who did not have prevalent CVD at baseline (defined as a history of stroke, MI, angina, congestive heart failure, peripheral arterial disease, percutaneous coronary angioplasty, or coronary artery bypass graft). We also conducted sensitivity analyses using data from the 70 072 (for SLE analyses) and 70 002 (for social strain analyses) participants who had complete data on all covariates. From the primary analysis samples, 8.1% were missing data on one or more sociodemographic characteristics (n=6661 for SLE analyses and n=6631 for social strain analyses) and 4.9% were missing data on one or more behavioral or biological risk factors (n=5374 for SLE analyses and n=5376 for social strain analyses). Compared with participants included in the analyses, those excluded from the analyses as a result of missing SLE and social strain were less likely to be white and more likely to be in the lowest education and income categories.
Multiple imputation (5 times) by chained equations was used to impute missing values on other covariates.33 Sensitivity analyses were conducted to assess the robustness of findings using data on participants with complete information. Sensitivity analyses were also conducted limiting analyses to follow‐up through the end of Extension Study I (2010), during which all stroke and CHD events were adjudicated. The multiple imputation was conducted using Stata 11.2 (StataCorp LP, College Station, TX). All other analyses were conducted using SAS 9.4 (SAS Institute Inc., Inc, Cary, NC).
Results
Women in the highest SLE category were younger and less likely to be married than those in the lowest category (Table 1). They were also more likely to be black or Hispanic, have lower educational attainment and income levels, have higher depressive symptoms scores, be current smokers, and have poor diets; they were less likely to be physically active. Patterns were similar by levels of social strain, with the exception of marital status, which did not differ across groups. All descriptive statistics were similar when analyses were restricted to participants with complete data on all study covariates (not shown).
Table 1.
Selected Baseline Characteristics of Study Participants by Stressful Life Events Category, Women's Health Initiative Observational Study
| Stressful Life Events Categories (n=82 107)* | Social Strain Categories (n=82 009)* | ||||||
|---|---|---|---|---|---|---|---|
| High (n=21 858) | Medium‐High (n=19 537) | Medium‐Low (n=22 229) | Low (n=18 483) | High (n=32 295) | Medium (n=25 136) | Low (n=24 578) | |
| Mean age, y (SE) | 62.1 (0.05) | 63.2 (0.05) | 63.7 (0.05) | 64.1 (0.05) | 62.1 (0.04) | 63.4 (0.05) | 64.5 (0.05) |
| Race/ethnicity, % | |||||||
| White | 79.6 | 85.2 | 86.4 | 87.8 | 81.4 | 87.5 | 86.3 |
| Black | 10.6 | 7.5 | 6.3 | 4.7 | 9.4 | 5.7 | 5.9 |
| Hispanic | 5.4 | 3.0 | 2.7 | 3.0 | 4.7 | 2.8 | 2.8 |
| Asian/Pacific Islander | 2.4 | 2.7 | 3.4 | 3.4 | 2.7 | 2.7 | 3.6 |
| Alaskan Native/American Indian | 0.6 | 0.4 | 0.3 | 0.3 | 0.5 | 0.3 | 0.4 |
| Other/unknown | 1.4 | 1.2 | 0.9 | 0.8 | 1.3 | 1.0 | 1.0 |
| Married or marriage‐like relationship, % | 56.5 | 65.2 | 66.1 | 66.6 | 63.2 | 64.1 | 62.7 |
| Education level, % | |||||||
| Less than high school completed | 5.8 | 4.2 | 3.6 | 4.0 | 5.4 | 3.3 | 4.1 |
| High school/GED | 26.6 | 26.2 | 23.5 | 25.1 | 26.0 | 24.1 | 25.5 |
| Some schooling after high school | 29.5 | 26.9 | 25.8 | 24.0 | 28.0 | 26.3 | 25.1 |
| College degree or more | 38.1 | 42.3 | 47.1 | 46.9 | 40.6 | 46.3 | 45.3 |
| Annual family income, % | |||||||
| <$35 000 | 44.6 | 36.7 | 34.5 | 32.5 | 39.6 | 35.0 | 36.1 |
| $35 000 to $49 999 | 19.6 | 20.5 | 20.5 | 20.9 | 19.9 | 21.2 | 20.3 |
| $50 000 to $74 999 | 18.7 | 20.9 | 21.7 | 22.1 | 20.4 | 21.5 | 20.9 |
| ≥$75 000 | 17.1 | 21.9 | 23.3 | 24.5 | 20.1 | 22.3 | 22.7 |
| Mean depressive symptoms, score (SE) | 0.09 (0.001) | 0.03 (0.0008) | 0.02 (0.0005) | 0.01 (0.0005) | 0.07 (0.0009) | 0.03 (0.0006) | 0.02 (0.0005) |
| Current smoker, % | 8.2 | 6.1 | 5.0 | 4.7 | 7.3 | 5.7 | 4.7 |
| Alcohol use*, % | |||||||
| Heavy | 11.5 | 12.9 | 13.8 | 14.0 | 11.8 | 14.2 | 13.6 |
| Moderate | 45.2 | 46.9 | 47.7 | 47.5 | 45.5 | 48.5 | 46.9 |
| None | 43.3 | 40.2 | 38.5 | 38.5 | 42.7 | 37.3 | 39.5 |
| Recreational physical activity*, % | |||||||
| None | 15.5 | 13.4 | 12.2 | 11.3 | 15.2 | 12.1 | 11.6 |
| Intermediate | 54.8 | 53.9 | 53.9 | 52.4 | 54.7 | 53.7 | 52.6 |
| High | 29.7 | 32.7 | 33.9 | 36.3 | 30.1 | 34.2 | 35.8 |
| Dietary quality, score* (SE) | 67.9 (0.08) | 69.3 (0.07) | 69.9 (0.07) | 70.4 (0.08) | 68.2 (0.06) | 69.8 (0.07) | 70.4 (0.06) |
| Hypertension, % | 41.2 | 40.8 | 39.8 | 39.4 | 40.8 | 39.5 | 40.4 |
| Mean waist circumference, cm (SE) | 86.1 (0.1) | 84.2 (0.1) | 83.7 (0.09) | 82.8 (0.09) | 85.7 (0.08) | 83.6 (0.08) | 82.9 (0.08) |
| High cholesterol, % | 13.2 | 12.8 | 12.3 | 12.8 | 13.1 | 12.8 | 12.3 |
| Diabetes, % | 5.9 | 4.3 | 4.0 | 3.4 | 5.5 | 3.8 | 3.6 |
GED indicates general equivalency diploma.
The stressful life events score is based on participant responses to questions assessing whether 11 major life changes occurred over the past year and the extent to which the event upset them. The categories low, medium‐low, medium‐high, and high correspond to scores of 0, 1 to 2, 3 to 4, and 5 or more, respectively.
Social strain was evaluated at baseline with 4 items derived from a previously validated measure of negative aspects of social relationships. High, medium, and low social strain corresponds to scores of 4, 5 to 6, and 7 or more, respectively.
Heavy alcohol use is defined as self‐reported consumption of >7 drinks per week; moderate alcohol is defined as ≤7 drinks per week; and none is defined as being a nondrinker.
High recreational physical activity was defined as ≥1000 metabolic equivalent (MET)‐minutes per week of energy expenditure from recreational activity, intermediate was defined as <1000 MET‐minutes per week), and none was defined as no self‐reported activity.
Dietary quality was measured using the Healthy Eating Index.28
Age‐adjusted incidence rates (per 10 000 person‐years) for all outcomes were highest for women in the highest stress categories and lowest for those in the lowest stress categories Figure. CHD incidence rates ranged from 34.7 (95% CI, 32.3, 37.4) among those in the highest SLE category to 28.6 (95% CI, 26.3, 31.0) for those in the lowest category, and incident stroke rates ranged from 28.7 (95% CI, 26.5, 31.2) to 24.0 (95% CI, 21.9, 26.3). Similar findings were obtained for age‐adjusted incidence rates across social strain categories. CHD incidence rates ranged from 33.8 (95% CI, 31.7, 36.0) in the high‐strain group to 29.0 (95% CI, 27.0, 31.2) in the low‐strain group. Stroke incidence rates went from a high of 28.8 (95% CI, 26.9, 30.9) to a low of 25.5 (95% CI, 23.5, 27.6).
Figure 1.
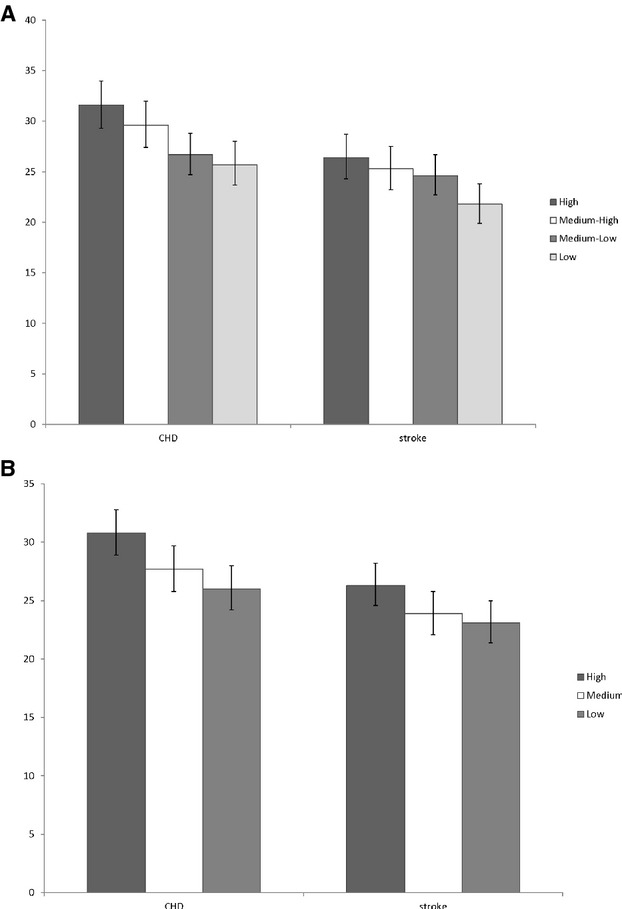
Age‐adjusted rates (per 10 000 person‐years) of incident coronary heart disease (CHD) and stroke according to (A) stressful life events categories and (B) social strain categories.
In analyses adjusted for age, race/ethnicity, education, income, marital status, and depressive symptoms, higher SLE scores and social strain scores were significantly associated with higher incident CHD (P for trend=0.01 for each; Table 2). The adjusted hazard of incident CHD was 12% (95% CI, 1.01, 1.25) higher for women in the high, and 11% (95% CI, 0.99, 1.23) higher for the medium‐high, compared to the low, SLE category. CHD incidence did not differ between those in the medium‐low versus low SLE category. For incident CHD, similar patterns were seen across social strain categories (high strain HR, 1.12; 95% CI, 1.03, 1.23 and medium strain HR, 1.05; 95% CI, 0.96, 1.15). The relationships between these 2 stressors and incident CHD appeared independent of each other. For example, in models further adjusted for both stressors, HRs for the relationships between high SLE and CHD (HR, 1.10; 95% CI, 0.99, 1.23) as well as high strain and CHD (HR, 1.10; 95% CI, 1.00, 1.20) were similar (not shown in table). Associations of SLE and social strain with incident CHD were attenuated with further adjustment for alcohol use, cigarette smoking, hypertension, high cholesterol, diabetes, abdominal obesity, physical activity, and diet; trends were no longer statistically significant (P for trend=0.21 and 0.33, respectively).
Table 2.
Hazard Ratios (95% Confidence Intervals) for Associations of Stressful Life Events and Social Strain Categories With Incident Coronary Heart Disease and Stroke
| Incident CHD | Incident Stroke | |||||
|---|---|---|---|---|---|---|
| No. of Events | Model 1* | Model 2* | No. of Events | Model 1* | Model 2* | |
| Stressful life events* | ||||||
| High | 760 | 1.12 (1.01, 1.25) | 1.05 (0.94, 1.17) | 632 | 1.14 (1.01, 1.28) | 1.09 (0.97, 1.23) |
| Medium‐high | 714 | 1.11 (0.99, 1.23) | 1.07 (0.96, 1.19) | 610 | 1.13 (1.01, 1.27) | 1.11 (0.99, 1.25) |
| Medium‐low | 771 | 1.02 (0.92, 1.14) | 1.00 (0.90, 1.11) | 714 | 1.12 (1.00, 1.25) | 1.10 (0.98, 1.23) |
| Low | 639 | 1.00 (ref) | 1.00 (ref) | 544 | 1.00 (ref) | 1.00 (ref) |
| P for trend | 0.01 | 0.21 | 0.04 | 0.16 | ||
| Social strain* | ||||||
| High | 1097 | 1.12 (1.03, 1.23) | 1.05 (0.96, 1.15) | 930 | 1.10 (1.00, 1.21) | 1.06 (0.96, 1.16) |
| Medium | 886 | 1.05 (0.96, 1.15) | 1.03 (0.94, 1.13) | 766 | 1.02 (0.93, 1.13) | 1.01 (0.92, 1.12) |
| Low | 892 | 1.00 (ref) | 1.00 (ref) | 800 | 1.00 (ref) | 1.00 (ref) |
| P for trend | 0.01 | 0.33 | 0.06 | 0.27 | ||
Adjusted for age, race/ethnicity, education, income, marital status, and depressive symptoms.
Adjusted for model 1, alcohol use, cigarette smoking, hypertension, waist circumference, high cholesterol, diabetes, physical activity, and dietary quality.
The stressful life events score is based on participant responses to questions assessing whether 11 major life changes occurred over the past year and the extent to which the event upset them. The categories low, medium‐low, medium‐high, and high correspond to scores of 0, 1 to 2, 3 to 4, and 5 or more, respectively.
Social strain was evaluated at baseline with 4 items derived from a previously validated measure of negative aspects of social relationships. High, medium, and low social strain corresponds to scores of 4, 5 to 6, and 7 or more, respectively.
Women in the higher SLE categories also had higher hazards of incident stroke, compared to those in the low category (HRs ranged from 1.12 to 1.14; P for trend=0.04). Women in the high social strain category were more likely to have a stroke than those in the low‐strain category (HR, 1.10; 95% CI, 1.00, 1.21). There was no difference in incident stroke between women in the medium‐ and low‐strain categories (HR, 1.02; 95% CI, 0.93, 1.13). Associations of both SLE and social strain with incident stroke were attenuated with further adjustment for behavioral and biological risk factors, and trends were no longer significant (P for trend=0.16 and 0.27, respectively).
Adjustments for each risk factor separately showed that waist circumference was the strongest mediator of the high SLE‐CHD and high social strain‐CHD associations followed by diabetes status (Table 3). Waist circumference attenuated the relationship between high SLE and CHD by 28.6% and the relationship between high strain and CHD by 29.2%. Diabetes attenuated associations of high SLE and social strain with CHD by 20.1% and 19.6%, respectively. This attenuation was even stronger in models simultaneously adjusted for waist circumference and diabetes status (42.5% for SLE and 43.1% for strain), suggesting that they were independent mediators. As with CHD, waist circumference was the strongest mediator of the associations of high SLE (17.3% attenuation) and social strain (24.7% attenuation) with stroke. Diabetes status was once again the second strongest mediator for stroke (8.6 and 13.5% attenuation for SLE and strain, respectively).
Table 3.
Role of Behavioral and Biological Risk factors in Explaining the Associations of High Stressful Life Events and Social Strain With Incident Coronary Heart Disease and Stroke*
| HR (95% CI) | % Attenuation | HR (95% CI) | % Attenuation | |
|---|---|---|---|---|
| Incident Coronary Heart disease | Incident Stroke | |||
| Stressful life events | ||||
| Model 1 | 1.12 (1.01, 1.25) | 1.14 (1.01, 1.28) | ||
| Plus alcohol consumption | 1.12 (1.01, 1.25) | 0.7 | 1.14 (1.01, 1.28) | 0.4 |
| Plus smoking | 1.11 (0.99, 1.23) | 11.5 | 1.13 (1.00, 1.27) | 5.5 |
| Plus diet | 1.11 (0.99, 1.23) | 11.2 | 1.13 (1.00, 1.27) | 6.0 |
| Plus physical activity | 1.11 (1.00, 1.24) | 6.0 | 1.13 (1.00, 1.27) | 4.3 |
| Plus hypertension | 1.12 (1.00, 1.24) | 5.1 | 1.13 (1.00, 1.27) | 4.1 |
| Plus waist circumference | 1.09 (0.97, 1.21) | 28.6 | 1.11 (0.99, 1.25) | 17.3 |
| Plus high cholesterol | 1.12 (1.00, 1.25) | 1.7 | 1.14 (1.01, 1.28) | –0.3 |
| Plus diabetes | 1.10 (0.98, 1.22) | 20.1 | 1.12 (1.00, 1.26) | 8.6 |
| Social strain | ||||
| Model 1 | 1.12 (1.03, 1.23) | 1.10 (1.00, 1.21) | ||
| Plus alcohol consumption | 1.12 (1.02, 1.23) | 1.0 | 1.10 (1.00, 1.21) | 0.5 |
| Plus smoking | 1.11 (1.01, 1.21) | 10.2 | 1.09 (0.99, 1.20) | 6.0 |
| Plus diet | 1.11 (1.01, 1.21) | 11.6 | 1.09 (0.99, 1.20) | 7.4 |
| Plus physical activity | 1.11 (1.01, 1.22) | 7.6 | 1.09 (0.99, 1.20) | 7.3 |
| Plus hypertension | 1.11 (1.01, 1.22) | 7.9 | 1.09 (0.99, 1.20) | 7.8 |
| Plus waist circumference | 1.08 (0.99, 1.19) | 29.2 | 1.07 (0.97, 1.18) | 24.7 |
| Plus high cholesterol | 1.12 (1.02, 1.22) | 2.8 | 1.10 (1.00, 1.21) | −0.09 |
| Plus diabetes | 1.10 (1.00, 1.20) | 19.6 | 1.08 (0.98, 1.20) | 13.5 |
HR indicates hazard ratio.
The mediating role of each behavioral and biological risk factor was calculated as the percentage reduction in the beta coefficient for high (vs low) stressful life events/social strain after inclusion of each factor, using the formula: 100×([βModel 1−βModel 1+risk factor]/βModel 1).
Table 4 shows associations of SLE and social strain with incident hemorrhagic and ischemic stroke. Findings were similar for high versus low SLE with hemorrhagic (HR, 1.21; 95% CI, 0.91, 1.61) and ischemic stroke (HR, 1.13; 95% CI, 0.99, 1.30) in models adjusted for sociodemographic characteristics and depressive symptoms. High social strain was associated with higher incident ischemic stroke (HR, 1.15; 95% CI, 1.02, 1.28), but not incident hemorrhagic stroke (HR, 0.89; 95% CI, 0.70, 1.14). Associations were attenuated with further adjustment for behavioral and biological risk factors. All HRs were similar when analyses were restricted to participants with complete data on all study covariates and when follow‐up time was limited to the end of the WHI Extension I study period (data not shown).
Table 4.
Hazard Ratios (95% Confidence Intervals) for Associations of Stressful Life Events and Social Strain Categories With Incident Hemorrhagic and Ischemic Stroke
| Incident Hemorrhagic Stroke | Incident Ischemic Stroke | |||||
|---|---|---|---|---|---|---|
| No. of Events | Model 1* | Model 2* | No. of Events | Model 1* | Model 2* | |
| Stressful life events* | ||||||
| High | 110 | 1.21 (0.91, 1.61) | 1.22 (0.92, 1.62) | 466 | 1.13 (0.99, 1.30) | 1.09 (0.95, 1.25) |
| Medium‐high | 98 | 1.08 (0.81, 1.44) | 1.08 (0.82, 1.44) | 447 | 1.12 (0.98, 1.29) | 1.10 (0.96, 1.26) |
| Medium‐low | 111 | 1.02 (0.77, 1.34) | 1.02 (0.77, 1.35) | 527 | 1.12 (0.99, 1.28) | 1.10 (0.97, 1.26) |
| Low | 93 | 1.00 (ref) | 1.00 (ref) | 399 | 1.00 (ref) | 1.00 (ref) |
| P for trend | 0.17 | 0.15 | 0.10 | 0.27 | ||
| Social strain* | ||||||
| High | 134 | 0.89 (0.70, 1.14) | 0.90 (0.71, 1.15) | 709 | 1.15 (1.02, 1.28) | 1.10 (0.98, 1.23) |
| Medium | 136 | 1.02 (0.81, 1.30) | 1.03 (0.81, 1.31) | 547 | 1.00 (0.89, 1.13) | 0.99 (0.88, 1.11) |
| Low | 140 | 1.00 (ref) | 1.00 (ref) | 582 | 1.00 (ref) | 1.00 (ref) |
| P for trend | 0.37 | 0.41 | 0.02 | 0.09 | ||
Adjusted for age, race/ethnicity, education, income, marital status, and depressive symptoms.
Adjusted for model 1, alcohol use, cigarette smoking, hypertension, waist circumference, high cholesterol, diabetes, physical activity, and dietary quality.
The stressful life events score is based on participant responses to questions assessing whether 11 major life changes occurred over the past year and the extent to which the event upset them. The categories low, medium‐low, medium‐high, and high correspond to scores of 0, 1 to 2, 3 to 4, and 5 or more, respectively.
Social strain was evaluated at baseline with 4 items derived from a previously validated measure of negative aspects of social relationships. High, medium, and low social strain corresponds to scores of 4, 5 to 6, and 7 or more, respectively.
Discussion
In this study, we found that higher SLE and social strain were both associated with higher incident CHD and stroke independent of sociodemographic characteristics and depressive symptoms. Associations of SLE and social strain with CHD and stroke were attenuated after adjustment for behavioral and biological risk factors hypothesized to mediate relationships between stress and CVD. Waist circumference was the strongest mediator for associations of SLE and social strain with both CHD and stroke. We also found that associations of SLE with stroke were similar by stroke subtype; however, social strain was only associated with ischemic stroke.
Our results for stroke are generally consistent with findings in the literature. A study of major life events and incident CVD in a Danish cohort found that accumulation of events in adulthood was associated with incident stroke, but not incident myocardial infarction.34 A case‐control study in Sweden found that psychological stress, assessed with a single‐item questionnaire asked retrospectively, was significantly associated with ischemic stroke.1 Another case‐control study of participants from 22 countries found that psychological stress, assessed using a combined measure of general stress at home and work, was significantly associated with ischemic stroke, but not hemorrhagic stroke.3 We found that SLE and social strain were both significantly associated with incident stroke. Stroke subtype analyses revealed similar point estimates for associations of SLE with incident hemorrhagic and ischemic stroke, but social strain was only associated with ischemic stroke. This may reflect the different pathways through which different types of stressors act to influence CV risk. However, more research is needed in studies with multiple measures of stress exposure to confirm these findings.
Although several studies have reported significant associations between chronic stress and CHD, recent publications have challenged these findings, suggesting that these findings may be a result of reporting bias.9–11 Specifically, these researchers found strong associations of multiple stress measures with angina pectoris, an outcome that is more heavily dependent on self‐reported symptoms, and no associations with MI. In our study, we found significant associations between our 2 different stress measures with CHD (excluding angina pectoris) independent of sociodemographic characteristics and depressive symptoms. Given the diverse ways stress is measured and the various domains in which stress can arise (eg, workplace, interpersonal relationships, and major life events), these mixed findings may more likely reflect differences in the pathways linking different stressors to CVD than the way the outcome is measured.
There are several behavioral and biological pathways that may link psychosocial stress exposure to incident CVD.14,17,35 Individuals may adopt certain behaviors as a result of chronic exposure to psychosocial stressors that are related to CVD risk, including cigarette smoking, high fat and carbohydrate food consumption, physical inactivity, and heavy alcohol use.13–14 In addition, chronic stress may result in physiologic dysregulation of the sympathetic nervous system and the hypothalamic‐pituitary‐adrenal (HPA) axis, which, in turn, may increase CVD risk through inflammation, endothelial dysfunction, and atherosclerosis.16–18,24
In this study, our findings for CHD and stroke were attenuated after adjustment for behavioral risk factors, hypertension, high cholesterol, diabetes, and waist circumference, supporting the role of these factors in mediating the relationships between stress and CVD. In particular, we found that waist circumference was the strongest mediator for associations of both stressors with both outcomes and that diabetes was a substantial mediator as well. These findings are consistent with several animal and human studies that have implicated chronic stress in the accumulation of visceral fat and development of metabolic syndrome through increased secretion of cortisol and adrenal androgens in response to HPA axis activation.24,36 This is also consistent with the stress literature that suggests that women are more likely than men to consume high‐fat, high‐sugar foods that may lead to increased central adiposity and metabolic disorders.37
This study has several strengths. The use of 2 previously validated stress measures allowed us to examine the robustness of our findings to different measures of stress. The large sample size and prospective study design allowed for the evaluation of several CV outcomes, including stroke subtypes. In addition, the clinical adjudication of endpoints provided a more objective examination of associations of stress with the different CV outcomes. One limitation of this study is that we do not have repeat measures of stress. Given the long follow‐up period, repeat measures would provide a more comprehensive picture of the longitudinal role of stress exposure and stress coping behaviors in the development of CVD. In addition, our stress measures and hypothesized mediators were measured at the same time, which precludes us from being able to definitively determine whether these stressors caused increases in the risk factors associated with incident CHD and stroke.
In summary, we found that higher accumulation of stressful life events over a 1‐year period and social strain were both significantly associated with incident CHD and stroke independent of sociodemographic factors and depressive symptoms. All associations were explained by hypothesized mediating behavioral and biological factors, particularly waist circumference and diabetes status. By improving our understanding of the relationship between stress and CVD, the findings of this study may spur the development of more targeted interventions to promote stress management and lower CVD risk in women.
Sources of Funding
Dr Kershaw is funded by NIH grant N01‐HC‐95164. The WHI program is funded by the National Heart, Lung and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Disclosures
None.
References
- 1.Jood K, Redfors P, Rosengren A, Blomstrand C, Jern C. Self‐perceived psychological stress and ischemic stroke: a case‐control study. BMC Med. 2009; 7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kornerup H, Osler M, Boysen G, Barefoot J, Schnohr P, Prescott E. Major life events increase the risk of stroke but not of myocardial infarction: results from the Copenhagen City Heart Study. Eur J Cardiovasc Prev Rehabil. 2010; 17:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez‐Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf SInvestigators I. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case–control study. Lancet. 2010; 376:112-123. [DOI] [PubMed] [Google Scholar]
- 4.Iso H, Date C, Yamamoto A, Toyoshima H, Tanabe N, Kikuchi S, Kondo T, Watanabe Y, Wada Y, Ishibashi T, Suzuki H, Koizumi A, Inaba Y, Tamakoshi A, Ohno Y. Perceived mental stress and mortality from cardiovascular disease among Japanese men and women: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk Sponsored by Monbusho (JACC study). Circulation. 2002; 106:1229-1236. [DOI] [PubMed] [Google Scholar]
- 5.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case–control study. Lancet. 2004; 364:953-962. [DOI] [PubMed] [Google Scholar]
- 6.Rosengren A, Tibblin G, Wilhelmsen L. Self‐perceived psychological stress and incidence of coronary artery disease in middle‐aged men. Am J Cardiol. 1991; 68:1171-1175. [DOI] [PubMed] [Google Scholar]
- 7.Schnall PL, Landsbergis PA, Baker D. Job strain and cardiovascular disease. Annu Rev Public Health. 1994; 15:381-411. [DOI] [PubMed] [Google Scholar]
- 8.De Vogli R, Chandola T, Marmot MG. Negative aspects of close relationships and heart disease. Arch Intern Med. 2007; 167:1951-1957. [DOI] [PubMed] [Google Scholar]
- 9.Macleod J, Davey Smith G, Heslop P, Metcalfe C, Carroll D, Hart C. Psychological stress and cardiovascular disease: Empirical demonstration of bias in a prospective observational study of Scottish men. BMJ. 2002; 324:1247-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore L, Meyer F, Perusse M, Cantin B, Dagenais GR, Bairati I, Savard J. Psychological stress and incidence of ischaemic heart disease. Int J Epidemiol. 1999; 28:652-658. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen NR, Kristensen TS, Prescott E, Larsen KS, Schnohr P, Gronbaek M. Perceived stress and risk of ischemic heart disease: causation or bias? Epidemiology. 2006; 17:391-397. [DOI] [PubMed] [Google Scholar]
- 12.Andersen I, Diderichsen F, Kornerup H, Prescott E, Rod NH. Major life events and the risk of ischaemic heart disease: does accumulation increase the risk? Int J Epidemiol. 2011; 40:904-913. [DOI] [PubMed] [Google Scholar]
- 13.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003; 100:11696-11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson JS, Knight KM. In: Schaie KW, Cartensen L. (eds.). Race and self‐regulatory health behaviors: the role of the stress response and the HPA axis in physical and mental health disparities. Social Structures, Aging, and Self‐Regulation in the Elderly. 2006New York, NY: Springer; 189-207. [Google Scholar]
- 15.Marniemi J, Kronholm E, Aunola S, Toikka T, Mattlar CE, Koskenvuo M, Ronnemaa T. Visceral fat and psychosocial stress in identical twins discordant for obesity. J Intern Med. 2002; 251:35-43. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998; 338:171-179. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993; 153:2093-2101. [PubMed] [Google Scholar]
- 18.Pickering TG. Stress, inflammation, and hypertension. J Clin Hypertens. 2007; 9:567-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The women's health initiative recruitment methods and results. Ann Epidemiol. 2003; 13:S18-S77. [DOI] [PubMed] [Google Scholar]
- 20.Women's Health Initiative Study Group. Design of the women's health initiative clinical trial and observational study. Control Clin Trials. 1998; 19:61-109. [DOI] [PubMed] [Google Scholar]
- 21.Ruberman W, Weinblatt E, Goldberg JD, Chaudhary BS. Psychosocial influences on mortality after myocardial infarction. N Engl J Med. 1984; 311:552-559. [DOI] [PubMed] [Google Scholar]
- 22.Antonucci TA, Kahn RC, Akiyama H. In: Yanick R, Yaes JW. (eds.). Psychosocial factors and the response to cancer symptoms. Cancer in the Elderly: Approaches to Early Detection and Treatment. 1989New York, NY: Springer Publishing Company; 40-52. [Google Scholar]
- 23.Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Med Care. 1988; 26:775-789. [DOI] [PubMed] [Google Scholar]
- 24.Bjorntorp P. Visceral fat accumulation: the missing link between psychosocial factors and cardiovascular disease? J Intern Med. 1991; 230:195-201. [DOI] [PubMed] [Google Scholar]
- 25.US Department of Health and Human Services. Physical activity guidelines for Americans. 2008.
- 26.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the women's health initiative study design. Ann Epidemiol. 2003; 13:S5-S17. [DOI] [PubMed] [Google Scholar]
- 27.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs‐Collins T. Measurement characteristics of the women's health initiative food frequency questionnaire. Ann Epidemiol. 1999; 9:178-187. [DOI] [PubMed] [Google Scholar]
- 28.Guenther PM, Reedy J, Krebs‐Smith SM, Reeve BB, Basiotis PP. Development and evaluation of the healthy eating index‐2005, Technical report. 2007.
- 29.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx‐Burns L, Pastore L, Criqui M, Daugherty S. Outcomes ascertainment and adjudication methods in the women's health initiative. Ann Epidemiol. 2003; 13:S122-S128. [DOI] [PubMed] [Google Scholar]
- 30.Women's Health Initiative. Data preparation and use of whi investigator data sets posted on the study operations website. 2011.
- 31.Women's Health Initiative. Whi extension manual: Protocol. 2011.
- 32.Zhao D. Poisson regression adjustment of event rates and its macro procedure ADJ_POIS. SAS Users Group 24th International Annual Conference. 1999.
- 33.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011; 30:377-399. [DOI] [PubMed] [Google Scholar]
- 34.Truelsen T, Nielsen N, Boysen G, Gronbaek MCopenhagen City Heart S. Self‐reported stress and risk of stroke: the Copenhagen City Heart Study. Stroke. 2003; 34:856-862. [DOI] [PubMed] [Google Scholar]
- 35.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999; 99:2192-2217. [DOI] [PubMed] [Google Scholar]
- 36.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001; 2:73-86. [DOI] [PubMed] [Google Scholar]
- 37.Grunberg NE, Straub RO. The role of gender and taste class in the effects of stress on eating. Health Psychol. 1992; 11:97-100. [DOI] [PubMed] [Google Scholar]


