Introduction
Cardiovascular disease complicates ≈1% to 3% of all pregnancies and is responsible for 10% to 15% of maternal mortality. Although its prevalence is considered relatively low in pregnant women, heart disease is the most common cause of maternal mortality. Because more women with congenital or acquired heart disease are reaching childbearing age due to improved medical and surgical care, and desire children, the incidence of cardiovascular disease in pregnancy is increasing. The onset of pregnancy in patients with significant congenital or acquired valvular heart disease (VHD) presents challenges to their management. Despite significant advances in diagnosis, medical and surgical therapy of VHD, the course for many of these patients during and after pregnancy can be fraught with significant adverse events for both the mother and fetus. Many patients with significant VHD often are not aware of their diagnosis prior to pregnancy and the diagnosis is made when the hemodynamic challenges of pregnancy presents clinical symptoms. The objective of this review is to address preconception counseling, risk assessment, and management issues related to the care of women of childbearing age with VHD who are pregnant or may become pregnant.
Valvular Heart Disease in Women of Childbearing Age
The prevalence and distribution of VHD differs depending on the location of the patient's origin (Table 1). In the developed world, progress in the medical and surgical management of patients with complex congenital heart disease (CHD) has resulted in an increase in the number of those reaching adulthood and childbearing age. CHD currently accounts for ≈30% to 50% of all cardiac diseases during pregnancy.1 Rheumatic heart disease (RHD), once the most common cause of valvular disease in the developed world, is still a common disease worldwide with ≈90% of all heart disorders in women of child‐bearing age being of rheumatic origin in non‐industrialized regions.2 However, the clinical suspicion for RHD among expectant mothers in developed nations remains heightened given the expansion of immigration patterns worldwide. Although accurate statistics are lacking, the estimated incidence of rheumatic fever in sub‐Saharan Africa is ≈13 cases per 100 000 per year based on clinical screening,3 while estimations between 21.5 and 30.4 per 1000 have been reported in Cambodia and Mozambique when using echocardiographic screening.4 Besides its high prevalence, rheumatic heart disease in developing countries is characterized by the occurrence of severe VHD at a younger age than in developed countries. Mitral stenosis, which affects women more frequently than men, is relatively common among women of childbearing age with VHD. In the European Registry on Pregnancy and Heart Disease,5 which included women from North America, Europe, the Russian Federation, Egypt, and others, mitral stenosis and/or regurgitation were the most common types of valvular pathologies (63%), followed by aortic valve disease (23%). In this registry, patients with VHD had a higher maternal mortality rate compared with patients with CHD.5 Women with severe symptomatic mitral stenosis hospitalized in a tertiary care center in Africa experienced a nearly 50% mortality rate, most of which occurred postpartum.6 Therefore, cardiologists worldwide must maintain awareness of the possibility of rheumatic valve disease, which remains prevalent in migrant populations.
Table 1.
Etiology of Valvular Heart Disease in Women of Childbearing Age
| Aortic valve lesions |
|
|
|
|
|
|
|
| Mitral valve lesions |
|
|
|
|
|
|
|
| Pulmonic valve lesions |
|
|
|
| Co‐existing lesions/problems that affect outcome |
|
|
|
|
|
|
|
|
LVEF indicates left ventricular ejection fraction.
Cardiac Physiology During Pregnancy, Labor, and Delivery
Hemodynamic changes during pregnancy
The onset of pregnancy marks the beginning of progressive and profound changes in the physiology of the cardiovascular system, which includes marked increases in cardiac output and intravascular volume, as well as lowered systemic vascular resistance (Table 2, Figure 1).7–9 Cardiovascular hemodynamic changes begin very early in pregnancy. Initially, marked increases in circulating blood volume are met with an increase in stroke volume, a 15% to 20% increase in heart rate, and increases in left ventricular end‐diastolic volume, which peak during the third trimester. The net effect is a 30% to 50% increase in cardiac output by the end of the first trimester, an effect that peaks between the second and third trimesters. During the early stages of pregnancy, increases in stroke volume are largely responsible for the observed increase in cardiac output, while later in pregnancy an increased heart rate accounts for these changes. Another important hemodynamic consideration is the maturation of the placental circulation, which provides a substantial reduction in systemic vascular resistance. Vascular resistance decreases by 30% to 50% by the end of the second trimester, before rising toward the end of the third trimester.10–11 As soon as 20 weeks gestation, preload reduction may occur due to compression of the inferior vena cava (IVC) by the gravid uterus, thus reducing cardiac output. Moreover, afterload may increase due to aortic compression at this time. A substantial increase in blood volume causes all 4 cardiac chambers to increase in size; these changes are often noted on echocardiography and decrease to baseline levels in the postpartum period.
Table 2.
| Part A: changes during gestation |
|
|
|
|
|
|
|
| Part B: changes during labor & delivery |
|
|
|
|
Figure 1.

Hemodynamic changes in pregnancy, labor and postpartum. Time on the x‐axis changes scale. Adapted from Cornetter and Ross‐Hesselink,9 with permission from Springer. CO indicates cardiac output; MAP, mean arterial pressure; PP, postpartum; SVR, systemic vascular resistance.
Pregnancy‐related volume load contributes to progression of diastolic dysfunction in women with structural and valvular heart disease.12 As early as 20 weeks gestation, the enlarging uterus can obstruct venous return and may cause stasis, leading to a further rise in the risk of thromboembolism.13 In patients on medical therapy, close monitoring and dosing adjustments are important throughout pregnancy as pharmacokinetics of drugs vary during different stages of pregnancy.14 Notably, pregnancy induces a series of hemostatic changes leading to hypercoagulability and an increased risk of thromboembolic events particularly relevant in the presence of underlying valve disease and chamber dilatation.
Each of these hemodynamic alterations may have effects that can be beneficial or detrimental depending on the characteristics of the existing valvular abnormality. As will be discussed in more detail later, pregnant women with severe mitral or aortic stenosis, when faced with an increase in cardiac output and decrease in systemic vascular resistance, will likely experience clinical decompensation. Conversely, women with advanced mitral or aortic regurgitation and a preserved ejection fraction, may benefit from a decrease in afterload provided by a low resistance circuit, and remain relatively asymptomatic until late in pregnancy. Specific valve lesions will be discussed in later sections of this review.
Hemodynamic changes during labor, delivery, and postpartum
The cardiovascular system of women with valvular heart disease is limited in its ability to accommodate the demands of pregnancy. These limitations become more evident during labor and delivery, where several changes in the circulatory system could result in hemodynamic decompensation.15 The abrupt nature of these changes can be a challenge particularly for women with limited cardiovascular reserve. At the time of labor and delivery, maternal hemodynamics are influenced by pain, anxiety, maternal position, blood loss, Valsalva maneuver, uterine contractions, and analgesia if used. There are catecholamine‐induced increases in heart rate and stroke volume, due to pain and anxiety, which lead to marked changes in cardiac output. Arterial blood pressure increases during each contraction by up to 15 to 20 mm Hg. Cardiac output further increases from pre‐labor values, particularly in the second stage of labor. Some intravascular volume is lost at delivery, where variable blood loss will occur; ≈500 mL with a normal vaginal delivery and 1000 mL for a routine Cesarean section. The use of epidural anesthesia and analgesia can blunt many of the hemodynamic alterations associated with labor and delivery. Alleviating pain reduces heart rate, blood pressure, and oxygen consumption.15 Compared with no anesthesia, the presence of regional analgesia limits the overall increase in cardiac output at the time of labor and delivery. Induction of general anesthesia can exacerbate hypertension and tachycardia.
The gradual hemodynamic adaptation of pregnancy is rapidly reversed in the early postpartum period. Dramatic alterations in hemodynamics occur within the first 12 to 24 hours postpartum. Within the first hour of delivery, cardiac output remains high compared with pre‐labor values (as much as 80% above pre‐labor values), due to the relief of caval compression and the massive autotransfusion from the uterine blood volume.16 The use of anesthesia and analgesia can cause hypotension as a result of venous pooling and decreased systemic vascular resistance. Changes in fluid balance resulting from decompression of the inferior vena cava (IVC), as well as the redistribution of blood from the lower limbs, can result in a rapid increase in preload and possibly pulmonary congestion with clinical heart failure. Further fluctuations in hemodynamics result from the loss of the low resistance placenta and a relative increase in systemic vascular resistance, as well as the mobilization of dependent edema and interstitial fluid. Importantly, the hemodynamic changes reverse most prominently within the first 2 weeks postpartum, but will continue for 6 months postpartum or longer.
Mode of Delivery
The type of delivery mainly depends on the hemodynamic status of the patient as well as obstetric indications.17 In women at elevated risk for cardiac complications at the time of labor and delivery, a multidisciplinary team should be formed that consists of the patient's obstetrician, obstetrical anesthesiologist and a cardiologist. This team should coordinate the timing and mode of delivery, discuss any precautions, the type of anesthesia, the necessity for specific medical therapies and the need for additional hemodynamic monitoring. Deliveries in women with cardiac disease are best accomplished in a tertiary care hospital. A multidisciplinary team is crucial to adequate management of patients at the time of labor and delivery and consultation is recommended prior to the onset of labor and delivery. In addition, patients with clinical indications, such as advanced VHD, clinical heart failure or underlying left ventricular systolic dysfunction, should be monitored carefully throughout labor and delivery, as well as in the early postpartum period, when hemodynamic decompensation is most likely to occur. Pulmonary artery catheterization is rarely required, with invasive hemodynamic monitoring only necessary in select cases of complex and severe hemodynamic compromise according to one's own best clinical judgment.18 According to the European guidelines,19 primary Cesarean section should be considered in pregnant patients with severe heart failure, aortic root >45 mm, acute aortic dissection, or patients on oral anticoagulants in pre‐term labor.
Vaginal delivery is the preferred mode of delivery in most women, even in those with established cardiovascular disease. Virtually all pregnant women with cardiac disease should expect an attempt at vaginal delivery, unless obstetric contraindications exist. For women with pre‐existing cardiac disease, a vaginal delivery poses less cardiac risk, as Cesarean delivery is accompanied by approximately twice as much blood loss. Patients who are considered stable from a cardiac perspective can be allowed to spontaneously progress through the stages of labor. The advantages of vaginal delivery include less blood loss, absence of abdominal surgery, more rapid recovery, and decreased thrombogenic risk. The presence of epidural anesthesia allows for a controlled descent of the fetus to the pelvic floor, diminishing the frequency and intensity of the Valsalva maneuver. Of note, epidural catheter placement is contraindicated in patients on anticoagulants. An assisted second stage of delivery, which shortens the time to delivery (by vacuum or forceps), may be required in women who cannot tolerate a long second stage of labor or excessive maternal efforts with the Valsalva maneuver for cardiac reasons.
Cesarean delivery eliminates the hemodynamic insults associated with labor. However, it is associated with more significant blood loss and more abrupt hemodynamic changes. In addition, it increases the risk of venous thromboembolism, infection, and postpartum hemorrhage. It usually allows for more invasive hemodynamic monitoring if required. General anesthesia is rarely required in the setting of a Cesarean section and is mainly reserved for hemodynamically unstable patients.
Maternal Risk Stratification
Risk assessment of adverse maternal and fetal outcomes during pregnancy complicated by valvular heart disease remains difficult. Ongoing risk assessment and a tailored management approach during pregnancy in patients with VHD according to baseline disease severity are essential. A pre‐pregnancy evaluation and discussion is emphasized by the American Heart Association/American College of Cardiology Valvular Heart Disease guidelines.20 The Task Force on the Management of Cardiovascular Disease in Pregnancy from the European Society of Cardiology (ESC) now recommends an integrated risk stratification scheme, which incorporates all known maternal cardiovascular risk factors (Figure 2).18–19,21 This scheme is the most comprehensive risk stratification system to date. Non‐cardiac maternal predictors of neonatal events include smoking during pregnancy and multiple gestation pregnancy. Maternal cardiac factors directly related to valvular disease include the presence of a mechanical valve prosthesis, the use of oral anticoagulants during pregnancy, moderate or severe left‐sided cardiac obstruction in the mother, maternal cyanosis, and baseline maternal New York Heart Association (NYHA) heart failure of class II or above.19,22–24 Potential adverse fetal events include the fetus being small for gestational age, intracranial hemorrhage, and death. In the European Registry on Pregnancy and Heart Disease,5 heart failure was the most commonly observed maternal complication in pregnant women with VHD. Hospital admission was common, seen in 38% of patients with VHD, and supraventricular arrhythmias were more commonly observed in VHD patients than in any other patient subgroup, which included those with congenital heart disease, cardiomyopathy, and ischemic heart disease. Furthermore, postpartum hemorrhage was frequently encountered in the VHD group, likely related to the use of anticoagulant medications.5 Maternal mortality varies widely in patients with valvular heart disease, and is considered a relatively rare cause of maternal mortality in developed societies.5,22,25 Patients at prohibitively high risk who should be counseled against pregnancy are those in World Health Organization (WHO) Class IV (Figure 2, right‐sided column), and include patients with severe symptomatic aortic or mitral stenosis, cardiomyopathy with a left ventricular ejection fraction of ≤30%, Marfan syndrome with aortic root ≥45 mm, and advanced pulmonary hypertension (two‐thirds of systemic pressure), and/or Eisenmenger syndrome. In addition, women with a history of peripartum cardiomyopathy in whom left ventricular systolic function has not fully recovered are also at prohibitively high risk. Particularly in these categories of patients, who are symptomatic early in pregnancy, termination of pregnancy needs to be considered.19,21
Figure 2.
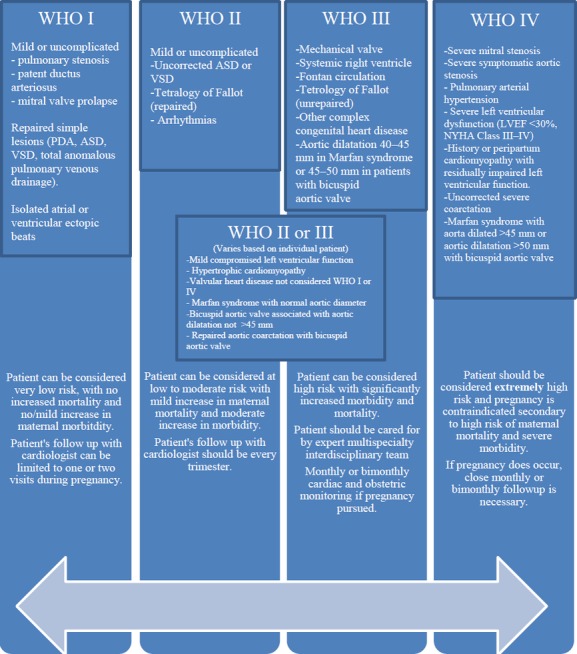
Risk stratification: the modified World Health Organization (WHO) approach. Adapted from Regitz‐Zagrosek et al19 and Thorne et al,21 with permission of Oxford University Press (UK) © European Society of Cardiology, www.escardio.org/guidelines and BMJ Group, respectively. ASD indicates atrial septal defect; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PDA, patent ductus arteriosis; VSD, ventricular septal defect.
Exercise testing can be valuable in patients with various cardiac lesions. Exercise testing is useful to objectively assess the functional capacity of any individual patient, as well as their hemodynamic response and possible presence of exercise‐induced arrhythmias. It has become an integral part of the follow‐up of nonpregnant asymptomatic patients with VHD, as well as those with CHD.26–27 Pre‐pregnancy symptoms can often predict the likelihood of serious adverse outcomes during pregnancy or labor and delivery.28–29 It can be performed in women in order to aid in risk assessment, preferably prior to pregnancy (Level of Evidence C).19 However, consensus on specific cutoff values for metabolic equivalents, heart rate, or blood pressure responses are not available for guidance. One study suggested that women who achieve pre‐pregnancy peak heart rate ≥150 beats per minute and/or peak oxygen uptake ≥25 mL kg−1 min−1 may be considered to have safer pregnancy outcomes.29 In another study, peak heart rate and percentage of maximum heart rate were associated with pregnancy‐related cardiac events.28
Preconception Counseling
Physicians caring for female patients of reproductive age that have a history of VHD or prior cardiac valve surgery have largely underutilized preconception counseling. A substantial number of women present for the first time with symptoms during pregnancy, without an opportunity for preconception counseling and timely treatment before pregnancy. From risk stratification, to contraception counseling and pursuit of the appropriate therapeutic course prior to conception, preconception counseling has the potential to simplify the clinical course for many of these patients at high risk (Figure 3).20,30 The American College of Cardiology/American Heart Association adult congenital heart disease guidelines,27 valvular heart disease guidelines,20 and European Society of Cardiology guidelines31 all endorse preconception counseling and discussion of contraception as the duty of the cardiologist. Individual counseling by experts, advice on contraception, and ultimately close follow‐up between the patient and a multidisciplinary care team which includes a cardiologist and obstetrician can potentially impact the lives of both mother and baby. As part of these complex discussions, pregnancy‐related complications should include those related to the pregnancy, as well as potential late adverse maternal cardiac outcomes, and the potential for fetal prematurity and its consequences. In addition, attention to discontinuation and possibly replacement of medications that are teratogenic should take place. Commonly used medications that are contraindicated in pregnancy (FDA Class D) include atenolol, amiodarone, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, spironolactone, statin medications, and anticoagulants including warfarin.
Figure 3.
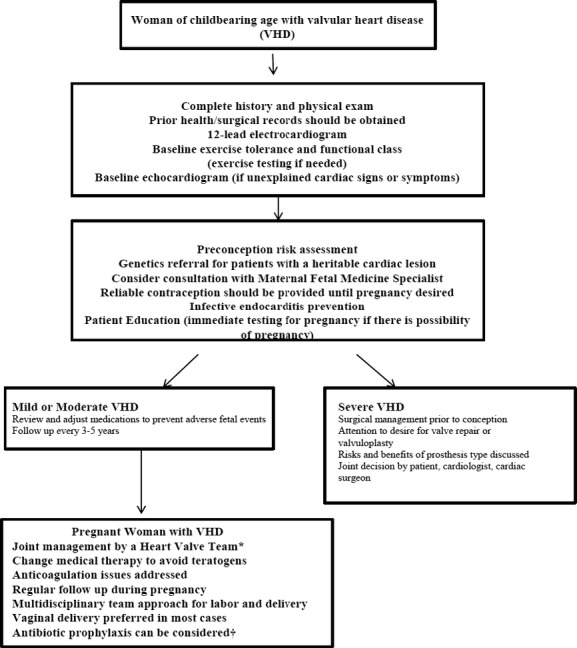
Algorithm of preconception counseling and evaluation. *A heart valve team includes monitoring in a tertiary care center with a dedicated a team of cardiologists, surgeons, anesthesiologists, and obstetricians with expertise in the management of high‐risk cardiac patients during pregnancy (Level of Evidence: C).20 †Infective endocarditis (IE) prophylaxis may be considered for high risk lesions, but is not required for vaginal delivery.30
Contraceptive Choices in Women With Heart Disease
Choice of contraception requires consideration of pregnancy risk, available contraception options as well as their risks and benefits, failure rates, understanding the consequences of unplanned pregnancy, and the preferences of the woman. Cardiologists are responsible for educating women about safe contraceptive options as they relate to their cardiac condition, a position recently endorsed by professional societies.19,27 Furthermore, it is now a Class I recommendation from the ESC guidelines that pre‐pregnancy and post‐conception risk assessments and counseling are performed in all women with known or suspected congenital or acquired heart or aortic disease.19 There is considerable room for improvement in this area.
Contraceptive options include: (1) combined hormonal contraceptives (COCs; estrogen/progestin formulations); (2) progestin‐only formulations; (3) intrauterine devices; (4) barrier methods, and (5) sterilization/permanent forms of contraception.32 The most comprehensive guidance is provided by a British working group that developed guidelines for the use of COCs in women with heart disease using the World Health Organization (WHO) format.32–36 Both estrogen and progestins have adverse cardiac effects. However, the most clinically important are those of estrogen, which can cause thromboembolic events and hypertension. Due to the potential for thromboembolic complications, combined hormonal contraceptives in the form of pills, transdermal patches or vaginal rings are not recommended in women with mechanical heart valves (due to the risk of valve thrombosis), Eisenmenger syndrome (due to the risk of pulmonary embolism), and women with intracardiac shunts (due to paradoxical emboli).21 Monthly injectable formulations that contain medroxyprogesterone acetate are no longer appropriate for patients with heart failure because of the tendency for fluid retention. Barrier methods and the levonorgestrel‐releasing intrauterine devices are the safest and most effective options that can be used in women with cardiomyopathy and reduced systolic ventricular function, cyanotic heart disease, and advanced pulmonary hypertension. In women at prohibitively high risk for pregnancy, permanent forms of contraception can be considered. A detailed discussion regarding family planning coordinated with the patient's obstetrician is indicated so that patients can better understand the risks of pregnancy and contraception.
Specific Valve Lesions and Therapies
Treatment of a specific valvular lesion during pregnancy may be required in the presence of heart failure, arrhythmia, or hemodynamic deterioration, in order to prevent significant maternal or fetal morbidity or mortality. However, the optimal treatment pathway to improve maternal and fetal outcomes, whether medical or interventional, remains unclear.37 A brief review of the medical therapies used during pregnancy for heart failure symptoms can be found in Table 3.38 A more comprehensive review of medical therapies used in pregnancy is discussed in the most recent ESC guidelines on the management of cardiovascular disease during pregnancy.19 Often, recommendations are made with level of evidence C.27,31 Factors independently associated with maternal complications are the presence of left heart obstruction, mechanical valve replacement, and systemic or pulmonic atrioventricular valve regurgitation, among others.22,24
Table 3.
Medical Management of Heart Failure in Pregnancy
| Drug/Class | Purpose | Comment |
|---|---|---|
| Diuretics | ||
| Furosemide |
|
|
| Digoxin |
|
|
| Vasodilators | ||
| Hydralazine |
|
|
| ACE Inhibitors/ARB |
|
|
| Amlodipine |
|
|
| Nitrates |
|
|
| Beta‐blockers | ||
| Carvedilol, Labetalol, Metoprolol, Propranolol |
|
|
| Aldosterone antagonists | ||
| Spironolactone, Epleronone |
|
|
| Warfarin |
|
|
ACE inhibitor indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; IUGR, intrauterine growth retardation; SVR, systemic vascular resistance.
Food and Drug Administration (FDA) Class: A (controlled studies show no risk), B (no evidence of human risk in controlled studies), C (risk cannot be ruled out), D (positive evidence of risk), X (contraindicated in pregnancy).
Adapted from Stergiopoulos et al.38 Copyright 2011, with permission from Elsevier.
Aortic stenosis
The most common etiology of aortic stenosis (AS) in women of childbearing age is a congenital bicuspid aortic valve, often with an associated aortopathy.31 If provided with a timely diagnosis and appropriate follow up, carefully chosen aortic stenosis patients can tolerate pregnancy well when the obstruction is of mild or moderate severity (aortic valve area >1.0 cm2).39 Recent data suggests that the overall maternal mortality is <1% even in patients with severe aortic stenosis40–41; however, the incidence of other complications for both mother and fetus rises dramatically with increasing severity of aortic stenosis. Nearly a third of mothers with aortic valve area <1.5 cm2 require hospitalization, while also experiencing an increased incidence of unfavorable outcomes for the fetus, including fetal growth restriction, respiratory distress, preterm birth, and low birth weight.39 Studies in this population of patients have had small sample sizes but estimates suggest that heart failure occurs in ≈7% to 17% of pregnant women with severe aortic stenosis, while arrhythmias occur in 3% to 33%.39–41 This phenomenon highlights the need for preconception counseling, as well as careful monitoring and follow‐up in this particular subset of patients. Interestingly, some patients with severe AS can sustain a pregnancy well, particularly if they remain asymptomatic during exercise testing prior to pregnancy.31,40
While there is no optimal medical treatment for AS, patients may respond to a restriction in activities as well as preload reduction with cautious use of diuretics if they are symptomatic. Options for patients unresponsive to medical therapy include termination of pregnancy in the early stages of pregnancy, catheter‐based valvuloplasty and surgical valve replacement in later stages depending on the clinical status and availability.40,42–43 Symptomatic patients with severe aortic stenosis should undergo surgery prior to pregnancy.44 Even asymptomatic patients with severe stenosis and LV dysfunction with an ejection fraction <50% or exercise‐induced symptoms are at higher risk for adverse outcomes and should be considered for surgery prior to pregnancy.40–41,45 In women with severe, symptomatic AS, Cesarean delivery is usually preferred.19 In non‐severe AS, vaginal delivery is preferred with avoidance of epidural anesthesia secondary to the accompanying marked decrease in peripheral vascular resistance.
Patients with bicuspid aortic valve disease often have a concomitant aortopathy. Aortic coarctation, aortic dilatation, or aneurysms may coexist in patients with bicuspid aortic valve disease. Therefore, all women with bicuspid aortic valve should undergo imaging of the ascending aorta and proximal descending aorta prior to pregnancy as part of their pre‐pregnancy risk assessment. Women with unrepaired native coarctation and those with residual coarctation and/or aneurysms and residual hypertension have an increased risk of aortic dissection and rupture, or rupture of a cerebral aneurysm.46 If the ascending aorta measurement is >50 mm (or 27.5 mm/m2), aortic surgery should be considered prior to pregnancy.19
Mitral stenosis
Mitral stenosis (MS), the most common manifestation of rheumatic heart disease, remains the most common acquired valvular lesion in pregnant women and the most common cause of maternal death from cardiac causes worldwide.8,18 Although mortality remains low in women from developed societies, the rate of fetal morbidity, including fetal growth restriction and preterm birth, rises with the severity of MS from ≈14% in pregnant patients with mild MS, to 28% and 33% in pregnant patients with moderate and severe MS.47–48 Moderate or severe mitral stenosis is poorly tolerated during pregnancy. Direct planimetry of the mitral valve is probably the most reliable measurement of the mitral valve area during pregnancy.49 During changing hemodynamic conditions, such as pregnancy, determination of mitral valve area by the continuity equation likely provides a better estimation over the pressure half‐time method.50 However, the functional significance of the degree of mitral stenosis is likely more closely related to the mean gradient across the valve, which usually increases during pregnancy, as well as the effects on pulmonary artery systolic pressure.51 Exercise testing is useful prior to pregnancy to determine exercise tolerance and maternal risk.19 The risk of clinical decompensation depends on the severity of MS. Heart failure occurs frequently in pregnant women with moderate or severe mitral stenosis (area <1.5 cm2), even in previously asymptomatic patients, in the second and third trimesters, when maternal blood volume and cardiac output peak. The rates of prematurity are 20% to 30%, fetal growth restriction 5% to 20%, and stillbirth (1% to 3%).39,48
Therapeutic options for patients with mitral stenosis include both medical and surgical alternatives, as well as catheter‐based interventions if available, with the choice dependent on the degree of stenosis and patient symptoms. Medical therapy includes limitation of activity, use of diuretics and beta‐blockers for symptom control if heart failure or atrial arrhythmias are present. Patients with severe MS are classified as high risk (World Health Organization class IV risk), with percutaneous balloon mitral valvuloplasty as the preferable option in symptomatic patients refractory to medical therapy and bedrest.8,48,52–55 Patients with atrial fibrillation, left atrial thrombus, and/or a history of embolism should receive therapeutic anticoagulation.26 Medically refractory pregnant patients with severe symptoms and/or pulmonary artery pressure >50 mm Hg may benefit from open mitral valve replacement surgery when percutaneous valvuloplasty is not an option.52–53 Fetal compromise remains a major issue with open surgery. All patients with moderate or severe MS (even when asymptomatic) should be counseled against pregnancy and intervention should be performed prior to pregnancy, favoring valvuloplasty.
Vaginal delivery is preferred in patients with mild MS, and in patients with moderate or severe MS in whom symptoms are NYHA Class I‐II without pulmonary hypertension. Cesarean section may be considered in patients with moderate or severe MS with Class III‐IV symptoms, or who have pulmonary hypertension despite medical therapy, in whom percutaneous mitral valvuloplasty cannot be performed, or has failed.
Mitral and aortic insufficiency
Mitral valve prolapse is the most common cause of mitral insufficiency in developed societies, surpassing rheumatic fever, which is still a prevalent cause in underdeveloped countries. Aortic insufficiency in women of reproductive age can be the result of a wide variety of pathologic processes including a history of endocarditis or rheumatic heart disease, bicuspid aortic valve disease, and aortic annular dilatation related to ascending aortic dilatation.8,56 The placental circulation provides a reduction in systemic vascular resistance, thereby reducing afterload, and interacts favorably with the hemodynamic characteristics of both mitral and aortic insufficiency. In the absence of significant left ventricular dysfunction, significant chronic left‐sided valvular insufficiency can be well tolerated during pregnancy.39,47 Again, evaluation should be performed prior to pregnancy. In the presence of moderate or severe regurgitation, exercise testing is recommended prior to pregnancy, although the level of evidence supporting this statement is based on committee consensus (Level of Evidence C).19 In the presence of left ventricular dysfunction, the volume load of pregnancy may induce symptoms of pulmonary congestion, necessitating restriction of activities, dietary interventions aimed at lowering sodium intake, and medical therapy for heart failure including diuretics, beta‐blockers, and/or vasodilators. Vasodilators may only be used cautiously in the pregnant woman, with concern for avoidance of uteroplacental hypoperfusion. Commonly used vasodilators such as angiotensin converting enzyme inhibitors and angiotensin receptor blockers are contraindicated at any stage of pregnancy due to their teratogenicity57; preload and afterload reducing effects can be obtained using hydralazine and nitrates in combination, which have an established record of safety during pregnancy.26 Other commonly used medications in pregnancy, with established efficacy in the treatment of hypertension in pregnancy, include methyldopa, labetalol, and amlodipine. Women without severe regurgitation, normal left ventricular systolic function, and no clinical symptoms are at low risk for pregnancy‐related complications. Women with severe regurgitation and symptoms, or compromised left ventricular function, should be referred for valve surgery prior to pregnancy, favoring valve repair. Treatment of severe valvular regurgitation surgically is rarely indicated in pregnancy, with the exception of treatment of infective endocarditis, due to its life‐threatening nature.58 Vaginal delivery is the preferred mode of delivery in women with severe regurgitant valve disease, with an epidural and shortened second stage of labor.
Tricuspid regurgitation
Tricuspid regurgitation (TR) is usually functional, related to annular dilatation due to RV pressure or volume overload (often in association with mitral valve disease), previous endocarditis, or rheumatic heart disease. Isolated TR, without right ventricular dysfunction, seldom causes any clinical sequelae during pregnancy, labor or delivery, and rarely requires correction prior to pregnancy. An exception may be women with uncorrected Ebstein's anomaly if heart failure or cyanosis are present. Ebstein's anomaly is associated with the presence of an atrial septal defect and Wolff‐Parkinson‐White syndrome. Pregnant women with interatrial shunt reversal can develop cyanosis. Due to severe right atrial dilatation and right heart failure, arrhythmias may develop, and are associated with a worse prognosis in pregnancy.59 In this setting, correction of Ebstein's anomaly and its associated conditions can be considered prior to pregnancy.
Pulmonic stenosis and insufficiency
Pulmonic stenosis (PS) is most often congenital and valvular in location, though subvalvular or supravalvular congenital forms may also be present. Homograft deterioration after a Ross procedure may result in PS or pulmonic regurgitation. Pulmonic stenosis, even when severe, is generally well tolerated during pregnancy, though some patients experience systemic hypertension‐related disorders, including preeclampsia during their pregnancy.60 Severe pulmonic stenosis may result in right ventricular failure and arrhythmias. Relief of pulmonic stenosis obstruction prior to pregnancy is ideal, using balloon valvuloplasty, and should be performed when the peak forward flow gradient is higher than 64 mm Hg.27 Patients with mild or moderate pulmonic stenosis are regarded as low risk (Figure 4). Balloon valvuloplasty is only rarely indicated in pregnancy in those patients unresponsive to medical therapy and bed rest.61–62 Uncorrected severe PS is associated with a number of serious complications to the fetus, including preterm birth in 17% of patients and a high offspring mortality of 4.8%.60 Vaginal delivery is usually possible and preferable in patients with mild or moderate PS and severe PS who have class I or II symptoms. Women with severe PS and class III/IV symptoms, in whom percutaneous intervention has failed or cannot be performed, should be delivered by Cesarean section.39 Vaginal delivery is most commonly the preferred method of delivery, even for patients with severe pulmonic stenosis.
Figure 4.
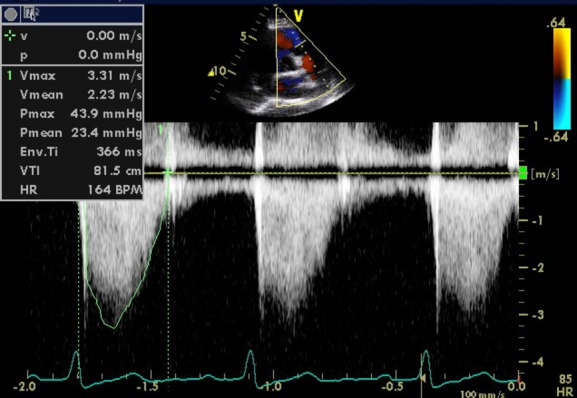
Valvular pulmonic stenosis in a pregnant woman. Moderate pulmonic stenosis is noted in this woman, previous to pregnancy was asymptomatic, now presenting with shortness of breath near term. Figure demonstrates the forward flow spectral Doppler of the pulmonic valve with a peak gradient of 44 mm Hg, consistent with moderate pulmonic stenosis. No significant pulmonic regurgitation was noted.
Severe pulmonic regurgitation (PR) can be due to previous balloon valvuloplasty secondary to PS, or previously repaired Tetralogy of Fallot. Severe PR has been shown to be an independent predictor of maternal complications, particularly in patients with impaired right ventricular function.23 Pregnancy may be associated with a progressive dilatation in right ventricular size, which may persist after pregnancy. In symptomatic patients with severe PR and reduced right ventricular function and/or dilatation, pulmonic valve replacement, preferably with a bioprosthesis, should be considered prior to pregnancy.
Anticoagulation Options in Pregnancy
Patients of childbearing age with mechanical prosthetic valves pose unique challenges since there is no optimal anticoagulation agent considered completely safe at all stages of pregnancy.63 Each anticoagulant option has its drawbacks, whether increased risk of bleeding, increased risk of thromboembolism, or both. Another concern is that the current data includes heterogeneous populations, different risk factors for thromboembolism, different valve types and position, unreported or unknown time in therapeutic range, and unknown compliance.64–65 In the hypercoagulable setting of pregnancy, the risks of maternal thromboembolism and death when anticoagulation is not adequately maintained are a constant concern. However, whether it be potential maternal hemorrhage or fetal complications such as spontaneous abortion or embryopathy, the risks of anticoagulation are both numerous and considerable. Immediate echocardiography is indicated in patients with a mechanical heart valve and any change in symptoms or physical examination findings (Figure 5). Extensive counseling of the patient is required prior to embarking on this challenging aspect of care (Figures 3 and 6; Table 4).19–20,66
Figure 5.

Mechanical aortic valve thrombosis in a pregnant woman. A 38‐year‐old woman gravida 1 para 0, presents with new onset shortness of breath at 12 weeks of pregnancy. She has a history of a bileaflet mechanical aortic valve placed 15 years prior, secondary to endocarditis and a bicuspid aortic valve. Transthoracic echocardiogram prior to pregnancy revealed a normally functioning aortic valve mechanical prosthesis. She was counseled prior to pregnancy and opted to use subcutaneous heparin during weeks 6 to 12, and then use warfarin until week 36. Physical examination at the time of symptoms revealed muffled mechanical closing clicks, and a new grade III/VI systolic murmur in the aortic position. Transthoracic echocardiogram reveals a newly elevated forward flow mean gradient of 52 mm Hg (A). Subsequently, a transesophageal echocardiogram revealed thrombus (B, arrows) attached to the valve leaflets and the annulus (midesophageal view, 120°). Spontaneous abortion occurred (prior to transesophageal echocardiogram was performed), diagnosed at week 12. CW indicates continuous wave Doppler.
Figure 6.

Anticoagulation algorithm for women with valve prosthesis desiring pregnancy.19–20 Adapted from Regitz‐Zagrosek et al19 with permission of Oxford University Press (UK) © European Society of Cardiology, www.escardio.org/guidelines. aPTT indicates activated partial thromboplastin time; LMWH, low molecular weight heparin; OAC, oral anti‐coagulation; UFH, unfractionated heparin.
Table 4.
American College of Chest Physicians Guidelines on the Use of Antithrombotic Therapies in Pregnant Women With Mechanical Valves
| Recommendation | Grade of Recommendation |
|---|---|
|
Grade 2C |
|
Grade 1A |
|
Grade 2C |
|
|
|
Grade 2C |
|
Grade 1C |
Grade 1A—Strong recommendation, high‐quality evidence. Benefits clearly outweigh risks. Recommendation can apply to most patients. Grade 1B—Strong recommendation, moderate‐quality evidence. Benefits clearly outweigh risks. Recommendations can apply to most patients. Grade 1C—Strong recommendation, low‐ or very low‐quality evidence. Benefits clearly outweigh risks. Recommendations can apply to most patients. Grade 2A—Weak recommendation, high‐quality evidence. Benefits closely balanced with risks. Best action may differ depending on patient circumstances. Grade 2B—Weak recommendation, moderate‐quality evidence. Benefits closely balanced with risks. Best action may differ depending on patient circumstances. Grade 2C—Weak recommendation, low‐ or very low‐quality evidence. Other alternatives may be equally reasonable. aPTT indicates activated partial thromboplastin time; LMWH, low molecular weight heparin; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Adapted from Bates et al,66 with permission from the American College of Chest Physicians.
Oral anticoagulants
The established efficacy of warfarin makes it the benchmark of the currently available treatment options, as it affords the greatest protection against maternal valvular thrombosis, thromboembolism, and death in this subset of patients.64 Mothers receiving warfarin with an international normalized ratio of 2.5 to 3.5 throughout pregnancy experience a much lower incidence of thromboembolic event (3.9%), compared with those patients receiving heparin in the first trimester followed by warfarin (9.2%) and those receiving heparin throughout pregnancy (25%), results that paralleled the incidence of maternal death as well (1%, 4.2%, and 6.7%, respectively).64 The risk of valve thrombosis with warfarin throughout pregnancy is low (2.4%) compared with unfractionated heparin (UFH) in the first trimester (10.3%).67 However, the efficacy record of warfarin is offset by its poor safety record with reported associations including fetal wastage, congenital fetal anomalies (nasal hypoplasia and epiphysis stippling), and a higher incidence of fetal intraventricular hemorrhage particularly during forceps extraction, all of which are unacceptable side effects for most women.64 The most feared potential complication is warfarin embryopathy, which occurs as a consequence of it crossing the placenta with a prevalence estimated at ≈0.6% to 6%.64,68–70 The dependency of the embryopathy risk on the dosage has also been a source of controversy with one school of thought suggesting that the incidence can be lowered if the total daily dose is maintained below 5 mg,71–73 while other investigators refute this conclusion and believe the risk to be independent of the warfarin dose.74 Newer agents, such as dabigatran, are considered Food and Drug Administration (FDA) Class X, and are not considered safe in pregnancy, nor are they considered safe in patients with mechanical valves who are not pregnant.75 A recent pilot study highlighted the impact of comprehensive preoperative counseling on women evaluating their options for valve replacement surgery and possible anticoagulation, while also demonstrating that low‐dose anticoagulation therapy for patients with third‐generation mechanical aortic valves was associated with no maternal or fetal complications in a small selected group of patients.76 While provocative, future studies are required to verify these outcomes in a larger group of patients.72,77
Low‐dose aspirin is often added to systemic anticoagulation in the pregnant woman, particularly if the risk of thromboembolism is high (ie, mechanical prosthesis in the mitral position) and in the presence of other risk factors, such as the presence of atrial fibrillation or prior thromboembolic event. This is considered a Level 2A, Level of Evidence C recommendation.66
Unfractionated heparin
The safety of unfractionated heparin (UFH) to the fetus is well established, as it does not cross the placenta and is not teratogenic. However, this increased safety profile for the fetus comes with a cost of an increase in maternal thromboembolism and death.68,72 This anticoagulation option is associated with the highest rate of thromboembolism (33%).66 The possibility of inadequate anticoagulation due to the difficulty in effectively monitoring activated partial thromboplastin time (aPTT) and the decreased aPTT response to heparin during pregnancy due to increased levels of factor VIII and fibrinogen may offer some explanation for these poor maternal outcomes. Continuous intravenous heparin is suggested in high‐risk patients,26 at the expense of an increased risk of infections and osteoporosis, and the possibility of infection from long‐term intravenous access and hospitalization. This option is used infrequently due to its impracticality and not mentioned in current issues of guidelines.66
Low molecular weight heparin
Low molecular weight heparin (LMWH) is an attractive alternative to heparin, in that it does not permeate the placenta while posing a lower risk of bleeding complications, spontaneous abortion, and osteoporosis. The pharmacokinetics of LMWH are also superior to UFH, providing a predictable dose response, superior bioavailability, and a longer half‐life. LMWH may also have higher efficacy than UFH in the first trimester.67 This favorable profile, however, is offset by a higher incidence of maternal valve thrombosis, thromboembolism, and death, though this could be due to inadequate dosing, failed monitoring, or subtherapeutic anti‐Xa levels.78–80 LMWH dosed twice daily with a target anti‐factor Xa level of 0.7 to 1.2 IU/mL at 4 hours after injection is associated with an embolic risk of 7% to 16%.66 Dose requirements for LMWH change during pregnancy and the current recommended target anti‐Xa level is 0.8 to 1.2 U/mL 4 to 6 hours post‐dosing, measured weekly.81 The close monitoring of dosing requirements is essential given the importance of measuring trough levels and the increased maternal thromboembolic risk associated with sub‐therapeutic anti‐Xa trough levels.82 The use of LMWH is restricted to those patients in whom compliance with measurement of anti‐Xa levels is possible.44 While the risk with dose adjusting according to anti‐Xa levels of thromboembolic events is lower at about 9%, it is still higher than the risk of OAC treatment.67,80–81,80–84 Some authors recommend measuring peak levels of anti‐Xa (goal <1.5 U/mL) to reduce bleeding risk.63 Though more frequent dosing seems to be the logical answer for raising pre‐dose levels and lowering peak levels, there is no current evidence to suggest that this actually impacts the therapeutic effect of the anticoagulation or reduces incidence of valve thrombosis or bleeding.82,84–85 Further studies are necessary to differentiate the proper peak and pre‐dose anti‐Xa levels. If epidural anesthesia is planned or likely, LMWH should be withdrawn 18 to 24 hours prior to elective delivery to prevent spinal hematoma.
New data on prosthetic valve thrombosis
Prosthetic valve thrombosis is a rare complication in the nonpregnant patient, with an estimated incidence of 0.1% to 5.7% per patient‐year.86 During pregnancy, the risk increases to up to 10%. Recently published literature suggests the efficacy of low‐dose thrombolysis in pregnant women with mechanical mitral valve thrombosis under transesophageal echocardiography guidance, with excellent efficacy and fewer complications for mother and fetus than cardiac surgery, and no maternal mortality.87 Re‐thrombosis in several patients in this study was reported after thrombolytic treatment. There were no maternal deaths, but neonatal mortality was 20%. Future studies are required to determine the durable efficacy of this therapy.88
Comparing anticoagulation options
When deciding on an anticoagulation regimen, one must consider the type and position of the valve, history of thromboembolism, concurrent atrial fibrillation, patient compliance, and the limitations of the available data on counseling patients. Warfarin is currently the safest option for mothers in preventing thromboembolic complications, while posing a risk for embryopathy when taken in the first trimester, especially in doses higher than 5 mg during weeks 6 to 12 (Figure 6).19–20 Ultimately the lack of randomized clinical trial data directly comparing different anticoagulation options emphasizes the necessity of conducting a thorough discussion regarding the choice of anticoagulation with patient and family, while providing full disclosure regarding the risks, benefits, and alternatives of each regimen and the option of choosing not to become pregnant and/or surrogacy. American and European Cardiology society recommendations for anticoagulation in pregnant women with a mechanical prosthesis are not in complete agreement.19,89 The European Society of Cardiology discourages the use of LMWH throughout the entire pregnancy due to the thrombotic risks, and considers the use of warfarin safest particularly when the dose is <5 mg daily (Figure 6).19 Table 4 summarizes the recommendations from the American College of Chest Physicians (ACCP).66 As do the new ACCP and AHA/ACC Valvular Heart Disease guidelines,20,66 we emphasize patient choice and planning prior to pregnancy, and present an individualized anticoagulation plan based on the overall risk/benefit assessment for that individual patient, taking into consideration the patient's own choices.
Choice of Prosthesis in Women of Childbearing Age
The contrasting needs for durability and antithrombotic therapy make the choice of valve prosthesis in women of childbearing age a difficult process when required. Whenever possible, a choice for valve repair is ideal if the lesion is suitable, even if transfer to a center with this surgical expertise is required. Choices for valve replacement include a bioprosthesis, a human tissue valve (homograft), a mechanical prosthesis and the Ross procedure (autograft). Several valve replacements require no long‐term anticoagulation, with the exception of mechanical prostheses. Accelerated or early structural valve deterioration had been a point of concern during pregnancy for bioprosthetic valve replacements necessitating re‐operation sooner than expected79; however, more recent studies have reported that pregnancy likely does not increase structural valve deterioration or reduce survival in patients with either homograft, pulmonary autograft valve, or bioprosthetic valves.90–94 A common strategy among women of childbearing age is to opt for a bioprosthesis in the aortic or mitral position if repair is not possible, without the need for long‐term anticoagulation, with the expectation that re‐operation would be required in ≈10 years. In contemporary practice, bileaflet mechanical valves are most often preferred by surgeons when a mechanical prosthesis is chosen (as compared with first generation mechanical valves such as the Bjork Shiley valve, a tilting disc prosthesis no longer in use in the United States, and the Starr‐Edwards caged‐ball valve), which possess established durability and an advantageous hemodynamic profile but obligate anticoagulation in some form at all times, including during pregnancy. The presence of mechanical prostheses during pregnancy carries the risk of potentially life‐threatening bleeding, thrombosis, thromboembolic complications, and death, despite careful anticoagulation management. The risk is higher when the mechanical prosthesis is in the mitral position. The presence of a mechanical valve in the aortic position was associated with the greatest incidence of cardiac and obstetric complications among all aortic valve substitutes,90 while others confirm the use of mechanical valves and anticoagulation as a risk.73 This is particularly true of older‐generation mechanical prostheses in the mitral position, not commonly used in contemporary practice. Incremental risk includes the presence of concomitant atrial fibrillation as well as the presence of multiple mechanical prostheses, with a theoretical additive risk in thromboembolic events with these factors.95 Ongoing clinical trials with newer‐generation mechanical valves made purely of pyrolytic carbon, may ultimately lead to the requirement of less anticoagulation, with interim results suggesting less bleeding with similar protection against thromboembolic events with reduced INR goals (1.5 to 2.0; PROACT Study at www.clinicaltrials.gov NCT00291525).96–97
Conclusions
Valvular heart disease in pregnancy is an increasingly common cause of adverse complications for both mother and baby, with medical and surgical advances allowing for many patients with VHD to survive to childbearing age. While rheumatic heart disease has become relatively rare in developed countries, it remains quite common worldwide and an important cause of VHD among immigrant populations. We recommend an integrated risk stratification scheme for pregnant patients with VHD, with WHO classification and an algorithmic approach to both preconception counseling and anticoagulation strategy as outlined here, as well as early referral to a cardiologist with expertise in the management of cardiac disease and pregnancy for these complex patients (Table 5).
Table 5.
Take Home Points
|
|
|
|
|
|
|
|
Disclosures
None.
References
- 1.Kaemmerer H, Hess J. Congenital heart disease. Transition from adolescence to adulthood. Internist (Berl). 2009; 50:1221-1222. [DOI] [PubMed] [Google Scholar]
- 2.Soler‐Soler J, Galve E. Worldwide perspective of valve disease. Heart. 2000; 83:721-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005; 5:685-694. [DOI] [PubMed] [Google Scholar]
- 4.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, Paquet C, Jacob S, Sidi D, Jouven X. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007; 357:470-476. [DOI] [PubMed] [Google Scholar]
- 5.Roos‐Hesselink JW, Ruys TP, Stein JI, Thilen U, Webb GD, Niwa K, Kaemmerer H, Baumgartner H, Budts W, Maggioni AP, Tavazzi L, Taha N, Johnson MR, Hall R, Investigators R. Outcome of pregnancy in patients with structural or ischaemic heart disease: results of a registry of the European Society of Cardiology. Eur Heart J. 2013; 34:657-665. [DOI] [PubMed] [Google Scholar]
- 6.Diao M, Kane A, Ndiaye MB, Mbaye A, Bodian M, Dia MM, Sarr M, Kane A, Monsuez JJ, Ba SA. Pregnancy in women with heart disease in sub‐Saharan Africa. Arch Cardiovasc Dis. 2011; 104:370-374. [DOI] [PubMed] [Google Scholar]
- 7.van Oppen AC, van der Tweel I, Alsbach GP, Heethaar RM, Bruinse HW. A longitudinal study of maternal hemodynamics during normal pregnancy. Obstet Gynecol. 1996; 88:40-46. [DOI] [PubMed] [Google Scholar]
- 8.Elkayam U, Bitar F. Valvular heart disease and pregnancy part I: native valves. J Am Coll Cardiol. 2005; 46:223-230. [DOI] [PubMed] [Google Scholar]
- 9.Cornette J, Roos‐Hesselink JW. In: Stergiopoulos K, Brown D. (eds.). Normal cardiovascular adaptation to pregnancy. Evidence Based Cardiology Consult. 2013London: Springer; 423-432. [Google Scholar]
- 10.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989; 256:H1060-H1065. [DOI] [PubMed] [Google Scholar]
- 11.Hunter S, Robson SC. Adaptation of the maternal heart in pregnancy. Br Heart J. 1992; 68:540-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornette J, Ruys TP, Rossi A, Rizopoulos D, Takkenberg JJ, Karamermer Y, Opic P, Van den Bosch AE, Geleijnse ML, Duvekot JJ, Steegers EA, Roos‐Hesselink JW. Hemodynamic adaptation to pregnancy in women with structural heart disease. Int J Cardiol. 2012; 168:825-831. [DOI] [PubMed] [Google Scholar]
- 13.Rossi A, Cornette J, Johnson MR, Karamermer Y, Springeling T, Opic P, Moelker A, Krestin GP, Steegers E, Roos‐Hesselink J, van Geuns RJ. Quantitative cardiovascular magnetic resonance in pregnant women: cross‐sectional analysis of physiological parameters throughout pregnancy and the impact of the supine position. J Cardiovasc Magn Reson. 2011; 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson GD. Pregnancy‐induced changes in pharmacokinetics: a mechanistic‐based approach. Clin Pharmacokinet. 2005; 44:989-1008. [DOI] [PubMed] [Google Scholar]
- 15.Silversides CK, Coleman JM. In: Oakley C, Warnes CA. (eds.). Physiologic changes in pregnancy. Heart Disease in Pregnancy. 20072nd edMalden, MA: Blackwell Publishing; 6-17. [Google Scholar]
- 16.Robson SC, Dunlop W, Boys RJ, Hunter S. Cardiac output during labour. Br Med J (Clin Res Ed). 1987; 295:1169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruys TP, Cornette J, Roos‐Hesselink JW. Pregnancy and delivery in cardiac disease. J Cardiol. 2013; 61:107-112. [DOI] [PubMed] [Google Scholar]
- 18.Stout KK, Otto CM. Pregnancy in women with valvular heart disease. Heart. 2007; 93:552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regitz‐Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke‐Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos‐Hesselink JW, Schaufelberger M, Seeland U, Torracca L, Bax J, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Baumgartner H, Deaton C, Aguiar C, Al‐Attar N, Garcia AA, Antoniou A, Coman I, Elkayam U, Gomez‐Sanchez MA, Gotcheva N, Hilfiker‐Kleiner D, Kiss RG, Kitsiou A, Konings KT, Lip GY, Manolis A, Mebaaza A, Mintale I, Morice MC, Mulder BJ, Pasquet A, Price S, Priori SG, Salvador MJ, Shotan A, Silversides CK, Skouby SO, Stein JI, Tornos P, Vejlstrup N, Walker F, Warnes C. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32:3147-3197. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, III, Thomas JD. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014. 10.1016/j.jacc.2014.1002.1536 [DOI] [PubMed] [Google Scholar]
- 21.Thorne S, MacGregor A, Nelson‐Piercy C. Risks of contraception and pregnancy in heart disease. Heart. 2006; 92:1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, Kells CM, Bergin ML, Kiess MC, Marcotte F, Taylor DA, Gordon EP, Spears JC, Tam JW, Amankwah KS, Smallhorn JF, Farine D, Sorensen S. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001; 104:515-521. [DOI] [PubMed] [Google Scholar]
- 23.Khairy P, Ouyang DW, Fernandes SM, Lee‐Parritz A, Economy KE, Landzberg MJ. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006; 113:517-524. [DOI] [PubMed] [Google Scholar]
- 24.Drenthen W, Boersma E, Balci A, Moons P, Roos‐Hesselink JW, Mulder BJ, Vliegen HW, van Dijk AP, Voors AA, Yap SC, van Veldhuisen DJ, Pieper PG. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010; 31:2124-2132. [DOI] [PubMed] [Google Scholar]
- 25.Huisman CM, Zwart JJ, Roos‐Hesselink JW, Duvekot JJ, van Roosmalen J. Incidence and predictors of maternal cardiovascular mortality and severe morbidity in The Netherlands: a prospective cohort study. PLoS One. 2013; 8:e56494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 Guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006; 48:e1-e148. [DOI] [PubMed] [Google Scholar]
- 27.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Page RL, Riegel B, Tarkington LG, Yancy CWAmerican College of C, American Heart Association Task Force on Practice G, American Society of E, Heart Rhythm S, International Society for Adult Congenital Heart D, Society for Cardiovascular A, Interventions, Society of Thoracic S. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008; 52:e143-e263. [DOI] [PubMed] [Google Scholar]
- 28.Lui GK, Silversides CK, Khairy P, Fernandes SM, Valente AM, Nickolaus MJ, Earing MG, Aboulhosn JA, Rosenbaum MS, Cook S, Kay JD, Jin Z, Gersony DRAlliance for Adult Research in Congenital C. Heart rate response during exercise and pregnancy outcome in women with congenital heart disease. Circulation. 2011; 123:242-248. [DOI] [PubMed] [Google Scholar]
- 29.Ohuchi H, Tanabe Y, Kamiya C, Noritake K, Yasuda K, Miyazaki A, Ikeda T, Yamada O. Cardiopulmonary variables during exercise predict pregnancy outcome in women with congenital heart disease. Circ J. 2013; 77:470-476. [DOI] [PubMed] [Google Scholar]
- 30.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DTAmerican Heart Association Rheumatic Fever E, Kawasaki Disease C, American Heart Association Council on Cardiovascular Disease in the Y, American Heart Association Council on Clinical C, American Heart Association Council on Cardiovascular S, Anesthesia, Quality of C, Outcomes Research Interdisciplinary Working G. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007; 116:1736-1754. [DOI] [PubMed] [Google Scholar]
- 31.Baumgartner H, Bonhoeffer P, De Groot NM, Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma ETask Force on the Management of Grown‐up Congenital Heart Disease of the European Society of C, Association for European Paediatric C, Guidelines ESCCfP. ESC Guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010; 31:2915-2957. [DOI] [PubMed] [Google Scholar]
- 32.Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep. 2009; 11:298-305. [DOI] [PubMed] [Google Scholar]
- 33.Canobbio MM, Perloff JK, Rapkin AJ. Gynecological health of females with congenital heart disease. Int J Cardiol. 2005; 98:379-387. [DOI] [PubMed] [Google Scholar]
- 34.Bulletins‐Gynecology ACoP. ACOG practice bulletin. No. 73: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2006; 107:1453-1472. [DOI] [PubMed] [Google Scholar]
- 35.Gaffield ME, Curtis KM, Mohllajee AP, Peterson HB. Medical eligibility criteria for new contraceptive methods: combined hormonal patch, combined hormonal vaginal ring and the etonogestrel implant. Contraception. 2006; 73:134-144. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 2010Geneva: Department of Reproductive Health and Research, World Health Organization [Google Scholar]
- 37.Henriquez DD, Roos‐Hesselink JW, Schalij MJ, Klautz RJ, Helmerhorst FM, de Groot CJ. Treatment of valvular heart disease during pregnancy for improving maternal and neonatal outcome. Cochrane Database Syst Rev. 2011; 5:CD008128. [DOI] [PubMed] [Google Scholar]
- 38.Stergiopoulos K, Shiang E, Bench T. Pregnancy in patients with pre‐existing cardiomyopathies. J Am Coll Cardiol. 2011; 58:337-350. [DOI] [PubMed] [Google Scholar]
- 39.Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW, Goodwin I, Zapadinsky N, Elkayam U. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. 2001; 37:893-899. [DOI] [PubMed] [Google Scholar]
- 40.Silversides CK, Colman JM, Sermer M, Farine D, Siu SC. Early and intermediate‐term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol. 2003; 91:1386-1389. [DOI] [PubMed] [Google Scholar]
- 41.Yap SC, Drenthen W, Pieper PG, Moons P, Mulder BJ, Mostert B, Vliegen HW, van Dijk AP, Meijboom FJ, Steegers EA, Roos‐Hesselink JW. Risk of complications during pregnancy in women with congenital aortic stenosis. Int J Cardiol. 2008; 126:240-246. [DOI] [PubMed] [Google Scholar]
- 42.Ben‐Ami M, Battino S, Rosenfeld T, Marin G, Shalev E. Aortic valve replacement during pregnancy. A case report and review of the literature. Acta Obstet Gynecol Scand. 1990; 69:651-653. [DOI] [PubMed] [Google Scholar]
- 43.Bhargava BAR, Yadav R, Bahl VK, Manchanda SC. Percutaneous balloon aortic valvuloplasty during pregnancy: use of the Inoue balloon and the physiologic antegrade approach. Cathet Cardiovasc Diagn. 1998; 45:422-425. [DOI] [PubMed] [Google Scholar]
- 44.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J. 2007; 28:230-268. [DOI] [PubMed] [Google Scholar]
- 45.Balint OH, Siu SC, Mason J, Grewal J, Wald R, Oechslin EN, Kovacs B, Sermer M, Colman JM, Silversides CK. Cardiac outcomes after pregnancy in women with congenital heart disease. Heart. 2010; 96:1656-1661. [DOI] [PubMed] [Google Scholar]
- 46.Immer FF, Bansi AG, Immer‐Bansi AS, McDougall J, Zehr KJ, Schaff HV, Carrel TP. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003; 76:309-314. [DOI] [PubMed] [Google Scholar]
- 47.Lesniak‐Sobelga A, Tracz W, KostKiewicz M, Podolec P, Pasowicz M. Clinical and echocardiographic assessment of pregnant women with valvular heart diseases—maternal and fetal outcome. Int J Cardiol. 2004; 94:15-23. [DOI] [PubMed] [Google Scholar]
- 48.Silversides CK, Colman JM, Sermer M, Siu SC. Cardiac risk in pregnant women with rheumatic mitral stenosis. Am J Cardiol. 2003; 91:1382-1385. [DOI] [PubMed] [Google Scholar]
- 49.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones MAmerican Society of E, European Association of E. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009; 22:1-23. [DOI] [PubMed] [Google Scholar]
- 50.Braverman AC, Thomas JD, Lee RT. Doppler echocardiographic estimation of mitral valve area during changing hemodynamic conditions. Am J Cardiol. 1991; 68:1485-1490. [DOI] [PubMed] [Google Scholar]
- 51.Bryg RJ, Gordon PR, Kudesia VS, Bhatia RK. Effect of pregnancy on pressure gradient in mitral stenosis. Am J Cardiol. 1989; 63:384-386. [DOI] [PubMed] [Google Scholar]
- 52.de Souza JA, Martinez EE, Jr, Ambrose JA, Alves CM, Born D, Buffolo E, Carvalho AC. Percutaneous balloon mitral valvuloplasty in comparison with open mitral valve commissurotomy for mitral stenosis during pregnancy. J Am Coll Cardiol. 2001; 37:900-903. [DOI] [PubMed] [Google Scholar]
- 53.Esteves CA, Munoz JS, Braga S, Andrade J, Meneghelo Z, Gomes N, Maldonado M, Esteves V, Sepetiba R, Sousa JE, Palacios IF. Immediate and long‐term follow‐up of percutaneous balloon mitral valvuloplasty in pregnant patients with rheumatic mitral stenosis. Am J Cardiol. 2006; 98:812-816. [DOI] [PubMed] [Google Scholar]
- 54.Fawzy ME, Kinsara AJ, Stefadouros M, Hegazy H, Kattan H, Chaudhary A, Williams E, Al Halees Z. Long‐term outcome of mitral balloon valvotomy in pregnant women. J Heart Valve Dis. 2001; 10:153-157. [PubMed] [Google Scholar]
- 55.Weiss BM, von Segesser LK, Alon E, Seifert B, Turina MI. Outcome of cardiovascular surgery and pregnancy: a systematic review of the period 1984–1996. Am J Obstet Gynecol. 1998; 179:1643-1653. [DOI] [PubMed] [Google Scholar]
- 56.Lind J, Wallenburg HC. The Marfan syndrome and pregnancy: a retrospective study in a Dutch population. Eur J Obstet Gynecol Reprod Biol. 2001; 98:28-35. [DOI] [PubMed] [Google Scholar]
- 57.Shotan A, Widerhorn J, Hurst AK, Elkayam U. In: Elkayam U, Gleicher N. (eds.). Angiotensin‐converting enzyme inhibitors and pregnancy. Cardiac Problems in Pregnancy: Diagnosis and Management of Maternal and Fetal Disease. 1998New York, NY: Wiley‐Liss; 339-450. [Google Scholar]
- 58.Montoya ME, Karnath BM, Ahmad M. Endocarditis during pregnancy. South Med J. 2003; 96:1156-1157. [DOI] [PubMed] [Google Scholar]
- 59.Donnelly JE, Brown JM, Radford DJ. Pregnancy outcome and Ebstein's anomaly. Br Heart J. 1991; 66:368-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drenthen W, Pieper PG, Roos‐Hesselink JW, Schmidt AC, Mulder BJ, van Dijk AP, Vliegen HW, Sollie KM, Voors AA, Ebels T, van Veldhuisen DJInvestigators Z. Non‐cardiac complications during pregnancy in women with isolated congenital pulmonary valvar stenosis. Heart. 2006; 92:1838-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hameed AB, Goodwin TM, Elkayam U. Effect of pulmonary stenosis on pregnancy outcomes—a case‐control study. Am Heart J. 2007; 154:852-854. [DOI] [PubMed] [Google Scholar]
- 62.Bruce CJ, Connolly HM. Right‐sided valve disease deserves a little more respect. Circulation. 2009; 119:2726-2734. [DOI] [PubMed] [Google Scholar]
- 63.Elkayam U, Bitar F. Valvular heart disease and pregnancy: part II: prosthetic valves. J Am Coll Cardiol. 2005; 46:403-410. [DOI] [PubMed] [Google Scholar]
- 64.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med. 2000; 160:191-196. [DOI] [PubMed] [Google Scholar]
- 65.Malik HT, Sepehripour AH, Shipolini AR, McCormack DJ. Is there a suitable method of anticoagulation in pregnant patients with mechanical prosthetic heart valves? Interact Cardiovasc Thorac Surg. 2012; 15:484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik POAmerican College of Chest P. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012; 141:e691S-e736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abildgaard U, Sandset PM, Hammerstrom J, Gjestvang FT, Tveit A. Management of pregnant women with mechanical heart valve prosthesis: thromboprophylaxis with low molecular weight heparin. Thromb Res. 2009; 124:262-267. [DOI] [PubMed] [Google Scholar]
- 68.Salazar E, Izaguirre R, Verdejo J, Mutchinick O. Failure of adjusted doses of subcutaneous heparin to prevent thromboembolic phenomena in pregnant patients with mechanical cardiac valve prostheses. J Am Coll Cardiol. 1996; 27:1698-1703. [DOI] [PubMed] [Google Scholar]
- 69.Schaefer C, Hannemann D, Meister R, Elefant E, Paulus W, Vial T, Reuvers M, Robert‐Gnansia E, Arnon J, De Santis M, Clementi M, Rodriguez‐Pinilla E, Dolivo A, Merlob P. Vitamin K antagonists and pregnancy outcome. A multi‐centre prospective study. Thromb Haemost. 2006; 95:949-957. [DOI] [PubMed] [Google Scholar]
- 70.van Driel D, Wesseling J, Sauer PJ, Touwen BC, van der Veer E, Heymans HS. Teratogen update: fetal effects after in utero exposure to coumarins overview of cases, follow‐up findings, and pathogenesis. Teratology. 2002; 66:127-140. [DOI] [PubMed] [Google Scholar]
- 71.Cotrufo M, De Feo M, De Santo LS, Romano G, Della Corte A, Renzulli A, Gallo C. Risk of warfarin during pregnancy with mechanical valve prostheses. Obstet Gynecol. 2002; 99:35-40. [DOI] [PubMed] [Google Scholar]
- 72.Sadler L, McCowan L, White H, Stewart A, Bracken M, North R. Pregnancy outcomes and cardiac complications in women with mechanical, bioprosthetic and homograft valves. BJOG. 2000; 107:245-253. [DOI] [PubMed] [Google Scholar]
- 73.Sillesen M, Hjortdal V, Vejlstrup N, Sorensen K. Pregnancy with prosthetic heart valves—30 years' nationwide experience in Denmark. Eur J Cardiothorac Surg. 2011; 40:448-454. [DOI] [PubMed] [Google Scholar]
- 74.Denbow CE, Matadial L, Sivapragasam S, Spencer H. Pregnancy in patients after homograft cardiac valve replacement. Chest. 1983; 83:540-542. [DOI] [PubMed] [Google Scholar]
- 75.Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, Van de Werf F, Investigators R‐A. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013; 369:1206-1214. [DOI] [PubMed] [Google Scholar]
- 76.De Santo LS, Romano G, Della Corte A, D'Oria V, Nappi G, Giordano S, Cotrufo M, De Feo M. Mechanical aortic valve replacement in young women planning on pregnancy: maternal and fetal outcomes under low oral anticoagulation, a pilot observational study on a comprehensive pre‐operative counseling protocol. J Am Coll Cardiol. 2012; 59:1110-1115. [DOI] [PubMed] [Google Scholar]
- 77.Shannon MS, Edwards MB, Long F, Taylor KM, Bagger JP, De Swiet M. Anticoagulant management of pregnancy following heart valve replacement in the United Kingdom, 1986–2002. J Heart Valve Dis. 2008; 17:526-532. [PubMed] [Google Scholar]
- 78.Elkayam U, Singh H, Irani A, Akhter MW. Anticoagulation in pregnant women with prosthetic heart valves. J Cardiovasc Pharmacol Ther. 2004; 9:107-115. [DOI] [PubMed] [Google Scholar]
- 79.Hung L, Rahimtoola SH. Prosthetic heart valves and pregnancy. Circulation. 2003; 107:1240-1246. [DOI] [PubMed] [Google Scholar]
- 80.Oran B, Lee‐Parritz A, Ansell J. Low molecular weight heparin for the prophylaxis of thromboembolism in women with prosthetic mechanical heart valves during pregnancy. Thromb Haemost. 2004; 92:747-751. [DOI] [PubMed] [Google Scholar]
- 81.Quinn J, Von Klemperer K, Brooks R, Peebles D, Walker F, Cohen H. Use of high intensity adjusted dose low molecular weight heparin in women with mechanical heart valves during pregnancy: a single‐center experience. Haematologica. 2009; 94:1608-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barbour LA, Oja JL, Schultz LK. A prospective trial that demonstrates that dalteparin requirements increase in pregnancy to maintain therapeutic levels of anticoagulation. Am J Obstet Gynecol. 2004; 191:1024-1029. [DOI] [PubMed] [Google Scholar]
- 83.McLintock C, McCowan LM, North RA. Maternal complications and pregnancy outcome in women with mechanical prosthetic heart valves treated with enoxaparin. BJOG. 2009; 116:1585-1592. [DOI] [PubMed] [Google Scholar]
- 84.Yinon Y, Siu SC, Warshafsky C, Maxwell C, McLeod A, Colman JM, Sermer M, Silversides CK. Use of low molecular weight heparin in pregnant women with mechanical heart valves. Am J Cardiol. 2009; 104:1259-1263. [DOI] [PubMed] [Google Scholar]
- 85.Friedrich E, Hameed AB. Fluctuations in anti‐factor Xa levels with therapeutic enoxaparin anticoagulation in pregnancy. J Perinatol. 2010; 30:253-257. [DOI] [PubMed] [Google Scholar]
- 86.Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KHAmerican College of Chest P. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012; 141:e576S-e600S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ozkan M, Cakal B, Karakoyun S, Gursoy OM, Cevik C, Kalcik M, Oguz AE, Gunduz S, Astarcioglu MA, Aykan AC, Bayram Z, Biteker M, Kaynak E, Kahveci G, Duran NE, Yildiz M. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low‐dose, slow infusion of tissue‐type plasminogen activator. Circulation. 2013; 128:532-540. [DOI] [PubMed] [Google Scholar]
- 88.Casais P, Rolandi F. Prosthetic valve thrombosis in pregnancy: a promising treatment for a rare and mostly preventable complication. Circulation. 2013; 128:481-482. [DOI] [PubMed] [Google Scholar]
- 89.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JSAmerican College of Cardiology/American Heart Association Task Force on Practice G. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008; 52:e1-e142. [DOI] [PubMed] [Google Scholar]
- 90.Heuvelman HJ, Arabkhani B, Cornette JM, Pieper PG, Bogers AJ, Takkenberg JJ, Roos‐Hesselink JW. Pregnancy outcomes in women with aortic valve substitutes. Am J Cardiol. 2013; 111:382-387. [DOI] [PubMed] [Google Scholar]
- 91.Avila WS, Rossi EG, Grinberg M, Ramires JA. Influence of pregnancy after bioprosthetic valve replacement in young women: a prospective five‐year study. J Heart Valve Dis. 2002; 11:864-869. [PubMed] [Google Scholar]
- 92.North RA, Sadler L, Stewart AW, McCowan LM, Kerr AR, White HD. Long‐term survival and valve‐related complications in young women with cardiac valve replacements. Circulation. 1999; 99:2669-2676. [DOI] [PubMed] [Google Scholar]
- 93.Arabkhani B, Heuvelman HJ, Bogers AJ, Mokhles MM, Roos‐Hesselink JW, Takkenberg JJ. Does pregnancy influence the durability of human aortic valve substitutes? J Am Coll Cardiol. 2012; 60:1991-1992. [DOI] [PubMed] [Google Scholar]
- 94.Cleuziou J, Horer J, Kaemmerer H, Teodorowicz A, Kasnar‐Samprec J, Schreiber C, Lange R. Pregnancy does not accelerate biological valve degeneration. Int J Cardiol. 2010; 145:418-421. [DOI] [PubMed] [Google Scholar]
- 95.Suri V, Keepanasseril A, Aggarwal N, Chopra S, Bagga R, Sikka P, Vijayvergiya R. Mechanical valve prosthesis and anticoagulation regimens in pregnancy: a tertiary centre experience. Eur J Obstet Gynecol Reprod Biol. 2011; 159:320-323. [DOI] [PubMed] [Google Scholar]
- 96.Moidl R, Simon P, Wolner EOn XPHVT. The On‐X prosthetic heart valve at five years. Ann Thorac Surg. 2002; 74:S1312-S1317. [DOI] [PubMed] [Google Scholar]
- 97.Puskas J, Gerdisch M, Nichols D, Quinn R, Anderson C, Rhenman B, Fermin L, McGrath M, Kong B, Hughes C, Sethi G, Wait M, Martin T, Graeve A. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on‐X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg. 2014; 4:1002-1011. [DOI] [PubMed] [Google Scholar]


