Abstract
Background
Up to 30% of acute stroke evaluations are deemed stroke mimics (SM). As telestroke consultation expands across the world, increasing numbers of SM patients are likely being evaluated via Telestroke. We developed a model to prospectively identify ischemic SMs during Telestroke evaluation.
Methods and Results
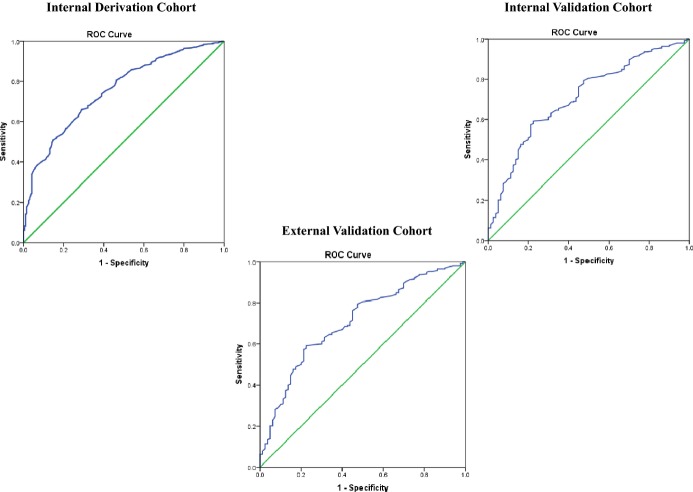
We analyzed 829 consecutive patients from January 2004 to April 2013 in our internal New England–based Partners TeleStroke Network for a derivation cohort, and 332 cases for internal validation. External validation was performed on 226 cases from January 2008 to August 2012 in the Partners National TeleStroke Network. A predictive score was developed using stepwise logistic regression, and its performance was assessed using receiver‐operating characteristic (ROC) curve analysis. There were 23% SM in the derivation, 24% in the internal, and 22% in external validation cohorts based on final clinical diagnosis. Compared to those with ischemic cerebrovascular disease (iCVD), SM had lower mean age, fewer vascular risk factors, more frequent prior seizure, and a different profile of presenting symptoms. The TeleStroke Mimic Score (TM‐Score) was based on factors independently associated with SM status including age, medical history (atrial fibrillation, hypertension, seizures), facial weakness, and National Institutes of Health Stroke Scale >14. The TM‐Score performed well on ROC curve analysis (derivation cohort AUC=0.75, internal validation AUC=0.71, external validation AUC=0.77).
Conclusions
SMs differ substantially from their iCVD counterparts in their vascular risk profiles and other characteristics. Decision‐support tools based on predictive models, such as our TM Score, may help clinicians consider alternate diagnosis and potentially detect SMs during complex, time‐critical telestroke evaluations.
Keywords: cerebrovascular disease, stroke mimics, telestroke, thrombolysis
Introduction
Telestroke has grown significantly in the past decade and has entered mainstream care for patients with acute stroke.1 Defined as the use of telecommunication technologies to provide medical information and services to stroke patients,2 telestroke has been adopted and implemented by multiple different types of healthcare organizations across the United States and abroad.3 Telestroke enables stroke patients to be remotely evaluated, thereby allowing optimal treatment and management in medically underserved areas, removing geographical disparities in access to expert care.1,4 Decision‐analytic models demonstrate that telestroke is cost‐effective from both a societal and a hospital perspective.5–6
While a significant percentage of stroke patients are now provided initial care through telestroke consultations,4 its ease of use, greater availability, and cost effectiveness have led to a high number of consults by emergency department (ED) physicians. However, it is estimated that 5% to 30% of ED patients suspected of having an acute stroke end up with a diagnosis of a stroke mimic (SM). Seizures, migraine, psychogenic disorders, and toxic/metabolic causes are the most common nonvascular conditions that mimic stroke.7–14 The use of telestroke consultations to evaluate SMs, and the use of intravenously administered tissue plasminogen activator (tPA) in these cases, while safe,12,14–16 may lessen the cost effectiveness of this method of stroke evaluation.
These observations motivated us to perform a retrospective analysis of all the patients managed in our large national telestroke network with a goal of developing a score to identify SMs based on presenting characteristics.
Methods
Patient Population
We report data from two mutually exclusive sources: our internal New England–based Partners TeleStroke Network and our Partners National TeleStroke Network (PNTN). The Partners TeleStroke Network comprises 2 hubs (Massachusetts General Hospital and Brigham and Women's Hospital) currently serving 31 spoke hospitals in Massachusetts, Maine, and New Hampshire. Partners TeleStroke Network was established in 2000 as one of the first telestroke systems in the country. All telestroke consults are entered into a database via an online web‐based portal by the neurologist, with the data stored centrally at a secure server at Massachusetts General Hospital. There were 8839 consecutive patients entered into the database from January 2004 to April 2013. Patients were excluded from this analysis if they were evaluated by telephone only (n=7044), leaving 1795 video‐telestroke consults for evaluation. After excluding those with unverified age data (n=463) or incomplete medical comorbidity data on admission (n=171), there were 1161 video‐telestroke consults available for analysis. Age was calculated and verified by subtracting the patient date of birth (DOB) from the date of consultation. Due to the urgent nature of telestroke consults, not all patients had a documented DOB in the database. To address any nonrandom incompleteness of age data, we compared patients with documented DOB and those missing DOB.
Of the 1161 patients with complete data included in the analysis, 70% (n=829) were randomly selected for the internal derivation cohort and the remaining were used for internal validation cohort. We extracted data on demographics (age, race, ethnicity, and gender), baseline clinical characteristics (hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, atrial fibrillation, heart failure, previous stroke, smoker, prior seizure, prior myocardial infarction), medication use (antiplatelet or anticoagulant use), initial symptoms documented by the referring emergency physician (presence of “weakness,” “speech problem,” or “altered level of consciousness”), and the evaluation performed by the telestroke consultant (initial National Institutes of Health Stroke Scale [NIHSS], diagnosis, and intravenous tPA eligibility and administration).
For external validation, we included 226 video‐telestroke consults from the external PNTN from January 2008 through August 2012. The PNTN centers included four Hubs serving a total of 22 spokes in their geographic regions: Yale–New Haven Hospital, New Haven, Conn; Swedish Medical Center, Seattle, Wash.; INTEGRIS Health, Oklahoma City, Okla., and the University of Virginia Health System, Charlottesville, Va. All PNTN Hub sites used the same shared database for recording patient information and documenting treatment timelines and decision‐making. The data are stored on a secure, HIPAA‐compliant central server located in Boston, Mass. housed behind the Massachusetts General Hospital firewall. Each PNTN Hub site granted permission to share de‐identified data on patient encounters. Analysis of this de‐identified data in our stroke database is approved by our Institutional Review Board.
TeleStroke Evaluation, Workflow, and Diagnostic Classification
Hospitals that wish to participate in the PNTN as referring spoke sites must first execute contracts with a PNTN Hub hospital, and obtain the necessary approved equipment for teleconsultation, adapt their acute stroke protocols, and engage in training for telestroke consultations. All Hub neurologists in our network are board certified in vascular neurology or have extensive experience in stroke. Spoke sites are instructed to contact the Hub by phone for any cases in which they suspect acute ischemic stroke and for advice or treatment guidance. Typically, spokes will have performed an initial assessment including basic diagnostics, blood work, and often brain imaging. If the clinical scenario requires videoconferencing and image review to be properly addressed, a secure, web‐based system of high‐quality commercial videoconferencing is initiated and image transfer via secure Internet protocols is performed. These methods have been described in detail in prior publications. They permit interactive dialogue, neurologic examination, discussion of risks and benefits of the available treatment options, and documentation of the consult in a secure, web‐enabled SQL database that can be used for reporting and analysis. At the conclusion of the teleconsultation, the spoke and Hub physicians come to agreement on the triage disposition of the patient (eg, remain at the spoke, discharge to home, transfer to the Hub, etc.). At this time, the Hub neurologist documents his/her findings and assigns a diagnosis based on review of all the available clinical and imaging data, picking this diagnosis from a list of prespecified options, or writing it in as a free text diagnosis. For these analyses, diagnoses were classified as either ischemic cerebrovascular disease (iCVD) if they were acute ischemic stroke (<9‐hour duration), subacute ischemic stroke (>9‐hour duration) or transient ischemic attack; or as a SM for all other diagnoses.
Statistical Analysis
Baseline demographics, clinical characteristics, initial presentation, and intravenous administration of tPA were compared between patients diagnosed with iCVD or SM in the derivation cohort. Chi‐square test was used to compare categorical variables, and an independent‐sample t test or Wilcoxon rank sum test was used to compare mean or median differences for continuous variables in baseline characteristics between groups. Variables significant in univariate testing at a level of P<0.10 were entered into a stepwise logistic regression analysis in the order of their significance of association. Those that remained significant in multivariable testing were then included in the prediction model scoring system. NIHSS was analyzed both as a continuous variable and as a categorical variable. In addition, the NIHSS was plotted as a distribution, and was found to be skewed with an inflection point at an NIHSS of 14 points. Based on this distribution, a dichotomous variable (NIHSS >14) was also created and both versions were tested in the multivariable model separately. In the stepwise logistic regression model, factors with P<0.05 were retained. We also explored interactions between these variables, and included any interaction terms that were significant at the 0.05 level.
The results of the multivariable analysis were then used to develop a prediction model for SMs using a standard regression coefficient‐based scoring method.17–18 Integer scores were assigned by dividing factors' coefficients by the model constant and rounding up to the nearest integer. The TeleStroke Mimic Score (TM‐Score) for any individual patient was determined by summing the points assigned for each factor present. Scores were divided into deciles and displayed accordingly. Model discrimination was assessed by the receiver‐operating characteristic area under curve, which is equivalent to the c‐statistic.19 The performance of the model was validated by comparing the receiver‐operating characteristic curve analysis in the derivation set with that in the internal validation and external validation sets.20 The resulting continuous distributions of total scores across all patients in the 2 validation cohorts were then stratified into the decile groups of the derivation cohort. Separate graphs were plotted with a trend line showing the relationship between the score and likeliness of being diagnosed as a SM in the derivation cohort and comparing that with internal and external validation cohorts. Finally, we constructed a simple graphic nomogram for use by physicians performing telestroke consults to quantify the likelihood of any individual case being a SM. All statistical analyses were performed using version 20.0 of the IBM Software Package for Statistical Analysis (SPSS) software package for statistical analysis (SPSS).
Results
Patients Characteristics
There were 829 patients with video‐telestroke consults in the derivation cohort, 332 patients in the internal validation cohort, and 226 in the external validation cohort. When we compared those with DOB missing versus present in the record, there were no significant differences in any measured variables, including demographics, baseline clinical characteristics, medication use, initial symptoms documented by the emergency physician, NIHSS, diagnoses, or use of intravenous tPA.
Derivation, internal validation, and external validation cohorts were similar in these characteristics (Table 1) without any significant differences between the derivation and internal validation cohorts other than the presence of facial weakness. When compared to the derivation cohort, patients in the external validation cohort were younger, with more often a history of hypertension, diabetes, smoking and prior seizure, less often atrial fibrillation, and more often the presence of altered level of consciousness (Table 1). The percentages of SM were not different in the 3 cohorts (22.9% versus 24.1% versus 21.7%).
Table 1.
Characteristics of Those Patients Evaluated by Telestroke Consultation, Comparing Those in the Derivation Cohort to Those in the Internal Validation and External Validation Cohorts, Respectively
| Variable | Derivation Cohort (n=829) | Int. Validation Cohort (n=332) | P Value | Ext. Validation Cohort (n=226) | P Value |
|---|---|---|---|---|---|
| Age, y | 68.3±16.2 | 67.8±16.8 | 0.580 | 65.5±16.0 | 0.020 |
| Gender—male | 46.9% | 48.8% | 0.564 | 46.0% | 0.809 |
| Ethnicity—Hispanic | 7.0% | 8.4% | 0.398 | ||
| Race—white | 88.2% | 86.4% | 0.417 | 87.2% | 0.679 |
| Medical history | |||||
| Hypertension | 52.2% | 51.2% | 0.752 | 63.3% | 0.003 |
| Diabetes mellitus | 18.3% | 18.1% | 0.917 | 25.7% | 0.014 |
| Hyperlipidemia | 23.4% | 20.5% | 0.282 | 26.5% | 0.327 |
| Coronary artery disease | 15.6% | 15.4% | 0.932 | 20.4% | 0.086 |
| Atrial fibrillation | 18.2% | 12.7% | 0.023 | 12.4% | 0.039 |
| Heart failure | 5.9% | 8.7% | 0.082 | 7.5% | 0.375 |
| Previous stroke | 24.7% | 24.7% | 0.992 | 30.1% | 0.103 |
| Smoker | 5.8% | 4.8% | 0.512 | 12.8% | 0.001 |
| Seizure | 3.0% | 2.7% | 0.781 | 6.2% | 0.025 |
| Prior MI | 7.2% | 5.7% | 0.354 | 6.2% | 0.586 |
| Clinical presentation | |||||
| NIHSS* | 6 (3 to 13) | 5 (2 to 11) | 0.246 | 5 (2 to 11) | 0.190 |
| Signs at presentation | |||||
| Facial weakness | 59.3% | 51.8% | 0.022 | 63.3% | 0.285 |
| Limb weakness | 69.5% | 65.4% | 0.173 | 72.1% | 0.442 |
| Speech problem | 63.1% | 60.2% | 0.366 | 65.9% | 0.431 |
| Altered mental status | 41.0% | 39.8% | 0.694 | 50.9% | 0.008 |
| Hypertensive crisis | 18.5% | 21.2% | 0.285 | 20.6% | 0.274 |
| Diagnosis—SM | 22.9% | 24.1% | 0.668 | 21.7% | 0.694 |
Ext. indicates external; Int., internal; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale; SM, stroke mimics.
Median (interquartile range).
Derivation Cohort
Mean age of patients in the derivation cohort was observed to be 68.3±16.2 years; 46.9% of patients were male and 88.2% were white. Seventy‐seven percent of patients had iCVD (acute ischemic stroke, 66.8%; transient ischemic attack, 8.6%; subacute ischemic stroke, 1.7%). The most common alternative diagnoses in SM group were seizure/epilepsy (3.2%), headache/migraine with or without aura (2.9%), encephalopathy (2.6%), conversion disorder (1.5%), hemorrhagic stroke (1.0%), and all other miscellaneous diagnoses combined (11.7%). Vascular comorbidities were frequent in the derivation cohort, with hypertension being the most common in 52.2%, hyperlipidemia in 23.4%, diabetes mellitus in 18.3%, coronary artery disease in 15.6%, atrial fibrillation in 18.2%, and a history of seizure in 3.0% of telestroke patients.
Among clinical presentations, facial weakness was documented in 59.3%, limb weakness in 69.5%, difficulty with speech in 63.1%, and altered level of consciousness in 41.0% of patients. The median NIHSS was 6 (interquartile range 3 to 13), and 18.5% of the telestroke patients were in hypertensive crisis (BP>180/110) at the time of the telestroke consult. Forty percent of patients were treated with intravenous tPA.
Patients with the diagnosis of SM as compared to those with iCVD differed significantly in many characteristics, as shown in Table 2. SM patients were approximately 10 years younger; were significantly less likely to have a history of hypertension, atrial fibrillation, and heart failure; and were more likely to have a history of seizure. SM patients presented with a 4‐point‐lower median NIHSS (7.0 versus 3.0). They were less likely to have facial weakness, limb weakness, speech problems, or hypertensive crisis (blood pressure >180/110) at presentation (Table 2).
Table 2.
Demographics and Clinical Characteristics of Patients With Ischemic Cerebrovascular Disease (iCVD) vs Stroke Mimics (ie, Those Without Ischemic Cerebrovascular Disease) in the Derivation Cohort Over the Study Period of 9 Years
| iCVD (n=639) | Stroke Mimic (n=190) | P Value | |
|---|---|---|---|
| Age, y | 70.5±15.0 | 61.1±18.2 | <0.0001* |
| Gender—male | 48.4% | 42.1% | 0.130 |
| Ethnicity—Hispanic | 6.7% | 7.9% | 0.580 |
| Race—white | 88.6% | 86.8% | 0.516 |
| Medical history | |||
| Hypertension | 56.2% | 38.9% | <0.0001* |
| Diabetes mellitus | 19.1% | 15.8% | 0.302 |
| Hyperlipidemia | 24.6% | 19.5% | 0.145 |
| Coronary artery disease | 16.6% | 12.1% | 0.134 |
| Atrial fibrillation | 21.6% | 6.8% | <0.0001* |
| Heart failure | 6.9% | 2.6% | 0.029* |
| Previous stroke | 25.8% | 21.1% | 0.181 |
| Smoker | 5.3% | 7.4% | 0.289 |
| Seizure | 2.2% | 5.8% | 0.011* |
| Prior MI | 7.2% | 7.4% | 0.937 |
| Clinical presentation | |||
| NIHSS* | 7 (3 to 15) | 3 (1 to 8) | <0.0001* |
| NIHSS <14 | 25.2% | 9.6% | <0.0001* |
| Signs at presentation | |||
| Facial weakness | 66.8% | 34.2% | <0.0001* |
| Limb(s) weakness | 73.6% | 55.8% | <0.0001* |
| Speech problem | 66.4% | 52.1% | <0.0001* |
| Altered LOC | 40.7% | 42.1% | 0.727 |
| Hypertensive crisis | 22.5% | 15.8% | 0.006* |
| Medications | |||
| Antiplatelet | 41.9% | 34.2% | 0.056* |
| Anticoagulation | 9.2% | 5.3% | 0.082* |
Ext. indicates external; iCVD, ischemic cerebrovascular disease; Int., internal; LOC, level of consciousness; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale.
Continuous NIHSS; median (interquartile range).
Stepwise logistic regression model.
Univariate and Multivariable Analysis
Factors significant in univariate correlates of SM in the derivation set included age, hypertension, atrial fibrillation, heart failure, history of seizures, initial NIHSS, presenting symptoms of facial weakness, limb weakness, speech problems, hypertensive crisis, and prior use of antiplatelet and anticoagulation medicines. In the stepwise logistic regression model for SM, the variables listed in Table 3 were retained as independent predictors (P<0.05) of SM. NIHSS was not significant in logistic regression when entered as a continuous variable but was significantly associated with iCVD when entered in the dichotomous form (NIHSS >14, Figure 1) (Table 3).
Table 3.
Factors Significantly Associated With Stroke Mimics in Derivation Cohort on Stepwise Logistic Regression Model
| SM Predictors | Multivariable Analysis | ||
|---|---|---|---|
| β | Adjusted OR (95% CI) | Points | |
| Age (per y) | −0.026 | 0.98 (0.96 to 0.99) | +0.2/y |
| Atrial fibrillation | −0.781 | 0.48 (0.26 to 0.90) | +6 |
| Hypertension | −0.399 | 0.67 (0.46 to 0.96) | +3 |
| Seizure | 0.885 | 2.70 (1.10 to 6.67) | −6 |
| Facial weakness | −1.245 | 0.32 (0.22 to 0.45) | +9 |
| NIHSS >14 | −0.591 | 0.56 (0.31 to 0.98) | +5 |
NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; SM, stroke mimics.
Figure 1.
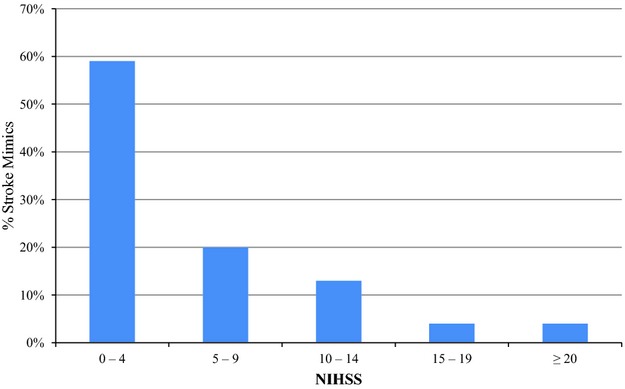
Stroke mimic patients classified according to initial NIHSS. NIHSS indicates National Institutes of Health Stroke Scale.
Development of the Prediction Rule: TM Score
To develop a prediction rule, we selected the 6 variables that remained significant after stepwise logistic regression (age, atrial fibrillation, hypertension, seizure, facial weakness, and NIHSS >14) and calculated an integer score proportional to their beta coefficients on logistic regression. Age was modeled as a continuous variable, while the other 5 variables were modeled as dichotomous variables. For each patient, all applicable scores were summed to attain a total TM Score for that patient. There was a graded decrease in the likelihood of having a SM disorder with increasing TM Score. Deciles were used to plot the data with a corresponding percentage of patients with SM in each point category and a trend‐line was plotted (Figure 2). The TM Score performed well on receiver‐operating characteristic curve analysis for predicting the likelihood of SM (area under the curve 0.75±0.02) (Figure 3).
Figure 2.
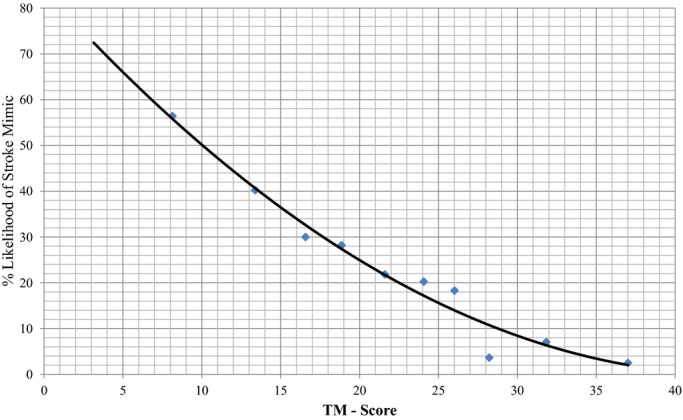
Scatter plot of the data with a trend line showing the relationship between TM‐Score and the likelihood of having a stroke mimic in derivation cohort. TM‐Score indicates TeleStroke Mimic Score.
Figure 3.
Response operator curve in the derivation cohort for the proposed score. ROC indicates receiver‐operating characteristic.
Model Validation
Validation sets consisted of an internal validation cohort and an external validation cohort. When the TM‐Score was applied to the internal validation cohort, the area under the curve was observed to be 0.72±0.03, only a slight degradation in performance. While applying the TM‐Score to the external validation cohort, we observed a greater area under curve of 0.77±0.03, showing that the prediction rule holds true on both internal and external validation (Figure 3). Similar to the derivation cohort, using the same deciles, we divided the data in the internal and external validation cohort and plotted as a trend line on the same graph. As shown by Figure 4, the 3 lines depict a similar trend and were not significantly different. For the ease of telestroke physicians using TM‐Score during their consult, we have included a nomogram showing the relationship between TM‐Score and the likelihood of having a SM based on the prediction rule (Figure 5).
Figure 4.
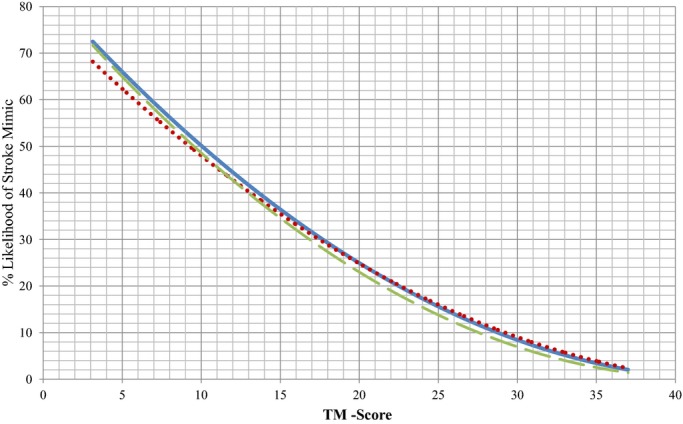
Scatter plot with a trend line showing the relationship between TM‐Score and the likelihood of having a stroke mimic in derivation cohort (solid blue line), internal validation cohort (dotted red line), and external validation cohort (dashed green line). TM‐Score indicates TeleStroke Mimic Score.
Figure 5.
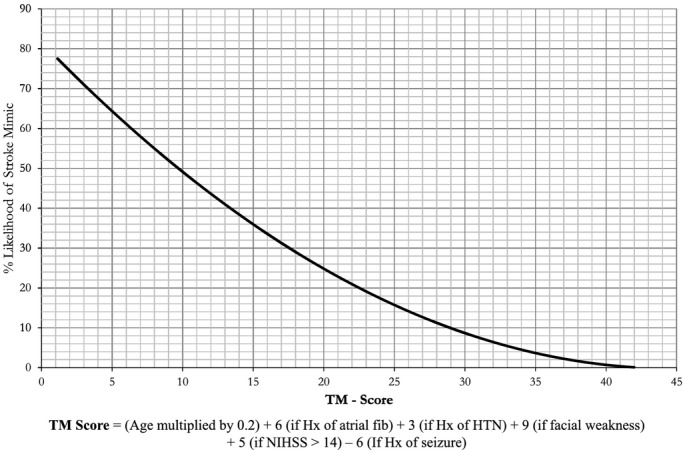
Recommended final prediction nomogram for Telestroke stroke mimics. TM‐Score=(Age multiplied by 0.2)+6 (if Hx of atrial fib)+3 (if Hx of HTN)+9 (if facial weakness)+5 (if NIHSS >14)−6 (if Hx of seizure). TM‐Score indicates TeleStroke Mimic Score.
Discussion
Using a national network of 52 spoke hospitals supported by 6 Hub hospitals in 7 states and evaluating a cohort of 1387 patients, we developed and validated a clinical prediction rule to calculate the likelihood of a patient being “tele”‐SM. A model based on the 6 factors that were associated with mimics allowed us to readily differentiate between SMs and patients with true iCVD on telestroke consults with reasonable model performance. The described predictive model represents a real‐time clinical decision‐making aid to prompt consideration of the possibility of a SM and is not intended as a diagnostic classification tool.
To our knowledge, this is the largest cohort study to date evaluating SM in a national telestroke network. We observed a similar rate of SMs in our telestroke cohort as that reported for patients presenting directly to stroke centers. In one of the earliest studies on SM patients by Harbison et al., the rate was 27% in a series of 487 consecutive patients who were directly admitted to a stroke service over a 6‐month period.21 Hand et al. reported that among 350 consecutive patients with focal brain dysfunction of sudden onset presenting to an urban teaching hospital, 31% were SMs.9 A recent series from a single stroke center found that 27% of patients referred from the ED as a stroke code did not have a cerebrovascular disorder, and the proportion of inpatient SMs was even higher.22 Other studies have reported lower rates of SMs. In a study by Libman et al., among 411 consecutive patients presenting to the ED, only 19% had a SM. It should be noted for this study that the ED physicians were specifically trained to identify stroke features since they were participating in an acute intervention trial.10 The SM rate was only 4.8% in a sample of 637 patients who were admitted to a stroke department after an initial evaluation, performed by a neurologist, that included computed tomography imaging.23
Several clinical factors that predict the presence of SM have been identified, and studies have noted that patients with SMs are younger and have lower vascular risk factors.9–10,12,15,24 The current study, however, is one of the first to explore the differences in demographics and clinical characteristics in patients managed during video‐telestroke consultations. We observed similar differences between SMs and those with cerebrovascular disease as those that were reported in the literature. Our results showed that SM patients were around 10 years younger with significantly fewer vascular risk factors. They were also observed to have fewer focal neurological symptoms and a lower median NIHSS at the time of consult. Chang et al. also reported a lower focal weakness and a lower median NIHSS in patients with SMs.25 Commensurate with the available literature, the SM group had a significantly higher percentage of patients with a medical history of seizure.9,12,14
Recognizing patient characteristics that differentiate iCVD patients from SM patients can be extremely useful when evaluating patients during telestroke consults. One interesting finding from our data is the very strong association between absence of facial weakness and a SM diagnosis. This suggests that patients with conditions that mimic stroke often do not have facial weakness, and this may be an important feature to focus on during the evaluation of patients with stroke‐like symptoms during telestroke evaluation. Similarly, the presence of a seizure disorder raises the likelihood that the current symptoms may be due to seizures with a postictal Todd's paralysis. However, a careful evaluation is still required because some of these patients will have had a new ischemic cerebrovascular event. Advanced imaging may be useful in these patients to definitively exclude ischemia. It is possible that this tool will also prove useful in detecting SMs in real time over telestroke, and potentially even in those patients who present in person to stroke centers. While it is possible that this tool is of greater value in a telestroke environment where some additional bedside clues may not be appreciated, most of the factors in the score are objective elements that are unlikely to be different when assessed in person versus remotely. The main difference would be facial weakness, which has been shown to be of good inter‐rated reliability on studies comparing in person to remote NIHSS.26 If one were to speculate on useful operational cutoffs for how best to use the score prospectively, based on our data it would be reasonable to use a score of ≤5 or the lack of facial weakness to strongly raise suspicion of a mimic (65% chance based on the score) and a score of ≥20 to strongly support the diagnosis of stroke. These cutoffs might be most useful in the decision of whether or not to mix intravenous tPA in advance of brain imaging, or to delay treatment until magnetic resonance imaging evidence of stroke, to most efficiently use these expensive resources.
As telestroke is rapidly being adopted,4 neurologists can anticipate an increased incidence of telestroke patients presenting with SM features. It may be beneficial to have a simple prediction rule that can be used to heighten awareness. However, a major issue with prediction rules is that physicians have found prediction rules difficult to implement in real‐time use.27 The prediction rule presented herein is based on the information easily available at the time of the initial ED evaluation. All the variables used to calculate the score are intuitive and biologically plausible, and the method of calculating the TM‐Score is straightforward. We have also produced a nomogram, which could be printed and carried on a pocket‐sized card and used to estimate the likelihood of a patient being a SM. If independently validated in other centers, the TM‐Score may help future ED physicians at spokes and tele‐neurologists at Hubs to be able to better prioritize and triage consultations, thus improving the overall system performance of telestroke consultations and saving valuable resources.
There are inherent limitations in the interpretations of the current study design. First, it is a retrospective analysis of a prospectively collected dataset. There may be incomplete data capture, and risk factors might have been abstracted with some variability across sites despite common definitions and a single, web‐based data report form. We may be missing some important patient characteristics that could be associated with SMs such as symptoms of vertigo, ataxia, dizziness, and visual changes that could add discriminating power to our prediction rule. The data were not subject to external audits, so there may be inaccuracies in data abstraction. Random measurement error and misclassification can lead to dilution bias and underestimation of the effects of the tested risk factors. However, the factors in our prediction rule were recorded regularly and are relatively unlikely to be influenced by the Hub neurologist judgment. The stroke neurologist's clinical diagnosis (iCVD versus SM) is the reference standard used in these analyses to classify patients. We had no alternative method available to further validate this diagnosis since only a fraction of patients were transferred to the Hub hospital postconsult for further evaluation or definitive magnetic resonance imaging. Since our approach to intravenous tPA eligibility is to err on the side of a diagnosis of ischemic stroke unless an alternative diagnosis is convincingly present, it is likely that some patients without true iCVD were classified as having iCVD; this result is more likely rather than the reverse, ie, patients with true iCVD would be classified as mimics. As this study included data from the spokes associated with these 6 urban tertiary care hospitals, it is possible that the proportion of SMs at other hospitals could differ. Therefore, we encourage the replication of this approach in other telestroke systems, especially those that serve very rural populations. Lastly, it is possible that SMs evaluated in person might have different characteristics than those seen over telestroke, where a physician has already applied some judgment as to the likelihood of iCVD. Therefore, our findings should also be replicated in non‐telestroke environments before being applied in these paradigms.
In conclusion, we believe that as telestroke consultation expands, increasing numbers of SM patients will be evaluated. These SM patients differ substantially from their counterpart iCVD patients in their vascular risk profiles and other characteristics. Decision‐making support tools based on predictive models, such as the TM‐Score we have proposed, may help clinicians consider alternative diagnoses and potentially identify SMs during complex, time‐critical telestroke evaluations.
Disclosures
Syed F. Ali, Anand Viswanathan, Aneesh B. Singhal, Natalia S. Rost, Pamela G. Forducey, Lawrence W. Davis, Joseph Schindler, William Likosky, Sherene Schlegel, and Nina Solenski all report no disclosures. Dr Schwamm serves as a consultant to the Massachusetts Department of Public Health, and is the Medical Director of the Mass General TeleHealth program and the Partners TeleStroke Center. Massachusetts General Hospital provides telehealth services to hospitals in the New England region, including telestroke. Dr Schwamm is the Principal Investigator of an National Institute of Neurological Disorders and Stroke–funded SPOTRIAS center clinical trial, MR WITNESS, for which Genentech provides alteplase and additional funding. His work is supported in part by Health Resource Services Administration Requisition 09‐HRS9923‐AB (Stroke and Traumatic Brain Injury Telehealth Services).
Acknowledgments
We would like to thank all the staff at our regional and Partners National TeleStroke Network sites for their continued effort and support. We gratefully acknowledge the contributions of John Johnson and Juan Estrada for their leadership and guidance in the technical and administrative aspects of the program, respectively.
References
- 1.Hess DC, Audebert HJ. The history and future of telestroke. Nat Rev Neurol. 2013; 9:340-350. [DOI] [PubMed] [Google Scholar]
- 2.Perednia DA, Allen A. Telemedicine technology and clinical applications. JAMA. 1995; 273:483-488. [PubMed] [Google Scholar]
- 3.Park S, Schwamm LH. Organizing regional stroke systems of care. Curr Opin Neurol. 2008; 21:43-55. [DOI] [PubMed] [Google Scholar]
- 4.Silva GS, Farrell S, Shandra E, Viswanathan A, Schwamm LH. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke. 2012; 43:2078-2085. [DOI] [PubMed] [Google Scholar]
- 5.Nelson RE, Saltzman GM, Skalabrin EJ, Demaerschalk BM, Majersik JJ. The cost‐effectiveness of telestroke in the treatment of acute ischemic stroke. Neurology. 2011; 77:1590-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Switzer JA, Demaerschalk BM, Xie J, Fan L, Villa KF, Wu EQ. Cost‐effectiveness of hub‐and‐spoke telestroke networks for the management of acute ischemic stroke from the hospitals' perspectives. Circ Cardiovasc Qual Outcomes. 2013; 6:18-26. [DOI] [PubMed] [Google Scholar]
- 7.Brunser AM, Illanes S, Lavados PM, Munoz P, Carcamo D, Hoppe A, Olavarria VV, Delgado I, Diaz V. Exclusion criteria for intravenous thrombolysis in stroke mimics: an observational study. J Stroke Cerebrovasc Dis. 2013; 22:1140-1145. [DOI] [PubMed] [Google Scholar]
- 8.Hemmen TM, Meyer BC, McClean TL, Lyden PD. Identification of nonischemic stroke mimics among 411 code strokes at the University of California, San Diego, stroke center. J Stroke Cerebrovasc Dis. 2008; 17:23-25. [DOI] [PubMed] [Google Scholar]
- 9.Hand PJ, Kwan J, Lindley RI, Dennis MS, Wardlaw JM. Distinguishing between stroke and mimic at the bedside: the brain attack study. Stroke. 2006; 37:769-775. [DOI] [PubMed] [Google Scholar]
- 10.Libman RB, Wirkowski E, Alvir J, Rao TH. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995; 52:1119-1122. [DOI] [PubMed] [Google Scholar]
- 11.Ay H, Buonanno FS, Rordorf G, Schaefer PW, Schwamm LH, Wu O, Gonzalez RG, Yamada K, Sorensen GA, Koroshetz WJ. Normal diffusion‐weighted mri during stroke‐like deficits. Neurology. 1999; 52:1784-1792. [DOI] [PubMed] [Google Scholar]
- 12.Winkler DT, Fluri F, Fuhr P, Wetzel SG, Lyrer PA, Ruegg S, Engelter ST. Thrombolysis in stroke mimics: frequency, clinical characteristics, and outcome. Stroke. 2009; 40:1522-1525. [DOI] [PubMed] [Google Scholar]
- 13.Uchino K, Massaro L, Hammer MD. Transient ischemic attack after tissue plasminogen activator: aborted stroke or unnecessary stroke therapy? Cerebrovasc Dis. 2010; 29:57-61. [DOI] [PubMed] [Google Scholar]
- 14.Chernyshev OY, Martin‐Schild S, Albright KC, Barreto A, Misra V, Acosta I, Grotta JC, Savitz SI. Safety of tpa in stroke mimics and neuroimaging‐negative cerebral ischemia. Neurology. 2010; 74:1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsivgoulis G, Alexandrov AV, Chang J, Sharma VK, Hoover SL, Lao AY, Liu W, Stamboulis E, Alexandrov AW, Malkoff MD, Frey JL. Safety and outcomes of intravenous thrombolysis in stroke mimics: a 6‐year, single‐care center study and a pooled analysis of reported series. Stroke. 2011; 42:1771-1774. [DOI] [PubMed] [Google Scholar]
- 16.Guillan M, Alonso‐Canovas A, Gonzalez‐Valcarcel J, Garcia Barragan N, Garcia Caldentey J, Hernandez‐Medrano I, Defelipe‐Mimbrera A, Sanchez‐Gonzalez V, Terecoasa E, Alonso de Lecinana M, Masjuan J. Stroke mimics treated with thrombolysis: further evidence on safety and distinctive clinical features. Cerebrovasc Dis. 2012; 34:115-120. [DOI] [PubMed] [Google Scholar]
- 17.Moons KG, Harrell FE, Steyerberg EW. Should scoring rules be based on odds ratios or regression coefficients? J Clin Epidemiol. 2002; 55:1054-1055. [DOI] [PubMed] [Google Scholar]
- 18.Tu JV, Naylor CD. Clinical prediction rules. J Clin Epidemiol. 1997; 50:743-744. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (roc) curve. Radiology. 1982; 143:29-36. [DOI] [PubMed] [Google Scholar]
- 20.Metz CE. Basic principles of roc analysis. Semin Nucl Med. 1978; 8:283-298. [DOI] [PubMed] [Google Scholar]
- 21.Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003; 34:71-76. [DOI] [PubMed] [Google Scholar]
- 22.El Husseini N, Goldstein LB. “Code stroke”: hospitalized versus emergency department patients. J Stroke Cerebrovasc Dis. 2013; 22:345-348. [DOI] [PubMed] [Google Scholar]
- 23.Vroomen PC, Buddingh MK, Luijckx GJ, De Keyser J. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis. 2008; 17:418-422. [DOI] [PubMed] [Google Scholar]
- 24.Scott PA, Silbergleit R. Misdiagnosis of stroke in tissue plasminogen activator‐treated patients: characteristics and outcomes. Ann Emerg Med. 2003; 42:611-618. [DOI] [PubMed] [Google Scholar]
- 25.Chang J, Teleb M, Yang JP, Alderazi YJ, Chapple K, Frey JL, Restrepo L. A model to prevent fibrinolysis in patients with stroke mimics. J Stroke Cerebrovasc Dis. 2012; 21:839-843. [DOI] [PubMed] [Google Scholar]
- 26.Shafqat S, Kvedar JC, Guanci MM, Chang Y, Schwamm LH. Role for telemedicine in acute stroke. Feasibility and reliability of remote administration of the NIH stroke scale. Stroke. 1999; 30:2141-2145. [DOI] [PubMed] [Google Scholar]
- 27.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules: a review and suggested modifications of methodological standards. JAMA. 1997; 277:488-494. [PubMed] [Google Scholar]