Abstract
Background
Endothelium‐derived acetylcholine (eACh) plays an important role in the regulation of vascular actions in response to hypoxia, whereas arterial baroreflex (ABR) dysfunction impairs the eACh system. We investigated the effects of ABR dysfunction on ischemia‐induced angiogenesis in animal models of hindlimb ischemia with a special focus on eACh/nicotinic ACh receptor (nAChR) signaling activation.
Methods and Results
Male Sprague‐Dawley rats were randomly assigned to 1 of 3 groups that received (1) sham operation (control group), (2) sinoaortic denervation (SAD)‐induced ABR dysfunction (SAD group), or (3) SAD rats on diet with an acetylcholinesterase inhibitor pyridostigmine (30 mg/kg per day, SAD+Pyr group). After 4 weeks of the SAD intervention, unilateral limb ischemia was surgically induced in all animals. At postoperative day 14, SAD rats exhibited impaired angiogenic action (skin temperature and capillary density) and decreased angiogenic factor expressions (vascular endothelial growth factor [VEGF] and hypoxic inducible factor [HIF]‐1α) in ischemic muscles. These changes were restored by acetylcholinesterase inhibition. Rats with ABR dysfunction had lower eACh levels than did control rats, and this effect was recovered in SAD+Pyr rats. In α7‐nAChR knockout mice, pyridostigmine improved ischemia‐induced angiogenic responses and increased the levels of VEGF and HIF‐1α. Moreover, nicotinic receptor blocker inhibited VEGF expression and VEGF receptor 2 phosphorylation (p‐VEGFR2) induced by ACh analog.
Conclusions
Thus, ABR dysfunction appears to impair ischemia‐induced angiogenesis through the reduction of eACh/α7‐nAChR‐dependent and ‐independent HIF‐1α/VEGF‐VEGFR2 signaling activation.
Keywords: acetylcholinesterase inhibitor, angiogenesis, arterial baroreflex, non‐neural cholinergic system, peripheral vascular disease
Introduction
Peripheral artery disease (PAD) is a growing health concern worldwide. Critical limb ischemia is a severe manifestation of PAD, characterized by intractable pain, ulcer, and gangrene. The clinical prognosis for patients with this form of artery disease is poor, because up to one third of PAD patients are not amenable to conventional interventions, such as percutaneous angioplasty or surgical bypass, and they will ultimately require amputation of the limb.1 Therapeutic neovascularization has been proposed as an alternative treatment in these “no”‐option patients, and both growth cytokines and cells have shown impressive efficacy in the laboratory.2 However, recent clinical trials of angiogenic therapies for ischemic disease have achieved only modest outcomes.3–4 The mechanisms by which vascular angiogenic action is impaired in PAD are not yet well understood.
Arterial baroreflex (ABR) is an important mechanism in the regulation of cardiovascular activities.5 It has been reported that decreased ABR sensitivity accelerates the progression of cardiovascular diseases (CVDs).6–9 ABR dysfunction has been shown to promote atherosclerotic plaque growth10–11 and it is indeed a target for treatment of atherosclerosis‐related cerebral artery and coronary artery disease.12–13 Recent evidence demonstrates that angiogenesis is essential in reestablishing blood supply to ischemic tissues and, consequently, to recovery of ischemic organ function in response to hypoxic injury.14–16 These data suggested that impaired atherogenic vascular angiogenic action was associated with ABR dysfunction.
ABR deficiency has been proven to impair coronary vascular supply and reduce vascular growth in myocardium after myocardial infarction (MI).12 The activation of vascular endothelial growth factor (VEGF)‐ and its receptor 2 (VEGFR2)‐dependent angiogenic pathways represents a critical molecular mechanism by which ABR stimulation triggers angiogenesis.17 Recently, several researches demonstrated that the non‐neuronal cholinergic system is a critical modulator for angiogenesis.18–23 Nicotine has been reported to stimulate angiogenesis as well.18,20 We were curious about whether endothelium‐derived acetylcholine (eACh) was involved in ischemia‐induced angiogenic response and modulated by ABR dysfunctional status.
In addition, the function of α7‐nicotinic ACh receptor (α7‐nAChR) has been proven to be involved in proangiogenic signaling pathways, and pharmacological intervention‐mediated ABR stimulation improves receptor signaling in infarcted myocardium and cultured vascular endothelial cells (ECs).19 However, there is limited evidence as to whether expression of α7‐nAChR in the lower extremities is also regulated by ABR sensitivity.
Accordingly, we investigated the role of ABR in ischemia‐mediated angiogenesis with and without ABR dysfunction induced by sinoaortic denervation, with a special focus on vascular action associated with eACh/α7‐nAChR‐depedent and ‐independent VEGF/VEGFR2 signaling.
Methods
Animals
Male, 8‐week‐old Sprague‐Dawley rats (250 to 300 g) and 10‐week‐old C57BL/6 mice (20 to 30 g) were obtained from the Sino‐British SIPPR/BK Laboratory Animal Ltd. (Shanghai, China), whereas age‐matched α7‐nAChR knockout (KO; C57BL/6 background) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All animals were housed with controlled temperature (22 to 25°C) and lighting (8:00 am to 8:00 pm light and 8:00 pm to 8:00 am dark) and had free access to tap water and standard rat chow. All animals used in this work received humane care in compliance with institutional guidelines of Shanghai Jiao Tong University (Shanghai, China) and Second Military Medical University (Shanghai, China).
Study Protocol
Rats were randomly assigned to 1 of 3 groups and received either (1) a sham operation (control group, n=18), (2) sinoaortic denervation (SAD)‐induced ABR dysfunction (SAD group, n=17), or (3) SAD rats on the diet with an acetylcholinesterase inhibitor, pyridostigmine (Pyr; 30 mg/kg per day, SAD+Pyr group, n=17; Selleck Chemicals, Houston, TX). At 4 weeks of the SAD intervention, unilateral hindlimb ischemia was surgically induced among all groups, as previously described.14 A schematic diagram of the study protocol is shown in Figure 1. For mice experiments, all mice were subjected to unilateral femoral artery resection at day 0 to establish hindlimb ischemia16 and fed by Pyr (30 mg/kg per day) until day 14. For the biological analysis, the animals were perfused with isotonic saline at physiological pressure after euthanization at an indicated time point after ischemic surgery. Thigh adductor muscles were then isolated and kept in liquid nitrogen (for the protein assay). For morphometry, after being immersed in fixative for 16 hours (4°C), muscles were embedded in paraffin.
Figure 1.
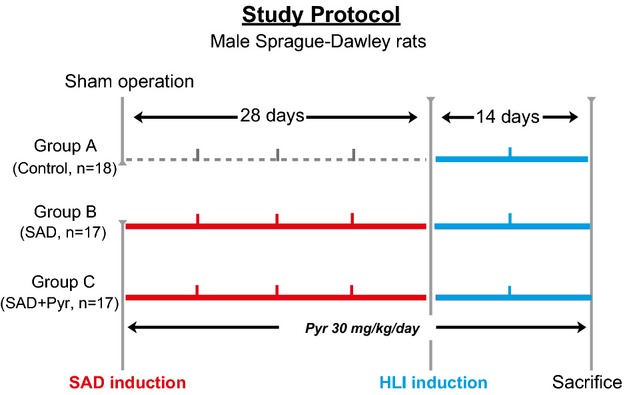
Study protocol and sampling time points. HLI indicates hindlimb ischemia; Pyr, pyridostigmine; SAD, sinoaortic denervation.
Animal Model of SAD
SAD has already been proven as an established baroreflex dysfunction animal model.10,12–13,24 Rats were anesthetized with ketamine (50 mg/kg; Daiichi‐Sankyo, Tokyo, Japan) and xylazine (10 mg/kg; Kyoritsu Seiyaku, Tokyo, Japan), and carotid sinus baroreceptors were surgically denervated bilaterally to establish an experimental ABR dysfunctional model.
Infrared Spectrum Imaging Analysis
The temperatures of both hindlimbs were measured by the infrared spectrum imaging analyzer (FLIR Systems, Wilsonville, OR). In this method, a color‐coded image representing temperature distribution is displayed to evaluate blood perfusion. Low or no temperature is displayed as dark blue, and high temperature is displayed as red to white colors. At every time point (before and after ischemic surgery at day 14), we performed 2 consecutive infrared spectrum scans over the same region of interest, and average perfusion values were computed from histograms of the colored pixels. To minimize variations resulting from ambient light, calculated temperature (relative units) is expressed as the ischemic/normal limb temperature ratio.
Capillary Density
At postoperative day 14, capillary ECs in each cross‐section (5 μm) were identified by immunohistochemistry with anti‐CD31 monoclonal antibody (Ab; Becton Dickinson, Franklin Lakes, NJ). Capillaries were counted in 5 random microscopic fields (×400 magnification) from 4 independent cross‐sections of the adductor skeletal muscle in each animal. Capillary density is expressed as the number of capillaries per field.
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA) and cultured in endothelial basal medium 2 (EBM‐2) plus 10% FBS and EGM‐2 MV SingleQuotes (Clonetics) in a humidified atmosphere of 5% CO2 and 95% air. HUVECs were seeded into 6‐well plates at 5×105 cells per well and cultured overnight in serum‐free EBM‐2. HUVECs at passage 5 to 8 were used. After washing twice with PBS, cells were treated with carbachol (Sigma‐Aldrich, St. Louis, MO) and/or donepezil (Selleck Chemicals, Houston, TX) at indicated concentrations and time points. In some experiments, HUVECs were treated with a phosphoinositide 3‐kinase (PI3K) inhibitor (LY294002; Cell Signaling Technology, Beverly, MA) at indicated concentrations. Lysates and conditioned media were then collected for biological analysis.
siRNA Transfection Protocol
Specific siRNA against hypoxic inducible factor 1 alpha (HIF‐1α; 15345‐001 F#569) was purchased from Sigma‐Aldrich. HUVECs were grown on a 6‐cm dish until they reached 30% confluence. The siRNA solution was mixed with Opti‐MEM reduced‐serum media containing Lipofectamine RNAiMax reagent (Invitrogen, Carlsbad, CA). The culture medium was then removed and switched to antibiotics‐free EGM‐2, together with 500 μL of the transfection mixture, at a final concentration of siRNA in 100 nmol/L and were cultured for 48 hours.
Western Blotting
Equal amounts of total protein from extracts of thigh muscle and HUVECs were resolved on SDS 10% PAGE and transferred to nitrocellulose membranes for Western blotting with HIF‐1α (1:200 dilution), phospho‐VEGFR2 (p‐VEGFR2; Tyr996, 1:100 dilution), phosphor‐protein kinase B (p‐Akt; Ser473, 1:100 dilution), and β‐actin (1:200 dilution; Cell Signaling Technology), as well as with choline acetyltransferase (ChAT; 1:100 dilution), vesicular acetylcholine transporter (VAChT; 1:100 dilution), and α7‐nAChR (1:200 dilution; Sigma‐Aldrich), followed by incubation with HRP‐conjugated secondary Ab (GE Healthcare, Fairfield, CT), as described previously.16 Positive signals were detected with an enhanced chemiluminescence (ECL) system (GE Healthcare). Relative protein levels were quantified using an ECL Western blotting detection kit (GE Healthcare) and ImageJ software (National Institutes of Health, Bethesda, MD).
Real‐Time Polymerase Chain Reaction
Total RNA was extracted from HUVECs lysates using TRIzol Reagent (Life Technologies, Carlsbad, CA) and were then reverse transcribed as previously described.16 Quantitative gene expression was studied using the BIO‐RED C1000 Real‐Time Polymerase Chain Reaction (PCR) System with SYBR Green I agents (Life Technologies). Serial dilutions of a control sample of cDNA were used as the standard curve for each reaction. All experiments were performed in triplicate. The primers used (Sigma‐Aldrich) were 5′‐ TACCCTACGGAGAGCGAAGA ‐3′ and 5′‐CTGTAGAGGCGAACATGACG‐3′ for VAChT; 5′‐AAGACGCCCATCCTGGAAAAG‐3′ and 5′‐GTACGTGGCCAGGGTCTG‐3′ for ChAT; 5′‐ATCACCATCTTCCAGGAGCG‐3′ and 5′‐CCTGCTTCACCACCTTCTTG‐3′ for GADPH. Each RNA quantity was normalized to its respective GAPDH mRNA quantity.
Enzyme‐Linked Immunosorbent Assay
The concentration of VEGF, basic fibrotic growth factor (bFGF), and stromal‐derived factor 1 (SDF‐1) in the conditioned medium of cultured HUVECs were determined using ELISA kits according to the manufacturers' instructions (R&D Systems, San Diego, CA). The concentration of VEGF contained in ischemic tissue was measured with a kit purchased from Raybiotech (Norcross, GA).
Immunofluorescence
After attachment of HUVECs (cell density at 2×104 cells/well) to coverslips, cells were cultured in the presence or absence of donepezil (10 μmol/L) overnight. Then, cells were fixed for 10 minutes with 4% paraformaldehyde and washed with 1% glycerol. After perforation with 0.3% Triton X‐100 (Sigma‐Aldrich) and blocking with 5% BSA/PBS, cells were treated with 1 μg/mL of mouse anti‐ChAT or 3 μg/mL of rabbit anti‐human VAChT (Sigma‐Aldrich), respectively, overnight at 4°C. ChAT and VAChT were visualized using goat anti‐mouse (1:1000 dilution, Alexa 568; Life Technologies) or chicken anti‐rabbit secondary Abs (1:1000 dilution, Alexa 488; Life Technologies), respectively. Coverslips were treated with VECTASHIELD mounting medium containing 4′,6‐diamidino‐2‐phenylindole (Vector Laboratories, Burlingame, CA) and analyzed by confocal microscopy.
Statistical Analysis
Data are expressed as means± SE. Student t tests (for comparisons between 2 groups) or one‐way ANOVA (for 3 groups), followed by Tukey's post‐hoc tests, were used for statistical analyses. SPSS software (version 17.0; SPSS, Inc., Chicago, IL) was used. A value of P<0.05 was considered significant.
Results
Acetylcholinesterase Inhibition Improves ABR Dysfunction‐Related Impaired Angiogenic Responses
As the first step in examining whether ABR dysfunction affects angiogenic actions in response to hypoxic injury, all rats were applied to hindlimb ischemia, and the ischemic/normal hindlimb temperature ratio and capillary density of ischemic muscles were evaluated. On day 14 after induction of ischemia, SAD rats exhibited a lower skin temperature ratio than controls, suggesting that ABR dysfunction delayed angiogenesis in response to hypoxia (Figure 2A and 2B). This was further supported by the quantitative data of capillary density, which indicated that capillary formation was impaired more in SAD rats than in control rats (Figure 2C and 2D). Furthermore, acetylcholinesterase inhibition by Pyr resulted in recovery of both skin temperature ratio and capillary formation (Figure 2A through 2D).
Figure 2.
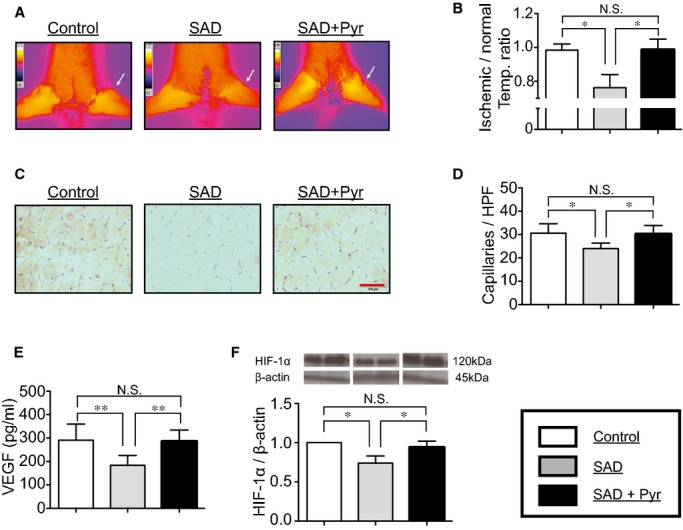
Hindlimb ischemia‐induced angiogenesis in vivo. At day 14 after induction of ischemia, (A and B) infrared spectrum imaging assay, (C and D) anti‐CD31 staining, (E) ELISA to the content of vascular endothelial growth factor (VEGF), and (F) Western blotting assay to expression of hypoxia‐inducible factor 1 alpha (HIF‐1α) in the ischemic area were performed to evaluate angiogenic actions. Infrared spectrum imaging data were analyzed and illustrated as an ischemic/normal hindlimb temperature ratio. Data of Western blotting are represented as fold of control. n=6 in each group. *P<0.05, **P<0.01; N.S., no significant difference; Tukey's post‐hoc test. Temp, temperature. Scale bar: 50 μm. HPF indicates high power field; Pyr, pyridostigmine; SAD, sinoaortic denervation.
To further examine the mechanism underlying the impairment of ischemia‐induced angiogenic action in rats with ABR dysfunction, we evaluated levels of VEGF and HIF‐1α protein expressions in local tissue among the 3 groups of rats at day 14 after induction of ischemia. Protein levels of VEGF and HIF‐1α were both significantly lower in SAD rats than in control rats (Figure 2E and 2F). Pyr restored not only tissue VEGF concentration, but also HIF‐1α expression in ischemic tissue of SAD rats (Figure 2E and 2F). There were no differences of α7‐nAChR expressions in ischemic muscle of rats among the 3 experimental groups (Figure 3A).
Figure 3.
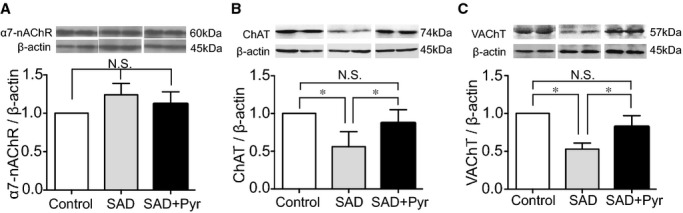
Acetylcholine (ACh)‐related protein expression in vivo. Protein expressions of (A) α7‐nicotinic acetylcholine receptor (α7‐nAChR), (B) choline acetyltransferase (ChAT), and (C) vesicular acetylcholine transporter (VAChT) in the ischemic area were determined among 3 experimental groups. Data of Western blotting are represented as fold of control. n=6 in each group. *P<0.05; N.S., no significant difference; Tukey's post‐hoc test. Pyr indicates pyridostigmine; SAD, sinoaortic denervation.
Effects of Acetylcholinesterase Inhibition on ChAT and VAChT Expressions In Vivo and In Vitro
In order to test whether the endogenous ACh system was affected under the condition of dysfunctional ABR status, we checked 2 key components involved in synthesis and secretion of endogenous ACh after hindlimb ischemia. Protein expressions of ChAT and VAChT declined in SAD rats, compared to controls; however, Pyr magnified expressions of ChAT and VAChT, even under ABR dysfunctional status (Figure 3B and 3C). In vitro, expressions of ChAT and VAChT genes and proteins in cultured HUVECs were analyzed by real‐time PCR, Western blotting, and immunofluorescence. We observed that both targeted genes and protein expressions were enhanced in HUVECs when exposed to donepezil (Figure 4A through 4F).
Figure 4.
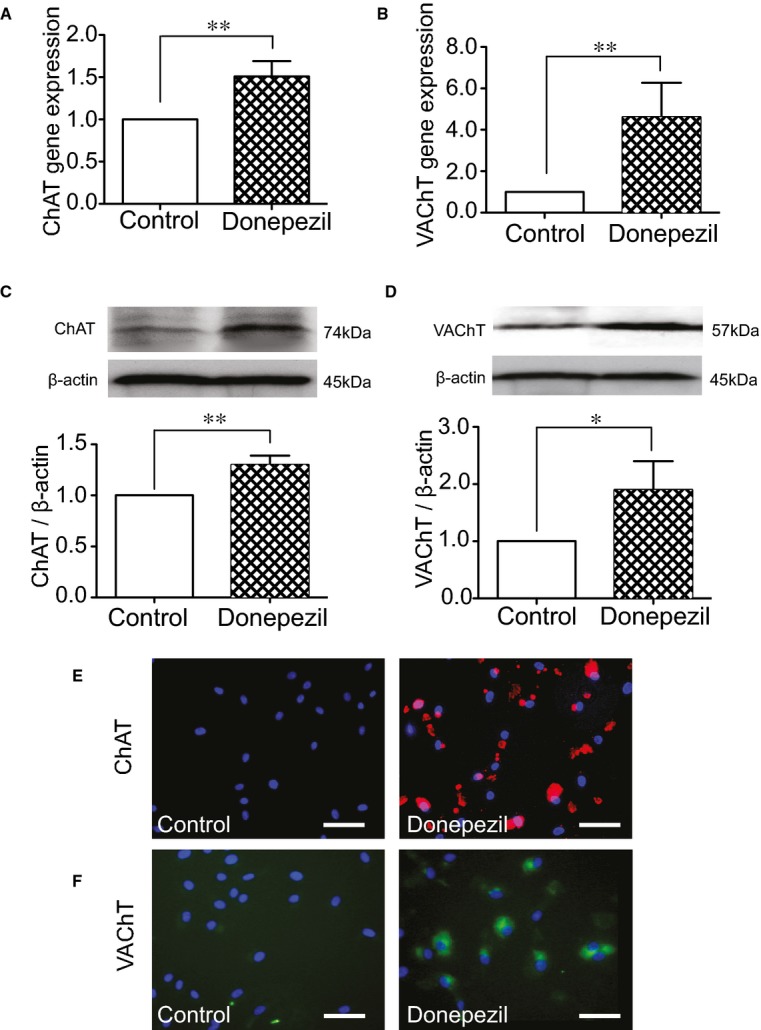
Donepezil‐mediated eACh activity in vitro. After being cultured in serum‐free EBM‐2, HUVECs were treated with donepezil at a concentration of 10 μmol/L for 24 hours. (A and B) Results of ChAT and VAChT gene expressions determined with real‐time PCR. (C and D) Results of ChAT and VAChT protein expressions determined with Western blotting. Data of real‐time PCR and Western blotting are represented as fold of control. n=4 in each group. *P<0.05; **P<0.01; Student t test. (E and F) Representative images (×400 magnification) of anti‐ChAT and ‐VAChT immunofluorescence. Scale bar: 50 μm. ChAT indicates choline acetyltransferase; eACh, endothelium‐derived acetylcholine; EBM‐2, endothelial basal medium 2; HUVECs, human umbilical vein endothelial cells; PCR, polymerase chain reaction; VAChT, vesicular acetylcholine transporter.
Effects of Donepezil‐Mediated Acetylcholinesterase Inhibition on Expression of Angiogenic Factors In Vitro
After treatment with donepezil for 24 hours, the conditioned medium and lysates of HUVECs were collected, and several angiogenic factors were quantified using ELISA and Western blotting. Donepezil treatment resulted in increased levels not only of HIF‐1α protein in lysates (Figure 5A), but also VEGF, bFGF, and SDF‐1 proteins in the conditioned medium (Figure 5C through 5E). Next, HUVECs were treated with a PI3K inhibitor (LY294002). LY294002 reduced donepezil‐mediated HIF‐1α expression (Figure 5A). Moreover, HIF‐1α/RNA interference decreased the concentration of VEGF in the HUVEC conditioned medium after donepezil treatment (Figure 5B and 5C). These results indicate that autocrine secretion of eACh stimulates the angiogenic signal pathway of ECs.
Figure 5.
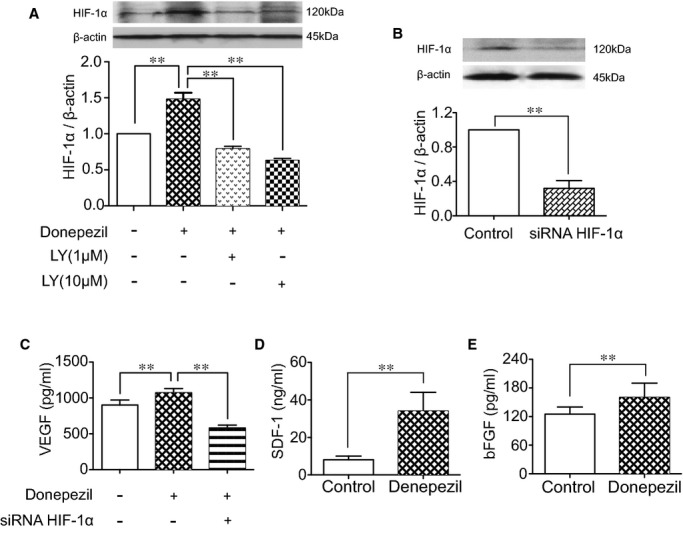
Donepezil‐mediated angiogenic effect in vitro. (A) Quantitative analysis of HIF‐1α expression in lysates of HUVECs treated with donepezil (10 μmol/L) and LY294002 (1 and 10 μmol/L; Tukey's post‐hoc test). (B) HIF‐1α expression in lysates of HUVECs when treated with nontargeting siRNA and siRNA HIF‐1α (100 nmol/L; Student t test). (C) ELISA for VEGF in the supernatant of HUVECs when treated with donepezil (10 μmol/L) and HIF‐1α (100 nmol/L; Tukey's post‐hoc test). (D and E) Quantitative analysis of stromal derived factor 1 (SDF‐1) and basic fibroblastic growth factor (bFGF) contained in the supernatant of HUVECs (Student t test). n=4 in each group. Data of real‐time PCR and Western blotting are represented as folds of control. **P<0.01. HIF‐1α indicates hypoxia‐inducible factor 1 alpha; HUVECs, human umbilical vein endothelial cells; PCR, polymerase chain reaction; VEGFR, vascular endothelial growth factor receptor.
Cross‐Talk Between ACh/nAChR and VEGF/VEGFR2 Systems In Vitro
To examine whether there is a potential relationship between the ACh/nAChR and VEGF/VEGFR2 systems, an analog of ACh (carbachol) and its receptor blockers were applied to HUVEC culture. As shown in Figure 6A and 6B, carbachol increased the levels of VEGF protein contained in the conditioned medium in a dose‐dependent manner, whereas VEGFR2 phosphorylation followed a time‐dependent manner. Profound suppression of carbachol‐mediated VEGF secretion and VEGFR2 activation were observed in HUVECs treated with a nicotinic receptor blocker (methyllycaconitine; MLA). Pretreatment by atropine, in contrast, conferred no detectable change (Figure 6C and 6D). Furthermore, enhanced expression of HIF‐1α was detected in a time‐dependent manner when HUVECs were exposed to carbachol (Figure 6E). Carbachol also stimulated p‐Akt in a time‐dependent manner, peaking at 30 minutes after administration (Figure 6F).
Figure 6.
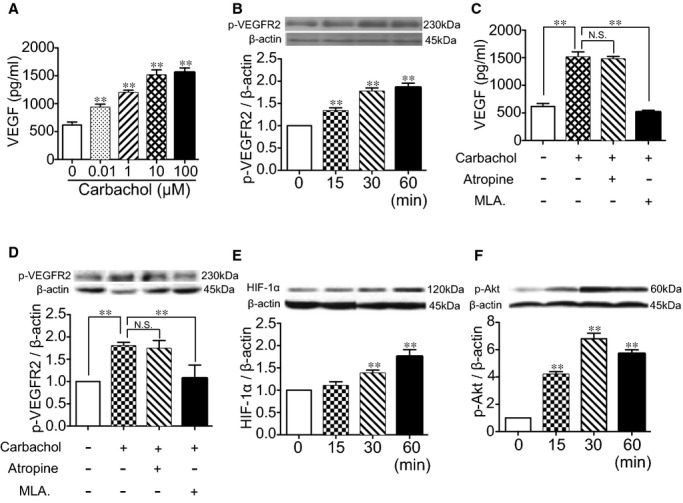
Effects of carbachol on HUVECs. HUVECs were exposed to different concentration gradients of carbachol (0 to 100 μmol/L) for 24 hours. (A) Concentrations of VEGF contained in the supernatant were measured by ELISA. After HUVECs were stimulated by carbachol (10 μmol/L), (B) levels of phosphorylation of VEGFR2 (p‐VEGFR2; Tyr 996) were measured by Western blotting (**P<0.01 versus corresponding control; Tukey's post‐hoc test). HUVECs were pretreated by atropine or methyllycaconitine (MLA) for 1 hour before carbachol (10 μmol/L) was added. Then, protein levels of (C) VEGF in supernatant and (D) p‐VEGFR2 in cell lysates were quantified (**P<0.01; Tukey's post‐hoc test). HUVECs were then exposed to carbachol (10 μmol/L) for 24 hours, and expression of (E) HIF‐1α and (F) phospho‐Akt (p‐Akt; Ser473) were measured by Western blotting (**P<0.01 versus corresponding control; Tukey's post‐hoc test). Data of Western blotting are represented as folds of control. n=4 in each group. HIF‐1α indicates hypoxia‐inducible factor 1 alpha; HUVECs, human umbilical vein endothelial cells; p‐VEGFR2, phosphorylated vascular endothelial growth factor receptor 2.
Acetylcholinesterase Inhibition Accelerates Angiogenesis Even in α7‐nAChR KO with Hindlimb Ischemia
Previous reports using α7‐nAChR KO mice indicated that α7‐nAChR is highly involved in ischemia‐induced angiogenesis. Therefore, to investigate whether the angiogenic effect of Pyr‐mediated acetylcholinesterase inhibition is mediated through α7‐nAChR, we studied the effects of Pyr on hindlimb ischemia using α7‐nAChR KO mice. At first, we observed that α7‐nAChR KO mice had a lower ischemic/normal limb temperature ratio and capillary formation in ischemic muscles than in wild‐type (WT) mice (Figure 7A through 7D). Levels of VEGF were also lower in α7‐nAChR KO mice than WT mice. Compared to untreated α7‐nAChR KO mice, Pyr‐treated α7‐nAChR KO mice surprisingly exhibited an improved ischemic/normal limb temperature ratio and capillary density. Quantitative ELISA revealed that tissue VEGF levels were elevated in Pyr‐treated α7‐nAChR KO mice than in untreated control mice (Figure 7E). In addition, Pyr augmented HIF‐1α expression in ischemic muscles (1.33‐fold increasing versus untreated group).
Figure 7.
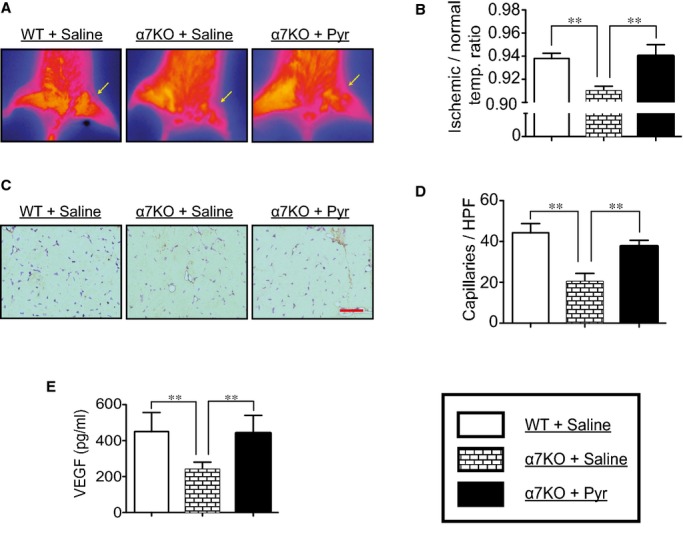
Pyridostigmine‐mediated inhibition of acetylcholinesterase on angiogenesis. Using wild‐type (WT) and α7‐nAChR KO mice, unilateral hindlimb ischemia was surgically induced and fed by either pyridostigmine or saline to evaluate (A and B) the ischemic/normal limb temperature ratio, (C and D) the capillary density, and (E) levels of VEGF in ischemic muscles. n=6 in each group. **P<0.01; N.S., no significant difference; Tukey's post‐hoc test. α7‐nAChR indicates α7‐nicotinic acetylcholine receptor; HPF, high power field; KO, knockout; Pyr, pyridostigmine; VEGFR, vascular endothelial growth factor receptor.
Discussion
In a previous study, impairment of ABR sensitivity led to sympathovagal imbalance and attenuated the adaption capacity of the cardiovascular system.10 Moreover, dysfunctional ABR led to the acceleration of atherosclerosis in both apolipoprotein E KO and WT animals10–11 and also decreasing the regulatory capacity of endothelial nitric oxide synthase.24–25 In contrast, stimulation of the carotid sinus baroreflex prevents ventricular remodeling and improves endothelial function.26–27 In our previous study, an enlarged infarcted area and declined ejection fraction were observed in the ischemic myocardium of SAD rats subjected to surgically induced MI.12 Clinically, decreased ABR sensitivity caused overaction of sympathetic tone and influenced the prognosis of CVDs.6–9 In the present study of unilateral hindlimb ischemia, suppressed skin temperature ratio, capillary density, as well as reduced expressions of tissue VEGF and HIF‐1α were observed in the local area of SAD rats, indicating that dysfunctional ABR weakened endothelial function, thus delaying functional recovery and collateral vessel formation in response to ischemia (Figure 8).
Figure 8.
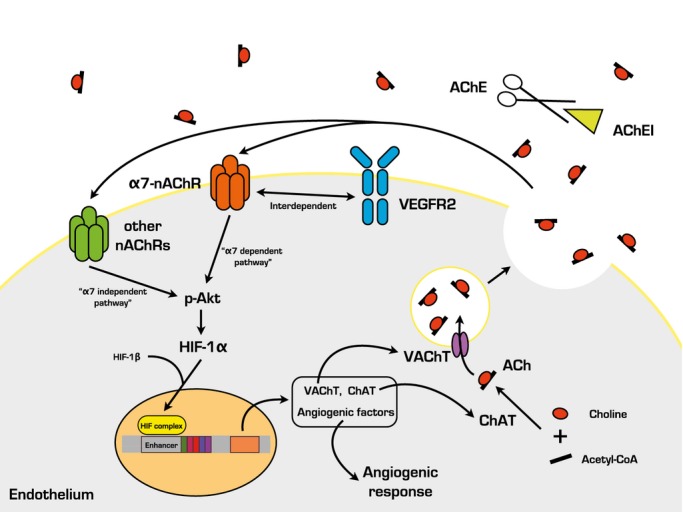
Possible mechanisms of acetylcholinesterase inhibitor‐mediated angiogenesis in the present study. ACh indicates acetylcholine; AChEI, acetylcholinesterase inhibitor; ChAT, choline acetyltransferase; HIF‐1α, hypoxia‐inducible factor 1 alpha; α7‐nAChR, α7‐nicotinic acetylcholine receptor; p‐Akt, phosphor‐Akt; VAChT, vesicular acetylcholine transporter; VEGFR, vascular endothelial growth factor receptor.
On the other hand, ACh is no longer considered to be exclusively a neurotransmitter, simply mediating rapid communication between the neuron and effector cells, but is now seen as a hormone‐like regulator that modulates various cell functions in such a way as to facilitate their adaption to new conditions.19–22,28 Increasing the level of ACh by vagal stimulation protected ischemic cardiomyocytes from apoptosis through the Akt/HIF‐1α axis.29 ACh also exhibited a proangiogenic capacity that accelerated tube‐like structure formation of HUVECs following a dose‐dependent manner in vitro.12 It has been clarified that in situ synthesis and secretion of ACh dominantly relies on the participation of ChAT and VAChT, respectively. Overexpression of either ChAT or VAChT raised the level of interstitial ACh.30–31 In the present study, expressions of ChAT and VAChT declined in ischemic thigh of SAD rats, suggesting that dysfunctional ABR impairs the synthesis and secretion of endogenous ACh.
We hypothesized that restored sympathovagal balance by “drawing back” the ACh level would reactivate the angiogenic pathway and compensate for the impaired neovascularization caused by SAD intervention. We and other researchers have demonstrated that in experimental MI models, increasing parasympathetic tone by acetylcholinesterase inhibition augments postnatal neovascularization, preserves heart function, and increases survival.30,32 In the present study, we applied Pyr, an acetylcholinesterase inhibitor medicated for myasthenia gravis, to intensify the effect of ACh in ischemic tissue. Augmented angiogenic responses (skin temperature ratio and capillary formation), accompanied by restored local VEGF and HIF‐1α levels, were observed in the ischemic area of SAD rats after Pyr treatment. This indicates that acetylcholinesterase inhibition has persevered the limited ACh production under the circumstance of dysfunctional ABR status and, consequently, rebuilding blood perfusion by activating the HIF‐1α/VEGF axis in ischemic tissue.
It is becoming progressively more evident that the cholinergic system is not confined to the nerve, but is nearly ubiquitous.19–23 Non‐neural parts of the cholinergic system are highly involved in physiological homeostasis and in modulating cell functions. We and other researchers have reported that myocardium‐derived ACh conferred resistance to ischemic heart disease and promoted survival.12,30 Moreover, several reports have demonstrated the existence of an intact eACh system, including synthesis, secretion, and utilization.23,33–35 The most significant observations of in vivo and in vitro studies were the enhancements of both gene and protein expressions of ChAT and VAChT under stimulation with an acetylcholinesterase inhibitor, suggesting the existence of positive feedback reinforcement in the production and utilization of eACh.
The receptor of ACh is also expressed on the surface of ECs, especially nAChR, the activation of which has been proven to exhibit proangiogenic potency.19–20,28 We and other researchers have previously demonstrated that administration of a nicotinic receptor blocker could cancel ACh‐mediated HUVEC vasculature formation in vitro; on the other hand, the muscarinic receptor blocker, atropine, caused only a modest effect.19 Our observation here shows that administration of carbachol, an ACh analog, enhanced VEGF release from HUVECs and augmented expressions of p‐VEGFR2 and HIF‐1α in vitro. Taken together, these results indicate that ACh not only triggers the paracrine function of ECs by activating the HIF‐1α/VEGF axis, but also promotes its autocrine function by activating VEGFR2 so that it magnifies the effect of VEGF. Moreover, we have shown that p‐Akt was enhanced in response to ACh stimulation in a time‐dependent manner in vitro, indicating that ACh triggers multiple pathways of ECs for the adaption of ischemic and hypoxic conditions. On the other hand, Heeschen et al. demonstrated exogenously that activation of nAChR by nicotine also triggers angiogenesis.18,20 In an in vitro model of fibrovascular growth, nicotine‐mediated angiogenic action was similar in magnitude to that of vascular growth factors, such as FGF.28 In 2002, Heeschen et al. attempted to determine whether nAChR‐mediated angiogenesis was mediated by VEGF or bFGF.20 Neutralizing Abs against VEGF, but not bFGF, resulted in a significant, but not complete, inhibition of nAChR‐mediated network formation. Further study showed that nAChR stimulation and selective stimulation of α7‐nAChR both resulted in a significant release of VEGF.18 Here, we have also demonstrated that in cultured HUVECs, nAChR stimulation by an ACh analog enhanced not only VEGF release, but also VEGFR‐2 phosphorylation. Notably, antagonists of nAChR have been shown to markedly attenuate VEGF‐induced angiogenesis.18 Collectively, nAChR and VEGFR2 appear to mediate distinct, but interdependent, pathways of angiogenesis in response to ischemic stress.
We then administrated donepezil to HUVECs to check the integration of eACh/nAChR with angiogenic signal pathways. Quantitative results demonstrated that donepezil treatment significantly raised the concentrations of several kinds of angiogenic cytokines (VEGF, bFGF, and SDF‐1) in the supernatant. Meanwhile, expression of HIF‐1α in HUVECs was augmented after treatment with donepezil. These data strongly suggest that the intact eACh/nAChR axis was conjugated with the angiogenic‐signaling pathway of ECs. In addition, administration of donepezil augmented angiogenesis by stimulating release of insulin‐like growth factor‐1 in the hippocampus.36 Consistent with results in vitro, Pyr increased protein levels of VEGF and HIF‐1α in rats with ABR dysfunction. Building on these findings, both ACh and its nicotinic receptor were present in ECs, and the eACh system was impaired by decreased ABR sensitivity. Therefore, pharmacological treatment by acetylcholinesterase inhibitor would maximize the effect eACh and enhance postnatal neovascularization.
α7‐nAChR expresses on the surface of ECs and is in charge of EC growth and migratory ability.19,28,37 We previously reported that acetylcholinesterase inhibition promoted α7‐nAChR expression in hypoxic myocardium through activation of the HIF‐1α/VEGF axis, which triggers angiogenesis.12 In this study, α7‐nAChR deletion impaired ischemia‐induced angiogenic action. This change was then recovered by acetylcholinesterase inhibition with Pyr in a mouse ABR dysfunction model, suggesting that acetylcholinesterase inhibitor‐related vascular activity may not solely act through α7‐nAChR in the lower extremities, but that other nAChRs are, at least partially, involved in the effect. It should be noted that there were no differences in α7‐nAChR protein expression in ischemic muscles of the 3 groups of rats, whether treated or untreated with Pyr. It seems that Pyr‐mediated vascular benefits in mice might not be attributable to quantitative change of α7‐nAChR‐dependent activation.
Besides α7‐nAChR, there exists several other functional nAChRs expressed on ECs, and interplay between them modulates different cell functions.18,20,28 Wu et al. described how nicotine remained capable of extending the tube length of HUVECs, even after α7‐nAChR had been knocked down by siRNA in vitro. In the present study, we applied α7‐nAChR KO mice to clarify the involvement of α7‐nAChR in the mice model of PAD. Despite the comparison with WT mice, α7‐nAChR KO exhibited decreased angiogenic response, and Pyr was still capable of triggering angiogenesis, even in the absence of α7‐nAChR, in the mice model of hindlimb ischemia. Taken together, these results indicate that Pyr‐mediated enhancement of ischemia‐induced angiogenesis acts through both α7‐nAChR‐dependent and ‐independent pathways.
Sources of Funding
This work was supported by the Shanghai Science and Technology Committee (11nm0503600), the China National Natural Science Foundation (11374213), and the Foundation of National Laboratory for Infrared Physics (200901).
Disclosures
None.
References
- 1.Aranguren XL, Verfaillie CM, Luttun A. Emerging hurdles in stem cell therapy for peripheral vascular disease. J Mol Med (Berl). 2009; 87:3-16. [DOI] [PubMed] [Google Scholar]
- 2.Emanueli C, Madeddu P. Therapeutic angiogenesis: translating experimental concepts to medically relevant goals. Vascul Pharmacol. 2006; 45:334-339. [DOI] [PubMed] [Google Scholar]
- 3.Tateishi‐Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone‐marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002; 360:427-435. [DOI] [PubMed] [Google Scholar]
- 4.Kinoshita M, Fujita Y, Katayama M, Baba R, Shibakawa M, Yoshikawa K, Katakami N, Furukawa Y, Tsukie T, Nagano T, Kurimoto Y, Yamasaki K, Handa N, Okada Y, Kuronaka K, Nagata Y, Matsubara Y, Fukushima M, Asahara T, Kawamoto A. Long‐term clinical outcome after intramuscular transplantation of granulocyte colony stimulating factor‐mobilized cd34 positive cells in patients with critical limb ischemia. Atherosclerosis. 2012; 224:440-445. [DOI] [PubMed] [Google Scholar]
- 5.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012; 33:1058-1066. [DOI] [PubMed] [Google Scholar]
- 6.Lohmeier TE, Iliescu R. Lowering of blood pressure by chronic suppression of central sympathetic outflow: insight from prolonged baroreflex activation. J Appl Physiol. 1985; 2012113:1652-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997; 96:3450-3458. [DOI] [PubMed] [Google Scholar]
- 8.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life‐threatening arrhythmias: implications for clinical trials. Circulation. 2001; 103:2072-2077. [DOI] [PubMed] [Google Scholar]
- 9.Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF. Cardiac baroreceptor sensitivity predicts long‐term outcome after acute ischemic stroke. Stroke. 2003; 34:705-712. [DOI] [PubMed] [Google Scholar]
- 10.Cai GJ, Miao CY, Xie HH, Lu LH, Su DF. Arterial baroreflex dysfunction promotes atherosclerosis in rats. Atherosclerosis. 2005; 183:41-47. [DOI] [PubMed] [Google Scholar]
- 11.Vasquez EC, Peotta VA, Meyrelles SS. Cardiovascular autonomic imbalance and baroreflex dysfunction in the apolipoprotein E‐deficient mouse. Cell Physiol Biochem. 2012; 29:635-646. [DOI] [PubMed] [Google Scholar]
- 12.Yu JG, Song SW, Shu H, Fan SJ, Liu AJ, Liu C, Guo W, Guo JM, Miao CY, Su DF. Baroreflex deficiency hampers angiogenesis after myocardial infarction via acetylcholine‐α7‐nicotinic ACh receptor in rats. Eur Heart J. 2011; 34:2412-2420. [DOI] [PubMed] [Google Scholar]
- 13.Liu AJ, Guo JM, Xia W, Su DF. New strategies for the prevention of stroke. Clin Exp Pharmacol Physiol. 2010; 37:265-271. [DOI] [PubMed] [Google Scholar]
- 14.Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide‐dependent mechanism. Circulation. 2000; 102:370-376. [DOI] [PubMed] [Google Scholar]
- 15.Duan J, Murohara T, Ikeda H, Sasaki K, Shintani S, Akita T, Shimada T, Imaizumi T. Hyperhomocysteinemia impairs angiogenesis in response to hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2000; 20:2579-2585. [DOI] [PubMed] [Google Scholar]
- 16.Cheng XW, Kuzuya M, Kim W, Song H, Hu L, Inoue A, Nakamura K, Di Q, Sasaki T, Tsuzuki M, Shi GP, Okumura K, Murohara T. Exercise training stimulates ischemia‐induced neovascularization via phosphatidylinositol 3‐kinase/Akt‐dependent hypoxia‐induced factor‐1 alpha reactivation in mice of advanced age. Circulation. 2010; 122:707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JG, Zhang EH, Liu AJ, Liu JG, Cai GJ, Su DF. Ketanserin improves cardiac performance after myocardial infarction in spontaneously hypertensive rats partially through restoration of baroreflex function. Acta Pharmacol Sin. 2013; 34:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001; 7:833-839. [DOI] [PubMed] [Google Scholar]
- 19.Cooke JP. Angiogenesis and the role of the endothelial nicotinic acetylcholine receptor. Life Sci. 2007; 80:2347-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non‐neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002; 110:527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non‐neuronal cholinergic system in humans. Br J Pharmacol. 2008; 154:1558-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawashima K, Fujii T. Basic and clinical aspects of non‐neuronal acetylcholine: overview of non‐neuronal cholinergic systems and their biological significance. J Pharmacol Sci. 2008; 106:167-173. [DOI] [PubMed] [Google Scholar]
- 23.Huang ZH, Guo W, Zhang LL, Song SW, Hao CN, Duan JL. Donepezil protects endothelial cells against hydrogen peroxide‐induced cell injury. CNS Neurosci Ther. 2012; 18:185-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen FM, Zhang SH, Xie HH, Jing Q, Wang DS, Su DF. Early structural changes of aortic wall in sinoaortic‐denervated rats. Clin Exp Pharmacol Physiol. 2006; 33:358-363. [DOI] [PubMed] [Google Scholar]
- 25.Feng J, Luo H, Qiu Y, Zhou W, Yu F, Wu F. Down‐regulation of DDAH2 and eNOS induces endothelial dysfunction in sinoaortic‐denervated rats. Eur J Pharmacol. 2011; 661:86-91. [DOI] [PubMed] [Google Scholar]
- 26.Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD, Rossing MA. Chronic baroreceptor activation enhances survival in dogs with pacing‐induced heart failure. Hypertension. 2007; 50:904-910. [DOI] [PubMed] [Google Scholar]
- 27.Sabbah HN, Gupta RC, Imai M, Irwin ED, Rastogi S, Rossing MA, Kieval RS. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 2011; 4:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke JP, Ghebremariam YT. Endothelial nicotinic acetylcholine receptors and angiogenesis. Trends Cardiovasc Med. 2008; 18:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakinuma Y, Ando M, Kuwabara M, Katare RG, Okudela K, Kobayashi M, Sato T. Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non‐hypoxic induction of HIF‐1alpha. FEBS Lett. 2005; 579:2111-2118. [DOI] [PubMed] [Google Scholar]
- 30.Kakinuma Y, Tsuda M, Okazaki K, Akiyama T, Arikawa M, Noguchi T, Sato T. Heart‐specific overexpression of choline acetyltransferase gene protects murine heart against ischemia through hypoxia‐inducible factor‐1α‐related defense mechanisms. J Am Heart Assoc. 2013; 2:e004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagy PM, Aubert I. Overexpression of the vesicular acetylcholine transporter increased acetylcholine release in the hippocampus. Neuroscience. 2012; 218:1-11. [DOI] [PubMed] [Google Scholar]
- 32.Lataro RM, Silva CA, Fazan R, Jr, Rossi MA, Prado CM, Godinho RO, Salgado HC. Increase in parasympathetic tone by pyridostigmine prevents ventricular dysfunction during the onset of heart failure. Am J Physiol Regul Integr Comp Physiol. 2013; 305:R908-R916. [DOI] [PubMed] [Google Scholar]
- 33.Kirkpatrick CJ, Bittinger F, Unger RE, Kriegsmann J, Kilbinger H, Wessler I. The non‐neuronal cholinergic system in the endothelium: evidence and possible pathobiological significance. Jpn J Pharmacol. 2001; 85:24-28. [DOI] [PubMed] [Google Scholar]
- 34.Kawashima K, Watanabe N, Oohata H, Fujimoto K, Suzuki T, Ishizaki Y, Morita I, Murota S. Synthesis and release of acetylcholine by cultured bovine arterial endothelial cells. Neurosci Lett. 1990; 119:156-158. [DOI] [PubMed] [Google Scholar]
- 35.Kawashima K, Oohata H, Fujimoto K, Suzuki T. Extraneuronal localization of acetylcholine and its release upon nicotinic stimulation in rabbits. Neurosci Lett. 1989; 104:336-339. [DOI] [PubMed] [Google Scholar]
- 36.Narimatsu N, Harada N, Kurihara H, Nakagata N, Sobue K, Okajima K. Donepezil improves cognitive function in mice by increasing the production of insulin‐like growth factor‐I in the hippocampus. J Pharmacol Exp Ther. 2009; 330:2-12. [DOI] [PubMed] [Google Scholar]
- 37.Wu JC, Chruscinski A, De Jesus Perez VA, Singh H, Pitsiouni M, Rabinovitch M, Utz PJ, Cooke JP. Cholinergic modulation of angiogenesis: role of the 7 nicotinic acetylcholine receptor. J Cell Biochem. 2009; 108:433-446. [DOI] [PMC free article] [PubMed] [Google Scholar]


