Abstract
Background
Increased carotid intima‐media thickness (IMT) is associated with subclinical left ventricular myocardial dysfunction, suggesting a possible role of carotid IMT in heart failure (HF) risk determination.
Methods and Results
Mean far wall carotid IMT, measured by B‐mode ultrasound, was available for 13 590 Atherosclerosis Risk in Communities study participants aged 45 to 64 years and free of HF at baseline. HF was defined using ICD‐9 428 and ICD‐10 I‐50 codes from hospitalization records and death certificates. The association between carotid IMT and incident HF was assessed using Cox proportional hazards analysis with models adjusted for demographic variables, major CVD risk factors, and interim CHD. There were 2008 incident HF cases over a median follow‐up of 20.6 years (8.1 cases per 1000 person‐years). Mean IMT was higher in those with HF than in those without (0.81 mm±0.23 versus 0.71 mm±0.17, P<0.001). Unadjusted rate of HF for the fourth compared with the first quartile of IMT was 15.4 versus 3.9 per 1000 person‐years; P<0.001. In multivariable analysis, after adjustment, each standard deviation increase in IMT was associated with incident HF (HR 1.20 [95% CI: 1.16 to 1.25]). After adjustment, the top quartile of IMT was associated with HF (HR 1.60 [95% CI: 1.37 to 1.87]). Results were similar across race and gender groups.
Conclusions
Increasing carotid IMT is associated with incident HF in middle‐aged whites and blacks, beyond risks explained by major CVD risk factors and CHD. This suggests that carotid IMT may be associated with HF through mechanisms different from myocardial ischemia or infarction.
Keywords: carotid intima‐media thickness, heart failure, subclinical atherosclerosis
Introduction
Heart failure (HF) represents an increasing clinical and public health burden in the United States as its prevalence and incidence continue to rise,1–3 especially among elderly Americans.4–5 HF has emerged as the single leading diagnosis for hospitalization in the elderly and hospitalization rates are higher among blacks than among whites, with 83% of HF patients hospitalized at least once and 43% hospitalized at least 4 times over a mean duration of 4.7 years.6–7 The burden of HF is likely to increase because of the increasing age of the American population, earlier age of onset of HF and improved treatment and survival from cardiovascular (CV) diseases, including myocardial infarction (MI) and hypertension.8–9
Carotid IMT is a well established marker of subclinical atherosclerosis, which indicates early manifestation of atherosclerosis in the carotid arteries,10–11 and is associated with future CV events,12–13 asymptomatic myocardial ischemia,14 and changes in risk factors induced by therapeutic interventions.15 There is evidence of a direct relationship between increased carotid IMT and reduced left ventricular (LV) systolic and diastolic function assessed by myocardial strain in asymptomatic individuals without previous clinical CV disease.16 Elderly patients with HF differ from younger patients with HF in terms of several biologic characteristics, including the relatively large proportion of elderly patients with HF who have preserved systolic function.17–18 Using data from the Cardiovascular Health Study, which included subjects 65 years or older, atherosclerosis as measured by carotid IMT was shown to predict overt systolic and diastolic HF.19 The association of carotid IMT with incident HF in middle‐aged adults, who are at a lower risk of HF than older adults, is not well known. Previous cross‐sectional analysis using data from the ARIC cohort showed that participants with HF had a higher mean carotid IMT than participants without HF.20 These findings were not adjusted for CVD risk factors.
Subclinical vascular disease as determined by an increase in carotid IMT may be a determinant of risk for future HF, but this has not been studied. In the present study, we examine the hypothesis that subclinical atherosclerosis, assessed by mean carotid IMT, is associated with incident HF beyond what is explained by major CVD risk factors in middle‐aged whites and blacks. We tested this hypothesis using data from the Atherosclerosis Risk in Communities (ARIC) Study cohort.
Methods
Selection and Description of Participants
The ARIC study is community‐based prospective study which enrolled 15 792 participants aged 45 to 64 years at the time of their baseline assessment (1987–1989) from 4 communities: Forsyth County, North Carolina; suburban Minneapolis, Minnesota; Washington County, Maryland; and Jackson, Mississippi.21 In the cohort, response rates at baseline were 46% in Jackson and 65% to 67% for the other communities. Successful contact with living cohort members through an annual phone interview was above 93%. During the follow‐up period, annual phone interviews were conducted with participants to inquire about events (including HF), and hospital records were surveyed for identification and classification of HF events. The institutional review boards from each site approved the ARIC study.
Our analysis involved the use of data from the baseline examination. Excluded from the sample were: (1) participants who were missing all far wall values of carotid IMT at baseline (n=607);22 (2) racial groups other than black or white because of their limited numbers (n=48); (3) participants with missing criteria needed to define baseline glucose status (n=148); (4) participants with missing data needed to define HF at baseline (n=287); (5) prevalent cases of HF at baseline, either by self‐reported current intake of HF medication, or those with stage 3 or manifest HF by Gothenburg criteria (n=752).20,23 The Gothenburg criterion (Table 1) is composed of 3 scores, (1) cardiac, (2) pulmonary, and (3) therapy. To have stage 3 or manifest HF, the participant must have a point from each category. All current medications (taken within the last 2 weeks) were brought into the clinic and documented. Use of digoxin and diuretics was determined from these medication lists.20 Atrial fibrillation was diagnosed by visual inspection of a 2‐minute rhythm strip from leads V1, II, and V5 using standardized methodology.24 All other components were determined by participant self‐report.
Table 1.
Description of the Gothenburg Score
| Category | Gothenburg Components | Score |
|---|---|---|
| Cardiac | Coronary heart disease | 1 point if ever, 2 points if within the last year |
| Angina | 1 point if ever, 2 points if within the last year | |
| Leg edema | 1 point | |
| Shortness of breath at night | 1 point | |
| Rales on lung exam | 1 point | |
| Atrial fibrillation on ECG | 1 point | |
| Pulmonary | History of bronchitis | 1 point |
| History of asthma | 1 point | |
| Cough, phlegm, or wheezing | 1 point | |
| Rhonchi on lung exam | 1 point | |
| Therapy | Treatment with digoxin | 1 point |
| Treatment with diuretics | 1 point |
Modified with permission from Loehr et al.20 ECG indicates electrocardiogram.
Data Collection and Study Variables
ARIC study participants provided information on demographic and behavioral variables and medical history to a trained interviewer at each visit. Race, gender, educational level, current alcohol use, and smoking status were determined by self‐report at baseline. Medication use for dyslipidemia and hypertension were coded by trained personnel.
Measurement of Carotid IMT
Atherosclerosis of the common carotid arteries was measured by non‐invasive, high‐resolution B‐mode ultrasound.21 In this study, technicians scanned 3 specified segments of the extra cranial carotid arteries bilaterally: the common carotid, the bifurcation, and the internal carotid. The common carotid intima and media was assessed in a 1‐cm segment proximal to the dilatation of the carotid bulb. The intima and media of the bifurcation and internal carotid were assessed over the 1‐cm segments proximal and distal to the flow divider, respectively.25 A total of 6 sites were examined, 3 measurements in each carotid. The mean intimal‐medial thickness of the far wall of the 6 segments was used as a general indicator of atherosclerosis in the carotid artery.
The ultrasound examinations were performed according to a standardized protocol by trained, certified sonographers subject to semiannual evaluation.26 Ultrasound images were recorded on a videotape and forwarded to a central reading center for interpretation. The ultrasound readers were blinded from patient characteristics. For each of the 6 images, the IMT was measured over a 1‐cm segment at 1‐mm increments (total of 11 measurements). A mean wall thickness for each segment was then calculated. A mean carotid IMT adjusted for reader differences and reading date was then calculated. This protocol produces a single index of atherosclerosis with improved precision provided by the averaging of multiple IMT measurements.25
In a randomly selected subset of 855 participants, the between‐reader reliability coefficients ranged from 0.78 to 0.93 and coefficients of variation ranged from 13.1% to 18.3% (≥80% of duplicate scans differed by <0.267 mm).26–27
Ascertainment of Heart Failure
Incident HF was defined as the first occurrence of either (1) an HF hospitalization that included an International Classification of Diseases (ICD), ninth revision, discharge code of 428.x (428.0 to 428.9) in any position, or (2) a death certificate with a 428 (HF) or ICD, 10th revision, code I50 (HF) in any position. All hospitalizations occurring in cohort participants were identified either through generated computer programs, or review of hospital discharge indexes or information elicited during the annual follow‐up interview. Hospitalizations eligible based on set criteria (discharge codes, discharge dates, race sex, and age) but were found upon review to have been hospitalized for less than 24 hours were not abstracted. Abstractors made copies of sections of the medical record (discharge summary, history and physical report, admission note, and imaging reports) for use by the ARIC Heart Failure Mortality and Morbidity Classification Committee (HF MMCC). The inter‐abstractor agreement rate for determining whether or not to conduct detailed abstraction in a quality control sample was 99%. The ARIC community surveillance database was also searched for possible HF events occurring among cohort participants that were not reported at the annual follow‐up visit or may be otherwise missed.
Beginning in 2005, the ARIC Study conducted continuous, retrospective surveillance of hospital discharges for HF for all residents aged 55 and older in the 4 ARIC communities via annual hospital discharge indices. Each hospitalization eligible for full abstraction was independently reviewed by centrally trained and certified physicians on the ARIC HF MMCC and classified into 1 of 5 categories: definite acute decompensated HF, possible acute decompensated HF, chronic stable HF, HF unlikely, or unclassifiable.28 Differences among these reviewers are adjudicated by the Chair of the HF MMCC. HF cases occurring prior to 2005 were not adjudicated. The % agreement between any HF event adjudicated by the HF MMCC and ICD‐9 code 428 was 75%, similar to that with the Framingham HF criteria (71%).28
Follow‐up time for those with incident HF events was defined by the time from their baseline exam (visit 1) until the incident event. The end of follow‐up time for those without HF was (1) December 31, 2009, (2) date of last contact for those lost to follow‐up, or (3) date of death, whichever occurred first.
Measurement of Other Study Variables
Prevalent CHD at baseline was defined as a self‐reported history of MI, MI from adjudicated baseline ECG data, a history of physician‐diagnosed MI, a prior coronary reperfusion procedure. Incident CHD was defined as any case of hospitalized MI, fatal CHD, or ECG‐diagnosed MI by the end of the study period.
Three systolic and diastolic blood pressures were taken with participants in the sitting position after 5 minutes of rest using a random‐zero sphygmomanometer. The average of the second and third readings was recorded. Certified technicians measured height, weight, and waist circumference. Body mass index was calculated as weight (in kg) divided by the square of the height (in meters). Waist circumference was measured at the umbilicus.
Fasting serum glucose was measured by the optimized direct analysis in real time (DART) GLUCOSE reagent method. Diabetes mellitus was defined according to measured fasting glucose level of ≥7.0 mmol/L (≥126 mg/dL), self‐reported previous physician diagnosis, or use of diabetes medication (oral hypoglycemic agents and/or insulin), or a non‐fasting glucose of ≥11 mmol/L (≥200 mg/dL). Impaired fasting glucose was defined by a fasting glucose level between 5.6 and 6.9 mmol/L (100 to 125 mg/dL) in accordance with the 2004 American Diabetes Association definition. Normal fasting glucose was defined as any other participant who does not meet the criteria for DM and IFG. Triglycerides and HDL cholesterol were determined using enzymatic methods. LDL cholesterol was calculated using the Friedewald equation.29 Serum creatinine concentration was measured using a modified kinetic Jaffe method.
Statistical Analysis
Unadjusted differences in baseline characteristics between participants with and without HF were assessed using the Student's t test (for continuous variables) and chi‐square test (for categorical variables). The association of carotid IMT with incident heart failure was assessed using Cox proportional hazard models with sequential adjustment for covariates. Hazard ratios and 95% confidence intervals for Cox regression analysis are presented per unit standard deviation increase and by quartiles of IMT. The base model was an unadjusted model.
In multivariable analysis, the base model (unadjusted model) was sequentially adjusted for covariates of interest. A second model was adjusted for age, gender, race, and level of education (demographic model). A third model was further adjusted for systolic and diastolic blood pressures, BMI, waist circumference, HDL cholesterol, LDL cholesterol, triglycerides, smoking status and amount in pack‐years, alcohol consumption, serum creatinine, and medications for hypertension and dyslipidemia (clinical and biologic model). Finally, a fourth model was adjusted for prevalent and incident CHD (CHD model). For the fourth model, incident CHD was modeled as a time‐varying covariate. Covariates were chosen based on their associations in the present cohort and in prior published studies.13,30
Effect modification between race, gender, and carotid IMT was examined using 2‐way interaction. This was achieved by including the product the IMT×race and IMT×gender terms in the model. In the presence of a significant interaction (P for interaction <0.05), we performed a stratified analysis for the covariate in question.
Separate Cox regression models included carotid IMT as a continuous variable (Z‐scores) and as a categorical variable (quartiles). The proportional hazard assumption was assessed by visually examining the log (‐log survival) plots and time‐covariate interaction terms. Kaplan‐Meier plots were used to illustrate the overall survival probability and cumulative incidence of HF by subgroups, and group comparison was done using the log‐rank test. All analyses were performed using SAS 9.2 (SAS Institute Inc, Cary, NC). Statistical significance was inferred at 2‐sided P<0.05.
Results
Baseline Characteristics of Participants
Among the 13 590 participants in this study, there were 2008 (14.8% of total cohort) incident cases of HF over a median follow‐up period of 20.6 years. The overall unadjusted incidence rate of HF was 8.1 cases per 1000 person‐years. Table 2 compares the baseline demographic and clinical characteristics of participants with and without incident HF. Compared with participants without HF, participants with HF were older (56.6 versus 53.7 years), more likely to be male (52.5% versus 44.1%), and more likely to be hypertensive (50.3% versus 28.6%). Participants who developed HF over the course of follow‐up were also more likely, at baseline, to have an abnormal lipid profile, were current smokers but less likely to be current drinkers and to have completed a college education. The baseline prevalence of CHD and incidence of CHD were higher among subjects with HF (P<0.0001).
Table 2.
Baseline Demographic and Clinical Characteristics by Incident Heart Failure Status of ARIC Participants
| Characteristic | Overall (N=13 590) | Incident Heart Failure Status | |
|---|---|---|---|
| Subjects With HF (N=2008) | Subjects Without HF (N=11 582) | ||
| Age, y | 54.1±5.8 | 56.6±5.5 | 53.7±5.7 |
| Males, n (%) | 6166 (45.3) | 1057 (52.5) | 5109 (44.1) |
| White, n (%) | 10 227 (75.2) | 1378 (68.5) | 8849 (76.4) |
| Hypertension, n (%) | 4320 (31.8) | 1010 (50.3) | 3310 (28.6) |
| Systolic blood pressure, mm Hg | 120.8±18.6 | 128.0±20.6 | 119.5±18.0 |
| Diastolic blood pressure, mm Hg | 73.5±11.2 | 74.9±12.7 | 73.2±10.9 |
| Brachial pulse pressure, mm Hg | 47.3±13.4 | 53.1±15.5 | 46.3±12.7 |
| Blood pressure medication, n (%) | 3619 (26.6) | 893 (44.4) | 2,726 (23.5) |
| BMI, kg m−2 | 27.3±5.0 | 29.1±5.7 | 27.0±4.8 |
| Waist circumference, cm | 96.1±13.4 | 101.7±14.1 | 95.1±13.0 |
| Education level | |||
| Less than college education | 8688 (64.0) | 1512 (75.3) | 7176 (62.0) |
| Completed at least college | 4894 (36.0) | 497 (24.7) | 4397 (38.0) |
| Smoking status, % | |||
| Never | 5669 (41.7) | 605 (30.1) | 5064 (43.7) |
| Former smoker | 4381 (32.2) | 677 (33.7) | 3704 (32.0) |
| Current smoker | 3542 (26.1) | 728 (36.2) | 2814 (24.3) |
| Alcohol consumption, % | |||
| Never | 3315 (24.5) | 546 (27.3) | 2769 (24.0) |
| Former drinker | 2472 (18.2) | 521 (26.0) | 1951 (16.9) |
| Current drinker | 7761 (57.3) | 932 (46.6) | 6829 (59.1) |
| Total Cholesterol, mmol/L | 5.6±1.1 | 5.7±1.2 | 5.5±1.1 |
| LDL cholesterol, mmol/L | 3.6±1.0 | 3.7±1.1 | 3.5±1.0 |
| HDL cholesterol, mmol/L | 1.3±0.4 | 1.2±0.4 | 1.4±0.5 |
| Triglycerides, mmol/L* | 1.2 (0.9 to 1.7) | 1.4 (1.0 to 2.0) | 1.2 (0.9 to 1.7) |
| Lipid‐lowering medication, n (%) | 2937 (21.7) | 728 (36.5) | 2209 (19.2) |
| Fasting glucose level, mmol/L | 5.96±2.11 | 5.7±1.4 | 5.5±0.8 |
| Blood glucose category, n (%) | |||
| Normal fasting glucose | 7594 (55.8) | 835 (41.5) | 6,759 (58.3) |
| Impaired fasting glucose | 4574 (33.6) | 648 (32.2) | 3,926 (33.9) |
| Diabetes mellitus | 1431 (10.5) | 529 (26.3) | 902 (7.8) |
| Prevalent CHD, n (%) | 555 (4.1) | 240 (12.1) | 315 (2.7) |
| Carotid IMT, mm | 0.72±0.18 | 0.81±0.23 | 0.71±0.17 |
| Incident CHD, n (%) | 1727 (12.7) | 827 (41.1) | 900 (7.8) |
| Prevalent LVH, n (%) | 271 (2.0) | 109 (5.6) | 162 (1.4) |
Data are mean±standard deviation (SD), or number (percentages). All comparisons were significant at P<0.0001. BMI indicates body mass index; CHD, coronary heart disease; HDL, high density lipoprotein; HF, heart failure; IMT, intima‐media thickness; LDL, low density lipoprotein; LVH, left ventricular hypertrophy.
Data for triglycerides is presented as median (Q1 to Q3).
Association of Carotid IMT With Incident HF
Mean carotid IMT was significantly higher for subjects with HF (0.81±0.23 versus 0.71±0.17; P<0.0001), Table 2. Compared with subjects in the first (lowest) quartile of carotid IMT (IMT≤0.61 mm), the unadjusted incidence rates of HF increased across the second, third, and fourth quartiles (Table 3). The rate in the highest quartile of carotid IMT was 15.42 cases per 1000 person‐years (about 4 times the rate in the first quartile, and twice the rate in the third quartile). Figure 1 shows a Kaplan‐Meier plot of the overall survival probability of HF over time as a function of carotid IMT quartiles. There was a significant difference in event‐free survival between quartiles of IMT (P<0.0001) (Figure 1). Of note, participants in the fourth quartile had a significantly lower survival; 74% of those in the fourth quartile were HF‐free at 20 years, compared with 92% in the first quartile.
Table 3.
Unadjusted and Adjusted HRs (95% CI) for Incident Heart Failure across Quartiles of Carotid IMT
| Models | Quartiles of IMT (mm) | |||
|---|---|---|---|---|
| Q1 (<0.62) | Q2 (0.62 to 0.69) | Q3 (0.70 to 0.79) | Q4 (>0.79) | |
| Number of HF events | 268 | 389 | 482 | 869 |
| Rate/1000 pyrs (95% CI) | 3.9 (3.4, 4.4) | 6.0 (5.4, 6.6) | 8.3 (7.6, 9.0) | 15.4 (14.4, 16.5) |
| Model 1, HR (95% CI) | 1.0 (ref.) | 1.57 (1.35, 1.84)* | 2.20 (1.89, 2.55)* | 4.26 (3.71, 4.88)* |
| Model 2, HR (95% CI) | 1.0 (ref.) | 1.30 (1.11, 1.52)† | 1.59 (1.37, 1.85)* | 2.59 (2.23, 2.99)* |
| Model 3, HR (95% CI) | 1.0 (ref.) | 1.10 (0.93, 1.29) | 1.14 (0.98, 1.34) | 1.65 (1.42, 1.93)* |
| Model 4, HR (95% CI) | 1.0 (ref.) | 1.09 (0.92, 1.28) | 1.14 (0.97, 1.33) | 1.60 (1.37, 1.87)* |
CI indicates confidence interval; HF, heart failure; HR, hazard ratio; IMT, intima‐media thickness; pyrs, person‐years; Q, quartile; Ref., reference.
*P<0.0001, †P=0.001; P<0.0001 for trend in all 4 models. For each model, the HR for Q2, Q3, and Q4 are in comparison to the reference model, Q1. Model 1: unadjusted; Model 2: adjusted for age, gender, race, education; Model 3: model 2 plus systolic and diastolic blood pressure, BMI, waist circumference, LDL, HDL, triglycerides, smoking, alcohol, hypertension and cholesterol medication, serum creatinine; Model 4: model 3 plus prevalent and incident CHD.
Figure 1.
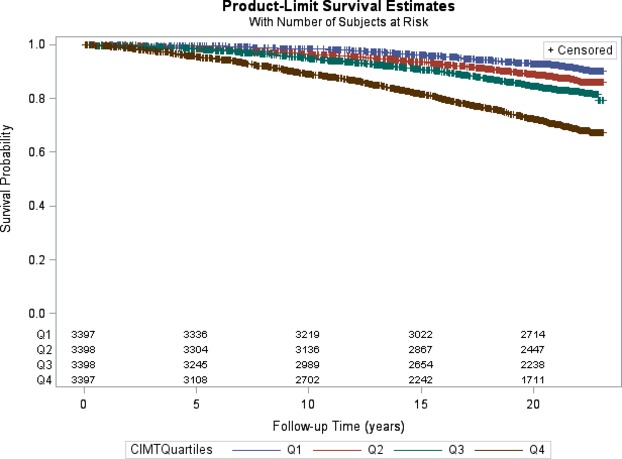
Kaplan‐Meier curves illustrating the HF‐free survival probability over time as a function of quartiles of carotid IMT. CIMTQuartiles indicates quartiles of carotid IMT; Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile. Logrank P<0.0001. HF indicates heart failure; IMT, intima‐media thickness.
In the multivariable analysis, we estimated the HR of HF across quartiles of carotid IMT (Table 3). After adjustment for demographic factors, the second, third, and fourth quartiles of carotid IMT were significantly associated with HF compared with the first quartile of IMT (HR for fourth quartile 2.59, 95% CI: 2.23 to 2.99). The strength of this association was reduced, but significantly persisted for the fourth quartile of carotid IMT after further adjustments for CVD risk factors (HR 1.65, 95% CI: 1.42, 1.93, <0.0001) and CHD (HR 1.60, 95% CI: 1.37 to 1.87, P<0.0001). There was a significant trend in the relationship between carotid IMT and HF across quartiles of IMT (P<0.0001) in all 4 models. Even after accounting for death from other causes as a competing risk event, the cumulative incidence functions of HF across quartiles of carotid IMT remained significant (P<0.0001) (Figure 2).
Figure 2.
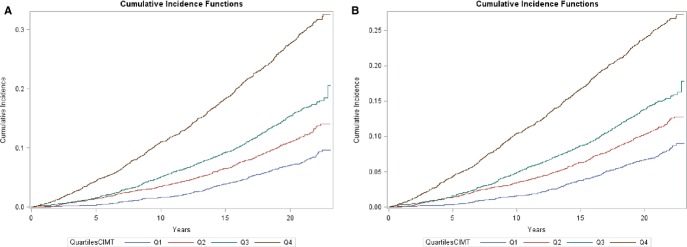
Cumulative incidence function curves of HF by quartiles of carotid IMT. A, Analysis does not take into account death from other causes as a competing risk. B, Analysis accounts for death from other causes as a competing risk. QuartilesCIMT indicates quartiles of carotid IMT; Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile. Gray's test for equality (both plots): P<0.0001. HF indicates heart failure; IMT, intima‐media thickness.
Similar results were observed with carotid IMT modeled as a continuous variable. The hazard ratios (HRs) and 95% confidence intervals of HF per unit SD (0.18 mm) increase in IMT are given in Table 4. No interactions were observed by gender or race (the P values for the IMT×race and IMT×gender interaction terms in the unadjusted model were 0.97 and 0.11, respectively).
Table 4.
Unadjusted and Adjusted HRs (95% CI) for Incident Heart Failure per 1 SD (0.18 mm) Increase in Carotid IMT
| Clinical Outcome: Heart Failure | HR | 95% CI | P Value |
|---|---|---|---|
| Model 1 (unadjusted) | 1.54 | 1.50, 1.58 | <0.0001 |
| Model 2 | 1.38 | 1.33, 1.42 | <0.0001 |
| Model 3 | 1.21 | 1.16, 1.25 | <0.0001 |
| Model 4 | 1.20 | 1.16, 1.25 | <0.0001 |
BMI indicates body mass index; CHD, coronary heart disease; CI confidence interval; HDL, high density lipoprotein; HR, hazard ratio; HF, heart failure; IMT, intima‐media thickness; LDL, low density lipoprotein.
Model 2: adjusted for age, gender, race, education; Model 3: model 2 plus systolic and diastolic blood pressure, BMI, waist circumference, LDL, HDL, triglycerides, smoking, alcohol, hypertension and cholesterol medication, serum creatinine; Model 4: model 3 plus prevalent and incident CHD.
Relationship Between Carotid IMT and Brachial Pulse Pressure (PP)
Mean brachial PP was higher among those with HF than those without the condition (53.1±15.5 mm Hg versus 46.3±12.7 mm Hg; P<0.0001, respectively). In univariate analysis, carotid IMT correlated directly with brachial PP (R=0.25, P<0.0001). When brachial PP was included in multivariable models, no significant modification of HF risk was observed.
Association of Carotid IMT with Incident HF by Race
Overall mean carotid IMT was not significantly different between blacks and whites (0.73 mm±0.16 versus 0.72 mm±0.19, respectively, P=0.16). The unadjusted incidence rate of HF was higher for blacks than whites (10.8 versus 7.22 cases per 1000 person‐years) (Table 5).
Table 5.
Unadjusted and Adjusted HRs (95% CI) for Incident Heart Failure Per 1 SD (0.18 mm) Increase in Carotid IMT by Race
| Models | Race | ||
|---|---|---|---|
| Whites (N=10 221) | Blacks (N=3369) | ||
| Number of HF cases | 1376 | 632 | |
| Rate/1000 person‐years (95% CI) | 7.22 (6.86, 7.62) | 10.80 (9.99, 11.67) | |
| Model 1 (unadjusted) | HR (95% CI) | 1.56 (1.51, 1.61) | 1.54 (1.45, 1.65) |
| Model 2 | HR (95% CI) | 1.38 (1.33, 1.43) | 1.35 (1.25, 1.45) |
| Model 3 | HR (95% CI) | 1.19 (1.14, 1.25) | 1.23 (1.14, 1.34) |
| Model 4 | HR (95% CI) | 1.20 (1.14, 1.25) | 1.22 (1.12, 1.32) |
CI indicates confidence interval; HF, heart failure; HR, hazard ratio; IMT, intima‐media thickness.
Model 2: adjusted for age, gender, race, education; Model 3: model 2 plus systolic and diastolic blood pressure, BMI, waist circumference, LDL, HDL, triglycerides, smoking, alcohol, hypertension and cholesterol medication, serum creatinine; Model 4: model 3 plus prevalent and incident CHD.
In multivariable analysis, carotid IMT was associated with incident HF in both blacks and whites. Although the strength of the association decreased progressively in both blacks and whites with adjustment of more covariates, the association was only slightly attenuated; for example, in blacks the HR was 1.54 per unit SD increase in IMT (95% CI, 1.45 to 1.65) for the unadjusted model and 1.23 (95% CI, 1.12 to 1.32) after adjustment for demographic, clinical, and biological variables, and 1.22 (95% CI, 1.12 to 1.32) after adjustment for CHD.
The results of the association of increasing carotid IMT and incident HF by gender paralleled that by race.
Discussion
The objective of this study was to investigate the association of carotid IMT with incident HF among middle‐aged adults. Carotid IMT was significantly associated with incident HF among whites and blacks, after adjusting for demographic and major CVD risk factors, and CHD. This relationship was comparable in both blacks and whites, as well as in males and females.
Carotid IMT is a well validated measure of pre‐clinical atherosclerotic lesions.10–11 In this population‐based study, we found mean far wall carotid IMT to be 0.72±0.18 mm. Similar mean far wall estimates have been reported in other populations of similar age groups; in the Carotid Atherosclerosis Progression Study31 and Malmo Diet and Cancer Study,32 mean far wall IMT was 0.73±0.16 mm and 0.77±0.15 mm, respectively. The present study has shown that carotid IMT is associated with incident HF, with IMT modeled as both a continuous and categorical variable, even after taking into account associations explained by age, gender, race, blood pressure, BMI, waist circumference, HDL cholesterol, LDL cholesterol, triglycerides, cigarette smoking, alcohol, and serum creatinine, as well as medications for dyslipidemia and hypertension. Our result is consistent with that described by Engstrom et al,30 who reported a significant association of increased IMT and HF hospitalizations in a sample of 4691 subjects with 75 cases of HF. Moreover, our results remained significant even after adjustment for prevalent and incident cases of CHD, which has been reported to be a major cause of HF.
Our study's finding that the association of carotid IMT with HF remained significant after adjustment for prevalent and incident CHD, as well as blood pressure, and other traditional CVD risk factors suggests that carotid IMT may be associated with HF through a mechanism that is different from that causing discrete clinical episodes of myocardial ischemia or infarction. This concept is strengthened by demonstrating that results were only slightly attenuated after adjustment for CHD. There are a number of potential mechanisms that could explain this association. First, increasing carotid IMT leads to structural changes of the artery wall, which result in the deposition of collagen in the intracellular matrix33 and a decrease in arterial distensibility,34 causing increased pressure afterload, pressure wave propagation, and eventually diastolic dysfunction.33,35 We found carotid IMT to be directly correlated with brachial pulse pressure (PP), but no significant change was observed when brachial PP was added to the multivariable models. Boutouyrie et al36 reported a similar correlation between brachial PP and carotid PP with carotid IMT. However, only carotid PP significantly influenced carotid wall thickness, indicating that carotid PP (a measure of arterial stiffening) may be superior to brachial PP in exploring the mechanistic relationship between IMT and HF. A second potential mechanism relates to the finding from prior studies that increasing common carotid IMT was associated with reduced myocardial flow reserve in adults with37 and without38–40 CHD. Some prospective and cross‐sectional studies have established an association between increasing carotid IMT and regional LV myocardial systolic and diastolic dysfunction,16,41 a subclinical marker and strong predictor of HF.42 It may be necessary to further characterize HF cases (HFpEF versus HFrEF) in their relation to carotid IMT to fully understand the different mechanisms that could contribute to HF in subjects with increased carotid IMT. Baseline echocardiographic data is not available in the ARIC study and cardiac ultrasound data at the time of incident HF (obtained by review of medical records) is available only for events occurring after January 2005. As such, HF type in our sample could not be classified in more than 86% cases.
There are significant differences in the etiology of HF among blacks and whites. Bourassa et al, using data from the Studies of Left Ventricular Dysfunction (SOLVD), reported that ischemic heart disease accounts for a majority of HF cases (73%) in whites than in blacks (36%). Another third of cases in blacks was due to hypertensive heart disease, compared with only 4% in whites.43 Despite these known racial differences in the etiology of HF, we found that an association of increasing carotid IMT with incident HF was observed in both whites and blacks, and to comparable degrees.
To the best of our knowledge, our study is the first to investigate the association of carotid IMT with incident HF in a large middle‐aged cohort with more than 2 decades of follow‐up and more than 2000 cases of incident HF. The use of a middle‐aged population that is at a lower risk of HF compared with the elderly is particularly important. There are nonetheless a number of limitations to this study that should be mentioned. First, we did not adjust our analyses for novel biomarkers that could potentially influence the relationship between carotid IMT and HF, such as N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) and high‐sensitivity C‐reactive protein. However, in a prior published study, the association of carotid IMT with HF remained significant, even after adjustment for these markers.30 Second, the use of hospital discharge codes and death registers to recruit HF cases may have underestimated HF incidence in the population. Also, these may have been severe cases of HF with complications. However, the fact that increased carotid IMT has been shown to be associated with reduced systolic and diastolic myocardial strain in asymptomatic individuals16 suggests an association of carotid IMT with less severe cases of HF. Our analyses included both adjudicated and non‐adjudicated cases of HF. In a separate sensitivity analysis (data not shown), there was no significant difference in the hazard ratios of HF prior to and after the adjudication process. Third, our cohort comprised of only blacks and whites, so caution should be made when generalizing these results to other race/ethnic groups. Finally, in our study, carotid IMT estimates used were based on mean far wall measurements only. Depending on the segment of the carotid artery measured (common, bifurcation, or internal carotid), the portion of the artery wall measured (far or near wall), or the variable used (mean or maximum IMT), different studies may report conflicting results on the association between IMT and CVD risk.44–45 Therefore caution should be exercised when comparing our findings to other studies employing a different ultrasound protocol.
Conclusion
In this cohort of middle‐aged blacks and whites, we have demonstrated that increasing thickness of the carotid intima and media is associated with the incidence of HF even beyond the risks accounted for by traditional CVD risk factors and CHD.
Implications
Carotid IMT is appealing because it has been shown in several population‐based studies to predict future risk of myocardial infarction and stroke, and our study shows that HF is also predicted by increasing carotid IMT. Carotid IMT is a reliable, low‐cost, low‐risk indicator of early atherosclerosis currently used in cardiovascular research. Measurement of carotid IMT can be quickly and easily accomplished in a clinical setting, and with modern ultrasound scanners this procedure becomes more efficient and reproducible.
Findings from our study indicate that carotid IMT may mediate HF through a mechanism that is different from myocardial ischemia or infarction. Carotid IMT may be useful in future HF risk prediction among middle‐aged blacks and whites.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1.Opasich C, Ambrosino N, Felicetti G, Aquilani R, Pasini E, Bergitto D, Mazza A, Cobelli F, Tavazzi L. Heart failure‐related myopathy. Clinical and pathophysiological insights. Eur Heart J. 1999; 20:1191-1200. [DOI] [PubMed] [Google Scholar]
- 2.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997; 133:703-712. [DOI] [PubMed] [Google Scholar]
- 3.Tavazzi L. Practical advice on the treatment of cardiac insufficiency with beta‐blockers. Announcement by the Italian Association of Hospital‐Based Cardiologists. Rev Port Cardiol. 1999; 18:556-557. [PubMed] [Google Scholar]
- 4.O'connell AM, Crawford MH, Abrams J. Heart failure disease management in an indigent population. Am Heart J. 2001; 141:254-258. [DOI] [PubMed] [Google Scholar]
- 5.Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc. 1997; 45:968-974. [DOI] [PubMed] [Google Scholar]
- 6.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins‐Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med. 2009; 169:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009; 54:1695-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagard R. Reversal of left ventricular hypertrophy. JAMA. 1996; 276:1636-1637. [PubMed] [Google Scholar]
- 9.Gillum RF. Epidemiology of heart failure in the United States. Am Heart J. 1993; 126:1042-1047. [DOI] [PubMed] [Google Scholar]
- 10.Adams MR, Celermajer DS. Detection of presymptomatic atherosclerosis: a current perspective. Clin Sci (Lond). 1999; 97:615-624. [PubMed] [Google Scholar]
- 11.Poli A, Tremoli E, Colombo A, Sirtori M, Pignoli P, Paoletti R. Ultrasonographic measurement of the common carotid artery wall thickness in hypercholesterolemic patients. A new model for the quantitation and follow‐up of preclinical atherosclerosis in living human subjects. Atherosclerosis. 1988; 70:253-261. [DOI] [PubMed] [Google Scholar]
- 12.Hodis HN, Mack WJ, Labree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med. 1998; 128:262-269. [DOI] [PubMed] [Google Scholar]
- 13.O'leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999; 340:14-22. [DOI] [PubMed] [Google Scholar]
- 14.Nagai Y, Metter EJ, Earley CJ, Kemper MK, Becker LC, Lakatta EG, Fleg JL. Increased carotid artery intimal‐medial thickness in asymptomatic older subjects with exercise‐induced myocardial ischemia. Circulation. 1998; 98:1504-1509. [DOI] [PubMed] [Google Scholar]
- 15.De Groot E, Jukema JW, Montauban van Swijndregt AD, Zwinderman AH, Ackerstaff RG, Van der Steen AF, Bom N, Lie KI, Bruschke AV. B‐mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: a report of the Regression Growth Evaluation Statin Study (REGRESS). J Am Coll Cardiol. 1998; 31:1561-1567. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes VR, Polak JF, Edvardsen T, Carvalho B, Gomes A, Bluemke DA, Nasir K, O'leary DH, Lima JA. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2006; 47:2420-2428. [DOI] [PubMed] [Google Scholar]
- 17.Hogg K, Swedberg K, Mcmurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004; 43:317-327. [DOI] [PubMed] [Google Scholar]
- 18.Senni M, Redfield MM. Heart failure with preserved systolic function. A different natural history? J Am Coll Cardiol. 2001; 38:1277-1282. [DOI] [PubMed] [Google Scholar]
- 19.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000; 35:1628-1637. [DOI] [PubMed] [Google Scholar]
- 20.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008; 101:1016-1022. [DOI] [PubMed] [Google Scholar]
- 21. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The Aric Investigators. Am J Epidemiol. 1989; 129:687-702. [PubMed] [Google Scholar]
- 22.Stevens J, Tyroler HA, Cai J, Paton CC, Folsom AR, Tell GS, Schreiner PJ, Chambless LE. Body weight change and carotid artery wall thickness. The Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1998; 147:563-573. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical‐epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987; 8:1007-1014. [DOI] [PubMed] [Google Scholar]
- 24.Vitelli LL, Crow RS, Shahar E, Hutchinson RG, Rautaharju PM, Folsom AR. Electrocardiographic findings in a healthy biracial Population. Atherosclerosis Risk In Communities (Aric) Study Investigators. Am J Cardiol. 1998; 81:453-459. [DOI] [PubMed] [Google Scholar]
- 25.Howard G, Wagenknecht LE, Burke GL, Diez‐Roux A, Evans GW, Mcgovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998; 279:119-124. [DOI] [PubMed] [Google Scholar]
- 26. High‐resolution B‐mode ultrasound scanning methods in the Atherosclerosis risk in Communities Study (ARIC). The Aric Study Group. J Neuroimaging. 1991; 1:68-73. [PubMed] [Google Scholar]
- 27. High‐resolution B‐mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. The Aric Study Group. J Neuroimaging. 1991; 1:168-172. [PubMed] [Google Scholar]
- 28.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012; 5:152-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499-502. [PubMed] [Google Scholar]
- 30.Engstrom G, Melander O, Hedblad B. Carotid intima‐media thickness, systemic inflammation, and incidence of heart failure hospitalizations. Arterioscler Thromb Vasc Biol. 2009; 29:1691-1695. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz MW, Von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima‐media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (Caps). Stroke. 2006; 37:87-92. [DOI] [PubMed] [Google Scholar]
- 32.Rosvall M, Janzon L, Berglund G, Engstrom G, Hedblad B. Incident coronary events and case fatality in relation to common carotid intima‐media thickness. J Intern Med. 2005; 257:430-437. [DOI] [PubMed] [Google Scholar]
- 33.Benetos A, Laurent S, Asmar RG, Lacolley P. Large artery stiffness in hypertension. J Hypertens Suppl. 1997; 15:S89-S97. [DOI] [PubMed] [Google Scholar]
- 34.Lage SG, Kopel L, Monachini MC, Medeiros CJ, Pileggi F, Polak JF, Creager MA. Carotid arterial compliance in patients with congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 1994; 74:691-695. [DOI] [PubMed] [Google Scholar]
- 35.Cuspidi C, Lonati L, Macca G, Sampieri L, Fusi V, Michev I, Severgnini B, Salerno M, Magrini F, Zanchetti A. Prevalence of left ventricular hypertrophy and carotid thickening in a large selected hypertensive population: impact of different echocardiographic and ultrasonographic diagnostic criteria. Blood Press. 2001; 10:142-149. [DOI] [PubMed] [Google Scholar]
- 36.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large‐artery remodeling. Circulation. 1999; 100:1387-1393. [DOI] [PubMed] [Google Scholar]
- 37.Sonoda M, Yonekura K, Yokoyama I, Takenaka K, Nagai R, Aoyagi T. Common carotid intima‐media thickness is correlated with myocardial flow reserve in patients with coronary artery disease: a useful non‐invasive indicator of coronary atherosclerosis. Int J Cardiol. 2004; 93:131-136. [DOI] [PubMed] [Google Scholar]
- 38.Dayanikli F, Grambow D, Muzik O, Mosca L, Rubenfire M, Schwaiger M. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. 1994; 90:808-817. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama I, Ohtake T, Momomura S, Nishikawa J, Sasaki Y, Omata M. Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis. Circulation. 1996; 94:3232-3238. [DOI] [PubMed] [Google Scholar]
- 40.Raitakari OT, Toikka JO, Laine H, Ahotupa M, Iida H, Viikari JS, Hartiala J, Knuuti J. Reduced myocardial flow reserve relates to increased carotid intima‐media thickness in healthy young men. Atherosclerosis. 2001; 156:469-475. [DOI] [PubMed] [Google Scholar]
- 41.Parrinello G, Colomba D, Bologna P, Licata A, Pinto A, Paterna S, Scaglione R, Licata G. Early carotid atherosclerosis and cardiac diastolic abnormalities in hypertensive subjects. J Hum Hypertens. 2004; 18:201-205. [DOI] [PubMed] [Google Scholar]
- 42.Yan RT, Bluemke D, Gomes A, Burke G, Shea S, Liu K, Bahrami H, Sinha S, Wu C, Fernandes V, Mcclelland R, Lima JA. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (multi‐ethnic study of atherosclerosis). J Am Coll Cardiol. 2011; 57:1735-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourassa MG, Gurne O, Bangdiwala SI, Ghali JK, Young JB, Rousseau M, Johnstone DE, Yusuf S. Natural history and patterns of current practice in heart failure. The Studies Of Left Ventricular Dysfunction (SOLVD) Investigators. J Am Coll Cardiol. 1993; 22:14a-19a. [DOI] [PubMed] [Google Scholar]
- 44.Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engstrom G, Evans GW, De Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O'leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML. Common carotid intima‐media thickness measurements in cardiovascular risk prediction: a meta‐analysis. JAMA. 2012; 308:796-803. [DOI] [PubMed] [Google Scholar]
- 45.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation. 2007; 115:459-467. [DOI] [PubMed] [Google Scholar]


