Abstract
Background
New techniques of Tissue Doppler Imaging (TDI) enable the measurement of myocardial velocities and provide information about left ventricular (LV) diastolic function. Recent studies explored the prognostic role of TDI‐derived indexes. However, these studies considered only total mortality and did not provide information on cardiovascular mortality and morbidity. Therefore, we investigated in continuous and categorical analyses whether Doppler diastolic indexes contained any prognostic information over and beyond traditional cardiovascular risk factors in a general population.
Methods and Results
We measured early and late diastolic peak velocities of mitral inflow (E and A) by conventional Doppler, and the mitral annular velocities (e' and a') by TDI in 793 participants (mean age 50.9 years). We calculated multivariable‐adjusted hazard ratios for conventional and TDI Doppler indexes, while accounting for family cluster and cardiovascular risk factors. Median follow‐up was 4.8 years (5th to 95th percentile, 3.0 to 5.4). With adjustments applied for covariables, e' velocity was a significant predictor of fatal and nonfatal cardiovascular (n=59; P=0.004) and cardiac events (n=40; P=0.001). TDI e' yielded a net reclassification improvement of 54.2% for cardiovascular and 64.0% for cardiac events. Hazard ratios of all cardiovascular (2.21; P=0.042) and cardiac (4.50; P=0.002) events were significantly elevated in participants with increased LV filling pressure compared with subjects with normal diastolic function.
Conclusions
TDI e' velocity is a significant predictor of fatal and nonfatal cardiovascular events in a general population. Furthermore, we observed an increase in all cardiovascular events in the diastolic dysfunction group characterized by elevated LV filling pressure.
Keywords: diastole, echocardiography, epidemiology, survival, tissue Doppler imaging
Introduction
Tissue Doppler Imaging (TDI) enables the measurement of myocardial velocities and provide valuable information about left ventricular (LV) diastolic function in addition to classical M‐mode, 2D echocardiography and pulsed‐wave Doppler (PWD). Impaired myocardial relaxation, an early stage of LV diastolic dysfunction, is characterized by decreased transmitral early (E peak), and enhanced atrial (A peak) LV filling as well as less vigorous mitral annulus motion (e') during early diastole. Moreover, combining early transmitral flow velocity with mitral annular velocity (E/e' ratio) reflects elevated LV filling pressure, another feature of LV diastolic dysfunction. Community‐based studies revealed a high‐prevalence (up to 34.7%) LV diastolic dysfunction using comprehensive conventional and TDI echocardiographic imaging.1–4 In the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO), the frequency was 27.3%.3
Recent clinical studies explored the prognostic role of the TDI‐derived indexes in patients with different cardiovascular diseases.5 TDI‐derived e' velocity and the E/e' ratio have independent prognostic value in patients with overt heart failure,6–9 hypertension,10–11 or myocardial infarction.12 On the other hand, population‐based studies are essential to determine the prognostic significance of subclinical LV diastolic dysfunction. To our knowledge, only 2 community‐based studies4,13 explored the predictive value of TDI velocities13 or LV diastolic dysfunction grades based on these new indexes.4 However, outcome in these studies was confined to total mortality.4,13 We, therefore, investigated in the FLEMENGHO cohort whether Doppler diastolic indexes analyzed as continuous or categorical measures contained prognostic information over and beyond traditional cardiovascular risk factors.
Methods
Study Participants
The Ethics Committee of the University of Leuven approved the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO). From August 1985 until December 2005, we identified a random population sample‐stratified by sex and age from a geographically defined area in northern Belgium.3,14 Households, defined as those who lived at the same address, were the sampling unit. We numbered households consecutively, and generated a random‐number list by use of SAS random function. Households with a number matching the list were invited; household members older than 18 years were eligible. From 2005 to 2009, we invited 1031 former participants for a re‐examination at our field center, including echocardiography (Figure 1). We obtained informed written consent from 828 subjects (participation rate, 80%). To study the incidence of mortality and morbidity in relation to baseline LV diastolic dysfunction, we invited these participants for a follow‐up examination on average 5 years after their first echocardiographic examination. For this analysis, we excluded 16 subjects, because of atrial fibrillation (n=8) or the presence of an artificial pacemaker (n=3), or because diastolic function could not be reliably determined (n=4). We additionally excluded 19 participants, because they were lost to follow‐up (Figure 1). Thus, the outcome cohort included 793 participants.
Figure 1.
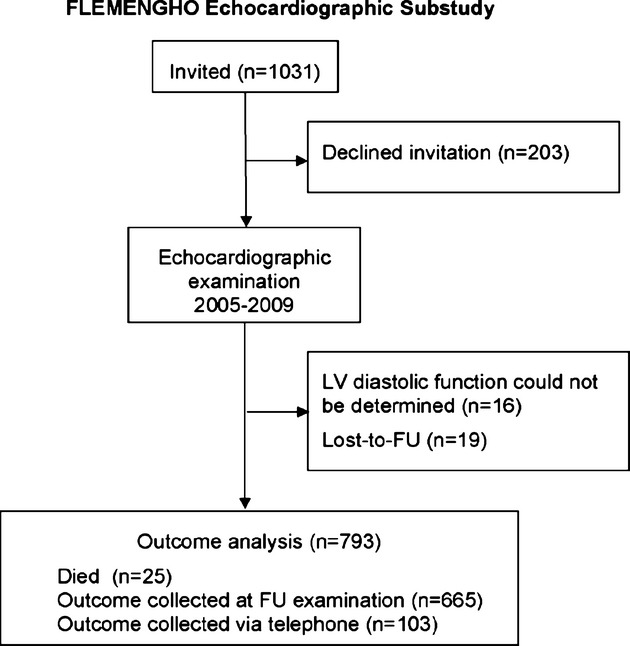
Flowchart for participants in the study. FLEMENGHO indicates Flemish Study on Environment, Genes and Health Outcomes; FU, follow‐up; LV, left ventricular.
Echocardiography
The participants refrained from smoking, heavy exercise, and drinking alcohol or caffeine‐containing beverages for at least 3 hours before echocardiography. The blood pressure during echocardiography was the average of 2 readings, obtained with a validated OMRON 705IT device (Omron Corp) at the end of the echocardiographic examination.
Data acquisition
One experienced physician (T.K.) did the ultrasound examination,3 using a Vivid7 Pro (GE Vingmed) interfaced with a 2.5‐ to 3.5‐MHz phased‐array probe, according to the recommendations of the American Society of Echocardiography.15 With the subjects in partial left decubitus and breathing normally, the observer obtained images, together with a simultaneous ECG signal, along the parasternal long and short axes and from the apical 4‐ and 2‐chamber long‐axis views. All recordings included at least 5 cardiac cycles and were digitally stored for offline analysis. M‐mode echocardiograms of the LV were recorded from the parasternal long‐axis view under control of the 2‐dimensional image. The ultrasound beam was positioned just below the mitral valve at the level of the posterior chordae tendineae. To record PWD mitral and pulmonary vein (PV) flow velocities from the apical window and the isovolumetric relaxation time (IVRT), the observer positioned the Doppler sample volume at the mitral valve tips, in the right superior PV, and between the LV outflow and mitral inflow, respectively.
Using TDI, the observer recorded low‐velocity, high‐intensity myocardial signals at a high frame rate (>190 FPS), while adjusting the imaging angle to ensure a parallel alignment of the ultrasound beam with the myocardial segment of interest. From the apical window, the sonographer placed a 5‐mm Doppler sample at the septal, lateral, inferior, and posterior sites of the mitral annulus.
Off‐line analysis
The post‐processing of echocardiograms was performed by an observer (TK) blinded to the participants' characteristics in a few weeks after the initial examination. Digitally stored images were analyzed using a workstation running the EchoPac, version 4.0.4 software package (GE Vingmed). All measurements were averaged over 3 heart cycles for statistical analysis. The LV internal diameter and interventricular septal and posterior wall thickness were measured at end‐diastole from the 2‐dimensionally guided M‐mode tracing. When optimal orientation of M‐mode ultrasound beam could not be obtained, the reader performed linear measurements on correctly oriented 2‐dimensional images. End‐diastolic LV dimensions were used to calculate LV mass by an anatomically validated formula according to the recommendations of the American Society of Echocardiography.15 We calculated LV ejection fraction (EF) from LV end‐systolic and end‐diastolic volumes measured from the apical 4‐ and 2‐chambers views, using the standard Simpson's method. We measured left atrial (LA) dimensions in 3 orthogonal planes: the parasternal long, lateral, and supero‐inferior axes. LA volume (LAVI) was calculated using the prolate‐elipsoid method and was indexed to body surface area.
From the transmitral flow signal, we measured peak early diastolic velocity (E), peak late diastolic velocity (A), the E/A ratio, and A flow duration. From the PV flow signal, we measured the duration of PV reversal time during atrial systole (AR). From the TDI recordings, we measured peaks systolic (s') and early (e') and late (a') diastolic mitral annular velocities, and the e'/a' ratio at the 4 acquisition sites (septal, lateral, inferior, and posterior). We calculated the E/e' ratio by dividing transmitral E peak by e' averaged from the 4 acquisition sites.
We combined the mitral inflow and TDI velocities to classify the stages of LV diastolic dysfunction at baseline as previously described.2–3 The first group included subjects with an abnormally low age‐specific transmitral E/A ratio indicative of impaired relaxation, but without evidence of increased LV filling pressures (E/e'≤8.5). The second group had mildly‐to‐moderately elevated LV filling pressure (E/e'>8.5), and E/A ratio within the normal age‐specific range. We also used the differences in durations between the mitral A flow and the reverse PV flow during atrial systole (Ad<ARd+10) and/or LA volume index (≥28 mL/m2) to confirm possible elevation of the LV filling pressures in group 2. Group 3 had an elevated E/e' ratio and an abnormally low age‐specific E/A ratio (combined dysfunction).
Assessment of Outcome
Outcomes were adjudicated against source documents, as described in previous publications.16 We ascertained the vital status of FLEMENGHO participants until December 31, 2012. During follow‐up, 25 participants died. We obtained the International Classification of Disease codes for the immediate and underlying cause of death.16 In all participants, we collected information on the incidence of nonfatal events via a follow‐up visit at the examination center (n=665) or a telephone interview (n=103) with repeat administration of the same standardized questionnaire used at baseline. To assess the symptoms associated with heart failure, we administered the standardized London School of Hygiene cardiovascular, dyspnea and respiratory questionnaires.17 Physician ascertained the diseases reported on the death certificates or by the questionnaires against the medical records of general practitioners or hospitals. Cardiac events included fatal and nonfatal myocardial infarction, coronary revascularization, fatal and nonfatal heart failure, new‐onset angina (stable or unstable), cor pulmonale, new‐onset atrial fibrillation and life‐threatening arrhythmias. Fatal and nonfatal cardiovascular events comprised cardiac endpoints, stroke, transient ischemic attacks, aortic aneurysm, arterial embolism, and revascularization of peripheral arteries. The diagnosis of symptomatic heart failure required the presence of symptoms or signs compatible with heart failure, such as dyspnea, peripheral edema, or pulmonary congestions. In the study participants who experienced cardiovascular events (n=59), we only considered the first event per participant.
Other Measurements
The conventional blood pressure was the average of 5 consecutive auscultatory readings obtained with the subject in the seated position. Hypertension was defined as a blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic or as the use of antihypertensive drugs. Body mass index was weight in kilograms divided by the square of height in meters. Venous blood samples were drawn for measurement of blood glucose, serum total cholesterol, and NT‐proBNP. Diabetes mellitus was determined by self‐reported diagnosis, fasting glucose level of at least 126 mg/dL, or use of antidiabetic agents. NT‐proBNP was measured in plasma samples by a competitive enzyme immunoassay for research use (Biomedica Gruppe). The standard range provided by the manufacturer of the EIA is from 0 to 1000 pmol/L (median, 208 pmol/L; 95th percentile, 300 pmol/L).
Statistical Methods
For database management and statistical analysis, we used SAS software, version 9.3 (SAS Institute). We compared means and proportions by means of a large sample z test and the χ2 statistics, respectively. Statistical significance was a 2‐sided significance level of 0.05.
In exploratory analyses, we plotted incidence rates by quartiles of the Doppler diastolic indexes at baseline, while standardizing for sex and age (age groups: <40 years; 40 to 60 years; >60 years) by the direct method. We used Cox regression to compute standardized hazard ratios, which express the risk associated with a 1‐standard deviation (SD) change in the Doppler diastolic indexes. The baseline characteristics considered as covariables in Cox regression were sex, age, body mass index, systolic blood pressure, smoking, serum cholesterol, diabetes mellitus, and a history of cardiac disease. We checked the proportional hazard assumption using the Kolmogorov‐type supremum test. Clustering of failure times within pedigrees was taken into account by fitting a sheared frailty model. In the categorical analyses, we used the Kaplan‐Meier method for estimation of cumulative incidence according to LV diastolic dysfunction group. The frailty Cox regression model was applied to calculate adjusted hazard ratios in the LV diastolic dysfunction groups using participants with normal diastolic function as the reference group.
Finally, we assessed the added ability of the selected Doppler diastolic indexes to predict fatal and nonfatal cardiovascular events, using the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI) as described by Pencina et al.18 IDI is the difference between the discrimination slopes of the basic model and the basic model extended with an echocardiographic variable. The discrimination slope is the difference in predicted probabilities (%) between subjects with and without composite endpoints. To calculate the continuous NRI,18 we predicted in each subject the 10‐year risk for the composite event from a Cox model with and without Doppler diastolic index included. Let P(up/event) the percentage of subjects with events whose predicted probability is increased by adding the echocardiographic variable to the model and P(up/nonevent) the percentage of subjects without events whose predicted probability is increased. The NRI was then calculated as 2×[P(up/event)‐P(up/nonevent)].
Results
Characteristics of Participants at Baseline
The study population consisted of 793 white Europeans. Of the participants, 405 (51.5%) were women, and 327 (41.2%) had hypertension of whom 200 (61.2%) were taking blood pressure‐lowering drugs. The mean age was 50.9 (SD 15.5) years. At baseline, 165 participants (20.8%) were current smokers. A total of 27 (3.4%) had a history of cardiac diseases and 27 (3.4%) had a history of diabetes mellitus. Six participants (0.8%) had an EF of ≤50%. Table 1 shows the baseline clinical and echocardiographic characteristics of the study participants by sex.
Table 1.
Characteristics of Participants
| Clinical Measurements | Echocardiographic Measurements | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Women (n=405) | Men (n=388) | P Value | Characteristic | Women (n=405) | Men (n=388) | P Value |
| Anthropometrics | Conventional echocardiography | ||||||
| Age, y | 51.8±15.3 | 50.0±15.7 | 0.09 | LA volume index, mL/m2 | 21.8±5.98 | 24.0±6.32 | <0.0001 |
| Height, cm | 162.5±6.9 | 175.4±7.2 | <0.0001 | LV internal diameter, cm | 4.83±0.39 | 5.25±0.52 | <0.0001 |
| Weight, kg | 69.7±13.9 | 81.9±12.1 | <0.0001 | Interventricular septum, cm | 0.93±0.15 | 1.04±0.17 | <0.0001 |
| Body mass index, kg/m2 | 26.3±4.7 | 26.6±3.71 | 0.33 | Posterior wall, cm | 0.85±0.13 | 0.95±0.14 | <0.0001 |
| Waist circumference, cm | 86.1±12.4 | 94.3±10.6 | <0.0001 | Relative wall thickness | 0.37±0.06 | 0.38±0.07 | 0.009 |
| Systolic pressure, mm Hg | 127.9±19.3 | 130.8±15.4 | 0.02 | LV mass index, g/m2 | 85.0±19.5 | 100.3±21.9 | <0.0001 |
| Diastolic pressure, mm Hg | 77.8±9.0 | 81.6±9.6 | <0.0001 | LVD volume index, mL/m2 | 48.0±8.65 | 58.2±10.7 | <0.0001 |
| Heart rate, beats/min | 62.7±9.2 | 59.1±9.8 | <0.0001 | LVS volume index, mL/m2 | 17.6±4.34 | 22.2±5.46 | <0.0001 |
| Ejection fraction, % | 64.2±6.4 | 62.4±6.8 | 0.0001 | ||||
| Questionnaire data | Doppler data | ||||||
| Current smoking, n (%) | 82 (20.3) | 83 (21.4) | 0.07 | E peak, cm/s | 78.8±15.7 | 71.8±15.8 | <0.0001 |
| Drinking alcohol, n (%) | 85 (21.0) | 230 (59.3) | <0.0001 | A peak, cm/s | 68.8±17.4 | 60.8±16.5 | <0.0001 |
| Hypertensive, n (%) | 159 (39.3) | 168 (43.3) | 0.25 | E/A ratio | 1.23±0.44 | 1.29±0.42 | 0.08 |
| Treated for hypertension, n (%) | 107 (26.4) | 93 (24.0) | 0.43 | s' peak*, cm/s | 8.63±1.37 | 9.49±1.42 | <0.0001 |
| History of CHD, n (%) | 9 (2.22) | 16 (4.12) | 0.13 | e' peak*, cm/s | 11.3±3.55 | 11.5±3.76 | 0.44 |
| History of diabetes, n (%) | 16 (3.95) | 11 (2.48) | 0.39 | a' peak*, cm/s | 9.89±1.98 | 10.3±2.19 | 0.004 |
| Biochemical data | e'/a' ratio* | 1.25±0.62 | 1.24±0.68 | 0.91 | |||
| Serum creatinine, μmol/L | 76.7±12.6 | 91.6±15.2 | <0.0001 | E/e' ratio | 7.51±2.35 | 6.65±1.88 | <0.0001 |
| Total cholesterol, mmol/L | 5.4±0.98 | 5.1±0.95 | <0.0001 | IVRT, ms | 96.6±15.9 | 98.9±17.2 | 0.06 |
| NT‐proBNP, pmol/L | 218 (106 to 488) | 192 (90 to 394) | <0.0001 | ∆(Adur‐ARdur), ms | 0.38±12.4 | 0.96±11.5 | 0.51 |
Values are mean (±SD), number of subjects (%) or geometric mean (10% to 90% percentile interval). Adur indicates mitral inflow A‐wave duration; ARdur, pulmonary vein reversal flow duration; CHD, coronary heart disease; IVRT, isovolumetric relaxation time; LA, left atrium; LV, left ventricle; LVD, left ventricular diastolic; LVS, left ventricular systolic; NT‐proBNP, amino terminal pro‐BNP.
Averaged of septum, lateral, inferior and posterior mitral annulus sites.
Incidence of Events
In the study population, the median follow‐up was 4.8 years (5th to 95th percentile, 3.0 to 5.4). During 3628 person‐years of follow‐up, 59 participants experienced either a fatal or nonfatal cardiovascular endpoint (16.3 events per 1000 person‐years). A fatal or nonfatal cardiac event occurred in 40 subjects (11.0 events per 1000 person‐years). Table 2 lists the cause‐specific cardiovascular mortality and morbidity for the study cohort. Only the first event within every category was considered in the outcome analyses.
Table 2.
Fatal and Nonfatal Cardiovascular Events
| Endpoint | Number of Events (n=793) | |
|---|---|---|
| Stroke | Fatal | 4 |
| Nonfatal | 3 | |
| Transient ischemic attack | Nonfatal | 5 |
| Myocardial infarction | Fatal | 2 |
| Nonfatal | 3 | |
| Ischemic heart disease | Fatal | 2 |
| Nonfatal | 15 | |
| Coronary revascularization | Nonfatal | 17 |
| Congestive heart failure | Fatal | 1 |
| Nonfatal | 16 | |
| Atrial fibrillation/arrhythmia | Fatal | 1 |
| Nonfatal | 6 | |
| Aortic aneurysm | Nonfatal | 2 |
| Cor pulmonale | Nonfatal | 1 |
| Arterial embolism | Fatal | 1 |
| Peripheral arterial diseases | Nonfatal | 7 |
| Total events | 86 |
Risk Associated With TDI e' Velocity and E/e' Ratio
Figure 2 shows the rates of sex‐and‐age standardized fatal and nonfatal cardiovascular endpoints across quartiles of TDI e' velocity and E/e' ratio. Table 3 shows the multivariable‐adjusted hazard ratios associated with every SD change in Doppler velocities and their ratios. With adjustments applied for family clusters, sex, age, body mass index, systolic blood pressure, serum cholesterol, current smoking, diabetes mellitus, and a history of cardiac disease, TDI e' velocity was a significant predictor of fatal and nonfatal cardiovascular (P=0.004) and cardiac events (P=0.001; Table 3). This finding was also noted in models, which were additionally adjusted for LVM index (Table 4). In adjusted models, the E/e' ratio was borderline associated with increased risk of cardiac events (P=0.05; Table 3). Because of the high intra‐correlation of TDI e' and s' velocities (r2=0.48; P<0.0001), s' velocity significantly predicted composite cardiovascular and cardiac events (P≤0.028; Table 3). However, in a Cox model including both e' and s' velocities, the s' velocity lost its prognostic significance for both cardiovascular and cardiac events (P≥0.16) whereas TDI e' velocity remained significant in predicting these events (P≤0.004). All Cox models complied with the proportional hazards assumption. None of the other Doppler diastolic variables significantly predicted combined cardiovascular events (Table 3).
Figure 2.
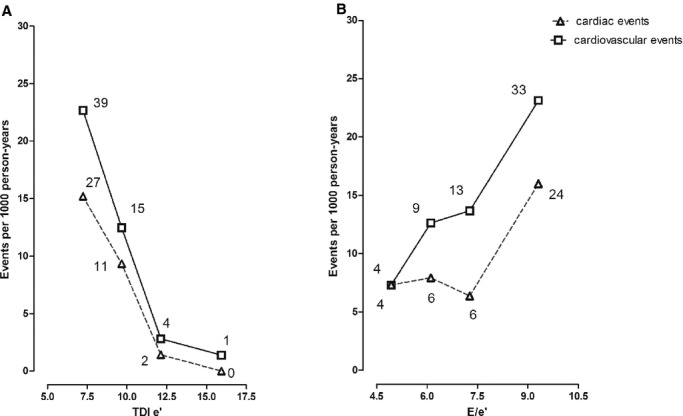
Incidence rates of fatal and nonfatal cardiovascular and cardiac events by quartiles of the distributions of the tissue Doppler imaging (TDI) e' velocity (A) and E/e' ratio (B) in 793 participants. Incidence rates were calculated as number of events per 1000 subject/year and were standardized for sex and age (age groups: <40 years; 40 to 60 years; >60 years) by the direct method. The number of endpoints contributing to the rates is shown. P values are for trend.
Table 3.
Adjusted Hazard Ratios for Fatal and Nonfatal Cardiovascular Events in Relation to LV Diastolic Doppler Indexes
| Predictor Variable | Cardiovascular Events (n=59) | Cardiac (n=40) | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Per SD decrease | ||||
| TDI e' | 2.50 (1.33 to 4.82) | 0.004 | 3.66 (1.68 to 7.76) | 0.001 |
| TDI a' | 1.20 (0.88 to 1.65) | 0.23 | 1.34 (0.94 to 1.94) | 0.10 |
| TDI e'/a' | 1.63 (0.73 to 3.71) | 0.22 | 1.75 (0.65 to 4.64) | 0.25 |
| TDI s' | 1.55 (1.04 to 2.30) | 0.028 | 1.90 (1.15 to 3.10) | 0.012 |
| Per SD increase | ||||
| E/A ratio | 0.79 (0.44 to 1.42) | 0.40 | 0.89 (0.45 to 1.75) | 0.72 |
| E/e' ratio | 1.23 (0.89 to 1.65) | 0.18 | 1.41 (1.00 to 1.98) | 0.050 |
Hazard ratios (HR) express the risk per standard deviation (SD) change in the Doppler diastolic velocities and its ratios. SDs were 3.7 cm/s, 2.10 cm/s, and 1.46 cm/s for TDI e', a', and s' velocities and 0.65, 0.50, and 2.17 for the e'/a', E/A, and E/e' ratios. All hazard ratios were adjusted for family clusters, sex, age, body mass index, systolic blood pressure, serum cholesterol, smoking, diabetes mellitus, and a history of cardiac disease. CI indicates confidence interval; LV, left ventricular; TDI, tissue Doppler imaging.
Table 4.
Hazard Ratios for Fatal and Nonfatal Cardiovascular Events in Relation to LV Diastolic Doppler Parameters Additionally Adjusted for LV Mass Index
| Predictor Variable | Cardiovascular Events (n=59) | Cardiac (n=40) | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Per SD decrease | ||||
| TDI e' | 2.35 (1.28 to 4.48) | 0.007 | 3.38 (1.52 to 7.28) | 0.002 |
| TDI a' | 1.11 (0.82 to 1.49) | 0.52 | 1.25 (0.86 to 1.79) | 0.24 |
| TDI e'/a' | 2.00 (0.79 to 3.72) | 0.17 | 1.80 (0.69 to 4.64) | 0.22 |
| Per SD increase | ||||
| E/A ratio | 0.76 (0.44 to 1.30) | 0.32 | 0.87 (0.45 to 1.66) | 0.66 |
| E/e' ratio | 1.11 (0.83 to 1.49) | 0.49 | 1.30 (0.92 to 1.83) | 0.15 |
Hazard ratios (HR) express the risk per SD change in the Doppler diastolic velocities and its ratios. SDs were 3.7 cm/s and 2.10 cm/s for TDI e' and a' velocities and 0.65, 0.50, and 2.17 for the e'/a', E/A and E/e' ratios, respectively. All hazard ratios were adjusted for family clusters, sex, age, body mass index, systolic blood pressure, serum cholesterol, smoking, diabetes mellitus, history of cardiac disease, and LV mass index. CI indicates confidence interval; LV, left ventricular; TDI, tissue Doppler imaging.
Risk Associated With LV Diastolic Dysfunction
At baseline, LV diastolic dysfunction groups 1, 2, and 3 included 76 (9.6%), 96 (12.1%), and 27 (3.4%) participants, respectively. The baseline clinical and echocardiographic characteristics of subjects by group of diastolic function appear in Tables 5 and 6, respectively. Because LV filling pressure (E/e'>8.5) was increased in both the second and third groups of LV diastolic dysfunction and because the third group represented only 3.4% of the study participants, we combined groups 2 and 3 in the categorical survival analysis.
Table 5.
Baseline Clinical Characteristics of Participants by Diastolic Function Group
| Characteristic | Normal Function (n=594) | Impaired Relaxation (n=76) | Elevated End‐Diastolic Pressure (n=96) | Combined Dysfunction (n=27) |
|---|---|---|---|---|
| Transmitral E/A ratio | N | ↓ | N | ↓ |
| E/e' ratio | N | N | ↑ | ↑ |
| Age, y | 46.5±13.8 | 58.4±14.9* | 67.3±9.4*† | 67.8±10.4*† |
| Women, n (%) | 291 (49.0) | 32 (42.1) | 62 (64.6)*† | 20 (74.1)*† |
| Body mass index, kg/m2 | 25.8±3.9 | 27.9±3.8* | 28.98±5.1* | 29.6±3.3* |
| Systolic pressure, mm Hg | 125.2±15.1 | 133.8±14.75* | 146.1±19.1*† | 148.8±17.8*† |
| Diastolic pressure, mm Hg | 79.0±9.2 | 83.2±9.2* | 79.4±9.6† | 85.5±10.5*‡ |
| Heart rate, beats/min | 60.4±9.2 | 67.3±12.1* | 58.16±8.8† | 64.1±8.6‡ |
| Questionnaire data | ||||
| Current smoking, n (%) | 131 (22.1) | 19 (25.0) | 13 (13.5)† | 2 (7.41) † |
| Drinking alcohol, n (%) | 261 (43.9) | 26 (34.2) | 24 (25.0)* | 4 (14.8)*† |
| Hypertensive, n (%) | 176 (29.6) | 51 (67.1)* | 77 (80.2)*† | 23 (85.2)* |
| Treated for hypertension, n (%) | 95 (16.0) | 36 (47.4)* | 53 (55.2)* | 16 (59.3)* |
| Beta‐blockers, n (%) | 53 (8.9) | 19 (25.0)* | 35 (36.5)* | 9 (33.3)* |
| ACE or ARB, n (%) | 30 (5.1) | 14 (18.4)* | 16 (16.7)* | 6 (22.2)* |
| Diuretics, n (%) | 31 (5.2) | 13 (17.1)* | 26 (27.1)* | 6 (22.2)* |
| CCB, n (%) | 14 (2.4) | 5 (6.6)* | 10 (10.4)* | 4 (14.8)* |
| History of CHD, n (%) | 7 (1.2) | 2 (2.6) | 11 (11.5)*† | 5 (18.5)*† |
| History of diabetes, n (%) | 13 (2.2) | 3 (3.95) | 6 (6.25)* | 5 (18.5)*†‡ |
| Biochemical data | ||||
| NT‐proBNP, pmol/L | 194 (92 to 398) | 237 (115 to 456) | 274 (136 to 582)* | 240 (73 to 509) |
| Serum creatinine, μmol/L | 82.7±13.47 | 89.6±27.97* | 86.7±15.2 | 85.4±13.9 |
| Total cholesterol, mmol/L | 5.2±0.96 | 5.4±0.90 | 5.6±1.0* | 5.5±1.0 |
Values are mean (±SD), geometric mean (10% to 90% percentile), or number of subjects (%). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blockers; CCB, calcium channel blockers; CHD, coronary heart disease; NT‐proBNP, amino terminal pro‐BNP.
Significance for between‐groups differences: *P≤0.05 vs normal; †P≤0.05 vs impaired relaxation group; ‡P≤0.05 vs elevated end‐diastolic pressure group.
Table 6.
Baseline Echocardiographic Characteristics of Participants by Diastolic Function Group
| Characteristic | Normal Function (n=594) | Impaired Relaxation (n=76) | Elevated End‐Diastolic Pressure (n=96) | Combined Dysfunction (n=27) |
|---|---|---|---|---|
| Transmitral E/A ratio | N | ↓ | N | ↓ |
| E/e' ratio | N | N | ↑ | ↑ |
| Conventional echocardiography | ||||
| LA volume index, mL/m2 | 21.8±5.3 | 27.1±6.6 | 28.4±7.4*† | 27.5±6.8*† |
| LV internal diameter, cm | 5.02±0.49 | 5.09±0.58 | 5.07±0.56 | 4.85±0.49 |
| Interventricular septum, cm | 0.95±0.16 | 1.03±0.17* | 1.07±0.15* | 1.18±0.18*†‡ |
| Posterior wall, cm | 0.87±0.14 | 0.94±0.14* | 0.97±0.13* | 1.04±0.15*† |
| LV mass index, g/m2 | 88.4±19.3 | 100.1±32.9* | 105.8±25.7* | 115.2±22.2*† |
| Ejection fraction, % | 63.4±6.2 | 61.8±9.0 | 64.0±7.1 | 64.0±6.9 |
| Transmitral Doppler | ||||
| E peak, cm/s | 77.7±14.8 | 54.7±10.2* | 80.8±13.7† | 62.2±14.0*‡ |
| A peak, cm/s | 59.2±13.9 | 76.8±12.4* | 82.3±15.5* | 95.4±16.9*†‡ |
| E/A ratio | 1.39±0.46 | 0.73±0.17* | 1.01±0.23*† | 0.65±0.10*‡ |
| IVRT, ms | 94.6±14.6 | 110.1±19.3* | 105.1±16.4* | 109.5±21.5* |
| Adur‐ARdur, ms | 1.43 (−4.3 to 7.1) | 11.4 (−2.8 to 28.5)* | −18.5 (−37.1 to 5.7)*† | 16.2 (−17.1 to 28.5)*‡ |
| Tissue Doppler§ | ||||
| e' peak, cm/s | 12.6±3.26 | 8.50±2.14* | 7.69±1.30* | 5.75±1.14*†‡ |
| a' peak, cm/s | 9.80±2.06 | 11.9±1.90* | 10.4±1.82†* | 11.4±1.24* |
| e'/a' ratio | 1.42±0.65 | 0.75±0.30* | 0.78±0.20* | 0.50±0.10* |
| E/e' ratio | 6.39±1.35 | 6.63±1.19 | 10.7±2.11*† | 11.0±2.45*† |
Values are mean (±SD), or median (10% to 90% percentile). Adur indicates mitral inflow A‐wave duration; ARdur, pulmonary vein reversal flow duration; IVRT, isovolumetric relaxation time; LA, left atrial; LV, left ventricle.
Significance for between‐groups differences: *P≤0.05 vs normal; †P≤0.05 vs impaired relaxation group; ‡P≤0.05 vs elevated LV filling pressure group.
§Averaged of septum, lateral, inferior, and posterior mitral annulus sites.
Figure 3 shows cumulative incidence estimates (1‐Kaplan‐Meier survival estimates) for fatal and nonfatal cardiovascular events by group of LV diastolic (dys‐)function. The risk for combined cardiovascular events increased with worsening of LV diastolic function: from 19 in the normal group (incidence rate, 6.9 per 1000 person‐years; 95% CI, 3.8 to 10.0), 12 in the impaired relaxation group (incidence rate, 34.6 per 1000 person‐years; 95% CI, 15.0 to 54.1), and 28 in participants with elevated LV filling pressure (incidence rate, 53.2 per 1000 person‐years; 95% CI, 33.5 to 72.9). The same trend was observed for combined cardiac events (Figure 3). Table 7 shows the multivariable‐adjusted hazard ratios expressing the risk in each LV diastolic dysfunction category compared with normal diastolic function. The risks of all cardiovascular and cardiac events were significantly elevated in participants with elevated LV filling pressure (Table 7).
Figure 3.
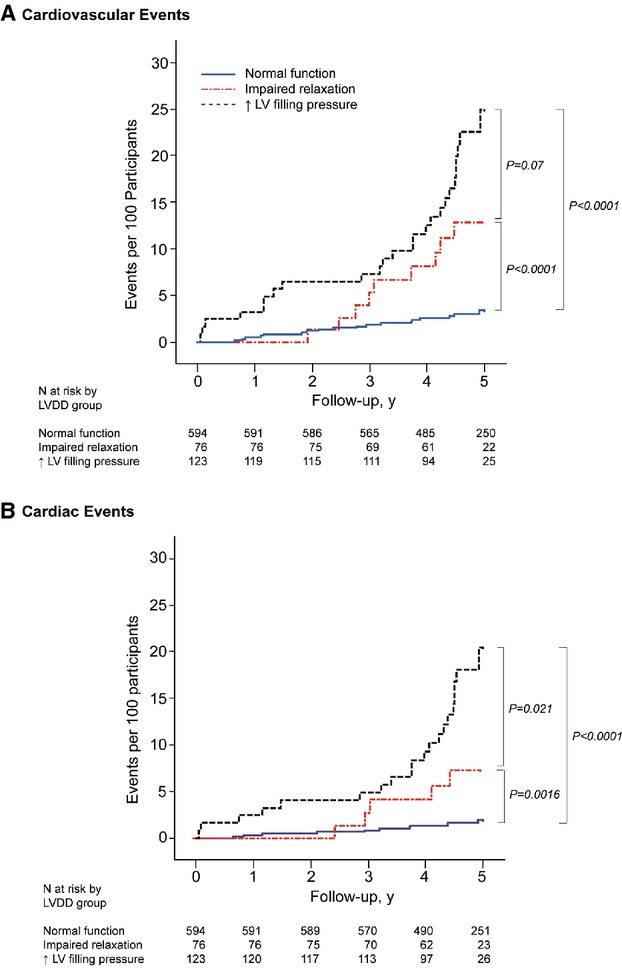
Cumulative incidence estimates (1‐Kaplan‐Meier survival estimates) for all cardiovascular and cardiac events. Normal left ventricular (LV) diastolic function is normal E/A and E/e'; impaired relaxation is low age‐specific E/A and normal E/e'; elevated LV filling pressure is high E/e'. P values are for the differences between groups by the log‐rank test. LVDD indicates left ventricular diastolic dysfunction.
Table 7.
Adjusted Hazard Ratios Associated With LV Diastolic Dysfunction Versus Normal Function at Baseline
| LV Diastolic Function Group | Total N | Fatal and Nonfatal Events | |||||
|---|---|---|---|---|---|---|---|
| All Cardiovascular (n=59) | Cardiac (n=40) | ||||||
| N of Events | HR (95% CI) | P Value | N of events | HR (95% CI) | P Value | ||
| Normal | 554 | 19 | Referent | 10 | Referent | ||
| Impaired relaxation (group 1) | 76 | 12 | 1.77 (0.75 to 4.17) | 0.17 | 7 | 2.13 (0.70 to 6.47) | 0.17 |
| Elevated LV filling pressure (group 2 and 3) | 123 | 28 | 2.21 (1.01 to 4.83) | 0.042 | 23 | 4.50 (1.73 to 11.7) | 0.002 |
Hazard ratios (HR) express the risk in each group of left ventricular diastolic dysfunction at baseline compared with the risk in the subjects with normal function at baseline. All hazard ratios were adjusted for family clusters, sex, age, body mass index, systolic blood pressure, serum cholesterol, smoking, diabetes mellitus, and history of cardiac disease. CI indicates confidence interval; LV, left ventricular.
Improvement of Prognostic Accuracy
For fatal and nonfatal cardiovascular (P=0.006) and cardiac outcome (P=0.004), IDI reached significance by adding TDI e' to the basic model (Table 8). According to the NRI, the ability of the Cox model to discriminate between subjects with and without cardiovascular and cardiac events significantly (P<0.0001) improved by adding TDI e' to a model already including the conventional cardiovascular risk factors (Table 8). We did not observe significant IDI and NRI for E/e' ratio (Table 8).
Table 8.
Integrated Discrimination Improvement and Net Reclassification Improvement by Adding the TDI e' and E/e' to a Model Including Covariables
| Integrated Discrimination Improvement | Net Reclassification Improvement | |||||
|---|---|---|---|---|---|---|
| IDI (%) | CI (%) | P Value | NRI (%) | CI (%) | P Value | |
| Cardiovascular events | ||||||
| TDI e' | 3.05 | 0.89 to 5.21 | 0.006 | 54.2 | 28.1 to 80.3 | <0.0001 |
| E/e' | −0.084 | −1.14 to 0.97 | 0.87 | 0.69 | −27.0 to 25.6 | 0.96 |
| Cardiac events | ||||||
| TDI e' | 5.03 | 1.57 to 8.48 | 0.004 | 64.0 | 32.9 to 95.0 | <0.0001 |
| E/e' | 0.48 | −2.31 to 3.27 | 0.74 | 7.30 | −24.5 to 39.1 | 0.65 |
The basic model includes as covariables sex, age, body mass index, systolic blood pressure, serum cholesterol, smoking, diabetes mellitus, and history of cardiac disease (see Table 3). The integrated discrimination improvement (IDI) is the difference between the discrimination slopes of basic models and basic models extended with a predictor variable. The discrimination slope is the difference in predicted probabilities (%) between subjects with and without event. The net reclassification improvements (NRI) reflect the improvement in discriminative power by adding a predictor variable to a Cox model already including important covariables. TDI indicates tissue Doppler imaging.
Discussion
The key findings of this study is that after adjustment for conventional cardiovascular risk factors, LV TDI e' velocity is a predictor of fatal and nonfatal cardiovascular events in the general population. We also found that TDI e' velocity improved the discrimination between subjects with and without events as compared with a model including only conventional cardiovascular risk factors, as quantified by the IDI and NRI analyses. Furthermore, we demonstrated that after full adjustment for important covariables, participants with elevated LV filling pressure (moderate diastolic dysfunction) had significantly increased risks of combined cardiovascular and cardiac events as compared with subjects with normal LV diastolic function.
Echocardiography plays a central role in the evaluation of LV diastolic function over the past 2 decades. Conventional echocardiography together with Doppler measurements of transmitral and pulmonary veins flows, and the TDI technique open up the possibility of non‐invasively evaluating diastolic function.11,19 Recent clinical studies explored the prognostic role of the new TDI‐derived indexes. Three studies6–8 in patients with symptomatic heart failure demonstrated that high E/e' independently predicted cardiac mortality and HF rehospitalization. These studies provided thresholds for the E/e' ratio in a range from 12.5 to 15. Moreover, in the ASCOT trial,10 E/e' was the strongest independent predictor of fatal and nonfatal cardiac events in a cohort of 980 high‐risk hypertensive patients. The authors demonstrated that in an adjusted model, a 1‐unit rise in the E/e' ratio was associated with a 17% increment in risk of cardiac events (P=0.003) 10 Wang et al11 reported that low TDI e' velocity independently predicted cardiac mortality in 174 patients with hypertension.
Only few community‐based studies explored in continuous analyses the prognostic role of the new TDI‐derived velocities13 or classical pulsed‐wave Doppler indexes.20–21 In 1036 participants (mean age, 60 years) enrolled in the Copenhagen City Heart study,13 low systolic (s') and a' myocardial velocities derived from Color Doppler imaging and averaged from 6 myocardial segments independently predicted total mortality. In this study, the averaged e', E/A and E/e' ratios had no independent predictive value.13 In our current study, low e' derived from pulsed TDI predicted combined cardiovascular and cardiac outcomes over and beyond conventional risk factors. Moreover, the E/e' ratio was borderline associated with an increased risk of cardiac events. Differences in recording of TDI velocities between the Danish and our study might explain this discrepancy. Indeed, Mogelvang et al13 reconstructed mean TDI velocities curves from 2D color‐coded TDI images, whereas, in our study, we derived maximal myocardial velocities from spectral pulsed TDI curves recorded at the level of mitral annulus. Overall, Color Doppler e' velocity reported by Mogelvang et al13 was about 38% lower than pulsed TDI e' velocity in our study (7.1 cm/s versus 11.4 cm/s). Moreover, the Mogelvang's study13 considered only total mortality, which includes noncardiovascular endpoints.
Two community‐based studies explored the prognostic role of transmitral Doppler E/A ratio. In the Cardiovascular Health Study (mean age, 73 years),20 the adjusted risk of symptomatic heart failure was highest at the extremes of the distribution of the E/A ratio. The relative risk was 1.88 (95% CI, 1.33 to 2.68) for an E/A ratio of less than 0.7 and 3.50 (CI, 1.80 to 6.80) for an E/A ratio higher than 1.5, compared with intermediate values. Moreover, among 3008 American Indians (mean age, 60 years) enrolled in the Strong Heart Study,21 all‐cause and cardiac mortality also had a U‐shaped relation with the E/A ratio. In our study, we did not observe significant associations between combined cardiovascular outcome and E/A. These findings have to be interpreted, keeping in mind that, in our study age averaged 50.9 years, and the proportion of participants with hypertension was 41.2%.
To our knowledge, only one community‐based study explored the predictive value of LV diastolic dysfunction grades based on conventional Doppler and new TDI velocities.4 In the Olmsted study4 mild LV diastolic dysfunction (hazard ratio, 8.31; P<0.001) and moderate or severe diastolic dysfunction (10.2; P<0.001) predicted all‐cause mortality, while controlling for sex, age, and ejection fraction. However, the Olmsted researchers did not adjust for other cardiovascular risk factors. In our study, we used a similar approach to grade LV diastolic dysfunction as in the Olmsted Study, but at variance with grading applied by Redfield et al we utilized age‐specific criteria for transmitral E/A ratio and also considered LAVI in grading LV diastolic function. Our fully adjusted hazard ratios indicated, compared with participants with normal diastolic function, a 2‐fold increased risk of a cardiovascular event and a 4‐fold higher risk of a cardiac event in participants with an elevated LV filling pressure. Furthermore, we noticed progressive lowering of TDI e' velocity in participants with an impaired relaxation pattern (8.50 cm/s), and in those with elevated end‐diastolic pressure (7.69 cm/s and 5.75 cm/s) compared to subjects with normal diastolic function (12.6 cm/s; Table 6).
Our study has to be interpreted within the context of its potential limitations and strengths. First, the Doppler blood flow measurements and the TDI velocities are prone to measurement error. In the present study, one experienced observer recorded all Doppler images using a highly standardized imaging protocol. All digitally stored images were centrally post‐processed by a single observer. Second, our sample size was smaller than in published community‐based studies. We, therefore, could not rule out that the associations that failed to reach statistical significance were due to a type II error. However, the research question addressed in this study and our conclusions were expanding the findings of previous publications in patients with heart failure6–9 and hypertension.11 Third, we included in our analysis 27 (3.4%) participants with a previous history of cardiac diseases from whom 14 participants experienced recurrent cardiovascular event during follow‐up. However, with adjustment for previous cardiac disease applied, TDI e' was significantly associated with increased risk of cardiovascular and cardiac events (Table 3). Previous community‐based studies, which explored the predictive value of TDI indexes13 or LV diastolic dysfunction grades4 included in the outcome analyses up to 11% participants with a previous coronary events. Furthermore, in a sensitivity analysis, after exclusion participants with previous cardiac diseases, our findings remained consistent (data not shown). Fourth, patterns of transmitral flow and mitral annulus velocities also depend on the compliance and contractile function of the left atrium. Thus, we did not evaluate LV diastolic function in participants with sustained atrial fibrillation. Fifth, because in our study we included only white European populations, the generalizability of the findings to other ethnicities is currently limited and should also be further explored by additional research.
In conclusion, low early diastolic mitral annulus velocity measured by TDI significantly predicted higher fatal and nonfatal cardiovascular events. TDI e' represents a simple echocardiographic measure which might be used for assessing cardiovascular risk in a general population. Furthermore, the risk of a cardiovascular or cardiac complication substantially increases in the diastolic dysfunction characterized by elevated LV filling pressure.
Sources of Funding
The European Union (grants HEALTH‐2011‐278249‐EU‐MASCARA and HEALTH‐F7‐305507‐HoMAGE), and the European Research Council (advanced grant 2011‐294713‐EPLORE) supported the Studies Coordinating Centre (Leuven, Belgium). The Studies Coordinating Centre also received grants from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (grants G.0734.09, G.0880.13, and G.0881.13).
Disclosures
None.
Acknowledgments
The authors gratefully acknowledge the expert assistance of Linda Custers, Marie‐Jeanne Jehoul, Daisy Thijs, and Hanne Truyens (Leuven, Belgium).
References
- 1.Abhayaratna W, Marwick TH, Smith WT, Becker NG. Characteristic of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006; 92:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloch‐Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, Gonzalez A, Lopez B, Thijs L, Jin Y, Malyutina S, Stolarz‐Skrzypek K, Casiglia E, Diez J, Narkiewicz K, Jawecka‐Jaszcz K, Staessen JA. Prevalences of left ventricular diastolic dysfunction in European populations based on cross‐validated diagnostic thresholds. Cardiovasc Ultrasound. 2012; 10:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, González A, Herregods MC, Fagard RH, Díez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009; 2:105-112. [DOI] [PubMed] [Google Scholar]
- 4.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. Appreciating the scope of the heart failure epidemic. JAMA. 2003; 289:194-202. [DOI] [PubMed] [Google Scholar]
- 5.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007; 49:1903-1914. [DOI] [PubMed] [Google Scholar]
- 6.Acil T, Wichter T, Stypmann J, Janssen F, Paul M, Grude M, Scheld HH, Breithardt G, Bruch C. Prognostic value of tissue Doppler imaging in patients with chronic congestive heart failure. Int J Cardiol. 2005; 103:175-181. [DOI] [PubMed] [Google Scholar]
- 7.Dokainish H, Zoghbi WA, Lakkis NM, Ambriz E, Patel R, Quinones MA, Nagueh SF. Incremental predictive power of B‐type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol. 2005; 45:1223-1226. [DOI] [PubMed] [Google Scholar]
- 8.Olson JM, Samad BA, Alam M. Prognostic value of pulse‐wave tissue Doppler parameters in patients with systolic heart failure. Am J Cardiol. 2008; 102:722-725. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Yip G, Yu CM, Zhang Q, Zhang Y, Tse D, Kong SL, Sanderson JE. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. 2005; 45:272-277. [DOI] [PubMed] [Google Scholar]
- 10.Sharp AS, Tapp RJ, Thom SA, Francis DP, Hughes AD, Stanton AV, Zambanini A, O'Brien E, Chaturvedi N, Lyons S, Byrd S, Poulter NR, Sever PS, Mayet JASCOT Investigators. Tissue Doppler E/E' ratio is a powerful predictor of primary cardiac events in a hypertensive population: an ASCOT substudy. Eur Heart J. 2010; 31:747-752. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, Yu CM, Sanderson JE. Tissue Doppler imaging provides incremental prognostic value in patients with systemic hypertension and left ventricular hypertrophy. J Hypertens. 2005; 23:183-191. [DOI] [PubMed] [Google Scholar]
- 12.Hillis GS, Møller JE, Pellikka PA, Gersh BJ, Wright RS, Ommen SR, Reeder GS, Oh JK. Noninvasive estimation of left ventricular filling pressure by E/e' is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol. 2004; 43:360-367. [DOI] [PubMed] [Google Scholar]
- 13.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, Goetze JP, Jensen JS. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009; 119:2679-2685. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zagato L, Kuznetsova T, Tripodi G, Zerbini G, Richart T, Thijs L, Manunta P, Wang JG, Bianchi G, Staessen JA. Angiotensin‐converting enzyme I/D and alpha‐adducin Gly460Trp polymorphisms: from angiotensin‐converting enzyme activity to cardiovascular outcome. Hypertension. 2007; 49:1291-1297. [DOI] [PubMed] [Google Scholar]
- 15.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJAmerican Society of Echocardiography. American Society of Echocardiography recommendation for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004; 17:1086-1119. [DOI] [PubMed] [Google Scholar]
- 16.Stolarz‐Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovský J, Kawecka‐Jaszcz K, Nikitin Y, Staessen JAEuropean Project on Genes in Hypertension (EPOGH) Investigators. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011; 305:1777-1785. [DOI] [PubMed] [Google Scholar]
- 17.Rose GA, Blackburn H. Cardiovascular Survey Methods. 1968Geneva: WHO; [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011; 30:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008; 51:679-689. [DOI] [PubMed] [Google Scholar]
- 20.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001; 37:1042-1048. [DOI] [PubMed] [Google Scholar]
- 21.Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle‐aged and elderly adults: the Strong Heart Study. Circulation. 2002; 105:1928-1933. [DOI] [PubMed] [Google Scholar]


