Abstract
Background
We evaluated temporal trends in ischemic stroke and warfarin use among demographic subsets of the US Medicare population that are not well represented in randomized trials of warfarin for stroke prevention in nonvalvular atrial fibrillation (AF).
Methods and Results
One‐year cohorts of Medicare–primary payer patients (1992–2010) were created using the Medicare 5% sample. International Classification of Diseases, Ninth Revision, Clinical Modification codes were used to identify AF and ischemic and hemorrhagic stroke; ≥3 consecutive prothrombin time claims were used to identify warfarin use. Ischemic stroke rates (per 1000 patient‐years) decreased markedly from 1992 to 2010. Among women, rates decreased from 37.1 to 13.6 for ages 65 to 74 years, from 55.2 to 16.5 for ages 74 to 84, and from 66.9 to 22.9 for age ≥85; warfarin use increased 31% to 59%, 27% to 63%, and 15% to 49%, respectively. Among men, rates decreased from 33.8 to 11.7 for ages 65 to 74 years, from 49.2 to 13.8 for ages 75 to 84, and from 51.5 to 18.0 for age ≥ 85; warfarin use increased 34% to 63%, 28% to 66%, and 15% to 55%, respectively. Rates decreased from 47.0 to 14.8 for whites and 73.0 to 29.3 for blacks; warfarin use increased 27% to 61% and 19% to 52%, respectively. In all age categories, the thromboembolic risk (CHADS [congestive heart failure, hypertension, age ≥75 years, diabetes, stroke]) score was significantly higher among women (versus men) and blacks (versus whites).
Conclusions
Ischemic stroke rates among Medicare AF patients decreased significantly in all demographic subpopulations from 1992–2010, coincident with increasing warfarin use. Ischemic stroke rates remained higher and warfarin use rates remained lower for women and blacks with AF, groups whose baseline CHADS scores were higher.
Keywords: anticoagulants, arrhythmia, stroke
Introduction
Patients with atrial fibrillation (AF) have a measurable and potentially modifiable risk of future cardioembolic stroke. Studies have demonstrated therapeutic benefit and cost‐effectiveness of warfarin in reducing the hazard of stroke in patients with nonvalvular AF.1–3 In a meta‐analysis involving 29 clinical studies and 28 044 patients, judicious antithrombotic therapy with adjusted‐dose warfarin was found to reduce ischemic stroke risk by nearly 60% and mortality by nearly 25% without significant increases in hemorrhagic stroke.1 Medicare beneficiaries may sustain a 27% reduction in ischemic stroke risk with warfarin use, with marked associated reduction in expenses.2 Using Medicare claims data, we previously showed a progressive decline in ischemic stroke over 15 years that was associated with greater diffusion of warfarin,3 reflecting the real‐life translation of evidence from randomized trials into daily practice by clinicians.
However, participants enrolled in randomized trials evaluating warfarin efficacy have been confined to limited demographic subsets,4 with conspicuous underrepresentation of elderly patients, women, and minority groups. Yet, these demographic subsets represent burgeoning populations routinely determined to be candidates for thromboprophylaxis in clinical practice. The burden of AF is significantly higher in the elderly,5 but the net benefit of anticoagulation is unclear because these patients are also at higher risk of hemorrhagic complications. Ischemic stroke rates are consistently higher in women than in men with AF, leading some researchers to question the effectiveness of warfarin in reducing stroke among elderly women with AF.6 Existing literature suggests that the population‐attributable risk of AF for ischemic stroke is significantly higher among white than among nonwhite populations,7 implying a higher incidence of noncardioembolic stroke among nonwhite patients with AF. This observation has led to questions about the benefit of warfarin in reducing ischemic strokes among nonwhite populations.
Thus, clinicians are increasingly faced with complex anticoagulation decisions for demographic subsets of patients in whom treatment with anticoagulation currently represents a “leap of faith” due to a lack of randomized data to support this strategy. This observational study aimed to evaluate ischemic stroke rates in demographic subsets (age, sex, and race) of US Medicare beneficiaries and to assess warfarin use for 1992–2010. Additionally, we sought to examine ischemic stroke risk among warfarin‐treated and nontreated Medicare beneficiaries with AF to better understand the contribution of warfarin to ischemic stroke reduction. These observational data reflect the diffusion of evidence‐based therapies into actual practice and the real‐life choices made by clinicians in addressing the evidence gap in extrapolation of guideline‐based recommendations to populations not adequately represented in randomized trials.
Methods
This observational study was performed using the 5% Medicare database; methodology has been previously described.3,5 The institutional review board at Hennepin County Medical Center approved the study.
We identified 1‐year cohorts of beneficiaries aged ≥65 years (excluding patients with end‐stage renal disease), for 1992–2010, with Medicare as their primary payer. Patients with ≥1 Medicare Part A inpatient or 2 outpatient or Part B claims with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for AF or atrial flutter (427.3x) during the cohort year were classified as having AF. Prevalence of AF was separately identified for each cohort year, ignoring claims from the previous year. Patients were identified as having incident AF if they were not identified as having AF in the previous cohort year. AF patients with cardiac valvular diseases identified using inpatient, outpatient, or Part B claims with an ICD‐9‐CM diagnosis code (394.x to 397.x, 424.1 to 424.4, 746.0 to 746.7) or inpatient claims with an ICD‐9‐CM procedure code (35.1, 35.2, 35.95, 35.96, 35.99) indicating valvular disease or valvular surgery were excluded. For prevalent and incident AF, an AF date was established as the date of the first AF claim during the year. Comorbid conditions were similarly identified from Medicare claims (1 inpatient or 2 outpatient/Part B).
Strokes and warfarin use were identified during the year after the AF date. Strokes were identified from inpatient Medicare claims with stroke as the principal diagnosis (ICD‐9‐CM codes 434.x, 436.x, ischemic stroke; 430.x, 431.x, hemorrhagic stroke). The usefulness and accuracy rates of ICD‐9‐CM codes for the detection of ischemic stroke and AF have been previously reported.8 The previously validated surrogate method of using ≥3 prothrombin time claims during the year after the AF date was implemented to identify warfarin‐treated patients with AF because direct information regarding warfarin prescriptions was not available.5 Patients with ≤2 prothrombin time claims were considered non–warfarin‐treated AF patients.
Patient characteristics in 1992 and 2010 were compared using χ2 tests. SEs of the prevalence and incidence of AF, and the percentage of patients using warfarin, were calculated as the square root of p×(1−p) divided by the sample size, where p represented the proportion of interest. Unadjusted stroke rates were calculated as the number of events divided by total time at risk. CI values for stroke rates were obtained by first calculating exact upper and lower confidence limits of the number of strokes assuming a Poisson distribution,9 which were then divided by the total time at risk. An estimation of thromboembolic risk was further calculated based on the CHADS score (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke), established from Medicare claims data during the 1 year before the date of AF diagnosis. Generalized logit models were fit to CHADS scores in 3 risk categories (low, 0; medium, 1 or 2; high, ≥3) for patients aged<75 years and ≥75 years within each cohort year. The associations between risk level and demographic characteristics (using sex and race as explanatory variables) were investigated.
Results
A consistent surge in AF prevalence in the Medicare population was evident; prevalence more than doubled over 18 years, from 40 255 (3.2%) in 1992 to 80 314 (7.6%) in 2010. This occurred for all age groups (Figure 1A), but the absolute increase was most marked in patients aged ≥85 years (6.6%, 14.6%) and less marked in patients aged 65 to 74 years (2.1%, 4.5%). Incident AF remained relatively unchanged: 22 191 (2%) in 1992 to 19 918 (2.2%) in 2010 (Figure 1B); incident AF was also steady across the age strata studied (about 1.5%, 2.5%, and 4.0% for patients aged 65 to 74, 75 to 84, and ≥85 years, respectively). Of note, a higher proportion of men than women were diagnosed with prevalent AF throughout the study period (3.7% versus 3.0% in 1992; 8.8% versus 6.7% in 2010). Similarly, a higher proportion of white than black patients had prevalent AF (3.4% versus 1.8% in 1992; 8.1% versus 4.0% in 2010). Rates of incident AF were also higher among men vs. women (2.3% versus 1.8% in 1993; 2.7% versus 2.0% in 2010) and whites vs. blacks (2.1% versus 1.3% in 1993; 2.4% versus 1.6% in 2010). P<0.0001 for all comparisons.
Figure 1.
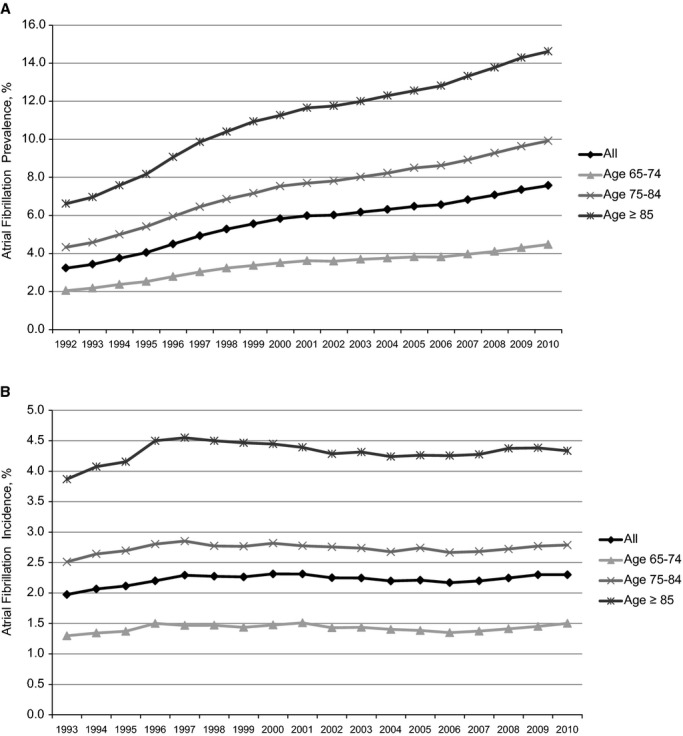
Age‐specific rates of prevalent (A) and incident (B) atrial fibrillation in the 5% Medicare cohort. SEs of incidence and prevalence are <0.1% for every estimate and therefore are not displayed in the figure.
The demographic profiles of the 1992 and 2010 AF cohorts are presented in the 1. Patients aged 75 to 84 years constituted the highest proportions in both cohorts (43.5%, 42.8%). Importantly, the proportion of patients aged ≥85 years increased from 20% to 25% between 1992 and 2010. Women (55.3%, 50.9%) and whites (94.1%, 93.0%) were majority groups. Prevalence of some conditions, such as atherosclerotic heart disease (44.4%, 41.0%) and congestive heart failure (38.1%, 30.3%), decreased. However, prevalence of other conditions, such as anemia (15.7%, 26.2%), chronic kidney disease (4.6%, 17.5%), hypertension (46.4%, 80.3%), and peripheral vascular disease (18.1%, 23.6%) increased noticeably. P<0.0001 for all comparisons.
Table 1.
Demographics of US Medicare Beneficiaries With Prevalent Atrial Fibrillation, 1992 and 2010
| Characteristics | 1992 | 2010 |
|---|---|---|
| n | 40 255 | 80 314 |
| Age 65 to 74 years | 14 747 (36.6) | 25 798 (32.1) |
| Age 75 to 84 years | 17 511 (43.5) | 34 397 (42.8) |
| Age ≥85 years | 7997 (19.9) | 20 119 (25.1) |
| Male | 18 004 (44.7) | 39 435 (49.1) |
| White | 37 862 (94.1) | 74 660 (93.0) |
| Black | 1637 (4.1) | 3265 (4.1) |
| Other race | 756 (1.9) | 2389 (3.0) |
| Diabetes | 8010 (19.9) | 25 833 (32.2) |
| Atherosclerotic heart disease | 17 888 (44.4) | 32 956 (41.0) |
| Chronic kidney disease | 1857 (4.6) | 14 037 (17.5) |
| Hypertension | 18 696 (46.4) | 64 521 (80.3) |
| Congestive heart failure | 15 320 (38.1) | 24 333 (30.3) |
| Anemia | 6314 (15.7) | 21 069 (26.2) |
| Peripheral vascular disease | 7275 (18.1) | 18 961 (23.6) |
Values are n (%) unless otherwise indicated.
Ischemic stroke rates in prevalent AF patients progressively declined, as previously described,3 and the trend appeared to plateau toward the end of the study period. Ischemic stroke rates decreased for men (42.5 to 13.7 per 1000 patient‐years), and warfarin use increased (29% to 63%), between 1992 and 2010 (Figure 2A). Among men aged 65 to 74 years, the ischemic stroke rate decreased 65% from 33.8 to 11.7 per 1000 patient‐years; warfarin use increased from 34% to 63%. The baseline ischemic stroke rate for men aged 75 to 84 years was higher; it progressively decreased 72% during the study period (49.2 to 13.8 per 1000 patient‐years), with a proportionately greater increase in warfarin use (28% to 66%). The baseline ischemic stroke rate was highest for men aged ≥85 years; it progressively decreased 65% from 51.5 to 18.0 per 1000 patient‐years, with a corresponding increase in warfarin use from 15% to 55% between 1992 and 2010.
Figure 2.
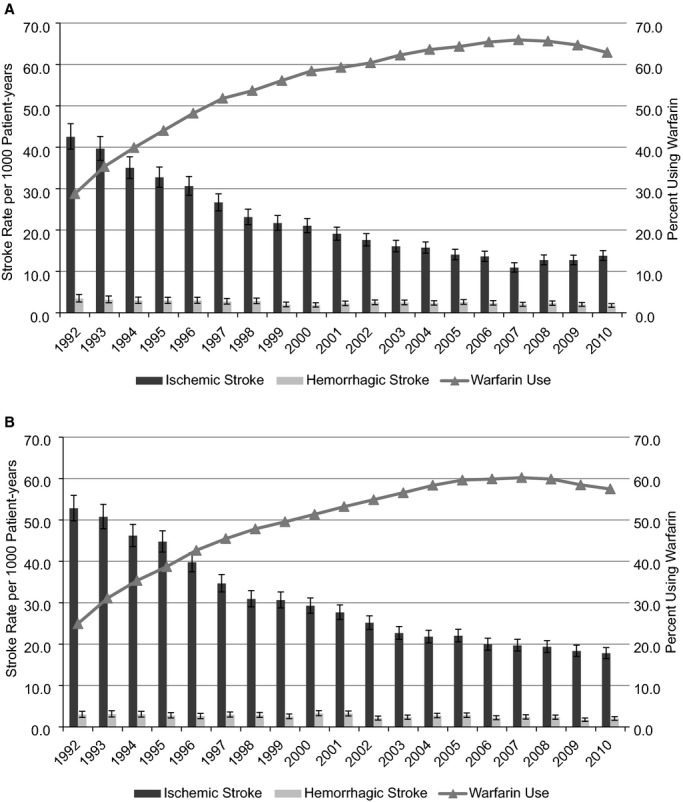
Rates of ischemic stroke, with 95% CIs, in patients with prevalent atrial fibrillation based on sex: (A) men, (B) women. CI values of the percentages using warfarin are not displayed in the figures to maintain readability; however, the SEEs were <0.5% for every estimate.
Ischemic stroke risk among women with AF was progressively higher with advancing age; however, the ischemic stroke rate decreased markedly in all age categories (Figure 2B). Among women aged 65 to 74 years, the ischemic stroke rate decreased 63% from 37.1 per 1000 patients‐years in 1992 to 13.6 in 2010, coincident with increase in warfarin use from 31% to 59%. Among women aged 75 to 84 years, the ischemic stroke rate decreased 70% from 55.2 per 1000 patients‐years to 16.5 in 2010; warfarin use increased from 27% to 63%. Among women aged ≥ 85 years, the ischemic stroke rate decreased 66% from 66.9 per 1000 patient‐years to 22.9; warfarin use increased from 15% to 49%. Importantly, the rate of hemorrhagic stroke in women was not significantly increased in any age category.
Among white patients, ischemic stroke rates decreased 69% from 47.0 to 14.8 per 1000 patient‐years between 1992 and 2010 (Figure 3A), correlated with increased warfarin use from 27% to 61%. Ischemic stroke rates among black patients were the highest among all the demographic subgroups studied; rates per 1000 patient‐years were 73.0 in 1992, peaked at 86.7 in 1994, and gradually decreased 60% (from 1992) to 29.3 in 2010 (Figure 3B). Warfarin use was lowest among black patients, 19% in 1992 and increasing to 52% in 2010. Among ethnic groups other than white and black, ischemic stroke rates decreased from 51.7 per 1000 patient‐years in 1992 to 29.7 in 2010; these rates varied considerably over the study period, reflecting lower numbers (Figure 3C). Warfarin use showed an increasing trend from 19% in 1992 to 49% in 2010. Hemorrhagic stroke risk was similar among the ethnic subgroups studied. Of note, black patients and members of other races represented small proportions of the Medicare population (4% and 3%, respectively, in 2010).
Figure 3.
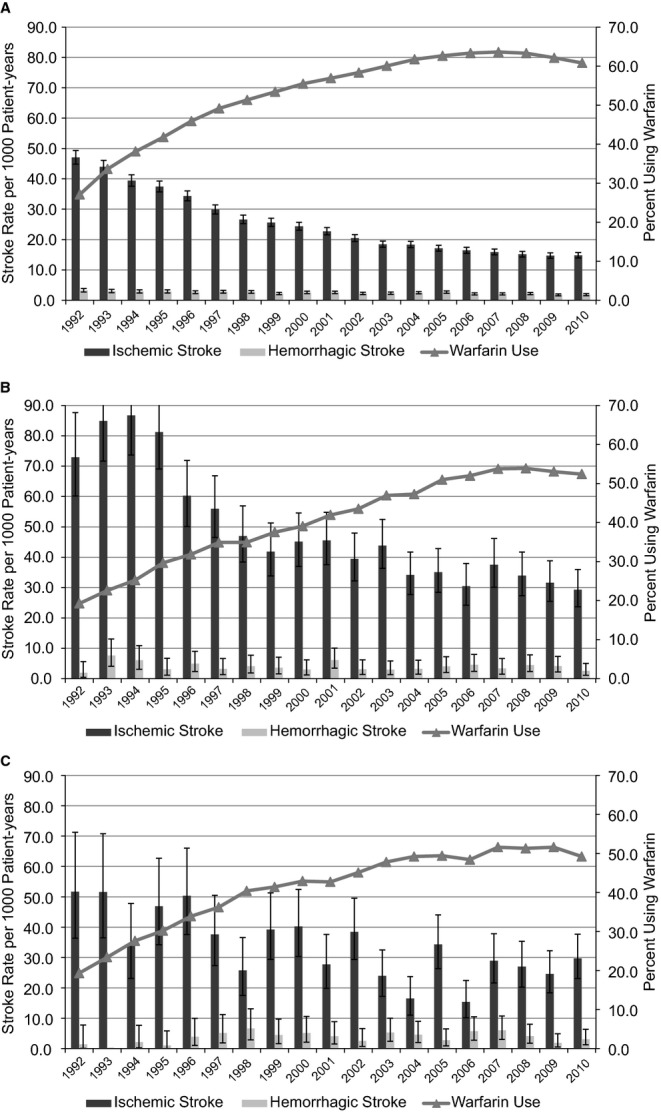
Rates of ischemic stroke, with 95% CIs, in patients with prevalent atrial fibrillation based on race: (A) white, (B) black, (C) other. CI values of the percentages using warfarin are not displayed in the figures to maintain readability; however, the SEEs ranged from 0.18% to 0.22% for white patients, 0.87% to 1.01% for black patients, and 1.02% to 1.52% for patients of other race.
We further calculated ischemic stroke rates in warfarin‐treated AF patients after warfarin initiation (with time at risk between AF diagnosis and start of warfarin therapy excluded, resulting in lower stroke rates than shown in Figures 2 and 3), and compared them with rates for non–warfarin‐treated AF patients. As expected, the ischemic stroke rate substantially decreased in warfarin‐treated patients (29 to 9 per 1000 patient‐years, 1992–2009; Figure 4). Interestingly, the ischemic stroke rate (although higher than in warfarin‐treated patients) also decreased in non‐warfarin‐treated patients (36 to 18 per 1000 patient‐years, 1992–2009), likely reflecting background trends in improved cardiovascular care.
Figure 4.
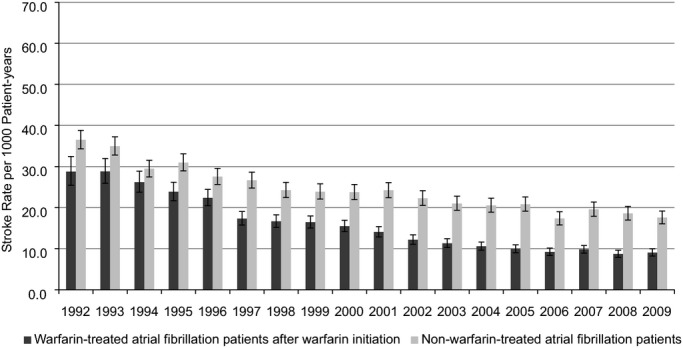
Rates of ischemic stroke, with 95% CIs, in patients with prevalent atrial fibrillation after starting warfarin therapy relative to patients not using warfarin.
When we evaluated the association between thromboembolic risk categories (based on CHADS score) and demographics, we found that in general, women and black patients were more likely to be in higher CHADS risk categories than were men and white patients. Specifically, based on the above modeling, for patients aged younger than 75 years, the adjusted odds ratio of being in a higher CHADS risk category was 1.05 to 1.50 times higher for women (versus men) and 1.5 to 5.5 times higher for black (versus white) patients. Among patients aged ≥75 years, corresponding adjusted odds ratios ranged from 1.15 to 1.35 for women and 1.9 to 2.2 for black patients.
Discussion
Randomized trials demonstrating the benefit of warfarin for stroke prevention in patients with nonvalvular AF were first published about 2 decades ago.10–12 Our study data provide a broad overview of ischemic stroke trends among various demographic subcategories in the Medicare population, and of warfarin use over nearly 2 decades. These real‐life epidemiological data reflect practice patterns in the United States, particularly in demographic subsets that have not been well represented in randomized trials. The study demonstrates an increase in baseline AF prevalence relative to prior studies,5,13 with relatively constant incidence rates,14 likely indicating improved longevity of patients with AF. Some important observations pertain to prevalent AF, ischemic stroke rates, and warfarin use among women, the elderly, and minority patients.
The adjusted hazard of ischemic stroke has consistently been demonstrated to be higher in women with AF than in men; the risk is especially higher with increasing age and consistent across all strata of thromboembolic risk factors.6,15–16 Even in the absence of other traditional thromboembolic risk factors, risk of stroke is higher for women with AF than for their male counterparts, but this difference has not been found to be consistently statistically significant.15,17 Our study results provide corroborative evidence that despite lower incidence/prevalence of AF compared with men, ischemic stroke rates are consistently higher in women. A pathobiological rationale for the increased hazard of ischemic stroke in women remains elusive; various explanations including hormonal factors and differences in hemodynamics between sexes have been postulated.18 Using a large population‐based cohort from Quebec, Avgil Tsadok et al6 found that although the hazard of stroke was significantly higher in women (14% higher than in men), prescription warfarin use was similar for the 2 groups. This observation led to lack of confidence in the effectiveness of warfarin in reducing stroke among elderly women with AF and to conjecture and debate as to whether newer anticoagulants may be more effective.6,18
Our study of ischemic stroke rates in US Medicare beneficiaries directly addresses this controversy. Of note, based on supplementary analysis, we identified a significantly higher thromboembolic (CHADS) score for women regardless of age, providing a plausible explanation for higher ischemic stroke rates among women relative to men. It is reassuring, however, that the proportional decline in ischemic stroke rates was similar for men and women, with the greatest reduction among patients aged 65 to 74 years. Nevertheless, this study raises concern about a “gender gap” in prescription of warfarin among women, despite a paradoxically higher hazard of ischemic stroke. We identified higher warfarin use among men than among women in each age category studied, especially in the most elderly subgroups. Various clinical factors could contribute to lower warfarin use rates among women, such as higher prevalence of clinical contraindications or higher perceived risk of bleeding complications. This area deserves further investigation.
Patients in all 3 age categories (65 to 74, 75 to 84, ≥85 years) experienced similar reductions in ischemic stroke rates (≈65% to 70%) between 1992 and 2010 (Figure 5A through 5C); moreover, the reduction in ischemic stroke rates was associated with a striking increase in warfarin use. The proportional increase in warfarin use over the study period was higher for more elderly patients (aged ≥75 years, ~35% to 40%) than for patients aged 65 to 74 years (~28%), with no significant impact on the hazard of hemorrhagic stroke. We found similar reductions in ischemic stroke rates among women and men aged ≥85 years with AF, without accompanying elevation in hemorrhagic stroke, although warfarin use was higher among men.
Figure 5.
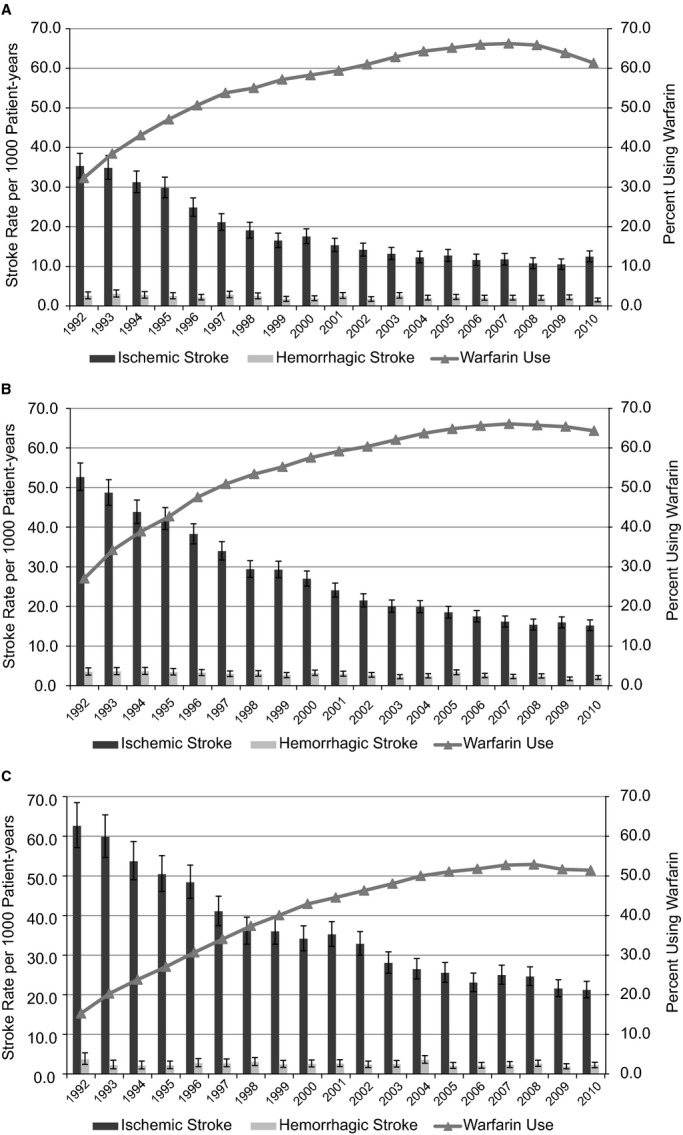
Rates of ischemic stroke, with 95% CIs, in patients with prevalent atrial fibrillation based on age: (A) 65 to 74 years, (B) 75 to 84 years, (C) ≥85 years. CI values of the percentages using warfarin are not displayed in the figures to maintain readability; however, the SEEs were <0.5% for every estimate.
The limited literature pertaining to efficacy of anticoagulation in octogenarians supports its use in well‐selected patients with AF. Mant et al19performed a randomized trial of 973 AF patients aged >75 years, demonstrating a significant reduction in fatal/disabling stroke with warfarin use compared with aspirin. In a single‐center study of 269 patients aged >80 years with nonvalvular AF, oral anticoagulation use was identified as the sole independent predictor of reduced embolic events and was associated with improved survival.20 Our data suggest increasing comfort among clinicians in prescribing warfarin to well‐selected elderly patients during the past 18 years, despite lack of systematic data from large randomized trials.
Although prevalence/incidence of AF was consistently lower than in white patients, ischemic stroke rates were consistently higher among black patients throughout the study period (Figure 3). Significantly higher ischemic stroke rates have been previously reported among Medicare beneficiaries of nonwhite ethnicities.21 Similarly, Shen et al22 reported significantly higher adjusted ischemic stroke rates among blacks (59% higher) and Hispanics (24% higher) compared with whites. This paradox between ischemic stroke rates in relation to prevalent/incident AF rates among black patients likely has multiple contributory mechanisms. Baseline disparities in risk factor control, with a higher contribution of usual risk factors toward AF burden,23 particularly hypertension24 and socioeconomic strata,25 may significantly influence ischemic stroke rates.
Lower population‐attributable risk of AF for ischemic stroke among nonwhite populations7 implies a higher incidence of noncardioembolic stroke, leading to questions regarding the benefit of warfarin in reducing ischemic strokes in these populations. Although limited by modest numbers in minority populations, Shen et al22 found no significant association between warfarin use and ischemic stroke rates among black and Hispanic patients, although the time in therapeutic range was lower for these groups. Similarly, among Medicare beneficiaries (circa 1998–1999), warfarin was considered less effective among black and Hispanic patients with AF relative to white patients.21 Additionally, it has been reported that nonwhite Medicare patients are less likely than white patients to be prescribed warfarin and less likely to undergo regular international normalization ratio monitoring.21
In this analysis, we noted that thromboembolic risk (CHADS) scores were consistently higher among black patients relative to white patients. The adjusted odds ratios for higher CHADS scores were much higher among patients aged younger than 75 years but consistent across all age‐groups. This observation provides a plausible explanation for higher ischemic stroke rates among black patients. Furthermore, we found that warfarin use among racial minorities varied significantly but increased consistently over time. Despite the highest observed ischemic stroke rates among black patients, the percentage using warfarin was lowest among all groups studied, albeit increasing over time. Although no firm conclusions can be derived from these data, one possible theory based on existing literature could be that inadequate risk factor control, particularly untreated hypertension, could dissuade clinicians from using warfarin due to safety concerns. Because we used a surrogate method to determine warfarin use, we cannot feasibly differentiate whether the lower warfarin use reflects clinical contraindications, lack of reliable international normalization ratio checks, or the perception among clinicians that stroke in this population is likely noncardioembolic. Because of high ischemic stroke rates in this population, this topic deserves further investigation.
Importantly, these observational data suggest 2 possibly synergistic mechanisms responsible for reduced ischemic stroke rates over the study period. While causality cannot be established with observational data, diffusion of warfarin into clinical practice likely contributed substantially to reductions in ischemic stroke. However, additional analysis (Figure 4) also demonstrated a steady reduction in ischemic stroke rates among non–warfarin‐treated AF patients, strongly suggesting alternative mechanisms contributing to ischemic stroke reduction, which likely reflect better control of modifiable atherosclerotic cardiovascular risk factors, particularly hypertension, diabetes, dyslipidemia, and cigarette smoking.26 A significant proportion of strokes in AF patients may not be cardioembolic in nature.27 This observation may be particularly important among black patients with AF, whose ischemic stroke rates are significantly higher than in other ethnic subpopulations, and higher than would be anticipated with AF alone, suggesting noncardioembolic mechanisms.
Our study is limited in several important ways. The data source was administrative with resultant lack of clinical data such as subtypes of ischemic stroke; we were unable to differentiate cardioembolic from noncardioembolic mechanisms. As is inherent to use of administrative data, this study does not include information about the effectiveness of anticoagulation with warfarin use (ie, time in therapeutic range). We employed a previously described surrogate method to ascertain warfarin use, which could underestimate use among patients undergoing testing in alternative settings and overestimate use among patients undergoing prothrombin testing for alternative reasons (such as liver failure). Although secondary analyses indicated that women and black patients were more likely at any age to have higher CHADS risk scores than men and white patients (reflecting higher risk‐factor burden), we suggest caution in over interpreting these findings given the observational nature of the data. The prevalence of several risk factors (most notably hypertension) increased markedly during the study period, possibly representing changing definitions and ascertainment during the study period rather than actual increases in prevalence. Ischemic stroke rates were likely under‐coded earlier in the study period (due to less focus on neuroimaging techniques), suggesting that the magnitude of reduction in ischemic stroke rates over time may be higher than we report in this study. Similarly, the accuracy of ICD‐9‐CM codes for diagnosis of various clinical factors may have been lower during the early years of the study. The strength of this study is its “bird's‐eye” perspective on real‐world use of warfarin and corresponding trends in ischemic stroke rates among various demographic subcategories.
In summary, ischemic stroke rates among US Medicare beneficiaries with prevalent AF have decreased markedly over the past 2 decades, coincident with increased warfarin use. Importantly, this reduction in ischemic stroke rates is evident in high‐risk populations with AF that have not been adequately represented in randomized trials, particularly the very elderly, women, and minority populations. It is additionally reassuring that warfarin use has increased in these demographic subgroups, despite percent utilization remaining lower among women and minority patients with AF. However, notably, based on these data, a significant proportion of the Medicare population is not yet receiving warfarin therapy in the contemporary era despite evidence for its effectiveness, likely reflecting systemic issues inherent to the efficient delivery of an effective pharmacotherapeutic agent. These trends, reflecting practice patterns in the United States over the past 2 decades, indicate gradually increasing comfort of clinicians in prescribing warfarin to all demographic subsets of Medicare patients with AF, and represent real‐life extrapolation of data from randomized trials to patients with AF.
Sources of Funding
This study was supported by a research contract with Ortho‐McNeil Janssen Scientific Affairs, LLC. Before submission for peer review, the manuscript was reviewed by the sponsor. Comments were sent to the authors, who are solely responsible for the final version. The analysis, interpretation, and reporting of these data are the responsibility of the authors.
Disclosures
Drs Shroff and Solid report no conflicts of interest. Dr Herzog has ownership interest in Johnson & Johnson.
Acknowledgments
The authors thank Chronic Disease Research Group colleagues Delaney Berrini, BS, for manuscript preparation and Nan Booth, MSW, MPH, ELS, for manuscript editing.
References
- 1.Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146:857-867. [DOI] [PubMed] [Google Scholar]
- 2.Mercaldi CJ, Ciarametaro M, Hahn B, Chalissery G, Reynolds MW, Sander SD, Samsa GP, Matchar DB. Cost efficiency of anticoagulation with warfarin to prevent stroke in medicare beneficiaries with nonvalvular atrial fibrillation. Stroke. 2011; 42:112-118. [DOI] [PubMed] [Google Scholar]
- 3.Shroff GR, Solid CA, Herzog CA. Temporal trends in ischemic stroke and anticoagulation therapy among Medicare patients with atrial fibrillation: a 15‐year perspective (1992–2007). JAMA Intern Med. 2013; 173:159-160. [DOI] [PubMed] [Google Scholar]
- 4.Dogliotti A, Paolasso E, Giugliano RP. Novel oral anticoagulants in atrial fibrillation: a meta‐analysis of large, randomized, controlled trials vs warfarin. Clin Cardiol. 2013; 36:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA. Atrial fibrillation and stroke in the general medicare population: a 10‐year perspective (1992 to 2002). Stroke. 2006; 37:1969-1974. [DOI] [PubMed] [Google Scholar]
- 6.Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012; 307:1952-1958. [DOI] [PubMed] [Google Scholar]
- 7.Hajat C, Tilling K, Stewart JA, Lemic‐Stojcevic N, Wolfe CD. Ethnic differences in risk factors for ischemic stroke: a European case‐control study. Stroke. 2004; 35:1562-1567. [DOI] [PubMed] [Google Scholar]
- 8.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005; 36:1776-1781. [DOI] [PubMed] [Google Scholar]
- 9.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol. 1990; 131:373-375. [DOI] [PubMed] [Google Scholar]
- 10.Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation. 1991; 84:527-539. [DOI] [PubMed] [Google Scholar]
- 11.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo‐controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989; 1:175-179. [DOI] [PubMed] [Google Scholar]
- 12.The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low‐dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990; 323:1505-1511. [DOI] [PubMed] [Google Scholar]
- 13.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370-2375. [DOI] [PubMed] [Google Scholar]
- 14.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012; 5:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friberg L, Benson L, Rosenqvist M, Lip GY. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012; 344:e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I‐III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke. 1999; 30:1223-1229. [DOI] [PubMed] [Google Scholar]
- 17.Chao TF, Liu CJ, Chen SJ, Wang KL, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Wu TJ, Chen TJ, Tsao HM, Chen SA. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2‐VASc score of 0 or 1? Stroke. 2012; 43:2551-2555. [DOI] [PubMed] [Google Scholar]
- 18.Hart RG, Eikelboom JW, Pearce LA. Sex, stroke, and atrial fibrillation. Arch Neurol. 2012; 69:1641-1643. [DOI] [PubMed] [Google Scholar]
- 19.Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmaurice D, Lip GY, Murray E. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007; 370:493-503. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz OM, Romo E, Mesa D, Delgado M, Ibanez CL, Anguita M, Castillo JC, Arizon JM, de Suarez LJ. Outcomes and safety of antithrombotic treatment in patients aged 80 years or older with nonvalvular atrial fibrillation. Am J Cardiol. 2011; 107:1489-1493. [DOI] [PubMed] [Google Scholar]
- 21.Birman‐Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006; 37:1070-1074. [DOI] [PubMed] [Google Scholar]
- 22.Shen AY, Yao JF, Brar SS, Jorgensen MB, Wang X, Chen W. Racial/ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke. 2008; 39:2736-2743. [DOI] [PubMed] [Google Scholar]
- 23.Lipworth L, Okafor H, Mumma MT, Edwards TL, Roden DM, Blot WJ, Darbar D. Race‐specific impact of atrial fibrillation risk factors in blacks and whites in the southern community cohort study. Am J Cardiol. 2012; 110:1637-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGruder HF, Malarcher AM, Antoine TL, Greenlund KJ, Croft JB. Racial and ethnic disparities in cardiovascular risk factors among stroke survivors: United States 1999 to 2001. Stroke. 2004; 35:1557-1561. [DOI] [PubMed] [Google Scholar]
- 25.Hanchate AD, Schwamm LH, Huang W, Hylek EM. Comparison of ischemic stroke outcomes and patient and hospital characteristics by race/ethnicity and socioeconomic status. Stroke. 2013; 44:469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:517-584. [DOI] [PubMed] [Google Scholar]
- 27.Hart RG, Pearce LA, Miller VT, Anderson DC, Rothrock JF, Albers GW, Nasco E. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis. 2000; 10:39-43. [DOI] [PubMed] [Google Scholar]


