Abstract
Background
Treatment with the combination of clopidogrel and aspirin taken soon after a transient ischemic attack (TIA) or minor stroke was shown to reduce the 90‐day risk of stroke in a large trial in China, but the cost‐effectiveness is unknown. This study sought to estimate the cost‐effectiveness of the clopidogrel‐aspirin regimen for acute TIA or minor stroke.
Methods and Results
A Markov model was created to determine the cost‐effectiveness of treatment of acute TIA or minor stroke patients with clopidogrel‐aspirin compared with aspirin alone. Inputs for the model were obtained from clinical trial data, claims databases, and the published literature. The main outcome measure was cost per quality‐adjusted life‐years (QALYs) gained. One‐way and multivariable probabilistic sensitivity analyses were performed to test the robustness of the findings. Compared with aspirin alone, clopidogrel‐aspirin resulted in a lifetime gain of 0.037 QALYs at an additional cost of CNY 1250 (US$ 192), yielding an incremental cost‐effectiveness ratio of CNY 33 800 (US$ 5200) per QALY gained. Probabilistic sensitivity analysis showed that clopidogrel‐aspirin therapy was more cost‐effective in 95.7% of the simulations at a willingness‐to‐pay threshold recommended by the World Health Organization of CNY 105 000 (US$ 16 200) per QALY.
Conclusions
Early 90‐day clopidogrel‐aspirin regimen for acute TIA or minor stroke is highly cost‐effective in China. Although clopidogrel is generic, Plavix is brand in China. If Plavix were generic, treatment with clopidogrel‐aspirin would have been cost saving.
Keywords: clopidogrel, cost‐effectiveness, quality‐adjusted life‐year, stroke
Introduction
The early (90‐day) risk of recurrence of stroke and other vascular events following index transient ischemic attack (TIA) and minor ischemic stroke is very high, even in patients treated with aspirin, the current standard of care.1–3 Treatment with the combination of clopidogrel and aspirin taken soon after a TIA or minor stroke was found to decrease the 90‐day risk of stroke (hazard ratio 0.68, 95% CI 0.57 to 0.81, P<0.001) but did not increase the risk of hemorrhage compared with aspirin alone in the CHANCE stroke trial (Clopidogrel in High‐risk patients with Acute Non‐disabling Cerebrovascular Events Trial).4 Although the clopidogrel‐aspirin regimen is a reasonable early management for TIA and minor ischemic stroke, the extent of its adoption and use in clinical practice will depend―to some extent―on its economic practicality. However, the cost‐effectiveness, which is very important for patients, clinicians, and policy‐makers, has not been evaluated. In this analysis, we sought to determine the cost‐effectiveness of adding clopidogrel to aspirin in patients with acute TIA or minor stroke.
Methods
Model Overview
We adhered to the recommendations of the Panel on Cost‐effectiveness in Health and Medicine.5 Markov models are generally used to estimate the long term costs and outcomes associated with a disease and a particular healthcare intervention, in which the disease is divided into distinct states and transits between these states over a discrete time period under assigned transitions probabilities.6 A Markov model was developed (Figure 1) to simulate the cost‐effectiveness of two antiplatelet treatment strategies for high‐risk patients with acute minor stroke or TIA: (1) clopidogrel‐aspirin strategy: a 300‐mg loading dose of clopidogrel followed by 75 mg clopidogrel per day on days 2 to 90 plus aspirin 75 to 300 mg on day 1 followed by 75 mg/day on days 2 to 21 or (2) aspirin‐alone strategy: aspirin 75 to 300 mg on day 1 followed by 75 mg/day on days 2 to 90. The base‐case was a cohort of 100 000 patients (33% female), with mean age of 63 years at the time of acute ischemic minor stroke or TIA, which is the sex distribution and mean age of the patients who were enrolled in the CHANCE trial (Clopidogrel in High‐risk patients with Acute Non‐disabling Cerebrovascular Events Trial). Patients in the two treatment arms entered the model at the Markov health state of minor or no disability and transited to other health states (ischemic stroke or intracranial hemorrhage with minor or no disability, moderate disability, severe disability, myocardial infarction, extracranial hemorrhage, and dead) in the next cycle. Death (by stroke or other causes) was the only absorbing state after which the patient was excluded from the model. Total direct medical costs and quality‐adjusted life‐years (QALYs) gained with each alternative were estimated for each health state at 90 days from onset of TIA or minor stroke and then estimated annually for the remaining 30 years. This analysis was conducted from the perspective of healthcare payers. The study protocol was approved by the ethics committee of Beijing Tiantan Hospital. All participants or their legal proxies provided written informed consent.
Figure 1.
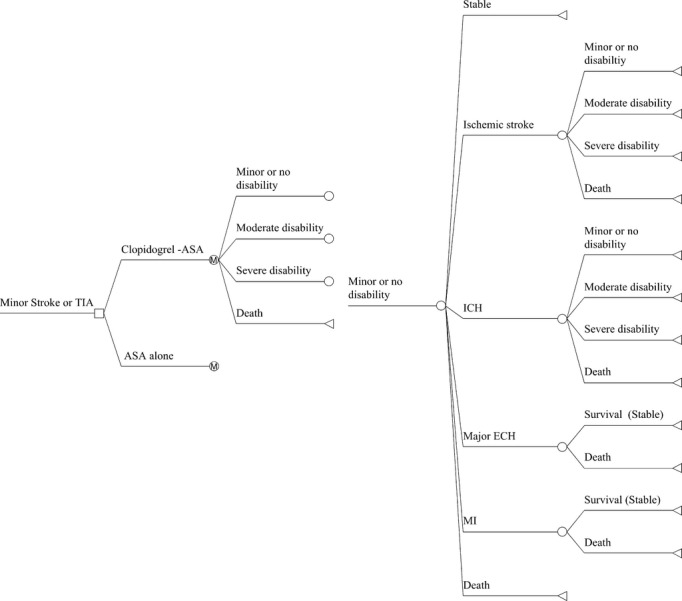
Markov model showing potential transitions between health states. Locations in the model where prescribers can make a decision (squares), chance nodes that are under the control of transition probabilities (circles), and terminal nodes (triangles) are presented. Transitions to future health states leading from the ASA‐alone branch are the same as for the Clopidogrel‐ASA branch. ASA indicates aspirin; ECH, extracranial hemorrhage; ICH, intracerebral hemorrhage; M, Markov node; MI, myocardial infarction; TIA, transient ischemic attack.
Input Parameters
Model input parameters were drawn from the published literature and directly from the results of the CHANCE trial (Tables 1 and 2). The death rates and the distribution of functional outcomes of patients treated with clopidogrel‐aspirin or aspirin alone in the first 90 days were based on results from the CHANCE trial, derived directly from the trial database. Major extracranial hemorrhage risk of the two treatment arms at 90 days were estimated by excluding intracranial hemorrhage from total hemorrhage defined by GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) criteria, because extracranial hemorrhage were not explicitly defined in CHANCE trial. We assumed all patients stopped the clopidogrel‐aspirin regimen and used aspirin alone after 90 days since dual‐antiplatelet therapy is not recommended for routine secondary stroke prevention7–8 and aspirin alone is more commonly used than clopidogrel alone in the long‐term setting after TIA and minor stroke.9–10 Recurrent rates of stroke and the proportion of intracranial hemorrhage (ICH) and fatal cases in recurrent stroke in years after the first 90 days of antiplatelet therapy were estimated from the China National Stroke Registry (CNSR), a nationwide registry for patients with acute cerebrovascular events in China between September 2007 and August 2008, recruiting 21 902 consecutive patients from 132 hospitals that cover all 27 provinces and four municipalities in China, and the Nanjing Stroke Registry Program (NSRP), a total of 1432 patients with first‐ever ischemic stroke registered from July 2002.11–12 We further assumed an increase in stroke recurrence rates by 1.017‐fold per life‐year, according to the relative risk estimated from CNSR. Patients remaining alive after recurrent stroke events were assumed to be reallocated equally among disability categories of equal and greater disability. For example, patients with minor or no disability who had a recurrent stroke and lived were allocated equally among minor or no disability, moderate disability, and severe disability categories. Age‐specific mortality rates for nonstroke death were derived from the most recent published census of China and adjusted by the causes of death of 2010 reported in the China Health Statistics Yearbook 2012.13–14
Table 1.
Efficacy of Clopidogrel‐Aspirin Regimen for TIA and Minor Stroke
| Model Input | Aspirin‐Alone Regimen | Clopidogrel‐Aspirin Regimen | Reference |
|---|---|---|---|
| Proportion of patients after 90 days | CHANCE trial database | ||
| Minor or no disability (mRS 0 to 2) | 0.9233 | 0.9376 | |
| Moderate disability (mRS 3 to 4) | 0.0670 | 0.0527 | |
| Severe disability (mRS 5) | 0.0058 | 0.0058 | |
| Death (mRS 6) | 0.0039 | 0.0039 | |
| 90‐day event risks | |||
| Recurrent stroke | 0.1172 | 0.0820 | |
| Proportion of ICH | 0.0264 | 0.0377 | |
| Major ECH | 0.0066 | 0.0108 | |
| MI | 0.0008 | 0.0012 |
TIA indicates transient ischemic attack; CHANCE, Clopidogrel in High‐risk patients with Acute Non‐disabling Cerebrovascular Events Trial; mRS, modified Rankin Score; ICH, intracerebral hemorrhage; ECH, extracranial hemorrhage; MI, myocardial infarction.
Table 2.
Model Parameters and the Range of Values Tested in Sensitivity Analyses
| Model Input | Base‐Case | Range | Reference |
|---|---|---|---|
| Cost inputs (2011 CNY) | |||
| Additional cost of 90‐day clopidogrel‐aspirin regimen | 1990 | 210 to 2388 | 22 |
| One‐time hospitalization costs | |||
| Ischemic stroke, mRS 0 to 2 | 9866 | 5542 to 12519 | CNSR14 |
| Ischemic stroke, mRS 3 to 5 or 6 | 13081 | 7383 to 17114 | |
| ICH, mRS 0 to 2 | 11118 | 5932 to 13902 | |
| ICH, mRS 3 to 5 or 6 | 14760 | 7665 to 19438 | |
| MI | 16793 | 6545 to 29434 | 14 |
| Major ECH | 7203 | 4391 to 14468 | |
| Annual posthospitalization costs | |||
| Stroke, mRS 0 to 2 | 6920 | 1728 to 8639 | CNSR |
| Stroke, mRS 3 to 5 | 10932 | 3240 to 14255 | |
| Utility inputs | |||
| Minor or no disability (mRS 0 to 2) | 0.75 | 0.70 to 0.90 | 18–19,23,25 |
| Moderate disability (mRS 3 to 4) | 0.39 | 0.10 to 0.50 | |
| Severe disability (mRS 5) | 0.20 | 0.00 to 0.32 | |
| Death (mRS 6) | 0 | 0.00 to 0.00 | |
| Utility of major ECH | 0.80 | 0.79 to 0.84 | 18–20 |
| Utility of MI | 0.84 | 0.67 to 0.96 | 18–19,21 |
| Probabilities inputs | |||
| Recurrent rate of stroke (per patient year) | 0.1219 | 0.1163 to 0.1276 | CNSR12 |
| Among patients with recurrent stroke, proportion with: | CNSR12 | ||
| ICH | 0.075 | 0.075 to 0.146 | |
| Death | 0.1933 | 0.1737 to 0.2128 | |
| Relative risk of stroke recurrence per life‐year | 1.017 | 1.013 to 1.022 | CNSR |
| Age‐specific nonstroke death rate* | 0.0089 to 0.1654 | 13–14 | |
| Rate of major ECH | 0.0038 | 0.0030 to 0.0048 | 15–16 |
| Mortality rate of major ECH | 0.06 | 16 | |
| Rate of MI | 0.0085 | 0.0060 to 0.0114 | 15–16 |
| Mortality rate of fatal MI | 0.150 | 0.103 to 0.246 | 15,17 |
| Discount rate inputs | |||
| Costs | 0.03 | 0.03 to 0.08 | 23 |
| Outcomes | 0.03 | ±20% | 23 |
All costs were converted to 2011 CNY by using the medical care component of consumer price index; to convert CNY to US dollars, divide by 6.5. CNY indicates Chinese Yuan Renminbi; mRS, modified Rankin Score; CNSR, China National Stroke Registry; MI, myocardial infarction; ECH, extracranial hemorrhage; ICH, intracerebral hemorrhage.
Age‐specific nonstroke death rate for only the number of 63‐year‐olds (0.0089) and 93‐year‐olds (0.1654) is presented.
In our model, myocardial infarction and major extracranial hemorrhages were considered temporary health states unless they resulted in death. These probabilities were derived from the literature.15–17 For nonfatal myocardial infarction and nonfatal major extracranial hemorrhage, we further assumed that all patients entering these health states would have a short‐term disutility of only 2 weeks for nonfatal major extracranial hemorrhage and 30 days for nonfatal myocardial infarction.18–21
Costs
All costs were total costs, including both out‐of‐pocket costs and reimbursement levels, and converted to 2011 Chinese Yuan Renminbi (CNY) by using the medical care component of consumer price index.14 One‐time hospitalization costs for major events and annual posthospitalization costs were based on CNSR and the China Health Statistics Yearbook 2012.14 Additional costs of the 90‐day clopidogrel‐aspirin regimen were based on the retail price of clopidogrel (brand) and aspirin according to Beijing Municipal Commission of Development and Reform.22 Indirect economic costs such as lost work productivity were not included in this analysis. All costs and utilities were discounted by 3% per year.23
Health States
In the model, patients could undergo transitions between four poststroke disability states based on the modified Rankin Scale (mRS): minor or no disability (mRS 0 to 2), moderate disability (mRS 3 to 4), severe disability (mRS 5), or death (mRS 6).24 At the end of each annual cycle, patients could remain in their current health state, transition to a lower health state due to recurrent stroke, or die due to a recurrent stroke or a nonvascular cause (see Figure 1).
Outcome Assessment
Health outcomes were measured in QALYs by multiplying years of life by utility scores derived from the literature. Utility estimates for stroke survivors were based on published utility values stratified by mRS category.18–19,23,25 Economic costs were measured as the difference of health care costs between the two treatment alternatives. The economic costs included the additional cost of the 90‐day clopidogrel‐aspirin regimen, hospitalization for stroke or TIA, myocardial infarction, extracranial hemorrhage, and posthospitalization long‐term care associated with each health state. The incremental cost‐effectiveness ratio (ICER) was calculated by dividing the cost difference by the difference in QALYs. Using the threshold of 3× GDP per capita of China in 2011 as the willingness‐to‐pay per QALY, a threshold recommended by the Commission on Macroeconomics and Health of World Health Organization,26 the intervention was considered cost‐effective if the ICER was <CNY 105 000 (3× GDP per capita of China in 2011,14 US$ 16 200) per QALY gained.
Sensitivity Analysis
One‐way sensitivity analyses were performed to test the robustness of model results on all variables across plausible ranges determined a priori. Plausible ranges were obtained from the literature or by varying estimates up to 20% in each direction (Table 2). To evaluate the impact of the uncertainty in all variables simultaneously, a probabilistic sensitivity analysis was performed using Monte Carlo simulation in Ersatz v1.3 (a bootstrap add‐in for Microsoft Excel for Windows; EpiGear International Pty Ltd). Costs were varied after assuming a lognormal distribution. Probabilities and utilities were varied according to a beta distribution. The simulation was run 10 000 times to capture stability of the results. Uncertainty was represented on a scatter‐plot and cost‐effectiveness acceptability curve.
Results
Base‐Case Analysis
In the base‐case scenario, for a 63‐year‐old patient with acute minor stroke or TIA, early 90‐day clopidogrel‐aspirin regimen would result in a lifetime gain of 0.037 QALY at an additional cost of CNY 1250 (US$ 192) (Table 3).Therefore, the ICER of early 90‐day clopidogrel‐aspirin regimen would be approximately CNY 33 800 (US$ 5200) per QALY gained. Using the threshold of CNY 105 000 (3× GDP per capita of China in 2011, US$ 16 200) as the willingness‐to‐pay per QALY, early 90‐day clopidogrel‐aspirin regimen was more cost‐effective in the base‐case scenario.
Table 3.
Cost and QALYs per Capita in Base‐Case Analysis
| Strategy | Cost (CNY) | QALYs | ICER (CNY/QALY) |
|---|---|---|---|
| Aspirin‐alone regimen | 136 850 | 6.461 | — |
| Clopidogrel‐aspirin regimen | 138 100 | 6.498 | 33 784 |
QALY indicates quality‐adjusted life‐year; CNY, Chinese Yuan Renminbi; ICER, incremental cost‐effectiveness ratio.
Sensitivity Analysis
One‐way sensitivity analyses indicated that the study results were robust. The tornado diagram in Figure 2 illustrates the effect of varying input parameters on the ICER. Overall, results were most sensitive to additional cost of 90‐day clopidogrel regimen and annual posthospitalization cost of disabling stroke (mRS 3 to 5). When additional cost of 90‐day clopidogrel regimen was decreased to CNY 210 (based on the generic clopidogrel price in the United States), the ICER of clopidogrel‐aspirin decreased to CNY −14 300/QALY, which represents a cost saving with improved health. When annual posthospitalization cost of disabling stroke was varied from CNY 14254.6 to CNY 3239.7, the dual‐antiplatelet therapy's ICER increased from CNY 25 000/QALY to CNY 53 500/QALY. The ICER was relatively insensitive to varying parameters of proportion of ICH in recurrent stroke, utility of major extracranial bleed, utility of myocardial infarction, and hospitalization cost of ICH.
Figure 2.
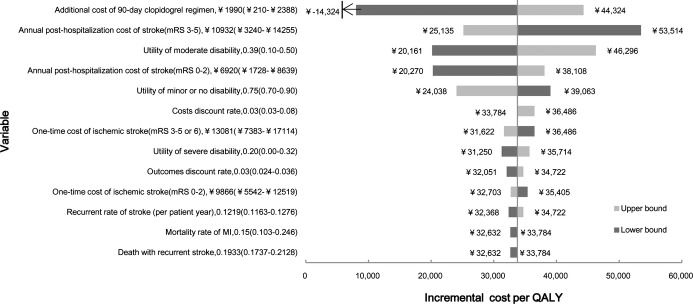
One‐way sensitivity analyses on incremental cost per quality‐adjusted life‐year (ICER) gained by 90‐day clopidogrel‐aspirin regimen. All parameters were analyzed, and only those with highest influence on ICER are displayed. The first number listed after the variable name is the base‐case value. Numbers listed in parentheses indicate the range of the variable. Dark‐shaded bars represent the lower bound of the variable range. Light‐shaded bars represent the upper bound. Solid vertical lines represent ICER of the clopidogrel‐aspirin regimen at the base‐case scenario (CNY 33 800). CNY indicates Chinese Yuan Renminbi; MI, myocardial infarction; QALYs, quality‐adjusted life‐years.
Results of 10 000 iteration probabilistic sensitivity analysis are shown in Figure 3. In 10.6% of simulation runs, the clopidogrel‐aspirin therapy was less costly and more effective than aspirin‐alone therapy. Clopidogrel‐aspirin therapy was cost‐effective in 95.7% of the simulations at a willingness‐to‐pay threshold of CNY 105 000 (3× GDP per capita of China in 2011, US$ 16 200) per QALY. It remained cost‐effective in 49.0% of the simulations at a willingness‐to‐pay threshold of CNY 35 100 (1× GDP per capita of China in 2011, US$ 5400) per QALY. A cost‐effectiveness acceptability curve shows the probability of cost‐effectiveness of the clopidogrel‐aspirin regimen as a function of the willingness‐to‐pay threshold (Figure 4).
Figure 3.
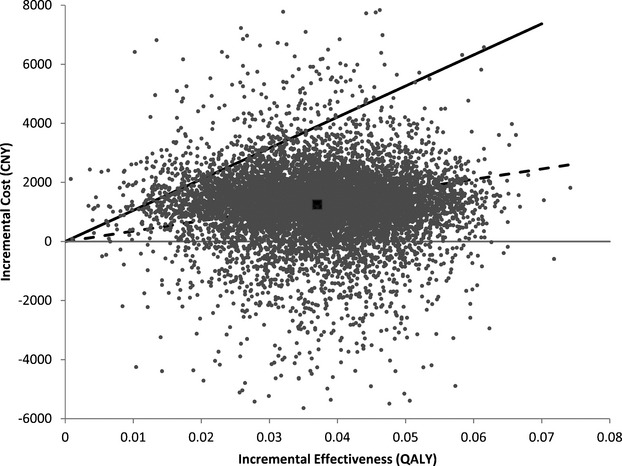
Incremental cost‐effectiveness scatterplot of the result of the probabilistic sensitivity analysis. Each point represents a simulation. The dark square represents the base‐case (0.037 QALY gained at an incremental cost of CNY 1250). The solid line represents the willingness‐to‐pay threshold of CNY 105 000 per QALY. The dashed line represents CNY 35 100 per QALY. CNY indicates Chinese Yuan Renminbi; QALYs, quality‐adjusted life‐years.
Figure 4.
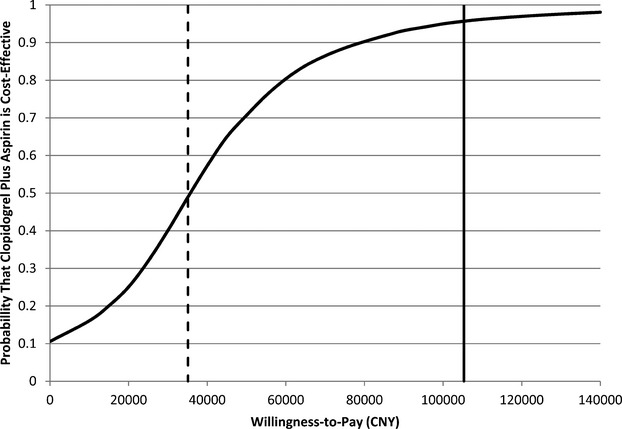
Cost‐effectiveness acceptability curve results based on 10 000 Monte Carlo simulations of the model. The curve presents the probability that the clopidogrel‐aspirin regimen is cost‐effective as a function of willingness‐to‐pay threshold. The solid vertical line represents the willingness‐to‐pay threshold of CNY 105 000 per QALY. The dashed vertical line represents CNY 35 100 per QALY. CNY indicates Chinese Yuan Renminbi; QALYs, quality‐adjusted life‐years.
Discussion
Based largely on the results of the CHANCE trial, this cost‐effectiveness analysis demonstrates that a 90‐day clopidogrel‐aspirin regimen increases costs but improves quality of life. A patient on dual antiplatelet therapy gained an additional 0.037 QALY over a lifetime but at an additional of CNY 1250 (US$ 192), resulting in an ICER of CNY 33 800 (US$ 5200) per QALY. The robustness of our overall conclusion that 90‐day clopidogrel‐aspirin regimen is cost‐effective is supported by the sensitivity analysis. The lifetime gain of 0.037 QALY (0.44 quality‐adjusted months) for early 90‐day clopidogrel‐aspirin regimen of TIA and minor stroke is comparable to that of most stroke treatments. For example, the lifetime QALY gain is 0.56 for tissue plasminogen activator treatment for acute ischemic stroke in the 3‐hour time window,27 0.17 for clopidogrel (ccompared with aspirin) for secondary prevention in stroke patients.15 The gain of QALYs associated with early 90‐day clopidogrel‐aspirin regimen of TIA and minor stroke is relatively smaller than that of other treatments mainly because, unlike other analyses did, our analysis referred to nondisabling cerebrovascular events (TIA and minor stroke) other than stroke with high severity.
Cost‐effectiveness was sensitive to the additional cost of 90‐day clopidogrel regimen and the annual posthospitalization cost of disabled stroke. When clopidogrel price was decreased to CNY 78 (US$ 12, the generic clopidogrel price in US) for a 1‐month supply, the additional cost of 90‐day clopidogrel regimen was decreased, and dual‐antiplatelet therapy became cost saving. If the annual posthospitalization cost of disabling stroke was reduced to approximately one‐third of the base‐case level, cost saving from dual antiplatelet therapy was decreased, but the resultant ICER was still substantially lower than the willingness‐to‐pay threshold.
In current guidelines for the early management of patients with acute ischemic stroke published by both the cerebrovascular disease group of Chinese Medical Association and by the American Heart Association/American Stroke Association, clopidogrel and aspirin combination therapy is not recommended for patients with minor ischemic stroke and TIA.28–29 Aspirin monotherapy is the current standard of care in China. However, guidelines have not incorporated results of the CHANCE trial and patients with aspirin monotherapy still have 90‐day stroke risk of 10% to 20% after minor ischemic stroke or TIA.1–3 Our study suggested that clopidogrel‐aspirin combination therapy is a cost‐effective alternative over aspirin monotherapy when taken soon after a TIA or minor stroke.
Our study has limitations that should be considered when interpreting the results. First, the external generalizability of our findings may be affected as it is based on the efficacy findings from a single trial performed in China (the CHANCE trial), whose participants were restricted to Chinese patients. It is not known whether combination regimen will be shown to be similarly effective in other populations or settings. Additionally, we used a base‐case cohort based on the CHANCE trial patient characteristics (mean age was 63 years and 33% were female), with a low proportion of female TIA patients. This was different from typical TIA population from population‐based cohorts, and may result in different events rates and medical resource use.30–31 Second, we used most estimates from CNSR and previously published literature for our input parameters. It is possible that these estimates are inaccurate. For example, we used the costs data from CNSR and inflate the costs to 2011 Chinese Yuan Renminbi to estimate the one‐time hospitalization costs of stroke. However, hospitalization costs of stroke differed widely by location and level of hospital.14,32 These estimates are higher than the official (mean hospital cost) figures from the Ministry of Health of China (CNY 7325.3, US$ 1127 for ischemic stroke; CNY 11 802.1 US$ 1816 for ICH),14 but comparable with those reported by the ChinaQUEST study (CNY 10 689, US$ 1527 for ischemic stroke; CNY 13 089, US$ 1870 for ICH, in 2006).32 This may have been because many of the patients in CNSR and ChinaQUEST studies were from urban locations where costs are greater. Additionally, several surveys explored poor rehabilitation and decreased adherence to secondary prevention of ischemic stroke in China after discharge,9,33–36 which may dramatically influence the annual posthospitalization costs but would do so similarly in both groups. We estimated annual posthospital costs from data of the real world registry of CNSR. Third, in the Markov model, we assumed future transitions of health status based on events related to stroke recurrence and total nonstroke death and functional status as a result of other cause were not considered in this model. Some studies have shown that disability status also affects survival,37–38 and this was not included in the present analysis. Fourth, we assumed that patients remaining alive after recurrent stroke events were reallocated equally among disability categories of equal and greater disability. Finally, this analysis was conducted from the perspective of healthcare payers. If a societal perspective was taken, indirect costs such as lost work productivity should be included and it may probably influence the results of the model.5 These assumptions may deviate from the real world, but are unlikely to affect the overall results of our study because our findings were robust over reasonable variations in all model inputs.
Our study reflects costs and event rates in China. Results may not be generalizable to other countries. For example, the monthly cost of clopidogrel (CNY 650, US$ 100) is substantially greater in China than in the United States, where generic clopidogrel is available for CNY 78 (US$ 12) for a month supply while the costs of hospitalization and posthospital care are much greater in the United States. Clopidogrel will be available as a generic in China in 2017. If generic is introduced, the price will come down dramatically and will likely be cost saving.
Conclusions
Early treatment with a 90‐day clopidogrel‐aspirin regimen for acute TIA or minor stroke is highly cost‐effective in China setting. Although clopidogrel is generic, Plavix is brand in China. If Plavix were generic, treatment with clopidogrel‐aspirin would have been cost saving.
The CHANCE Investigators
Yongjun Wang (BeijingTiantan Hospital, Principal Investigator); S.Claiborne Johnston (Departments of Neurology and Epidemiology, University of California, San Francisco, USA, Co‐Principal Investigator); Yilong Wang (BeijingTiantan Hospital, Executive Committee); Xingquan Zhao (BeijingTiantan Hospital, Site Investigator); Zhimin Wang (Taizhou First People's Hospital, Site Investigator); Haiqin Xia (Taiyuan Iron And Steel [Group] Co., Ltd., General Hospital, Site Investigator); (Dagang Oilfield Gengeal Hospital, Site Investigator); Guiru Zhang (Penglai People's Hospital, Site Investigator); Xudong Ren (The Third People's Hospital Of Datong, Site Investigator); Chunling Ji (The Fourth Central Hospital Of Tianjin, Site Investigator); Guohua Zhang (The Second Hospital Of Hebei Medical University, Site Investigator); Jianhua Li (The First Hospital Of Fangshan District, Beijing, Site Investigator); Bohua Lu (Beijing Puren Hospital, Site Investigator); Liping Wang (Tianjin Ninghe District Hospital, Site Investigator); Shutao Feng (The People's Hospital Of Zhengzhou, Site Investigator); Dali Wang (Affiliated Hospital Of North China Coal Medical College, Site Investigator); WeiguoTang (Zhejiang Zhoushan Hospital, Site Investigator); Juntao Li (Han Dan Central Hospital, Site Investigator); Hongtian Zhang (Zhecheng People's Hospital, Site Investigator); Guanglai Li (Shanxi Medical University Second Hospital, Site Investigator); Baojun Wang (Baotou Central Hospital, Site Investigator); Yuhua Chen (The General Hospital Of Changjiang River Shipping, Site Investigator); Ying Lian (Dalian Economic And Technological Development Zone Hospital, Site Investigator); Bin Liu (First Neurology Department, Affiliated Hospital Of North China Coal Medical College, Site Investigator); Junfang Teng (The First Affiliated Hospital Of Zhengzhou University, Site Investigator); Rubo Sui (First Affiliated Hospital Of Liaoning Medical, Site Investigator); Lejun Li (Lianyungang Municipal Hospital Of TCM, Site Investigator); Zhiling Yuan (Central Hospital In Qiu County, Site Investigator); Dawei Zang (Tianjin First Center Hospital, Site Investigator); Zuneng Lu (Renmin Hospital Of Wuhan University, Site Investigator); Li Sun (Qingdao Central Hospital, Site Investigator); Dong Wang (Baogang Hospital, Site Investigator); Liying Hou (Changzhi City People's Hospital Of Shanxi Province, Site Investigator); Dongcai Yuan (HaLixun International Peace Hospital, Site Investigator); Yongliang Cao (People's Hospital Of Linzi District, Zibo, Site Investigator); Hui Li (Yantai City Yantai Mountain Hospital, Site Investigator); Xiuge Tan (Beijing Pinggu District Hospital, Site Investigator); Huicong Wang (Taiyuan Central Hospital, Site Investigator); Haisong Du (Chengde Central Hospital, Site Investigator); Mingyi Liu (Shijiazhuang Central Hospital, Site Investigator); Suping Wang (First Neurology Department, Dalian Municipal Central Hospital, Site Investigator); Qiuwu Liu (Xian 141 Hospital, Site Investigator); Zhong Zhang (Chengdu Third Municipal People's Hospital, Site Investigator); Qifu Cui (Affiliated Hospital Of Chifeng University, Site Investigator); Runqing Wang (Zhengzhou Central Hospital, Site Investigator); Jialin Zhao (Ningbo City, Zhejiang Province Lihuili Hospital Medical Center, Site Investigator); Jiewen Zhang (Henan Provincial People's Hospital, Site Investigator); Jianping Zhao (Jinzhong City Second Hospital, Site Investigator); Qi Bi (Beijing Anzhen Hospital, Capital Medical University, Site Investigator); Xiyou Qi (Beijing Huairou District Chinese Medicine Hospital, Site Investigator); Junyan Liu (Hebei Medical University Third Hospital, Site Investigator); Changxin Li (First Affiliated Hospital Shanxi Medical Unversity, Site Investigator); Ling Li (Hebei Provincial People's Hospital, Site Investigator); Xiaoping Pan (Guangzhou First Municipal Peoples Hospital, Site Investigator); Junling Zhang (Central Hospital In Cangzhou, Site Investigator); Derang Jiao (The Chinese People's Armed Police Force Medical School Affiliated Hospital, Site Investigator); Zhao Han (Zhejiang Wenzhou Medical College First Affiliated Hospital, Site Investigator); Dawei Qian (Jilin Central Hospital, Site Investigator); Jin Xiao (Anhui Maanshan Central Hospital, Site Investigator); Yan Xing (Beijing Aviation Industry Central Hospital, Site Investigator); Huishan Du (Luhe Hospital, Tongzhou District, Beijing, Site Investigator); Guang Huang (Beijing Fuxing Hospital, Capital Medical University, Site Investigator); Yongqiang Cui (The 306th Hospital Of P.L.A, Site Investigator); Yan Li (The First Affiliated Hospital Of Tianjin University Of Chinese Medicine, Site Investigator); Lianyuan Feng (Baiqiuen International Peace Hospital Of People's Liberation Army, Site Investigator); Lianbo Gao (Fourth Affiliated Hospital Of China Medical University, Site Investigator); Bo Xiao (Xiangya Hospital Central‐South University, Site Investigator); Yibin Cao (Tangshan Worker's Hospital, Site Investigator); Yiping Wu (The 1st Hospital In Handan, Site Investigator); Jinfeng Liu (Yangquan Coal (Group) Co., Ltd. General Hospital, Site Investigator); Zhiming Zhang (Tianjin Tianhe Hospital, Site Investigator); Zhengxie Dong (Nantong First People's Hospital, Site Investigator); Limin Wang (The 1st Hospital Of Zhangjiakou City, Site Investigator); Li He (West China Hospital, Sichuan University, Site Investigator); Xinchen Wang (The Second Affiliated Hospital Of Shandong University Of TCM, Site Investigator); Xueying Guo (Fenyang Hospital Of Shanxi Province, Site Investigator); Ming Wang (Zhejiang Zhoushan Putuo District People's Hospital, Site Investigator); Xiaosha Wang (Xiyuan Hospital Of China Academy Of Chinese Traditional Medicine, Site Investigator); Jiandong Jiang (No.2 People's Hospital East In Lianyungang City, Site Investigator); Renliang Zhao (Affiliated Hospital Of Qingdao University Medical College, Site Investigator); Shengnian Zhou (Qilu Hospital Of Shandong University, Site Investigator); HaoHu (Zibo Hospital Of Traditional Chinese Medicine, Site Investigator); Maolin He (Beijing Shijitan Hospital, Site Investigator); Fengchun Yu (Beijing Haidian Hospital, Site Investigator); Quping Ouyang (Beijing Shunyi District Hospital, Site Investigator); Jingbo Zhang (Dalian Third Municipal Hospital, Site Investigator); Anding Xu (The First Affliated Hospital Of Jinan University, Site Investigator); Xiaokun Qi (Navy Genaral Hospital Of P.L.A, Site Investigator); Lei Wang (Beijing Second Artillery General Hospital, Site Investigator); Fuming Shi (Beijing Daxing District Hospital, Site Investigator); Fuqiang Guo (Sichuan Province People's Hospital, Site Investigator); Jianfeng Wang (Dalian Municipal Central Hospital, Site Investigator); Fengli Zhao (The Second Hospital In Baoding, Site Investigator); Ronghua Dou (The Hospital Combine Traditional Chinese And Western Medicine In Cang zhou, Site Investigator); Dongning Wei (The 309th Hospital Of P.L.A, Site Investigator); Qingwei Meng (Liangxiang Hospital Of Fangshan District, Beijing, Site Investigator); Yilu Xia (HuaXin Hospital First Hospital Of Tsinghua University, Site Investigator); ShiminWang (TianjinHuanhu Hospital, Site Investigator); Zhangcang Xue (Shijiazhuang First Hospital, Site Investigator); Yuming Xu (The First Affiliated Hospital Of Zhengzhou University, Site Investigator); Liping Ma (Xinzhou City People's Hospital, Site Investigator); Chun Wang (Sichuan Province People's Hospital Of Deyang City, Site Investigator); Jiang Wu (First Hospital, Jilin University, Site Investigator); Yifeng Du (Shandong Provincial Hospital, Site Investigator); Yinzhou Wang (Fujian Province Hospital, Site Investigator); Lijun Xiao (Liaoyang City Third People's Hospital, Site Investigator); Fucong Song (Handan City Center Hospital, Site Investigator); Wenli Hu (Beijing Chaoyang Hospital, Capital Medical University, Site Investigator); Zhigang Chen (Beijing University Of Chinese Medicine East Hospital, Site Investigator); Qingrui Liu (Hebei Medical University Fourth Hospital, Site Investigator); Jiemin Zhang (The Fourth Affiliated Hospital Of Soochow University, Site Investigator); Mei Chen (Zhejiang University Of Chinese Medicine Affiliated First Hospital, Site Investigator); Xiaodong Yuan (Affiliated Hospital Of Kailuan Company Ltd, Site Investigator); Zhihui Liu (Affiliated Hospital Of Weifang Medical University, Site Investigator); Guozhong Li (The First Hospital Of Harbin Medical University, Site Investigator); Xiaohong Li (Dalian Friendship Hospital, Site Investigator); Tingchen Tian (Tianjin Dagang Hospital, Site Investigator).
Sources of Funding
The CHANCE study, which was used to derive certain model parameters, is supported by grant from the Ministry of Science and Technology of the People's Republic of China. The grant no. is 2008ZX09312‐008, 2011BAI08B02, 2012ZX09303 and 200902004.
Disclosures
Dr Johnston is the principal investigator of the POINT trial, a NIH‐sponsored trial with clopidogrel and placebo donated by Sanofi.
References
- 1.Johnston SC, Gress DR, Browner WS, Sidney S. Short‐term prognosis after emergency department diagnosis of TIA. JAMA. 2000; 284:2901-2906. [DOI] [PubMed] [Google Scholar]
- 2.Coull AJ, Lovett JK, Rothwell PMOxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004; 328:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population‐based study. Neurology. 2004; 62:2015-2020. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SCCHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013; 369:11-19. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost‐effectiveness in health and medicine. JAMA. 1996; 276:1253-1258. [PubMed] [Google Scholar]
- 6.Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998; 13:397-409. [DOI] [PubMed] [Google Scholar]
- 7.Wang YJ, Zhang SM, Zhang L, Wang CX, Dong Q, Gao S, Huang RX, Huang YN, Lv CZ, Liu M, Qin HQ, Rao ML, Xiao Y, Xu YM, Yang ZH, Wang YJ, Wang CX, Wang JZ, Wang WZ, Wang J, Wang WJ, Wu J, Wu SP, Zeng JS, Zhang SM, Zhang L, Zhao XQ, Zhong LY. Chinese guidelines for the secondary prevention of ischemic stroke and transient ischemic attack 2010. CNS Neurosci Ther. 2012; 18:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil‐Smoller S, Turan TN, Wentworth DAmerican Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:227-276. [DOI] [PubMed] [Google Scholar]
- 9.Wei JW, Wang JG, Huang Y, Liu M, Wu Y, Wong LK, Cheng Y, Xu E, Yang Q, Arima H, Heeley EL, Anderson CSChinaQUEST Investigators. Secondary prevention of ischemic stroke in urban China. Stroke. 2010; 41:967-974. [DOI] [PubMed] [Google Scholar]
- 10.Wettermark B, Persson A, von Euler M. Secondary prevention in a large stroke population: a study of patients' purchase of recommended drugs. Stroke. 2008; 39:2880-2885. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Cui L, Ji X, Dong Q, Zeng J, Wang Y, Zhou Y, Zhao X, Wang C, Liu L, Nguyen‐Huynh MN, Claiborne Johnston S, Wong L, Li H, Li HChina National Stroke Registry Investigators. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. 2011; 6:355-361. [DOI] [PubMed] [Google Scholar]
- 12.Xu G, Liu X, Wu W, Zhang R, Yin Q. Recurrence after ischemic stroke in Chinese patients: impact of uncontrolled modifiable risk factors. Cerebrovasc Dis. 2007; 23:117-120. [DOI] [PubMed] [Google Scholar]
- 13.National Bureau of Statistics of China. The 2010 Population Census of the Peaple's Republic of China. [online] Available at: http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm. (in Chinese). (accessed October 25, 2012).
- 14.Ministry of Health of the People's Republic of China. China Health Statistics Yearbook 2012. Beijing: Peking Union Medical College Press; 2012. (in Chinese). [Google Scholar]
- 15.Schleinitz MD, Weiss JP, Owens DK. Clopidogrel versus aspirin for secondary prophylaxis of vascular events: a cost‐effectiveness analysis. Am J Med. 2004; 116:797-806. [DOI] [PubMed] [Google Scholar]
- 16.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996; 348:1329-1339. [DOI] [PubMed] [Google Scholar]
- 17.Bernheim SM, Grady JN, Lin Z, Wang Y, Wang Y, Savage SV, Bhat KR, Ross JS, Desai MM, Merrill AR, Han LF, Rapp MT, Drye EE, Normand SL, Krumholz HM. National patterns of risk‐standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010; 3:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Anglade MW, Pham D, Pisacane R, Kluger J, Coleman CI. Cost–effectiveness of rivaroxaban ccompared with warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012; 110:845-851. [DOI] [PubMed] [Google Scholar]
- 19.You JH, Tsui KK, Wong RS, Cheng G. Cost‐effectiveness of dabigatran versus genotype‐guided management of warfarin therapy for stroke prevention in patients with atrial fibrillation. PLoS One. 2012; 7:e39640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson R, Parkin D, Eccles M, Sudlow M, Robinson A. Decision analysis and guidelines for anticoagulant therapy to prevent stroke in patients with atrial fibrillation. Lancet. 2000; 355:956-962. [DOI] [PubMed] [Google Scholar]
- 21.Coleman CI, Straznitskas AD, Sobieraj DM, Kluger J, Anglade MW. Cost–effectiveness of clopidogrel plus aspirin for stroke prevention in patients with atrial fibrillation in whom warfarin is unsuitable. Am J Cardiol. 2012; 109:1020-1025. [DOI] [PubMed] [Google Scholar]
- 22.Beijing Municipal Commission of Development and Reform. The maximum retail price of western medicine [online]. Available at: http://service2.bjpc.gov.cn/bjpc/mediprice/WesternMedicine_qry.jsp. (in Chinese). (accessed October 12, 2012).
- 23.Tung CE, Win SS, Lansberg MG. Cost‐effectiveness of tissue‐type plasminogen activator in the 3‐ to 4.5‐hour time window for acute ischemic stroke. Stroke. 2011; 42:2257-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, Singer DE. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003; 349:1019-1026. [DOI] [PubMed] [Google Scholar]
- 25.Earnshaw SR, Jackson D, Farkouh R, Schwamm L. Cost‐effectiveness of patient selection using penumbral‐based mri for intravenous thrombolysis. Stroke. 2009; 40:1710-1720. [DOI] [PubMed] [Google Scholar]
- 26.Commission on Macroeconomics and Health of World Health Organization. CHOosing Interventions that are Cost Effective (WHO‐CHOICE) Available at: http://www.who.int/choice/costs/CER_thresholds/en/. (accessed October 17, 2012).
- 27.Fagan SC, Morgenstern LB, Petitta A, Ward RE, Tilley BC, Marler JR, Levine SR, Broderick JP, Kwiatkowski TG, Frankel M, Brott TG, Walker MD. Cost‐effectiveness of tissue plasminogen activator for acute ischemic stroke. NINDS rt‐PA Stroke Study Group. Neurology. 1998; 50:883-890. [DOI] [PubMed] [Google Scholar]
- 28.Cerebrovascular Disease Group of Chinese Medical Association. Chinese guidelines for the diagnosis and therapy of acute ischemic stroke 2010 (in Chinese). Chin J Neurol. 2010; 43:1-8. [Google Scholar]
- 29.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Summers DR, Wang DZ, Wintermark M, Yonas HAmerican Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44:870-947. [DOI] [PubMed] [Google Scholar]
- 30.Johnston SC, Fayad PB, Gorelick PB, Hanley DF, Shwayder P, van Husen D, Weiskopf T. Prevalence and knowledge of transient ischemic attack among US adults. Neurology. 2003; 60:1429-1434. [DOI] [PubMed] [Google Scholar]
- 31.Fonseca PG, Weiss PA, Harger R, Moro CH, Longo AL, Gonçalves AR, Whiteley WN, Cabral NL. Transient ischemic attack incidence in joinville, Brazil, 2010: a population‐based study. Stroke. 2012; 43:1159-1162. [DOI] [PubMed] [Google Scholar]
- 32.Wei JW, Heeley EL, Jan S, Huang Y, Huang Q, Wang JG, Cheng Y, Xu E, Yang Q, Anderson CSChinaQUEST Investigators. Variations and determinants of hospital costs for acute stroke in China. PLoS ONE. 2010; 5:e13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li AD, Huang ZQ, Liu HT, Deng YT, Xiao JW, Liang JW. Knowledge, attitude and behavior for stroke and rehabilitation in stroke patients and relatives.(in Chinese). Chin J Rehabil Theory and Pract. 2009; 15:252-254. [Google Scholar]
- 34.Gao YL, Chen LD, Tao J, Chen SM. Investigation on the investment and popularization of rehabilitation from cerebral apoplexy: in 2 communities of Xiamen City.(in Chinese). Chin J Convalescent Med. 2009; 18:472-474. [Google Scholar]
- 35.Wang Y, Wu D, Ma R, Wang C, Zhao W. A survey on adherence to secondary ischemic stroke prevention. Neurol Res. 2006; 28:16-20. [DOI] [PubMed] [Google Scholar]
- 36.Ma R, Wang C, Zhao X, Xu M, Lv Y, Wei M, Cai Y, Zhang Z, Wang L, Zhang W, Huang Y, Li Y, Li H, Wang Y. A survey on compliance with secondary stroke prevention guidelines and follow up for the inpatients with atherosclerotic cerebral infarction/transient ischemic attack. Neurol Res. 2008; 30:383-388. [DOI] [PubMed] [Google Scholar]
- 37.Slot KB, Berge E, Dorman P, Lewis S, Dennis M, Sandercock POxfordshire Community Stroke Project, the International Stroke Trial (UK); Lothian Stroke Register. Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ. 2008; 336:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eriksson M, Norrving B, Terént A, Stegmayr B. Functional outcome 3 months after stroke predicts long‐term survival. Cerebrovasc Dis. 2008; 25:423-429. [DOI] [PubMed] [Google Scholar]


