Abstract
Background
The prevalence, clinical features, and long‐term outcome of patients with non–ST‐segment elevation acute coronary syndrome (NSTE ACS) associated with coronary spasm are not fully investigated.
Methods and Results
This observational multicenter study enrolled 1601 consecutive patients with suspected NSTE‐ACS who underwent cardiac catheterization between January 2001 and December 2010. A culprit lesion was found in 1152 (72%) patients. In patients without a culprit lesion, the acetylcholine provocation test was performed in 221 patients and was positive in 175 patients. In the other patients, coronary spasm was verified in 145 patients during spontaneous attack. Spasm‐induced NSTE‐ACS was diagnosed in 320 (20%) patients. Multivariable analysis identified age <70 years (odds ratio [OR] 2.19, 95% CI 1.58 to 3.04), estimated glomerular filtration rate >60 mL/min per 1.73 m2 (OR 1.72, 95% CI 1.16 to 2.56), and lack of hypertension (OR 2.55, 95% CI 1.90 to 3.41), dyslipidemia (OR 2.76, 95% CI 2.05 to 3.73), diabetes mellitus (OR 2.49, 95% CI 1.78 to 3.48), previous myocardial infarction (OR 5.37, 95% CI 2.89 to 10.0), and elevated cardiac biomarkers (OR 2.84, 95% CI 2.11 to 3.83) as significant correlates of spasm‐induced NSTE‐ACS (P<0.01 for all variables). Transient ST‐segment elevation during spontaneous attack (variant angina) was observed in 119 patients with spasm‐induced NSTE‐ACS. Variant angina was more common in nondyslipidemic men among patients with spasm‐induced NSTE‐ACS.
Conclusions
The study showed frequent involvement of coronary spasm in the pathogenesis of NSTE‐ACS. Variant angina was observed in one third of patients with spasm‐induced NSTE‐ACS. Coronary spasm should be considered even in patients with less coronary risk factors and nonobstructive coronary arteries.
Keywords: acetylcholine provocation test, coronary spasm, NSTE‐ACS
Introduction
Disruption of the coronary atherosclerotic plaque complicated by thrombosis is the most common pathophysiological mechanism of acute coronary syndrome (ACS).1 Coronary artery spasm is also considered an important etiologic factor in the pathogenesis of ACS.2–3 In fact, it is not uncommon in the clinical setting to find no significant coronary artery disease in ACS patients who undergo urgent coronary angiography. Although the etiologic mechanisms of ACS in patients lacking obstructive coronary artery disease remain obscure, coronary spasm seems to be the main etiomechanism of nonobstructive ACS. Currently, there is little information on the prevalence and clinical features of coronary spasm in patients with ACS. A recent study reported that one fourth of whites with ACS had no culprit coronary lesion and ~50% of the ACS patients without culprit lesions develop coronary spasm in response to intracoronary administration of acetylcholine (ACh),4 supporting the hypothesis that coronary spasm is an important cause of ACS.
ACS includes a spectrum of clinical presentations and is classified into ST‐segment elevation ACS (STE‐ACS) and non–ST‐segment elevation ACS (NSTE‐ACS), based on ECG findings. The therapeutic approaches vary considerably between these 2 presentations. Immediate reperfusion therapy is the established treatment for STE‐ACS,5 while NSTE‐ACS requires initial medical stabilization and early invasive therapy based on risk stratification.6–7 Unlike the invasive percutaneous coronary intervention or intensive medical treatment for complicated coronary atherosclerotic plaques and thrombosis, coronary spasm can be suppressed effectively with calcium channel blockers or nitrates. Therefore, an accurate understanding of the pathological condition in NSTE‐ACS patients is required to provide better medical treatments.
Previous studies described the presence of racial differences in the frequency of coronary spasm, which is more frequent in Japanese subjects than in white subjects.8 In the present study, we investigated the prevalence, clinical features, and long‐term outcome of NSTE‐ACS associated with coronary spasm in a large Japanese study population.
Methods
Study Population
We retrospectively studied consecutive patients who were hospitalized with the diagnosis of NSTE‐ACS and underwent cardiac catheterization during the acute phase between January 2001 and December 2010 at 3 Japanese cardiovascular institutions (Kumamoto University Hospital, Saiseikai Kumamoto Hospital, and Yokohama City University Medical Center). NSTE‐ACS was defined as chest pain lasting for >5 minutes at rest during the 48 hours before visiting the hospital and ischemic changes on the ECG apart from persistent ST‐segment elevation lasting for >20 minutes and/or increase in the levels of cardiac biomarkers such as creatine kinase–MB fraction and cardiac troponin levels. Acute myocardial infarction was defined based on the universal definition of myocardial infarction.9 Patients with any of the following conditions were excluded from the study: (1) secondary myocardial ischemia, (2) angina with recent myocardial infarction reported >48 hours before admission, (3) end‐stage renal disease and on dialysis, (4) heart failure requiring mechanical ventilation support, (5) new or presumably new left bundle branch block, and (6) ST‐segment elevation on initial ECG at the emergency department. Thus, 1601 patients with NSTE‐ACS who met these inclusion and exclusion criteria were enrolled in the study. The data for eligible patients were collected from the medical registry and the medical records of each hospital. A written informed consent for cardiac catheterization and data utilization was obtained from each patient. The study was conducted in accordance with the guidelines approved by the ethics committees of our institutions.
The diagnosis of spasm‐induced NSTE‐ACS was based on the absence of culprit lesion on coronary angiography and the (1) presence of ischemic ECG changes, including transient ST‐segment elevation of ≥0.1 mV, ST‐segment depression of ≥0.1 mV, or new appearance of negative U waves, recorded in ≥2 contiguous leads on the 12‐lead ECG during rest angina attacks, (2) spontaneous coronary spasm during coronary angiography, which was relieved by the administration of intracoronary nitroglycerin (see Figure 1), or (3) a positive spasm provocation test (ie, spasm after the intracoronary injection of ACh). We defined the culprit lesion as the site of (1) acute coronary occlusion, (2) severe coronary artery disease of >90% stenosis, or (3) coronary artery disease with complex lesion morphology, which is responsible for ischemic symptoms, corresponding to the electrocardiographic changes or wall motion abnormality observed on transthoracic echocardiography or left ventriculography.
Figure 1.
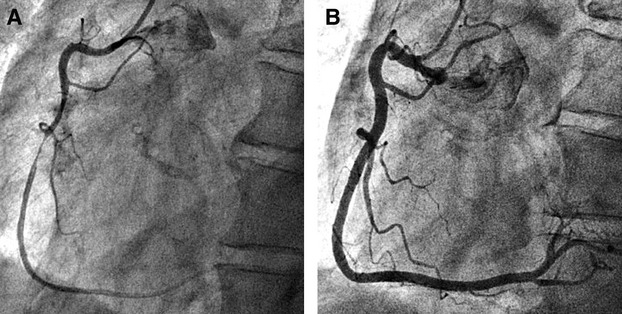
Representative case of spasm‐induced NSTE‐ACS diagnosed by spontaneous coronary spasm during coronary angiography. A, First coronary angiography showed severe stenosis of the middle right coronary artery. B, Intracoronary administration of nitroglycerin resulted in complete resolution of the stenosis and vasodilation of the entire right coronary artery. NSTE‐ACS indicates non–ST‐segment elevation acute coronary syndrome.
Cardiac Catheterization and the ACh Provocation Test
In our institutions, a spasm provocation test can be requested at the discretion of the attending physician. The test involves intracoronary injection of ACh in patients who show no culprit lesion on coronary angiography and are suspected to have coronary spasm as the cause of chest pain. The spasm provocation test was performed using the following procedure: Incremental doses of ACh were injected into the left coronary artery (20, 50, and 100 μg) and right coronary artery (20 and 50 μg) until the elicitation of coronary spasm. Patients with coronary artery spasm that did not resolve spontaneously within 5 minutes were treated with nitroglycerin injected into the responsible coronary artery. Positive findings of coronary spasm were defined as a total or subtotal obstruction or as severe diffuse vasoconstriction of epicardial coronary artery associated with transient myocardial ischemia as evidenced by ischemic ST‐segment changes on the ECG. Severe diffuse vasoconstriction was defined as 90% stenosis defined by the American Heart Association classification10 observed in >2 adjacent coronary segments of epicardial coronary arteries.
Definition of Coronary Risk Factors
Risk factors for cardiovascular disease were defined as current smoking (smoking within 1 year), hypertension (>140/90 mm Hg or taking antihypertensive medications), dyslipidemia (high‐density lipoprotein cholesterol <40 mg/dL, low‐density lipoprotein cholesterol ≥140 mg/dL, or receiving lipid‐lowering medications), and diabetes mellitus (fasting plasma glucose level of ≥126 mg/dL, 2‐hour value of ≥200 mg/dL in 75‐g oral glucose tolerance test, casual plasma glucose level of ≥200 mg/dL, hemoglobin A1c ≥6.5%, or taking medications for diabetes mellitus). The estimated glomerular filtration rate was calculated from the Japanese equation: glomerular filtration rate (mL/min per 1.73 m2)=194×serum creatinine−1.094×age−0.287 (if female, ×0.739).11
Follow‐Up Data
Follow‐up data were obtained by review of the medical records and phone calls to the patients or their families. Major adverse cardiovascular events were defined as cardiovascular mortality, hospitalization for acute myocardial infarction, unstable angina, stroke, or heart failure.
Statistical Analysis
Baseline features and clinical outcomes were compared between patients with culprit lesion and those with lesions induced by coronary spasm. Values were expressed as mean±SD or medians with interquartile ranges. Differences in continuous variables between groups were tested with the unpaired t tests for normally distributed variables and Mann–Whitney tests for skewed variables. Categorical variables were presented as proportions, and intergroup comparisons were analyzed by using the χ2 test or Fisher exact test. Simple and multiple logistic regression analyses were performed to determine the predictors of spasm‐induced NSTE‐ACS among patients of the obstructive and spasm groups and the presence of variant angina in patients with spasm‐induced NSTE‐ACS. Variables with a value of P<0.05 in univariate analysis were entered into multivariable analysis. Model discrimination was assessed by using C statistics, and model calibration was assessed by using the Hosmer–Lemeshow goodness‐of‐fit test. Differences were considered significant at P<0.05. Statistical analysis was performed with IBM SPSS Statistics 20 for Windows (SPSS Inc).
Results
Prevalence of Coronary Spasm in NSTE‐ACS in the Study Population
As shown in Figure 2, among the 1601 patients with NSTE‐ACS, 1152 patients (72%, obstructive group) were found to have culprit lesions on coronary angiography, while 449 did not have such lesions. In the latter group, the ACh provocation test was performed in 221 patients at the discretion of the attending physician and the results were positive in 175 (79%) patients. In patients who did not undergo the ACh provocation test (n=228), coronary spasm was verified in 145 (63.6%) patients, with ischemic ECG changes during rest angina attack or spontaneous coronary spasm during emergency coronary angiography. These findings indicated that coronary spasm was the underlying mechanism of NSTE‐ACS in at least 320 patients (20% of the spasm group) of our cohort. In this group, transient ST‐segment elevation during spontaneous attack (variant angina) before or after hospitalization was observed in 119 patients, including 38 patients with positive results from the ACh provocation test.
Figure 2.
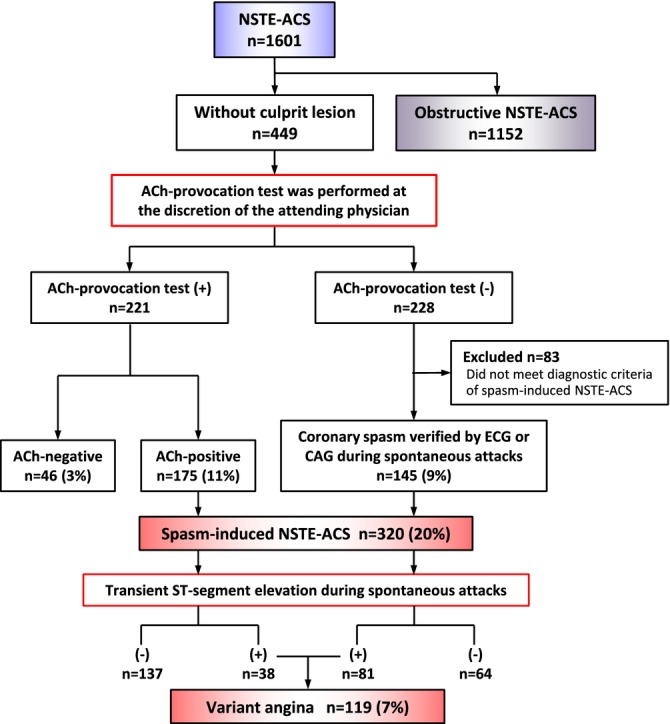
Flow chart of classification of the patients. ACh indicates acetylcholine; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome.
Characteristics of Patients With Spasm‐Induced NSTE‐ACS
Table 1 lists the characteristics of patients with obstructive and spasm‐induced NSTE‐ACS. Compared with the obstructive group, patients of the spasm group were younger and were less likely to be hypertensive or to have dyslipidemia, diabetes mellitus, or previous myocardial infarction. On the other hand, the proportion of current smokers was higher in the spasm group than in the obstructive group. Patients of the spasm group had lower levels of low‐density lipoprotein cholesterol, triglyceride, glucose, hemoglobin A1c, and creatine kinase–MB fraction and higher levels of high‐density lipoprotein cholesterol, glomerular filtration rate, and hemoglobin compared with the obstructive group. Furthermore, patients of the spasm group had lower Thrombolysis In the Myocardial Infarction (TIMI) risk scores and higher left ventricular ejection fraction compared with the obstructive group. Table 1 also lists the medication history at discharge for the 2 groups. In the spasm subgroups, the characteristics of the patients who had positive ACh provocation test results were almost identical to those with coronary spasm diagnosed on the basis of a spontaneous attack (data not shown).
Table 1.
Characteristics of Patients With Obstructive and Spasm‐Induced NSTE‐ACS
| Obstructive Group (n=1152) | Spasm Group (n=320) | P Value | |
|---|---|---|---|
| Age, y | 69±11 | 61±11 | <0.001 |
| Males, % | 73 | 71 | 0.25 |
| Current smoking, % | 32 | 42 | 0.002 |
| Body mass index, kg/m2 | 23.8±3.5 | 23.5±3.5 | 0.18 |
| Family history of CAD, % | 23 | 21 | 0.24 |
| Previous medical history, % | |||
| Hypertension | 76% | 48% | <0.001 |
| Dyslipidemia | 79% | 54% | <0.001 |
| Diabetes mellitus | 41% | 18% | <0.001 |
| Previous MI | 23% | 4% | <0.001 |
| Laboratory findings | |||
| Total cholesterol, mg/dL | 197±43 | 192 ± 37 | 0.06 |
| LDL cholesterol, mg/dL | 122±37 | 114 ± 32 | <0.001 |
| HDL cholesterol, mg/dL | 47±14 | 54 ± 17 | <0.001 |
| Triglycerides, mg/dL | 119 (84, 183) | 108 (73, 165) | 0.01 |
| Glucose, mg/dL | 149±60 | 136 ± 69 | 0.005 |
| HbA1c, % | 6.5±1.3 | 6.0 ± 1.3 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 68±22 | 78 ± 18 | <0.001 |
| Creatine kinase, U/L | 102 (71, 166) | 96 (65, 145) | 0.052 |
| Creatine kinase–MB, U/L | 11 (8, 16) | 10 (8, 14) | 0.01 |
| WBC, mm3 | 7166±2402 | 6899±2516 | 0.11 |
| Hemoglobin, g/dL | 13.5±2.0 | 14.0±1.6 | <0.001 |
| Elevated cardiac biomarkers | 54% | 30% | <0.001 |
| TIMI risk score | <0.001 | ||
| 1 to 2 | 34% | 76% | |
| 3 to 4 | 50% | 24% | |
| 5 to 7 | 16% | 1% | |
| LVEF, % | 58±12 | 65±7 | <0.001 |
| Medications at discharge, % | |||
| Aspirin | 98% | 60% | <0.001 |
| Thienopyridine | 70% | 3% | <0.001 |
| Calcium channel blockers | 47% | 97% | <0.001 |
| β‐Blockers | 55% | 2% | <0.001 |
| ACEI | 34% | 8% | <0.001 |
| ARB | 28% | 13% | <0.001 |
| Nitrates | 36% | 48% | <0.001 |
| Nicorandil | 39% | 30% | 0.005 |
| Statins | 65% | 38% | <0.001 |
Data are mean±SD, medians with interquartile ranges, or n (%). NSTE‐ACS indicates non–ST‐segment elevation acute coronary syndrome; CAD, coronary artery disease; MI, myocardial infarction; LDL, low‐density lipoprotein; HDL, high‐density lipoprotein; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate; WBC, white blood cell; TIMI, Thrombolysis In Myocardial Infarction; LVEF, left ventricular ejection fraction; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor antagonist.
In the spasm group, 243 (76%) of patients had normal coronary arteries, while the remaining 77 (24%) had mild‐to‐moderate fixed coronary artery disease. Patients with normal coronary arteries were significantly younger than those with mild‐to‐moderate coronary artery disease (60.4 vs 63.6 years, P=0.03). There were no significant differences in the proportions of other coronary risk factors and variant angina (36% vs 42%, P=0.4) between the group with normal coronary arteries and the group with mild‐to‐moderate coronary artery disease.
Table 2 shows the results of simple and multiple logistic regression analyses for the prediction of spasm‐induced NSTE‐ACS among patients of the obstructive and spasm groups. Multiple logistic regression analysis identified age <70 years (odds ratio [OR] 2.19, 95% CI 1.58 to 3.04, P<0.001), glomerular filtration rate >60 mL/min per 1.73 m2 (OR 1.72, 95% CI 1.16 to 2.56, P=0.009), and absence of hypertension (OR 2.55, 95% CI 1.90 to 3.41, P<0.001), dyslipidemia (OR 2.76, 95% CI 2.05 to 3.73, P<0.001), diabetes mellitus (OR 2.49, 95% CI 1.78 to 3.48, P<0.001), previous myocardial infarction (OR 5.37, 95% CI 2.89 to 10.0, P<0.001), and elevated cardiac biomarkers (OR 2.84, 95% CI 2.11 to 3.83, P<0.001) as significant correlates of spasm‐induced NSTE‐ACS (P<0.01 for all parameters). The C statistic for the prediction of spasm‐induced NSTE‐ACS was 0.82 (95% CI 0.79 to 0.84) when all of these factors were included. These models were reliable when these coronary risk factors were selected (P=0.70 by the Hosmer–Lemeshow test).
Table 2.
Logistic Regression Analyses for the Prediction of Spasm‐Induced NSTE‐ACS
| Simple Regression Analysis | Multiple Regression Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age <70 y | 3.04 | 2.31 to 3.98 | <0.001 | 2.19 | 1.58 to 3.04 | <0.001 |
| Male sex | 0.90 | 0.69 to 1.18 | 0.45 | |||
| Body mass index <25 kg/m2 | 0.99 | 0.77 to 1.28 | 0.96 | |||
| Current smoking | 1.51 | 1.17 to 1.95 | 0.002 | 1.03 | 0.76 to 1.40 | 0.83 |
| Family history of CAD | 1.13 | 0.84 to 1.53 | 0.43 | |||
| No hypertension | 3.49 | 2.69 to 4.51 | <0.001 | 2.55 | 1.90 to 3.41 | <0.001 |
| No dyslipidemia | 3.25 | 2.50 to 4.23 | <0.001 | 2.76 | 2.05 to 3.73 | <0.001 |
| No diabetes mellitus | 3.27 | 2.40 to 4.46 | <0.001 | 2.49 | 1.78 to 3.48 | <0.001 |
| No previous MI | 7.56 | 4.18 to 13.7 | <0.001 | 5.37 | 2.88 to 10.0 | <0.001 |
| eGFR >60 mL/min per 1.73 m2 | 3.32 | 2.35 to 4.70 | <0.001 | 1.72 | 1.16 to 2.56 | 0.009 |
| TIMI risk score ≤2 | 8.70 | 5.88 to 11.9 | <0.001 | |||
| LVEF >50% | 1.27 | 0.97 to 1.64 | 0.077 | |||
| Nonelevated cardiac biomarkers | 2.82 | 2.16 to 3.68 | <0.001 | 2.84 | 2.11 to 3.83 | <0.001 |
NSTE‐ACS indicates non–ST‐segment elevation acute coronary syndrome; OR, odds ratio; CAD, coronary heart disease; MI, myocardial infarction; eGFR, estimated glomerular filtration rate; TIMI, Thrombolysis In Myocardial Infarction; LVEF, left ventricular ejection fraction.
The characteristics of the patients with variant angina in the spasm group are shown in Table 3. Univariate analysis showed that age, sex, smoking habit, and dyslipidemia were significantly associated with variant angina in the spasm group. Multiple logistic regression analysis identified male sex (OR 1.94, 95% CI 1.08 to 3.49, P=0.03) and absence of dyslipidemia (OR 1.77, 95% CI 1.11 to 2.84, P=0.03) as significant correlates of variant angina (Table 4).
Table 3.
Characteristics of Patients With Spasm‐Induced NSTE‐ACS According to Variant Angina
| Variant Angina (n=119) | Nonvariant Angina (n=201) | P Value | |
|---|---|---|---|
| Age, y | 59±11 | 62±11 | 0.01 |
| Males, % | 82% | 64% | 0.001 |
| Current smoking, % | 50% | 37% | 0.02 |
| Body mass index, kg/m2 | 23.6±3.7 | 23.6±3.4 | 0.95 |
| Family history of CAD, % | 24% | 20% | 0.44 |
| Previous medical history, % | |||
| Hypertension | 49% | 48% | 0.93 |
| Dyslipidemia | 44% | 59% | 0.007 |
| Diabetes mellitus | 17% | 18% | 0.71 |
| Previous MI | 3% | 4% | 0.55 |
| Elevated cardiac biomarkers | 36% | 26% | 0.06 |
| LVEF, % | 66.3±7.0 | 64.5±7.2 | 0.11 |
| Medications at discharge, % | |||
| Aspirin | 61% | 58% | 0.62 |
| Calcium channel blockers | 98% | 96% | 0.22 |
| β‐Blockers | 0% | 3% | 0.09 |
| ACEI | 6% | 9% | 0.30 |
| ARB | 8% | 16% | 0.04 |
| Nitrates | 61% | 41% | <0.001 |
| Nicorandil | 35% | 27% | 0.17 |
| Statins | 36% | 38% | 0.74 |
Data are mean±SD or n (%).NSTE‐ACS indicates non–ST‐segment elevation acute coronary syndrome; CAD, coronary heart disease; MI, myocardial infarction; LVEF, left ventricular ejection fraction; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor antagonist.
Table 4.
Logistic Regression Analyses for the Presence of Variant Angina in Patients With Spasm‐Induced NSTE‐ACS
| Simple Regression Analysis | Multiple Regression Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age <70 y | 2.06 | 1.19 to 3.57 | 0.01 | 1.78 | 1.00 to 3.18 | 0.052 |
| Male sex | 2.46 | 1.43 to 4.25 | 0.001 | 1.94 | 1.08 to 3.49 | 0.03 |
| Body mass index <25 kg/m2 | 1.08 | 0.68 to 1.73 | 0.74 | |||
| Current smoking | 1.75 | 1.10 to 2.76 | 0.02 | 1.25 | 0.75 to 2.08 | 0.39 |
| Family history of CAD | 1.24 | 0.72 to 2.14 | 0.44 | |||
| No hypertension | 0.98 | 0.62 to 1.54 | 0.93 | |||
| No dyslipidemia | 1.87 | 1.18 to 2.96 | 0.007 | 1.77 | 1.11 to 2.84 | 0.02 |
| No diabetes mellitus | 1.12 | 0.61 to 2.03 | 0.72 | |||
| No previous MI | 1.81 | 0.48 to 6.83 | 0.38 | |||
| eGFR >60 mL/min per 1.73 m2 | 0.76 | 0.39 to 1.47 | 0.42 | |||
| Nonelevated cardiac biomarkers | 0.62 | 0.38 to 1.01 | 0.053 | |||
NSTE‐ACS indicates non–ST‐segment elevation acute coronary syndrome; OR, odds ratio; CAD, coronary heart disease; MI, myocardial infarction; eGFR, estimated glomerular filtration rate. The Hosmer–Lemeshow goodness‐of‐fit χ2 value was 1.04 (P=0.99).
Clinical Outcome of Patients With Spasm‐Induced NSTE‐ACS
During a mean follow‐up period of 20±8 months, major adverse cardiovascular events was registered in 157 (13.6%) patients of the obstructive group (cardiovascular death; n=9, myocardial infarction; n=23, unstable angina; n=83, stroke; n=14, and heart failure; n=28) and in 15 (4.7%) patients of the spasm group (cardiovascular death; n=1, myocardial infarction; n=4, unstable angina; n=9, stroke; n=1). On the other hand, major adverse cardiovascular events did not occur in those patients who were not classified into either the obstructive group or the spasm group.
Discussion
The present study demonstrated that coronary spasm is common in Japanese patients with NSTE‐ACS, especially those with few coronary risk factors. Furthermore, variant angina was observed in one third of patients with spasm‐induced NSTE‐ACS. The prevalence and clinical features of spasm‐induced NSTE‐ACS in this study were significantly different from those reported in a previous study of whites.4 To the best of our knowledge, this is the first report that shows high frequency and clinical features of spasm‐induced NSTE‐ACS in a large study population.
The pathogenic role of coronary spasm in ACS has already been discussed in previous studies.12–16 A severe attack of coronary spasm by itself can occlude the coronary artery and cause myocardial ischemia. Coronary spasm can also cause coronary plaque progression and rupture of vulnerable plaques.12 Furthermore, prolonged coronary spasm‐induced coronary flow limitation can trigger acute thrombus formation through the activation of platelets and various adhesion molecules,13 fibrin formation,14 and impairment of fibrinolytic activity.15 Using intravascular optical coherence tomography, Kobayashi et al16 examined the culprit lesion of a patient with ACS caused by coronary spasm and showed reduced luminal area with vascular contraction and thrombus formation without atherosclerotic plaque disruption.
Anderson and Pepine17 found nonobstructive coronary arteries in up to 30% of ACS patients. However, it should be noted that most studies concerning ACS without obstructive coronary artery disease did not evaluate the presence of coronary spasm. In the Coronary Artery Spasm in Patients with Acute Coronary Syndrome (CASPAR) study, the ACh provocation test was performed in consecutive ACS patients without culprit lesions, and a high prevalence of coronary spasm was found in the white population,4 showing that 138 (28%) of 488 ACS patients had no culprit lesions and 42 (49%) of 86 ACS patients who underwent the ACh provocation test had positive results. The present study demonstrated that 27% of Japanese patients with NSTE‐ACS had no culprit lesion, and coronary spasm was associated with NSTE‐ACS in at least 20% of patients. ACh‐induced coronary spasm was found in 79% patients during the ACh provocation test. Although we cannot directly compare the results of the CASPAR with those of the present study due to differences in the study population and definition of coronary spasm, the percentage of patients with positive ACh provocation test results and the prevalence of coronary spasm seem to be substantially higher in the Japanese population. Furthermore, one third of patients with spasm‐induced NSTE‐ACS in the present study presented with an angina attack with transient ST‐segment elevation before or after hospitalization. The presence of transient ST‐segment elevation during spontaneous attacks has been reported to be one of the significant correlates of major adverse cardiac events in patients with coronary spastic angina.18 Patients with spasm‐induced ACS seem to have enhanced coronary spasm activity, together with a high risk of life‐threatening complications, such as acute myocardial infarction and fatal arrhythmias. Although treatment with oral β‐blockers is recommended to be initiated within the first 24 hours of NSTE‐ACS,6 the use of β‐blockers without vasodilators might aggravate coronary spasm. In contrast, calcium channel blockers are highly effective in suppressing coronary spasm.19 Therefore, the precise diagnosis of coronary spasm and early appropriate pharmacological treatment are essential in NSTE‐ACS patients without culprit lesions.
We recently reported that ACh‐induced diffuse severe vasoconstriction was associated with better prognosis compared with ACh‐induced total or subtotal occlusion (ie, focal spasm) in coronary spastic angina.20 It is possible that ACh‐induced focal spasm and diffuse severe vasoconstriction have different features with respect to treatment and prognosis. The present study was retrospective and the ACh provocation test was not conducted in all patients of the spasm group. Further investigation is needed to define differences in the clinical features and prognosis of ACS patients according to the subtypes of spasm induced by ACh.
In the present study, cardiovascular risk factors were less frequently observed in patients with spasm‐induced NSTE‐ACS. These results are consistent with previous studies of Japanese patients with stable coronary artery disease and vasospastic angina, which demonstrated lower frequencies of hypertension, dyslipidemia, and diabetes mellitus in patients with vasospastic angina.21 The CASPAR study reported significant differences in some coronary risk factors between patients with and without culprit lesions, including age, sex, and diabetes mellitus, but not other cardiovascular risk factors. Coronary spasm occurs in both normal arteries and fixed atherosclerotic lesion of varying levels of severity. It has also been reported that the prevalence of organic stenosis in white patients with coronary spasm is higher than that in Japanese patients.19 Several clinical studies using intravascular ultrasonography and optical coherence tomography in an Asian population reported that coronary artery segments involved in coronary spasm are characterized by a smaller amount of plaque, diffuse intimal thickening with less calcium and lipid content, and negative remodeling.22–24 These data suggest that coronary spasm is associated with coronary risk factors and fixed coronary atherosclerotic lesion in the Western population, whereas Japanese patients can develop coronary spasm although they have fewer cardiovascular risk factors and nonsignificant stenotic lesion. Coronary computed tomography angiography is increasingly used to exclude the presence of coronary artery disease instead of cardiac catheterization.25–26 This may result in overlooking the diagnosis of coronary spasm. Coronary spasm should be considered even in ACS patients with less coronary risk factors and nonobstructive coronary arteries, especially in a Japanese population.
The present study has certain limitations. First, the ACh provocation test was not performed in all patients in whom no culprit lesion was identified. Because coronary spasm could be associated with ACS in some of these patients, it is possible that it might lead to the underestimation of the overall prevalence of coronary spasm in NSTE‐ACS. Second, about half of patients of the spasm group were not confirmed to have coronary spasm on coronary angiography. Third, the present study could not evaluate the involvement of coronary spasm in patients with culprit lesions. Coronary spasm also plays a key role in the development of ACS in patients with fixed atherosclerotic lesion. However, it is difficult to determine whether the coronary spasm induced ACS in patients with obstructive coronary artery diseases. Stent implantation for culprit lesion makes it impossible to evaluate coronary spasm at local site. On the other hand, regional treatment of coronary spasm at the stenotic site has been performed. Therefore, we investigated coronary artery spasm in patients without culprit lesions in the present study. Fourth, we did not evaluate microvascular spasm. In the present study, we adapted ACh‐provoked epicardial coronary artery spasm as the diagnostic criterion of spasm‐induced NSTE‐ACS. In addition, lactate production in the coronary circulation and quantitative coronary blood flow were not measured in all patients during the ACh provocation test. Therefore, patients with suspected microvascular spasm (provoked chest symptoms and ischemic ECG changes in response to intracoronary ACh in the absence of epicardial coronary artery spasm) were classified as ACh negative. Fifth, there was a lack of data from cardiac magnetic resonance imaging and endomyocardial biopsy samples. It was reported previously that myocarditis is a frequent cause of troponin‐positive acute chest pain in the absence of significant coronary artery disease.27 However, patients with suspected myocarditis (based on the findings of ECG, echocardiography, blood tests, and symptoms) were excluded in the present study. Sixth, the presence and morphological characteristics of coronary atherosclerotic plaque were not evaluated by using intravascular imaging, such as intravascular ultrasonography and optical coherence tomography or computed tomography coronary angiography. Disruption of an angiographically insignificant atherosclerotic plaque with transient thrombosis formation probably explains some cases of ACS without a culprit lesion on coronary angiography.28
In conclusion, the present study showed the frequent involvement of coronary spasm in the pathogenesis of NSTE‐ACS in Japanese patients, especially in patients with few cardiovascular risk factors. Coronary spasm should be considered even in patients with less coronary risk factors and nonobstructive coronary arteries, and detection of coronary spasm followed by treatment with calcium channel blockers is probably important for secondary prevention in patients with nonobstructive NSTE‐ACS.
Disclosures
None.
References
- 1.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992; 326:242-250. [DOI] [PubMed] [Google Scholar]
- 2.Maseri A, L'Abbate A, Baroldi G, Chierchia S, Marzilli M, Ballestra AM, Severi S, Parodi O, Biagini A, Distante A, Pesola A. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of “preinfarction” angina. N Engl J Med. 1978; 299:1271-1277. [DOI] [PubMed] [Google Scholar]
- 3.DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, Lang HT. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980; 303:897-902. [DOI] [PubMed] [Google Scholar]
- 4.Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: the caspar (coronary artery spasm in patients with acute coronary syndrome) study. J Am Coll Cardiol. 2008; 52:523-527. [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). Circulation. 2004; 110:e82-e292. [PubMed] [Google Scholar]
- 6.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non‐ST‐Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007; 50:e1-e157. [DOI] [PubMed] [Google Scholar]
- 7.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D, Bax JJ, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Achenbach S, Badimon L, Bertrand M, Botker HE, Collet JP, Crea F, Danchin N, Falk E, Goudevenos J, Gulba D, Hambrecht R, Herrmann J, Kastrati A, Kjeldsen K, Kristensen SD, Lancellotti P, Mehilli J, Merkely B, Montalescot G, Neumann FJ, Neyses L, Perk J, Roffi M, Romeo F, Ruda M, Swahn E, Valgimigli M, Vrints CJ, Widimsky P. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011; 32:2999-3054. [DOI] [PubMed] [Google Scholar]
- 8.Pristipino C, Beltrame JF, Finocchiaro ML, Hattori R, Fujita M, Mongiardo R, Cianflone D, Sanna T, Sasayama S, Maseri A. Major racial differences in coronary constrictor response between Japanese and Caucasians with recent myocardial infarction. Circulation. 2000; 101:1102-1108. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole‐Wilson PA, Gurfinkel EP, Lopez‐Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez‐Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio‐Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez‐Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al‐Attar N. Universal definition of myocardial infarction. Circulation. 2007; 116:2634-2653. [DOI] [PubMed] [Google Scholar]
- 10.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975; 51:5-40. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009; 53:982-992. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto S, Shiga Y, Hiramitsu S, Yamada K, Nomura S, Miyagi Y, Nomura M, Mizuno Y. Plaque rupture possibly induced by coronary spasm–an autopsy case of acute myocardial infarction. Jpn Circ J. 1988; 52:1286-1292. [DOI] [PubMed] [Google Scholar]
- 13.Kaikita K, Ogawa H, Yasue H, Sakamoto T, Suefuji H, Sumida H, Okumura K. Soluble P‐selectin is released into the coronary circulation after coronary spasm. Circulation. 1995; 92:1726-1730. [DOI] [PubMed] [Google Scholar]
- 14.Oshima S, Yasue H, Ogawa H, Okumura K, Matsuyama K. Fibrinopeptide A is released into the coronary circulation after coronary spasm. Circulation. 1990; 82:2222-2225. [DOI] [PubMed] [Google Scholar]
- 15.Misumi I, Ogawa H, Masuda T, Sakamoto T, Okumura K, Yasue H. Increased plasma plasminogen activator inhibitor activity after coronary spasm. Int J Cardiol. 1993; 41:21-29. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi N, Takano M, Hata N, Yamamoto M, Shinada T, Takahashi Y, Tomita K, Kitamura M, Mizuno K. Optical coherence tomography findings in a case of acute coronary syndrome caused by coronary vasospasm. Int Heart J. 2010; 51:291-292. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: bias or biology? Circulation. 2007; 115:823-826. [DOI] [PubMed] [Google Scholar]
- 18.Takagi Y, Yasuda S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa S, Kubo N, Momomura S, Ogawa H, Shimokawa H. Clinical characteristics and long‐term prognosis of vasospastic angina patients who survived out‐of‐hospital cardiac arrest: multicenter registry study of the Japanese Coronary Spasm Association. Circ Arrhythm Electrophysiol. 2011; 4:295-302. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa H, Akasaka T, Hattori R, Kawashima S, Kawasuji M, Kimura K, Miwa K, Mizuno K, Mohri M, Murohara T, Node K, Okumura K, Saito S, Shimokawa H, Sueda S, Takeyama Y, Tanabe Y, Tsuchihashi K, Yamagishi M, Yoshimura M, Ibuki C, Inoue T, Kaikita K, Kawano H, Kojima S, Kosuge M, Nakayama M, Oshita A, Soejima H, Takarada S, Yasuda S, Haze K, Kishida H, Tomoike H, Yokoyama M. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010; 74:1745-1762. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Kaikita K, Nakayama N, Horio E, Yoshimura H, Ono T, Ohba K, Tsujita K, Kojima S, Tayama S, Hokimoto S, Matsui K, Sugiyama S, Yamabe H, Ogawa H. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long‐term prognosis in 873 consecutive patients with coronary spasm: analysis of a single‐center study over 20 years. J Am Heart Assoc. 2013; 2:e000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaoka K, Yoshimura M, Ogawa H, Kugiyama K, Nakayama M, Shimasaki Y, Mizuno Y, Sakamoto T, Yasue H. Comparison of the risk factors for coronary artery spasm with those for organic stenosis in a Japanese population: role of cigarette smoking. Int J Cardiol. 2000; 72:121-126. [DOI] [PubMed] [Google Scholar]
- 22.Miyao Y, Kugiyama K, Kawano H, Motoyama T, Ogawa H, Yoshimura M, Sakamoto T, Yasue H. Diffuse intimal thickening of coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 2000; 36:432-437. [DOI] [PubMed] [Google Scholar]
- 23.Saito S, Yamagishi M, Takayama T, Chiku M, Koyama J, Ito K, Higashikata T, Seguchi O, Honye J, Kanmatsuse K. Plaque morphology at coronary sites with focal spasm in variant angina: study using intravascular ultrasound. Circ J. 2003; 67:1041-1045. [DOI] [PubMed] [Google Scholar]
- 24.Morikawa Y, Uemura S, Ishigami K, Soeda T, Okayama S, Takemoto Y, Onoue K, Somekawa S, Nishida T, Takeda Y, Kawata H, Horii M, Saito Y. Morphological features of coronary arteries in patients with coronary spastic angina: assessment with intracoronary optical coherence tomography. Int J Cardiol. 2011; 146:334-340. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the Romicat (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009; 53:1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012; 366:1393-1403. [DOI] [PubMed] [Google Scholar]
- 27.Baccouche H, Mahrholdt H, Meinhardt G, Merher R, Voehringer M, Hill S, Klingel K, Kandolf R, Sechtem U, Yilmaz A. Diagnostic synergy of non‐invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin‐positive patients without coronary artery disease. Eur Heart J. 2009; 30:2869-2879. [DOI] [PubMed] [Google Scholar]
- 28.Kovacic JC, Fuster V. Smoking gun theory: angiographically normal or mild coronary plaque as a cause of myocardial infarction. Circulation. 2012; 126:2918-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]


