Abstract
Background
Young women with coronary heart disease have high rates of depression and a higher risk of adverse events than men of similar age. Whether depression has a higher prognostic value in this group than in men and older women is not known. Our objective was to assess whether depression in young women is associated with higher risk of coronary artery disease (CAD) and adverse outcomes compared with similarly aged men and older women.
Methods and Results
We examined 3237 patients undergoing coronary angiography for evaluation of CAD and followed them for 2.9 years (median). Depressive symptoms were assessed with the Patient Health Questionnaire (PHQ)‐9, and CAD burden was dichotomized based on its presence or absence. After multivariable adjustment for CAD risk factors, depressive symptoms predicted CAD presence in women aged ≤55 years (odds ratio=1.07 95% confidence interval [CI] 1.02 to 1.13 per 1 point increase in PHQ‐9 score), but not in men aged ≤55 years or women aged >55 years. Depressive symptoms also predicted increased risk of death in women aged ≤55 years (adjusted hazard ratio=1.07, 95% CI 1.02 to 1.14, per 1 point increase in PHQ‐9 score), but not in men aged ≤55 years and women aged >55 years, with P=0.02 for the depression‐sex interaction and P=0.02 for depression‐sex‐age interaction.
Conclusions
Among patients with suspected or established CAD, depressive symptoms are associated with increased risk of death, particularly in young women. This group may be especially vulnerable to the adverse cardiovascular effects of depression.
Keywords: coronary artery disease, depression, sex differences
Introduction
Depression is a prevalent and increasingly recognized risk factor for both the development of and the outcome from coronary artery disease (CAD).1 Recent meta‐analyses have reported pooled estimates of cardiovascular risk between 50% to >100% higher for individuals with depressive symptoms or clinical depression compared to those without.2–3 All these reports, however, have pointed out substantial heterogeneity of results among individual studies.4–5
A potential explanation for the variability in previous results is heterogeneity of effects among various demographic groups. Limited evidence suggests that depression and other psychosocial risk factors might be more powerful risk factors in young individuals,6 and especially in young women.7–9 Although few women develop cardiovascular disease at a young age,10 the lifetime risk in women is over 50%,11 and therefore identification of risk factors in young populations may provide long‐term benefit by enabling early prevention. Furthermore, young women are traditionally under‐represented in cardiovascular disease studies,12 have higher rates of depression,13–14 and have higher mortality rates after acute myocardial infarction (MI) compared with men.15 Although coronary heart disease mortality rates have declined in the United States, this decline is less pronounced among young women in recent years.16,10
In this study, we sought to examine sex and age interactions in the association of depressive symptoms with both CAD severity and adverse events (death, MI, and revascularization) in a large sample of patients undergoing cardiac catheterization. We hypothesized that depression shows a more robust association with CAD and is a stronger predictor of adverse outcomes in younger women compared with men and older women.
Methods
Study Population
Study participants were recruited as part of the Emory Cardiovascular Biobank, consisting of 3911 patients enrolled before undergoing elective or emergent coronary angiography across 3 Emory healthcare sites between 2003 and 2010.17 A total of 674 patients (17.2%) were excluded because of missing depression data, leaving 3237 patients for analysis. For the CAD analysis, an additional 151 cardiac transplant recipients were excluded. The Emory Institutional Review Board approved the study and all patients provided informed consent.
Study Measures
Patients were enrolled and interviewed on the same day of their cardiac catheterizations, with most interviews occurring prior to catheterization. Depressive symptoms were assessed via the 9‐question Primary Care Evaluation of Mental Disorders Brief Patient Health Questionnaire (PHQ‐9),18 which, at a cutoff of 10 points or higher (out of 27), signifying at least moderate depression, has a sensitivity and specificity of 88% for major depression.19 Other measures, including lifestyle factors, medical comorbidities, revascularization during the index cardiac catheterization, and previous revascularization procedures were ascertained via patient interview and chart review.
CAD and Severity Scoring
Two of the authors (R.S.P. and I.J.N.) evaluated all coronary angiographies by visual estimation of luminal narrowing in multiple segments based on a modified form of the American Heart Association classification of the coronary tree,20 masked to depression status and other patient data. Reviewers were blinded to depression status, but not sex and age. Semi‐quantitative angiographic scoring was performed using the Gensini score, which quantifies CAD severity by a nonlinear points system for degree of luminal narrowing weighted by a multiplier for specific anatomical locations of any lesions.21 To determine inter‐rater reliability, both evaluators analyzed 25 random angiograms; the intra‐class correlation was 0.88 for CAD severity based on the Gensini classification scheme.
Outcomes and Follow‐Up
Follow‐up was conducted at 2 and 5 years post‐enrollment. Subsequent hospitalizations were assessed via telephone interview with the patient and/or a close family member who was familiar with the patient's health, and by monitoring (via chart review) admissions at Emory hospitals on all patients, regardless of patient self‐report. Specialized research staff confirmed all events via chart review if the hospital was within the Emory University system (80% of the admissions); hospital admissions outside of the Emory system were determined by self‐report, and, verified through chart review where possible. All‐cause mortality was assessed through verbal communications with family, Georgia vital records, and the Social Security Death Index. Major adverse cardiovascular events (MACE) were determined to occur if the patient died, was hospitalized for MI, or received new revascularization procedures (percutaneous coronary intervention [PCI] or coronary artery bypass surgery [CABG]).
Statistical Analysis
Analysis was conducted using SAS 9.3 for Windows (©2008). Patients were categorized by sex and age, with 3 main age groups of roughly equal proportion: ≤55, 56 to 64, and ≥65 years of age. Prevalence of various demographics and cardiovascular risk factors were compared between moderate‐to‐severe depression (PHQ‐9≥10) and none‐to‐mild depression (PHQ‐9<10). Risk ratios of each category between depressed and non‐depressed groups were also calculated, and chi‐square tests were used to calculate for statistical significance between groups with PHQ‐9 scores ≥10 and <10.
Because of the non‐normal distribution of the Gensini score, the association between depression and Gensini score was first attempted using ordinal logistic regression, with quartiles of the Gensini score as the outcome. For the first, second, third, and fourth quartiles, the Gensini scores were 0, 1 to 13, 14 to 53, and >54 units, respectively. However, the proportional odds assumption was not met (P=0.02) in women ≤55 years of age; all 3 upper quartiles of Gensini score had similarly increased depressive symptoms compared with the lowest quartile in which Gensini score=0. As a result, the Gensini score was used as a binary outcome in a logistic regression model such that patients were categorized as either having CAD (Gensini>0) or not having CAD (Gensini=0).
Cox proportional hazards models were performed with all‐cause death as the primary endpoint and MACE as a secondary endpoint. Censoring was applied after the date of the first MACE event or date of latest patient contact, whichever occurred first. Depressive symptoms were analyzed as a continuous predictor of death and MACE. The assumption of linearity was verified for each outcome. Additionally, to provide a clinically relevant interpretation of results, an additional analysis was performed with depression as a binary predictor (moderate/severe depression [PHQ‐9≥10] versus PHQ‐9<10). All multivariable models adjusted for pre‐specified potential confounding factors, including demographic factors (age, black race), CAD risk factors and cardiac medical history (hypertension, diabetes, hyperlipidemia, current or past smoking, body mass index, heart failure, history of stroke), and reason for coronary angiography (acute MI, angina [or angina‐equivalent], or positive stress test). Analyses were done in the entire group as well as after stratification for age and sex. In each of the 3 age categories, the interaction of depression with sex was tested. Additionally, the 3‐way interaction of depression, sex, and age category was tested in the pooled sample to evaluate whether the risk of depression differed by age category in addition to sex. The proportional hazards assumption was tested using visual inspection of the −2 log‐log plot, as well as Schoenfeld residuals for all independent variables. For the outcomes analysis (death and MACE), a sensitivity analysis was performed where the Gensini score and any planned revascularization (within 7 days for PCI and within 90 days for CABG) were added to the model to investigate the impact of CAD severity, and of any differences in likelihood of subsequent interventions, on the relationship between depressive symptoms and death/MACE. Because the validity of depression assessment in the acute setting is unknown, we also performed a subgroup analysis of elective cases, in which urgent cases (acute MI) were excluded.
Results
The 674 patients with missing depression data were similar to those without missing data with some exceptions; they were more likely to be women (39% versus 33%), never‐smokers (47% versus 38%), have heart failure (26% versus 20%), and less likely to have undergone coronary angiography because of a positive stress test (40% versus 46%).
Baseline Descriptive Analysis
In the sample that was left for analysis (n=3237) women comprised 34% of the sample, and the mean age±standard deviation (SD) was 62.5±11.8 years. In Table 1, we compared key characteristics between moderate to severe depression (PHQ‐9≥10) and none to mild depression (PHQ‐9<10), in 6 subgroups stratified by age and sex. With some exceptions, the moderate to severely depressed group showed a greater prevalence of cardiovascular risk factors and comorbidities compared with those with none to mild depression. Of note, significant differences in history of PCI and CABG, as well as in decision to performing stenting, were found only for women aged ≤55 years (higher in moderate to severe depression versus none to mild depression), and not in the other subgroups.
Table 1.
Baseline Characteristics of Population Undergoing Coronary Angiography According to Depressive Symptoms
| Age ≤55 Years | Age 56 to 64 Years | Age ≥65 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PHQ‐9≥10 | PHQ‐9<10 | RR | PHQ‐9≥10 | PHQ‐9<10 | RR | PHQ‐9≥10 | PHQ‐9<10 | RR | ||
| Women | n | 80 | 221 | 49 | 216 | 60 | 445 | |||
| Black race | 21% | 20% | 1.04 | 12% | 23% | 0.54 | 15% | 16% | 0.91 | |
| Current smoker | 31% | 18% | 1.72* | 22% | 14% | 1.56 | 14% | 8% | 1.77 | |
| Hypertension | 79% | 61% | 1.29* | 86% | 67% | 1.29* | 85% | 81% | 1.05 | |
| Diabetes | 36% | 24% | 1.48* | 51% | 38% | 1.34 | 32% | 32% | 1.00 | |
| Hyperlipidemia | 63% | 57% | 1.10 | 78% | 69% | 1.13 | 75% | 73% | 1.03 | |
| HF | 24% | 21% | 1.12 | 16% | 20% | 0.80 | 33% | 18% | 1.83* | |
| History of stroke | 11% | 6% | 1.91 | 18% | 13% | 1.47 | 10% | 11% | 0.95 | |
| History of PCI | 35% | 18% | 1.98* | 39% | 29% | 1.35 | 47% | 39% | 1.21 | |
| History of CABG | 15% | 6% | 2.37* | 12% | 11% | 1.15 | 25% | 13% | 1.85* | |
| Obese (BMI ≥30 kg/m²) | 58% | 52% | 1.10 | 65% | 50% | 1.29 | 30% | 39% | 0.78 | |
| Current MI | 5% | 6% | 0.90 | 8% | 6% | 1.47 | 5% | 9% | 0.60 | |
| Angina | 80% | 63% | 1.27* | 76% | 58% | 1.30* | 75% | 47% | 1.59* | |
| Reason for cath | ||||||||||
| Positive stress | 45% | 49% | 0.92 | 51% | 54% | 0.95 | 41% | 43% | 0.95 | |
| Angina (or equivalent) | 66% | 58% | 1.14 | 66% | 57% | 1.16 | 73% | 62% | 1.19 | |
| PCI after cath | 24% | 12% | 2.02* | 22% | 21% | 1.08 | 27% | 24% | 1.12 | |
| CABG after cath | 4% | 4% | 0.92 | 4% | 3% | 1.47 | 5% | 6% | 0.86 | |
| Men | n | 77 | 473 | 74 | 563 | 88 | 891 | |||
| Black race | 16% | 16% | 0.95 | 20% | 17% | 1.19 | 18% | 17% | 1.05 | |
| Current smoker | 26% | 21% | 1.25 | 31% | 16% | 1.89* | 14% | 6% | 2.28* | |
| Hypertension | 74% | 64% | 1.15 | 86% | 69% | 1.26* | 70% | 74% | 0.95 | |
| Diabetes | 29% | 26% | 1.11 | 46% | 29% | 1.58* | 44% | 33% | 1.33* | |
| Hyperlipidemia | 73% | 63% | 1.15 | 76% | 69% | 1.10 | 74% | 74% | 1.00 | |
| HF | 18% | 18% | 1.04 | 26% | 20% | 1.31 | 33% | 21% | 1.59* | |
| History of stroke | 6% | 4% | 1.46 | 15% | 7% | 2.26* | 22% | 9% | 2.47* | |
| History of PCI | 40% | 39% | 1.03 | 50% | 44% | 1.13 | 55% | 52% | 1.05 | |
| History of CABG | 21% | 12% | 1.69* | 26% | 22% | 1.17 | 42% | 35% | 1.19 | |
| Obese (BMI ≥30 kg/m²) | 52% | 49% | 1.07 | 53% | 44% | 1.20 | 32% | 30% | 1.05 | |
| Current MI | 9% | 12% | 0.73 | 15% | 12% | 1.30 | 13% | 12% | 1.08 | |
| Angina | 78% | 53% | 1.47* | 70% | 48% | 1.46* | 63% | 46% | 1.36* | |
| Reason for cath | ||||||||||
| Positive stress | 36% | 49% | 0.73* | 39% | 43% | 0.91 | 39% | 48% | 0.80 | |
| Angina (or equivalent) | 71% | 52% | 1.38* | 69% | 57% | 1.22* | 72% | 57% | 1.27* | |
| PCI after cath | 22% | 26% | 0.86 | 23% | 28% | 0.82 | 32% | 29% | 1.09 | |
| CABG after cath | 5% | 6% | 0.91 | 9% | 9% | 1.02 | 7% | 9% | 0.79 | |
BMI indicates body mass index; CABG, coronary artery bypass surgery; Cath, cardiac catheterization; HF, heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; PHQ‐9, Patient Health Questionnaire‐9; RR, risk ratio of risk factor in depressed group vs non‐depressed group.
P<0.05.
The prevalence of moderate to severe depression was compared among age and sex subgroups (Figure 1). Women aged ≤55 years were found to have the highest prevalence of moderate to severe depression (27%), while men ≥65 years had the lowest depression rates (9%).
Figure 1.
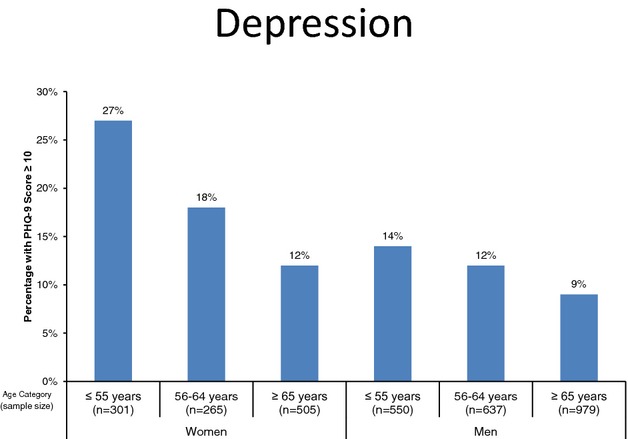
Proportion of patients with moderate or higher severity depressive symptoms (PHQ‐9≥10) according to age and sex. PHQ‐9 indicates Patient Health Questionnaire‐9.
Depression and Coronary Artery Disease
No association was found between depressive symptoms and the presence of CAD in the total sample (odds ratio [OR] for CAD per 1 point PHQ‐9 score increment, 1.02, 95% confidence interval [CI], 0.99 to 1.03). However, significant heterogeneity in this association was found across age and sex. Using logistic regression, Table 2 shows that depressive symptoms were associated with CAD only in women ≤55 years of age, such that each 1‐point increase in PHQ‐9 score was associated with a 1.08 increased odds (95% CI, 1.03 to 1.12) of CAD. Demographics, cardiovascular risk factors, and reason for coronary angiography did not explain this association; the adjusted OR was 1.07 (95% CI, 1.02 to 1.13). The relationship between PHQ‐9 score and CAD among women ≤55 years of age was approximately linear (Figure 2). No positive associations were found in the other subgroups. The adjusted 3‐way interaction between depression, sex and age category, which tests the difference in the association for depression according to both age category and sex, was significant (P=0.04, adjusted model). Among those ≤55 years of age, the depression‐sex interaction was also significant (P=0.01 in the adjusted model). Otherwise, no statistically significant differences in depression risk were found among age and sex subgroups.
Table 2.
Odds Ratio for Coronary Artery Disease Per 1‐Point Increase in Patient Health Questionnaire‐9 Score
| Model | Men | Women | Depression‐Sex Interaction | Depression‐ Sex‐Age Interaction* |
|---|---|---|---|---|
| Unadjusted models | ||||
| Age, y* | Odds ratio (95% confidence interval) | P value | P value | |
| ≤55 | 1.01 (0.97 to 1.04) | 1.08 (1.03 to 1.12) | 0.02 | 0.04 |
| 56 to 64 | 1.04 (0.99 to 1.09) | 1.06 (1.01 to 1.12) | 0.54 | |
| ≥65 | 1.02 (0.97 to 1.07) | 0.99 (0.95 to 1.04) | 0.46 | |
| Adjusted* model | ||||
| ≤55 | 1.00 (0.95 to 1.04) | 1.07 (1.02 to 1.13) | 0.01 | 0.04 |
| 56 to 64 | 1.02 (0.97 to 1.08) | 1.05 (0.99 to 1.12) | 0.68 | |
| ≥65 | 0.99 (0.94 to 1.04) | 0.98 (0.93 to 1.03) | 0.49 | |
P value derived from pooled model that included all age groups. The unadjusted P value for the 3‐way interaction was from a model that included PHQ‐9 score, sex, age group, and all the lower term interactions. The adjusted P value was from a model that also included the variables listed below.
Separate models were constructed for each age stratum and sex. The 2‐way interaction between depression and sex was from a model that pooled men and women within age strata.
Adjusted for age, black race, hypertension, hyperlipidemia, diabetes, heart failure, current/past smoking, body‐mass index, history of stroke, and reason for cardiac catheterization (acute myocardial infarction, symptoms, or positive stress test).
Figure 2.
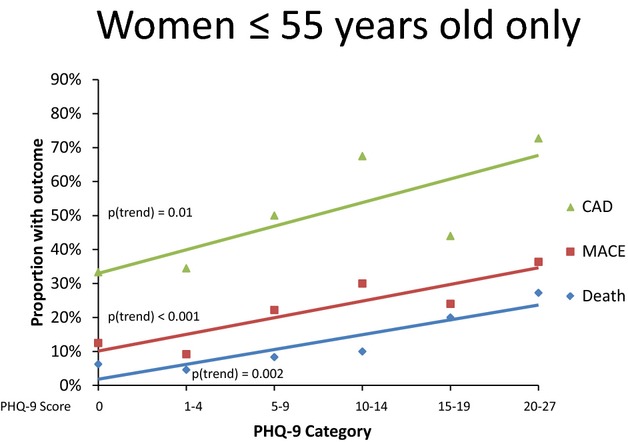
Prevalence of coronary artery disease, as well as risk of death and major adverse cardiac events according to PHQ‐9 score in women aged ≤55 years. CAD indicates coronary artery disease; MACE, major adverse cardiovascular events; PHQ‐9, Patient Health Questionnaire‐9.
When examining only elective cases (n=2760, with 326 acute MI cases excluded), the results were similar to those of the whole group. In women ≤55 years of age, the adjusted OR of CAD for each PHQ‐9 point increase was 1.07 (95% CI, 1.01 to 1.12), and the interaction between case urgency and depression was not significant (P=0.41). In the other demographic groups, no significant relationships between depression and CAD were found among elective cases, similar to the sample overall.
When using depression as a binary predictor of CAD (PHQ‐9≥10 versus PHQ‐9<10), moderate/severe depression was associated with an increased odds of CAD in women ≤55 years of age only, with adjusted OR=2.02 (95% CI, 1.03 to 3.97). The sex‐depression interaction of the OR for CAD was significant for those aged ≤55 years, P=0.02. The results for each sex and age subgroup are shown in Figure 3. When comparing the odds ratios of CAD among the other age and sex subgroups, no statistically significant differences were found.
Figure 3.
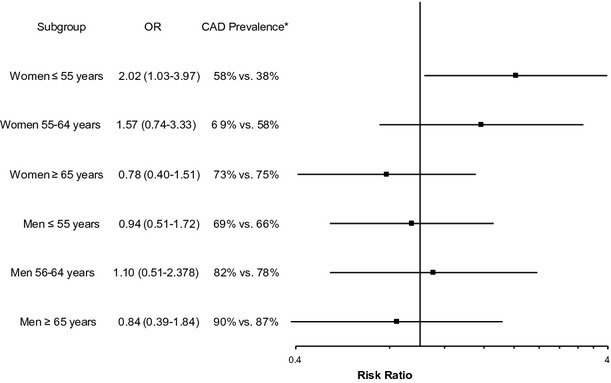
Adjusted odds ratio of coronary artery disease for moderate/severe depression (PHQ‐9≥10) compared with no or mild depression (PHQ<10). Models are adjusted for age, black race, hypertension, hyperlipidemia, diabetes, heart failure, current/past smoking, body‐mass index, history of stroke, and reason for cardiac catheterization (acute myocardial infarction, symptoms, or positive stress test), Gensini score, and post‐catheterization intervention. *CAD prevalence in depressed vs non‐depressed groups. Note: Sex‐depression interaction P=0.02, 0.36, and 0.45 for ages ≤55, 56 to 65, and ≥65 years, respectively. Sex‐age‐depression interaction P=0.03. CAD indicates coronary artery disease; OR, odds ratio; PHQ‐9, Patient Health Questionnaire‐9.
Depression and Major Adverse Cardiovascular Events
In the total sample, after 11 466 person‐years of follow‐up (median 2.9 years), at least 1 major adverse event occurred in 868 (27%) patients, including 467 deaths, 143 MIs, 402 PCIs, and 65 CABGs. Depressive symptoms predicted death and MACE in an approximately linear relationship. Each 1‐point increase in PHQ‐9 score was associated with a hazard ratio (HR) of 1.05 (95% CI, 1.03 to 1.06) for death and 1.04 (95% CI, 1.02 to 1.05) for MACE. Adjustment for covariates minimally impacted the results. The proportional hazards assumption was met for all independent variables.
When women and men were stratified by age (Table 3), depressive symptoms were found to have the highest adjusted hazard ratios of death and MACE in young women, with a HR of 1.08 (95% CI, 1.01 to 1.15) and 1.06 (95% CI, 1.01 to 1.11), respectively. The HRs for women decreased as age category increased. For death, but not MACE, the depression‐sex‐age interaction in the fully adjusted model was significant (P=0.02). Men with depressive symptoms aged ≥65 years were also noted to have a higher incidence of death and MACE relative to men of the same age without increased depressive symptoms, with HR=1.07 (95% CI, 1.03 to 1.09) for death and 1.04 (95% CI, 1.02 to 1.07) for MACE. Otherwise, no significant associations in the other subgroups were found. Sensitivity analysis revealed no appreciable change in HR when the Gensini score and post‐catheterization interventions were added to the models. When comparing the HR amongst age and sex subgroups, men aged ≥65 years with higher depressive symptoms had a significantly higher HR for death than men aged ≤55 years, with P<0.01. Otherwise, no significant differences in HR were found.
Table 3.
Hazard Ratios* for Death and Major Adverse Cardiovascular Events Per 1‐Point Increase in Patient Health Questionnaire‐9 Score
| Age, y* | Men | Women | Depression‐Sex Interaction | Depression‐ Sex‐Age Interaction* |
|---|---|---|---|---|
| Death | ||||
| Unadjusted | Hazard ratio (95% confidence interval) | P value | P value | |
| ≤55 | 1.02 (0.97 to 1.07) | 1.08 (1.03 to 1.14) | 0.11 | 0.02 |
| 56 to 64 | 1.05 (1.01 to 1.10) | 1.10 (0.96 to 1.07) | 0.28 | |
| ≥65 | 1.08 (1.06 to 1.11) | 1.03 (0.98 to 1.07) | 0.04 | |
| Adjusted* model | ||||
| ≤55 | 0.97 (0.91 to 1.03) | 1.07 (1.01 to 1.14) | 0.02 | 0.02 |
| 56 to 64 | 1.03 (0.98 to 1.09) | 1.10 (0.95 to 1.07) | 0.54 | |
| ≥65 | 1.07 (1.04 to 1.10) | 1.02 (0.97 to 1.07) | 0.08 | |
| Adjusted* model+Gensini score | ||||
| ≤55 | 0.97 (0.91 to 1.03) | 1.07 (1.07 to 1.14) | 0.02 | 0.03 |
| 56 to 64 | 1.03 (0.98 to 1.09) | 1.10 (0.99 to 1.07) | 0.58 | |
| ≥65 | 1.07 (1.04 to 1.11) | 1.02 (0.96 to 1.07) | 0.08 | |
| Adjusted* model+Gensini score+post‐catheterization intervention | ||||
| ≤55 | 0.97 (0.91 to 1.03) | 1.08 (1.01 to 1.15) | 0.02 | 0.03 |
| 56 to 64 | 1.04 (0.99 to 1.09) | 1.01 (0.95 to 1.98) | 0.57 | |
| ≥65 | 1.07 (1.03 to 1.09) | 1.02 (0.97 to 1.07) | 0.11 | |
| Myocardial infarction, revascularization, or death (composite) | ||||
| Unadjusted | Hazard ratio (95% confidence interval) | P value | P value | |
| ≤55 | 1.03 (1.00 to 1.64) | 1.07 (1.03 to 1.11) | 0.16 | 0.01 |
| 56 to 64 | 1.05 (1.02 to 1.08) | 1.02 (0.98 to 1.07) | 0.35 | |
| ≥65 | 1.05 (1.03 to 1.07) | 1.00 (0.96 to 1.04) | 0.03 | |
| Adjusted* model | ||||
| ≤55 | 1.01 (0.97 to 1.04) | 1.05 (1.01 to 1.110) | 0.18 | 0.07 |
| 56 to 64 | 1.03 (0.99 to 1.06) | 1.02 (0.98 to 1.07) | 0.62 | |
| ≥65 | 1.04 (1.02 to 1.07) | 0.99 (0.96 to 1.04) | 0.11 | |
| Adjusted* model+Gensini score | ||||
| ≤55 | 1.01 (0.97 to 1.04) | 1.05 (1.01 to 1.10) | 0.21 | 0.09 |
| 56 to 64 | 1.03 (0.99 to 1.06) | 1.02 (0.98 to 1.07) | 0.67 | |
| ≥65 | 1.04 (1.02 to 1.07) | 1.00 (0.96 to 1.04) | 0.13 | |
| Adjusted* model+Gensini score+post‐catheterization intervention | ||||
| ≤55 | 1.01 (0.97 to 1.04) | 1.06 (1.01 to 1.11) | 0.22 | 0.10 |
| 56 to 64 | 1.03 (0.99 to 1.06) | 1.03 (0.98 to 1.08) | 0.67 | |
| ≥65 | 1.04 (1.02 to 1.07) | 0.99 (0.95 to 1.04) | 0.13 | |
P value derived from pooled model that included all age groups. The unadjusted P value for the 3‐way interaction was from a model that included PHQ‐9 score, sex, age group, and all the lower term interactions. The adjusted P value was from a model that also included the variables listed below.
Separate models were constructed for each age stratum and sex. The 2‐way interaction between depression and sex was from a model that pooled men and women within age strata.
Adjusted for age black race, hypertension, hyperlipidemia, diabetes, heart failure, current/past smoking, body‐mass index, history of stroke, history of revascularization, and reason for cardiac catheterization (acute myocardial infarction, symptoms, or positive stress test).
When examining only elective cases (n=2910, with n=327 acute MI cases excluded) in multivariate analyses, the results were generally similar, with adjusted HR=1.05 (95% CI 1.03 to 1.07) for death and HR=1.03 (1.02 to 1.05) for MACE per PHQ‐point increase. The interactions between case urgency and PHQ‐9 scores were not significant for death (P=0.07) or MACE (P=0.36). In women ≤55 years, restriction of analysis to elective cases resulted in slight increase in the adjusted HR to 1.09 (95% CI, 1.03 to 1.17) for death and 1.08 (95% CI, 1.03 to 1.13) for MACE, P=0.98 and P=0.11, respectively, for the interaction with case urgency. Similarly, among men aged ≥65 years, the HR increased slightly to 1.08 (95% CI, 1.05 to 1.12) for death and 1.06 (95% CI, 1.03 to 1.09) for MACE; P=0.03 and P=0.02, respectively, for the interaction with case urgency.
When analyzing depression as a binary predictor (Figure 4), similar associations were found. In women aged ≤55 years, moderate to severe depression was associated with a HR of 2.45 (95% CI, 1.05 to 5.73) for death and 2.17 (95% CI 1.20 to 3.96) for MACE. In men aged ≥65 years, the results were also significant with similar effect size. For MACE, a significant sex‐depression interaction was found for those aged ≤55 years, P=0.049. Otherwise, no differences in depression risk were found among the other age and sex subgroups.
Figure 4.
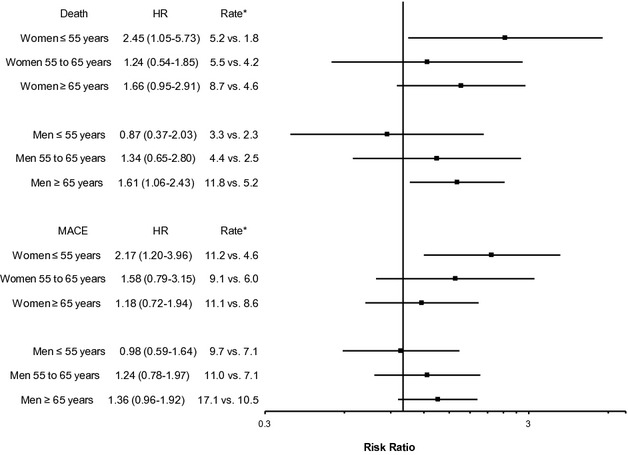
Adjusted hazard ratio of death or major adverse cardiac events for moderate/severe depression (PHQ‐9≥10) compared with no or mild depression (PHQ<10). Models are adjusted for age, black race, hypertension, hyperlipidemia, diabetes, heart failure, current/past smoking, body‐mass index, history of stroke, and reason for cardiac catheterization (acute myocardial infarction, symptoms, or positive stress test), Gensini score, and post‐catheterization intervention. *Event rates per 100 person‐years in depressed vs non‐depressed groups. Note: For death, sex‐depression interaction P=0.09, 0.98, and 0.93 for ages ≤55, 56 to 65, and ≥65 years, respectively. For MACE, sex‐depression interaction P=0.049, 0.90, and 0.77 for ages ≤55, 56 to 65, and ≥65 years, respectively. Sex‐age‐depression interaction P=0.24 and P=0.29 for death and MACE, respectively. CAD indicates coronary artery disease; HR, hazard ratio; MACE, major adverse cardiac events; PHQ‐9, Patient Health Questionnaire‐9.
Discussion
In this large sample of women and men undergoing coronary angiography, we found that the association between depressive symptoms and CAD, as well as death/MACE, varied by sex and age. As hypothesized, women <55 years of age emerged as the group with the highest risks associated with depressive symptoms. Of additional concern, this group also had the highest burden of depression as well, with 27% having at least moderate depressive symptoms or higher.
Our study is the first to systematically address the relationship of depression with both CAD severity and cardiovascular outcomes in women and men of various age groups, and points to depressed women ≤55 years as a subgroup with substantially higher CAD comorbidity and worse prognosis. Such interaction between sex and age on the adverse cardiovascular effects of depression has rarely been investigated before. Our findings are consistent with community data among nearly 7000 US adults age 17 to 39 years where women with depression or history of attempted suicide had a significant 14‐fold adjusted HR for ischemic heart disease death; the HR in men was also significant, but much lower, at 3.5.6 Wyman et al also noted disproportionately elevated cardiovascular disease death risk in women, but not men, with depression aged 18 to 39 years (HR=6.18, 95% CI, 1.39 to 27.46).9 Furthermore, in a cohort of young Finnish adults, anxiety was found to be a stronger risk factor for cardiovascular disease in women than in men.7 As a whole, these studies suggest that young women may be especially susceptible to the cardiovascular consequences of psychosocial risk factors, especially depression.
This is the first study to show such significant sex and age differences in young women with CAD, a potentially higher risk phenotype than similarly aged men.22 Other studies with CAD patients were similarly limited by small numbers of young subjects, particularly women.23–25 Two relatively large studies of post‐MI patients found no sex interaction of depression for cardiac events or mortality, but did not examine the results by age.5,26
Among patients with CAD, data are even more limited on whether psychosocial predictors of CAD severity or outcomes differ between women and men, and almost no information is available on young populations.1,27–30 Studies of the relationship between depression and angiographically verified CAD have been generally small, conflicting, and mostly in men.31–32 Some authors have reported significant associations between depression and coronary calcium score (a non‐invasive surrogate of CAD) in young and middle‐aged women, but did not have a male comparison group.28,33
Underlying reasons for why there are sex differences in the extent to which depression predicts death and MACE in young populations are unclear. Many potential mechanisms have been proposed to explain increased risk due to depression including inflammatory, neuroendocrine, autonomic, as well as behavioral pathways.1,27–30 Few studies have focused on sex differences, however. A previous study showed that in women, but not men, there was an association between depression and a haplotype of a leukotriene gene that is involved in inflammation.34 Women may also respond physiologically to stress and depression differently than men. Early life trauma, a risk factor for depression,35 is known to exacerbate the cortisol response due to acute stress in young women,36 which over time may lead to increased obesity, dyslipidemia, and other metabolic abnormalities.37 Depression may also affect hormonal regulation specifically in premenopausal women, potentially leading to ovulatory dysfunction and decreased hormonal cardioprotection.38–39 In addition to physiologic factors, behavioral mechanisms, such as sedentary lifestyle, smoking, and medication adherence, may also help explain the risk of adverse outcomes associated with depression.40 In following from the observation in Table 1 that more women with depression had previous revascularization procedures than those without depression, we must also consider a bidirectional relationship between depression and CAD. It may be possible, for example, that young women may develop depression in reaction to the diagnosis of CAD.41
Our results have important clinical implications, as young women have significantly higher post‐MI mortality than young men.15 More attention should be paid to depression in this high‐risk group, especially because <20% of depressed post‐MI patients (in general) receive pharmacologic treatment.14 Although treatment for depression has yet to show a significant benefit in the prognosis of CAD, particularly among women,42 emerging data suggest that stress reduction customized for women could be helpful in this respect.43
Of note, we found an opposite trend for outcomes in men, such that men ≥65 years of age with depressive symptoms also had increased risk of death and MACE; similar findings were also noted by Wyman et al.9 Although the reasons for this finding are not clear, previous literature suggests that in older men, as opposed to women, depression is more likely to be the result of underlying disability than stressful life events.44–45 Table 1 also shows a generally higher burden of risk factors in depressed men ≥65 years of age compared with depressed women ≤55 years of age, most notably in terms of history of PCI (55% versus 35%), CABG (42% versus 15%), and stroke (22% versus 11%). Therefore, not only may the etiology of depression differ in these 2 demographic groups, but also the increased risk of death in depressed older men may be, in part, secondary to unmeasured confounders related to frailty and disability.
Our study has a number of limitations worth noting. Since this is a single‐center study of patients referred for coronary angiography, our findings may be subject to selection bias and may not be generalizable to other centers or the general population. Nonetheless, it has the advantages of carefully measured and validated CAD severity assessment based on angiographic assessments, detailed information on previous medical history, and prospective outcome data, as well as a relatively large number of young women. Women with both CAD and depression may be over‐represented in our database due to referral differences, although whether referral bias might have occurred based on both sex and depression status is debatable. The associations found with CAD severity were cross‐sectional, and thus direction of causality with depression cannot be ascertained. Hospitalization outcomes, which were included as a component of MACE, could not be validated in the 20% of cases that occurred outside of Emory; because of this, this outcome was analyzed as a secondary endpoint. Finally, unmeasured confounders may exist in the relationship between depression and CAD/adverse events.
In conclusion, young women undergoing cardiac catheterization with depression are more likely to have CAD, and are at higher risk of cardiovascular events and death than men or older women. These findings stress the need for more research on CAD and psychosocial factors, particularly depression, in young women, who are often under‐represented in clinical studies of cardiovascular disease. Because ischemic heart disease is the most common cause of death in women, reducing the risk and/or consequences of depression in young women may have significant public health impact.
Sources of Funding
This work was supported by the National Institutes of Health (2K24 HL077506, KL2 TR000455, 2R01 HL68630, R01 AG026255, R01 HL109413, P01 HL101398, and R21 HL093665‐01A1S1); and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR000454.
Disclosures
None.
Acknowledgments
The authors thank the members of the Emory Cardiovascular Biobank Team, Emory Clinical Cardiovascular Research Institute, the Emory Program in Cardiovascular Outcomes Research and Epidemiology, Atlanta Veterans Affairs Medical Center, and the Atlanta Clinical and Translational Science Institute for recruitment of participants and compilation of data.
References
- 1.Rozanski A, Gransar H, Kubzansky LD, Wong N, Shaw L, Miranda‐Peats R, Thomson LE, Hayes SW, Friedman JD, Berman DS. Do psychological risk factors predict the presence of coronary atherosclerosis? Psychosom Med. 2011; 73:7-15. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta‐analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006; 27:2763-2774. [DOI] [PubMed] [Google Scholar]
- 3.Barth J, Schumacher M, Herrmann‐Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta‐analysis. Psychosom Med. 2004; 66:802-813. [DOI] [PubMed] [Google Scholar]
- 4.Lane D, Carroll D, Ring C, Beevers DG, Lip GY. In‐hospital symptoms of depression do not predict mortality 3 years after myocardial infarction. Int J Epidemiol. 2002; 31:1179-1182. [DOI] [PubMed] [Google Scholar]
- 5.Frasure‐Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one‐year prognosis after myocardial infarction. Psychosom Med. 1999; 61:26-37. [DOI] [PubMed] [Google Scholar]
- 6.Shah AJ, Veledar E, Hong Y, Bremner JD, Vaccarino V. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch Gen Psychiatry. 2011; 68:1135-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabi H, Hall M, Koskenvuo M, Singh‐Manoux A, Oksanen T, Suominen S, Kivimaki M, Vahtera J. Psychological and somatic symptoms of anxiety and risk of coronary heart disease: the health and social support prospective cohort study. Biol Psychiatry. 2010; 67:378-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korkeila J, Vahtera J, Korkeila K, Kivimaki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010; 96:298-303. [DOI] [PubMed] [Google Scholar]
- 9.Wyman L, Crum RM, Celentano D. Depressed mood and cause‐specific mortality: a 40‐year general community assessment. Ann Epidemiol. 2012; 22:638-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007; 50:2128-2132. [DOI] [PubMed] [Google Scholar]
- 11.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd‐Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel‐Smoller S, Hong Y. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007; 115:e69-e171. [DOI] [PubMed] [Google Scholar]
- 12.Kim ES, Carrigan TP, Menon V. Enrollment of women in National Heart, Lung, and Blood Institute‐funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J Am Coll Cardiol. 2008; 52:672-673. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005; 62:593-602. [DOI] [PubMed] [Google Scholar]
- 14.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med. 2006; 166:876-883. [DOI] [PubMed] [Google Scholar]
- 15.Champney KP, Frederick PD, Bueno H, Parashar S, Foody J, Merz CN, Canto JG, Lichtman JH, Vaccarino V. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart. 2009; 95:895-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedkoff LJ, Briffa TG, Preen DB, Sanfilippo FM, Hung J, Ridout SC, Knuiman M, Hobbs M. Age‐ and sex‐specific trends in the incidence of hospitalized acute coronary syndromes in Western Australia. Circ Cardiovasc Qual Outcomes. 2011; 4:557-564. [DOI] [PubMed] [Google Scholar]
- 17.Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel C, Nanjundappa RA, Sikora S, Malayter D, Wilson PW, Sperling L, Quyyumi AA, Epstein SE. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J Am Coll Cardiol. 2013; 62:329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self‐report version of PRIME‐MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999; 282:1737-1744. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001; 16:606-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975; 51:5-40. [DOI] [PubMed] [Google Scholar]
- 21.Gensini GG. Coronary Arteriography. 1975Austin, TX: Futura Publishing Company [Google Scholar]
- 22.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex‐based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999; 341:217-225. [DOI] [PubMed] [Google Scholar]
- 23.Blumenthal JA, Lett HS, Babyak MA, White W, Smith PK, Mark DB, Jones R, Mathew JP, Newman MF. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003; 362:604-609. [DOI] [PubMed] [Google Scholar]
- 24.Schleifer SJ, Macari‐Hinson MM, Coyle DA, Slater WR, Kahn M, Gorlin R, Zucker HD. The nature and course of depression following myocardial infarction. Arch Intern Med. 1989; 149:1785-1789. [PubMed] [Google Scholar]
- 25.Lauzon C, Beck CA, Huynh T, Dion D, Racine N, Carignan S, Diodati JG, Charbonneau F, Dupuis R, Pilote L. Depression and prognosis following hospital admission because of acute myocardial infarction. CMAJ. 2003; 168:547-552. [PMC free article] [PubMed] [Google Scholar]
- 26.Parashar S, Rumsfeld JS, Reid KJ, Buchanan D, Dawood N, Khizer S, Lichtman J, Vaccarino V. Impact of depression on sex differences in outcome after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009; 2:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutledge T, Linke SE, Krantz DS, Johnson BD, Bittner V, Eastwood JA, Eteiba W, Pepine CJ, Vaccarino V, Francis J, Vido DA, Merz CN. Comorbid depression and anxiety symptoms as predictors of cardiovascular events: results from the NHLBI‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Psychosom Med. 2009; 71:958-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews KA, Chang YF, Sutton‐Tyrrell K, Edmundowicz D, Bromberger JT. Recurrent major depression predicts progression of coronary calcification in healthy women: Study of Women's Health Across the Nation. Psychosom Med. 2010; 72:742-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diez Roux AV, Ranjit N, Powell L, Jackson S, Lewis TT, Shea S, Wu C. Psychosocial factors and coronary calcium in adults without clinical cardiovascular disease. Ann Intern Med. 2006; 144:822-831. [DOI] [PubMed] [Google Scholar]
- 30.Tiemeier H, van Dijck W, Hofman A, Witteman JC, Stijnen T, Breteler MM. Relationship between atherosclerosis and late‐life depression: the Rotterdam Study. Arch Gen Psychiatry. 2004; 61:369-376. [DOI] [PubMed] [Google Scholar]
- 31.Zyzanski SJ, Jenkins CD, Ryan TJ, Flessas A, Everist M. Psychological correlates of coronary angiographic findings. Arch Intern Med. 1976; 136:1234-1237. [PubMed] [Google Scholar]
- 32.Tennant CC, Langeluddecke PM. Psychological correlates of coronary heart disease. Psychol Med. 1985; 15:581-588. [DOI] [PubMed] [Google Scholar]
- 33.Agatisa PK, Matthews KA, Bromberger JT, Edmundowicz D, Chang YF, Sutton‐Tyrrell K. Coronary and aortic calcification in women with a history of major depression. Arch Intern Med. 2005; 165:1229-1236. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Quyyumi AA, Patel R, Zafari AM, Veledar E, Onufrak S, Shallenberger LH, Jones L, Vaccarino V. Sex‐specific association of depression and a haplotype in leukotriene A4 hydrolase gene. Psychosom Med. 2009; 71:691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briere J, Elliott DM. Prevalence and psychological sequelae of self‐reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003; 27:1205-1222. [DOI] [PubMed] [Google Scholar]
- 36.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary‐adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000; 284:592-597. [DOI] [PubMed] [Google Scholar]
- 37.Bjorntorp P. Neuroendocrine abnormalities in human obesity. Metabolism. 1995; 44:38-41. [DOI] [PubMed] [Google Scholar]
- 38.Amsterdam JD, Winokur A, Lucki I, Snyder P. Neuroendocrine regulation in depressed postmenopausal women and healthy subjects. Acta Psychiatr Scand. 1983; 67:43-49. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan JR, Manuck SB. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause. 2008; 15:768-776. [DOI] [PubMed] [Google Scholar]
- 40.Ye S, Muntner P, Shimbo D, Judd SE, Richman J, Davidson KW, Safford MM. Behavioral mechanisms, elevated depressive symptoms, and the risk for myocardial infarction or death in individuals with coronary heart disease: the REGARDS (Reason for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol. 2013; 61:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaCharity LA. The experiences of younger women with coronary artery disease. J Womens Health Gend Based Med. 1999; 8:773-785. [DOI] [PubMed] [Google Scholar]
- 42.Linden W, Phillips MJ, Leclerc J. Psychological treatment of cardiac patients: a meta‐analysis. Eur Heart J. 2007; 28:2972-2984. [DOI] [PubMed] [Google Scholar]
- 43.Orth‐Gomer K, Schneiderman N, Wang HX, Walldin C, Blom M, Jernberg T. Stress reduction prolongs life in women with coronary disease: the Stockholm Women's Intervention Trial for Coronary Heart Disease (SWITCHD). Circ Cardiovasc Qual Outcomes. 2009; 2:25-32. [DOI] [PubMed] [Google Scholar]
- 44.Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry. 1993; 150:1139-1148. [DOI] [PubMed] [Google Scholar]
- 45.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta‐analysis. Am J Psychiatry. 2003; 160:1147-1156. [DOI] [PubMed] [Google Scholar]


