Abstract
Background
In the general population, raised levels of inflammatory markers are stronger predictors of fatal than nonfatal cardiovascular disease (CVD) events. People with HIV have elevated levels of interleukin‐6 (IL‐6), high‐sensitivity C‐reactive protein (hsCRP), and D‐dimer; HIV‐induced activation of inflammatory and coagulation pathways may be responsible for their greater risk of CVD. Whether the enhanced inflammation and coagulation associated with HIV is associated with more fatal CVD events has not been investigated.
Methods and Results
Biomarkers were measured at baseline for 9764 patients with HIV and no history of CVD. Of these patients, we focus on the 288 that experienced either a fatal (n=74) or nonfatal (n=214) CVD event over a median of 5 years. Odds ratios (ORs) (fatal versus nonfatal CVD) (95% confidence intervals [CIs]) associated with a doubling of IL‐6, D‐dimer, hsCRP, and a 1‐unit increase in an IL‐6 and D‐dimer score, measured a median of 2.6 years before the event, were 1.39 (1.07 to 1.79), 1.40 (1.10 to 1.78), 1.09 (0.93 to 1.28), and 1.51 (1.15 to 1.97), respectively. Of the 214 patients with nonfatal CVD, 23 died during follow‐up. Hazard ratios (95% CI) for all‐cause mortality were 1.72 (1.28 to 2.31), 1.73 (1.27 to 2.36), 1.44 (1.15 to 1.80), and 1.88 (1.39 to 2.55), respectively, for IL‐6, D‐dimer, hsCRP, and the IL‐6 and D‐dimer score.
Conclusions
Higher IL‐6 and D‐dimer levels reflecting enhanced inflammation and coagulation associated with HIV are associated with a greater risk of fatal CVD and a greater risk of death after a nonfatal CVD event.
Clinical Trial Registration
URL: http://www.clinicaltrial.gov Unique identifier: SMART: NCT00027352, ESPRIT: NCT00004978, SILCAAT: NCT00013611.
Keywords: cardiovascular disease, inflammation
Introduction
Over 15 years ago, an association between enhanced inflammation, as demonstrated by higher plasma levels of C‐reactive protein (CRP), and risk of coronary heart disease (CHD) in middle‐aged men without previous cardiovascular disease (CVD) was reported using data from the Multiple Risk Factor Intervention Trial (MRFIT).1 In MRFIT, CRP was strongly related to CHD mortality, but was not related to nonfatal myocardial infarction (MI). Importantly, most of the CHD deaths occurred more than 10 years after the CRP measurement. This MRFIT observation that markers of inflammation are stronger predictors of fatal, as compared to nonfatal, CHD events has been confirmed in other studies.2–4 Reasons for this finding include the possibility that patients with higher inflammatory markers may have different underlying vascular disease. It has been speculated that patients with greater levels of inflammation might be more likely to experience fatal arrhythmias that result in sudden death or have greater levels of inflammation in unstable plaques.1–3 It is also possible that low‐grade inflammation results in activation of the coagulation system, increasing the likelihood of fatal outcomes subsequent to plaque rupture.4 Other reasons include a greater presence of other CVD risk factors among those with higher inflammatory biomarker levels that increased risk of death, or the presence of other nonvascular conditions that are associated with chronic inflammation and that lead to an increased risk of fatal events.
Patients with HIV may contribute to our understanding of these findings in the general population. They are in a sustained inflammatory state even when taking suppressive antiretroviral therapy (ART).5 Reasons for this inflammatory state and the associated chronic state of immune activation have been reviewed.6–7 Some key findings concerning HIV, inflammation, and CVD, which we and others have described, are: (1) compared to age‐ and gender‐matched people in the general population interleukin‐6 (IL‐6), high‐sensitivity CRP (hsCRP), and D‐dimer are elevated5; (2) patients with HIV appear to be at an increased risk of CVD, compared to individuals without HIV8–12; and (3) IL‐6, hsCRP, and D‐dimer measured several years beforehand are associated with all‐cause mortality and fatal or nonfatal CVD.1,13 The results in the reports by Kuller and Duprez, which utilized stored plasma specimens from the Strategies for Management of Antiretroviral Therapy (SMART) trial,14–15 also suggested that though the associations of these biomarkers with fatal or non‐fatal CVD was similar to associations reported in general population studies, the association with all‐cause mortality was stronger than for CVD, even though the deaths in SMART were attributed to a number of different causes.
Collectively, these findings led us to formulate 2 hypotheses: (1) HIV‐positive patients with higher levels of IL‐6, hsCRP, and D‐dimer, measured several years before the CVD event, are more likely to experience a fatal, as compared to a nonfatal, CVD event; and (2) mortality after nonfatal CVD events is higher among HIV‐positive patients with higher, as compared to lower, levels of IL‐6, hsCRP, and D‐dimer, also measured several years before the nonfatal event. To investigate these hypotheses, we used data from SMART and 2 other large international clinical trials of HIV treatments.
Methods
Study Populations
This investigation included patients from 3 large international HIV treatment trials conducted by the International Network for Strategic Initiatives in Global HIV Trials, the SMART trial,14–15 the Evaluation of Subcutaneous Proleukin® in a Randomized International Trial (ESPRIT),16–17 and the Subcutaneous Recombinant, Human Interleukin‐2 in HIV‐Infected Patients with Low CD4+ Counts under Active Antiretroviral Therapy (SILCAAT) trial.17 These trials were carried out in 33, 25, and 11 countries, respectively. The SMART study compared continuous ART with intermittent ART among HIV‐positive patients with a CD4+ cell count of more than 350 cells/mm3. ESPRIT and SILCAAT compared IL‐2 plus ART versus ART alone among HIV‐positive patients with CD4+ counts of 300 cells/mm3 or more and with CD4+ counts of 50 to 299 cells/mm3, respectively. All studies were approved by an institutional review committee and patients were included only after giving informed consent.
Biomarker Measurements
IL‐6, hsCRP, and D‐dimer were measured at baseline, before randomization, in each trial using stored plasma for patients who provided written consent. For patients in SMART, these biomarkers were measured at the Laboratory for Clinical Biochemistry Research at the University of Vermont (Burlington). In the ESPRIT and SILCAAT trials, laboratory measurements were performed by SAIC‐Frederick (Frederick, MD). All samples in both laboratories were analyzed blinded to treatment group and CVD event status. IL‐6 was measured by the same method at each laboratory (Chemiluminescent Sandwich ELISA; R&D Sytems, Minneapolis, MN). D‐dimer levels were measured by ELISA on the Sta‐R analyzer, Liatest D‐DI (Diagnostic Stago, Parsippany, NJ), for patients in SMART and on a VIDAS instrument (bioMérieux Inc, Durham, NC) for patients in ESPRIT and SILCAAT. hsCRP was measured by ELISA by both laboratories. For SMART, a NBTMII nephelometer, N Antiserum to Human CRP (Siemens Diagnostics, Deerfield, IL), was used. For ESPRIT and SILCAAT, an R&D Systems ELISA assay was used. Twenty samples were independently analyzed at each laboratory for these biomarker levels. Table S1 summarizes the measurements made at each laboratory. Lower limits of detection (LLOD) for IL‐6, hsCRP, and D‐dimer were 0.16 pg/mL, 0.16 μg/mL, and 0.01 μg/mL for SMART. In ESPRIT and SILCAAT, LLOD were 0.156 pg/mL, 0.078 μg/mL, and 0.045 μg/mL.
Baseline and Follow‐up Measurements
In all 3 studies, the following baseline measurements were obtained before randomization: age, sex, race, body mass index (BMI), CD4+ cell count, HIV‐RNA, duration of ART, and previous AIDS clinical event. In SMART and ESPRIT, additional baseline measurements were made, including hepatitis B/C coinfection, diabetes, and use of blood pressure and lipid‐lowering medication. For patients in SMART, smoking status was assessed and blood lipids were measured. During follow‐up, HIV RNA levels and CD4+ cell counts were recorded every 4 months in each study.
Events
CVD events considered were deaths attributed to CVD, unwitnessed deaths that were not attributed to suicide, drug abuse, or violence, nonfatal MI, nonfatal stroke, and coronary artery disease (CAD) requiring surgery. In SMART and ESPRIT, documentation of CVD events that was provided by the clinical sites were reviewed by an endpoint review committee using prespecified criteria.18 Criteria for acute MI followed the universal definition of MI.19 CAD requiring surgery required a procedure report, hospital discharge summary, or other medical record from the hospitalization during which the procedure was performed (coronary artery bypass graft, coronary artery stent implant, coronary artherectomy, or percutaneous transluminal angioplasty). For strokes, 5 criteria were considered: (1) acute onset with clinically compatible course, including unequivocal objective findings of a localizing neurological deficit; (2) computed tomography (CT) or magnetic resonance imaging (MRI) compatible with diagnosis of stroke and current neurologic signs and symptoms; (3) stroke diagnosed as cause of death at autopsy; (4) positive lumbar puncture compatible with subarachnoid hemorrhage; and (5) death certificate or death note from medical record listing stroke as the cause of death. A participant was considered to have experienced a stroke if the first and second criteria were met, the third criterion was met, the first and fourth criteria were met, or the first and fifth criteria were met. In SILCAAT, CVD events reported as serious adverse events were coded according to the Medical Dictionary for Regulatory Activities (MedRA; version 12.0). The following Standardized MedRA Query codes were used for nonfatal stroke (20000082), nonfatal MI (20000047), and CAD requiring surgery (10068176, 10052086, 10057787, or 10063025). In all 3 studies, cause of death was coded using documentation of the death provided by the clinical sites using the Coding of Death in HIV system.20
In addressing our first hypothesis, we defined fatal CVD events as: (1) deaths attributable to CVD or unwitnessed deaths for patients that did not experience a nonfatal MI or stroke before their death and (2) deaths within 28 days after a nonfatal MI, stroke, or CAD. Patients who experienced MI, stroke, or CAD events and survived at least 28 days were defined as having nonfatal CVD events.
For our second hypothesis, we considered any patient with an MI, stroke, or CAD event who survived at least 28 days. For this group of patients, we assessed subsequent risk of all‐cause mortality.
Statistical Analyses
Logistic regression, including indicators for study the patient was enrolled in (SMART, ESPRIT, SILCAAT), was used to study the association of each biomarker with fatal CVD. In addition to considering each biomarker individually, we also considered a combined IL‐6 and D‐dimer score used for predicting all‐cause mortality among HIV patients with a suppressed viral load. Because of their independent association with all‐cause mortality, the IL‐6 and D‐dimer score was created in order to account for the contribution of both markers in a single combined measure. This score was determined using the control arms of SMART, ESPRIT, and SILCAAT (144 deaths) and was adjusted for age, gender, and study; IL‐6 and D‐dimer were log2 transformed. The regression coefficients from this Cox model for IL‐6 and D‐dimer were then used to create the IL‐6 and D‐dimer score.21
In this investigation, the biomarkers were log2 transformed because their distributions were right‐skewed. With this approach, a 1 log2 higher level of a biomarker corresponds to a doubling of the marker. Results are also cited for tertiles of each biomarker, which were defined using all of the patients in the 3 studies that experienced a fatal or nonfatal CVD event. Other covariates, measured in each study, that were considered potential confounding factors were: time between biomarker measurement and the event, age, gender, race, baseline BMI, HIV RNA level, baseline CD4+ cell count, and earlier AIDS event at study entry. We also considered the interaction between time between biomarker measurement and the event with the log‐transformed biomarker. In sensitivity analyses, we adjusted for hepatitis B/C coinfection, diabetes, and use of blood pressure and lipid‐lowering medication (SMART and ESPRIT) and then added smoking and the ratio of total cholesterol to high‐density lipoprotein (HDL) cholesterol (SMART only). We also carried out separate analyses for patients in the control arms of the 3 studies. These patients were to receive continuous ART with a goal of suppressing HIV‐RNA levels during follow‐up. This is the recommended standard of care for patients with HIV.22
Cumulative mortality after a nonfatal CVD event was estimated using the Kaplan‐Meier method. Cox models that included study indicators were used to study factors related to mortality for the 214 patients who experienced a nonfatal CVD event. Models included the same covariates as the logistic model, as well as CD4+ cell counts and HIV‐RNA levels both proximal to the nonfatal event. Hazard ratios (HRs) and 95% confidence intervals (CIs) are cited. The proportional hazards assumption was tested by including an interaction term between each biomarker and log‐transformed follow‐up time.
All analyses were performed using SAS statistical software (version 9.2; SAS Institute Inc., Cary, NC). P<0.05 was considered significant.
Results
Among the 11 278 patients randomized in these trials (5472 SMART, 4111 ESPRIT, and 1695 SILCAAT), 10 001 (89%) had IL‐6, hsCRP, and D‐dimer measured on stored baseline plasma samples for consenting patients (5017 SMART, 3570 ESPRIT, and 1414 SILCAAT). Of these, 9764 (98%) did not report a history of CVD at study entry (Figure). Over a median (interquartile range; IQR) follow‐up of 5.0 (2.3, 7.1) years, 74 of these 9764 patients developed fatal CVD; for 68 of these patients, the CVD event reported was death (36 resulting from CVD and 32 unwitnessed deaths); for 6 additional patients, death occurred within 28 days of a nonfatal CVD event (3 after a stroke and 3 after an MI). Two hundred and fourteen patients experienced an MI, stroke, or CAD event and survived at least 28 days (102 MIs, 43 strokes, and 69 CADs requiring surgery). Thus, 26% of CVD events (74 of 288) were fatal. The median (IQR) time from biomarker measurement at baseline to the event was 2.6 (1.1, 4.7) for fatal and non‐fatal CVD events.
Figure 1.
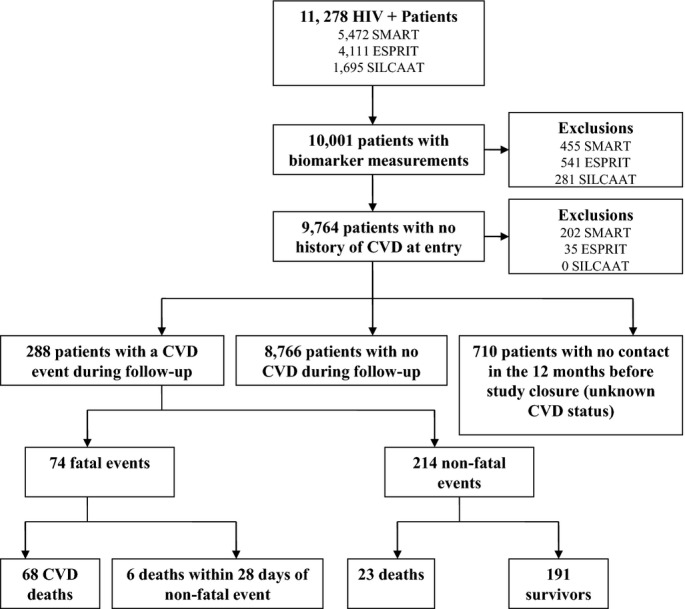
Flow diagram of patients included in analyses. CVD indicates cardiovascular disease; ESPRIT, Evaluation of Subcutaneous Proleukin® in a Randomized International Trial; SILCAAT, Subcutaneous Recombinant, Human Interleukin‐2 in HIV‐Infected Patients with Low CD4+ Counts under Active Antiretroviral Therapy; SMART, Strategies for Management of Antiretroviral Therapy.
Comparison of Fatal and Nonfatal CVD Events
Baseline characteristics for patients according to the development of a CVD event during follow‐up and its severity are summarized in Table 1. In these univariate analyses, IL‐6, D‐dimer, and the IL‐6 and D‐dimer score were significantly greater for those patients who experienced a fatal, as compared to a nonfatal, CVD event; hsCRP levels were also higher for those with fatal events, as compared to nonfatal events, but the difference was not significant (P=0.26). Consistent with a previous report,13 levels of all 3 of the biomarkers were higher for patients who developed CVD, as compared to those who did not. Difference in biomarker levels for those with and without a CVD event, and by the severity of the CVD event, was consistent across all 3 studies (Table 2). In regression models, we considered the interaction of study with each biomarker and none were significant (P>0.40 for all). In the summary below, we pool the results for the 3 studies.
Table 1.
Baseline Characteristics for HIV‐Positive Patients Who Experienced CVD Events According to Severity
| No Event* | Fatal CVD* | Nonfatal CVD* | P Value* | |
|---|---|---|---|---|
| Baseline characteristics, n | 9476 | 74 | 214 | |
| Age (y), median (IQR) | 42 (36 to 48) | 48 (41 to 54) | 49 (42 to 54) | 0.76 |
| Sex (% female) | 22 | 14 | 8 | 0.21 |
| Race (% black) | 19 | 23 | 16 | 0.20 |
| BMI (kg/m2), median (IQR) | 24.3 (22.1 to 27.0) | 24.0 (22.1 to 27.0) | 24.3 (22.1 to 27.1) | 0.58 |
| CD4+ cell count, (cells/mm3) median (IQR) | 487 (367 to 669) | 409 (331 to 644) | 469 (356 to 633) | 0.63 |
| HIV‐RNA<500 copies/mL, % | 77 | 74 | 77 | 0.64 |
| Earlier AIDS event, % | 26 | 39 | 29 | 0.10 |
| IL‐6, (pg/mL) median (IQR) | 1.8 (1.2 to 2.8) | 3.1 (1.9 to 4.5) | 2.3 (1.5 to 3.5) | 0.01* |
| % highest tertile (<1.88)* | 20 | 45 | 28 | |
| % middle tertile (1.88≤×<3.14)* | 27 | 31 | 36 | |
| % lowest tertile (≥3.14)* | 52 | 24 | 36 | 0.02* |
| D‐dimer, (μg/mL) median (IQR) | 0.24 (0.15 to 0.37) | 0.35 (0.24 to 0.61) | 0.27 (0.17 to 0.45) | 0.006* |
| % highest tertile (<0.22) | 21 | 45 | 29 | |
| % middle tertile (0.22≤×< 0.41) | 31 | 32 | 34 | |
| % lowest tertile (≥0.41) | 47 | 23 | 37 | 0.03* |
| hsCRP, (μg/mL) median (IQR) | 1.6 (0.7 to 3.6) | 3.1 (1.1. to 7.5) | 2.2 (1.1 to 5.6) | 0.26* |
| % highest tertile (<1.55) | 21 | 39 | 31 | |
| % middle tertile (1.55≤×<4.17) | 29 | 32 | 34 | |
| % lowest tertile (≥4.17) | 50 | 28 | 35 | 0.49* |
| IL‐6 and D‐dimer score median (IQR) | −0.02 (−0.60 to 0.61) | 0.85 (0.20 to 1.36) | 0.30 (−0.20 to 0.89) | 0.003 |
| % highest tertile (<1.04) | 19 | 51 | 27 | |
| % middle tertile (1.04≤×<1.75) | 28 | 26 | 36 | |
| % lowest tertile (≥1.75) | 53 | 23 | 37 | 0.001* |
BMI indicates body mass index; CVD, cardiovascular disease; hsCRP, high‐sensitivity C‐reactive protein; IQR, interquartile range.
No event measurements noted for completeness.
Deaths attributed to CVD and unwitnessed deaths not resulting from violence, suicide, or drug abuse that were not proceeded by a nonfatal event or deaths within 28 days of the nonfatal CVD event.
Nonfatal myocardial infarction, coronary artery disease requiring surgery, or nonfatal stroke for participants that survived at least 28 days.
From a univariate logistic model comparing fatal and nonfatal CVD events (n=288).
P values reported based on log2‐transformed biomarker measurement.
P value based on 2 df chi‐square test.
Tertiles were defined using all of the patients in the 3 studies that experienced a fatal or nonfatal CVD event.
Table 2.
Characteristics of SMART, ESPRIT, and SILCAAT Patients With and Without Fatal/Nonfatal CVD Events
| SMART | ESPRIT | SILCAAT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Event | Fatal CVD | Nonfatal CVD | No Event | Fatal CVD | Non‐Fatal CVD | No Event | Fatal CVD | Non‐Fatal CVD | |
| Baseline characteristics, n | 4697 | 32 | 86 | 3418 | 26 | 91 | 1361 | 16 | 37 |
| Age (y), median (IQR) | 43 (37 to 50) | 50 (47 to 54) | 50 (43 to 55) | 40 (35 to 46) | 44 (38 to 51) | 48 (41 to 54) | 40 (36 to 47) | 49 (43 to 58) | 47 (41 to 54) |
| Sex (% female) | 27 | 22 | 15 | 18 | 4 | 4 | 16 | 13 | 3 |
| Race (% black) | 29 | 41 | 34 | 9 | 8 | 4 | 9 | 13 | 5 |
| BMI, (kg/m2) median (IQR) | 25.0 (22.5 to 28.1) | 24.0 (21.8 to 30.1) | 25.7 (22.3 to 28.4) | 23.7 (21.9 to 25.9) | 24.4 (23.1 to 25.8) | 23.9 (22.0 to 26.0) | 23.8 (21.7 to 26.1) | 23.0 (21.3 to 26.9) | 23.5 (22.1 to 26.1) |
| BP‐lowering drug use, % | 17 | 47 | 37 | 5 | 0 | 10 | N/A | NA | NA |
| Lipid‐lowering drug use, % | 14 | 13 | 33 | 10 | 13 | 20 | N/A | NA | NA |
| Hepatitis B or C coinfected, % | 17 | 31 | 14 | 22 | 29 | 15 | N/A | NA | NA |
| CD4+ cell count, (cells/mm3) median (IQR) | 598 (467 to 794) | 649 (442 to 874) | 593 (450 to 838) | 453 (368 to 581) | 399 (341 to 516) | 470 (386 to 582) | 202 (150 to 255) | 200 (144 to 269) | 188 (131 to 249) |
| ART, % | 84 | 81 | 87 | 100 | 100 | 100 | 100 | 100 | 100 |
| Time since first ART, (y) median (IQR) | 6 (4 to 8) | 7 (5 to 9) | 6 (4 to 8) | 4 (2 to 6) | 5 (2 to 6) | 6 (4 to 8) | 4 (2 to 8) | 3 (1 to 6) | 5 (3 to 8) |
| Baseline HIV‐RNA <500 copies/mL, % | 72 | 72 | 72 | 81 | 69 | 81 | 82 | 88 | 78 |
| Earlier AIDS event, % | 24 | 41 | 34 | 27 | 58 | 23 | 32 | 6 | 32 |
| Diabetes, % | 6 | 13 | 21 | 2 | 8 | 6 | N/A | NA | NA |
| Smoker, % | 41 | 53 | 52 | N/A | NA | NA | N/A | NA | NA |
| Total/HDL cholesterol, (mmol/L) median (IQR) | 4.6 (3.6 to 5.9) | 4.1 (3.1 to 5.5) | 6.1 (4.2 to 8.1) | N/A | NA | NA | N/A | NA | NA |
| IL‐6, (pg/mL) median (IQR) | 1.7 (1.1 to 2.9) | 4.1 (2.0 to 6.5) | 2.6 (1.8 to 4.5) | 1.9 (1.3 to 2.7) | 2.7 (2.1 to 3.2) | 2.2 (1.5 to 3.1) | 1.80 (1.20 to 2.70) | 2.5 (1.6 to 3.6) | 1.9 (1.5 to 2.9) |
| D‐dimer, (μg/mL) median (IQR) | 0.20 (0.13 to 0.37) | 0.37 (0.21 to 0.72) | 0.27 (0.15 to 0.49) | 0.26 (0.19 to 0.37) | 0.33 (0.25 to 0.48) | 0.28 (0.19 to 0.42) | 0.25 (0.17 to 0.36) | 0.42 (0.30 to 0.70) | 0.27 (0.18 to 0.42) |
| hsCRP, (μg/mL) median (IQR) | 1.7 (0.7 to 4.0) | 5.9 (1.3 to 8.9) | 2.6 (1.4 to 6.7) | 1.5 (0.7 to 3.2) | 2.4 (1.0 to 3.7) | 1.7 (1.0 to 4.2) | 1.4 (0.6 to 3.3) | 3.5 (2.3 to 5.0) | 2.3 (1.0 to 4.6) |
| IL‐6 and D‐dimer score, median (IQR) | −0.12 (−0.81 to 0.64) | 1.17 (0.16 to 1.95) | 0.51 (−0.08 to 1.14) | 0.09 (−0.39 to 0.59) | 0.56 (0.29 to 0.92) | 0.18 (−0.21 to 0.78) | −0.03 (−0.53 to 0.51) | 0.61 (−0.22 to 1.19) | 00.15 (−0.25 to 0.56) |
ART indicates antiretroviral therapy; BP, blood pressure; BMI, body mass index; CVD, cardiovascular disease; ESPRIT, Evaluation of Subcutaneous Proleukin® in a Randomized International Trial; HDL, high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein; SILCAAT, Subcutaneous Recombinant, Human Interleukin‐2 in HIV‐Infected Patients with Low CD4+ Counts under Active Antiretroviral Therapy; SMART, Strategies for Management of Antiretroviral Therapy.
When considered as continuous log2‐transformed measurements, higher levels of IL‐6, D‐dimer, and the IL‐6 and D‐dimer score were associated with greater odds of a fatal CVD event (Table 3). This was evident in uni‐ and multivariate analyses. When tertiles were considered, significant differences between the third and first tertiles were found for IL‐6, D‐dimer, and the IL‐6 and D‐dimer score. The risk gradient, as judged by the odds ratio (OR) for a doubling of the biomarker and based on the tertile analysis, tended to be strongest for D‐dimer and the IL‐6 and D‐dimer score and weakest for hsCRP. Interactions of each biomarker and time between biomarker measurement and the event were also considered and none were significant. Sensitivity analyses were performed, excluding the unwitnessed deaths, and results were similar to those in Table 3. For example, univariate ORs (95% CI) associated with a doubling of IL‐6, D‐dimer, hsCRP, and the IL‐6 and D‐dimer score were 1.41 (1.03 to 1.92), 1.36 (1.01 to 1.82), 1.13 (0.92 to 1.37), and 1.50 (1.08 to 2.07), respectively.
Table 3.
Unadjusted and Covariate Adjusted* Odds Ratios for Fatal CVD* (Versus Nonfatal CVD*) According to Tertile and Associated With a Doubling of Each Biomarker or 1‐Unit Increase of IL‐6 and D‐Dimer Score
| Biomarker | Lowest Tertile | Middle Tertile | Highest Tertile | OR Associated With Doubling of Biomarker | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | OR | 95% CI | P Value | OR | 95% CI | P Value | Omnibus P Value* | OR | 95% CI | P Value | |
| IL‐6, pg/mL | |||||||||||
| Univariate | 1.0 | 1.32 | (0.66 to 2.64) | 0.44 | 2.46 | (1.25 to 4.87) | 0.01 | 0.02 | 1.39 | (1.07 to 1.79) | 0.01 |
| Adjusted | 1.0 | 1.41 | (0.69 to 2.88) | 0.34 | 2.62 | (1.26 to 5.46) | 0.01 | 0.02 | 1.41 | (1.07 to 1.86) | 0.01 |
| D‐dimer (μg/mL) | |||||||||||
| Univariate | 1.0 | 1.63 | (0.80 to 3.33) | 0.18 | 2.47 | (1.25 to 4.85) | 0.009 | 0.03 | 1.40 | (1.10 to 1.78) | 0.007 |
| Adjusted | 1.0 | 1.74 | (0.80 to 3.75) | 0.16 | 2.70 | (1.27 to 5.75) | 0.01 | 0.05 | 1.45 | (1.10 to 1.92) | 0.008 |
| hsCRP (μg/mL) | |||||||||||
| Univariate | 1.0 | 1.17 | (0.60 to 2.29) | 0.65 | 1.49 | (0.77 to 2.88) | 0.24 | 0.49 | 1.09 | (0.93 to 1.28) | 0.31 |
| Adjusted | 1.0 | 1.17 | (0.59 to 2.33) | 0.65 | 1.55 | (0.78 to 3.10) | 0.21 | 0.39 | 1.10 | (0.93 to 1.30) | 0.29 |
| IL‐6 and D‐dimer score | |||||||||||
| Univariate | 1.0 | 1.15 | (0.56 to 2.38) | 0.71 | 3.07 | (1.57 to 6.00) | 0.001 | 0.001 | 1.51 | (1.15 to 1.97) | 0.003 |
| Adjusted | 1.0 | 1.20 | (0.57 to 2.54) | 0.64 | 3.67 | (1.74 to 7.72) | <0.001 | <0.001 | 1.58 | (1.17 to 2.13) | 0.003 |
hsCRP indicates high‐sensitivity C‐reactive protein; OR, odds ratio.
Covariates include: study indicators, log‐transformed time to event, age, gender, race, body mass index, HIV‐RNA, baseline CD4+ cell count, and earlier AIDS at baseline.
Number of fatal CVD events=74.
Number of nonfatal CVD events=214.
Based on 2 df chi‐square test.
Considering the 206 patients (54 fatal and 152 nonfatal events) in SMART and ESPRIT that had measurements recorded at baseline, we adjusted for 4 additional covariates: diabetes, hepatitis B/C coinfection, and use of blood pressure and lipid‐lowering medication. Adjusted ORs were similar to those shown in Table 3: for IL‐6, D‐dimer, hsCRP, and IL and D‐dimer score, ORs (95% CIs) were 1.33 (95% CI, 0.95 to 1.86), 1.43 (95% CI, 1.02 to 2.00), 1.18 (95% CI, 0.95 to 1.47), and 1.49 (95% CI, 1.03 to 2.15), respectively.
For the 115 patients (31 fatal and 84 nonfatal events) in SMART for whom we could also adjust for smoking and total/HDL cholesterol, adjusted ORs were 1.10 (95% CI, 0.72 to 1.67), 1.39 (95% CI, 0.90 to 2.15), 1.25 (95% CI, 0.91 to 1.70), and 1.22 (95% CI, 0.78 to 1.93) for IL‐6, D‐dimer, hsCRP, and the IL‐6 and D‐dimer score, respectively.
In SMART, the OR associated with a doubling of IL‐6 was lower than in analyses based on all 3 studies or based on SMART and ESPRIT. For all 5017 patients in SMART, median (IQR) levels of IL‐6 for smokers and nonsmokers were 1.98 (1.19 to 3.23) and 1.65 (1.05 to 2.86), respectively (P<0.001 for difference). To investigate whether the adjustment for smoking led to the lower OR for SMART, we compared unadjusted and smoking adjusted ORs for SMART participants. In the unadjusted model, the OR associated with a doubling of IL‐6 was 1.29 (95% CI, 0.93 to 1.80); in the model adjusting for smoking, the OR was 1.30 (95% CI, 0.93 to 1.81).
There were 134 patients in the control arms of the 3 studies that experienced a CVD event (32 fatal and 102 nonfatal). ORs were similar to those cited for all patients in Table 3, but CIs were wider as a result of the smaller number of events. Adjusted ORs (fatal/nonfatal events) were 1.62 (95% CI, 0.96 to 2.74), 1.40 (95% CI, 0.88 to 2.25), 1.11 (95% CI, 0.82 to 1.51), and 1.89 (95% CI, 1.05 to 3.41) for IL‐6, D‐dimer, hsCRP, and the IL‐6 and D‐dimer score, respectively. Interaction P values for treatment/control and each biomarker were all >0.59.
Biomarker Association With Mortality After a Nonfatal CVD Event
Among the 214 patients who had a nonfatal CVD event, there were 23 deaths (10.7%). Cumulative mortality 6 and 12 months after the nonfatal event for these patients, all of whom survived at least 28 days, was 2.9% and 4.0%, respectively.
In univariate analyses, higher levels of each of the biomarkers were associated with an increased risk of death (Table 4). HRs were reduced with covariate adjustment, yet remained significant for all 4 biomarkers. There was no evidence that the proportional hazards assumption did not hold for each biomarker (interaction P values all >0.73).
Table 4.
Unadjusted and Covariate Adjusted* Hazard Ratios for All‐Cause Mortality After Nonfatal CVD* (n=214) Events Associated With a Doubling of Biomarker or 1‐Unit Increase in IL‐6/D‐Dimer Score
| Biomarker | Median (IQR) | Univariate | Adjusted | |||||
|---|---|---|---|---|---|---|---|---|
| Deaths (n=23) | Survivors (n=191) | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| IL‐6, pg/mL | 3.1 (2.3 to 6.2) | 2.2 (1.5 to 3.2) | 1.72 | (1.28 to 2.31) | <0.001 | 1.85 | (1.25 to 2.72) | 0.002 |
| D‐dimer, μg/mL | 0.47 (0.29 to 0.59) | 0.27 (0.17 to 0.42) | 1.73 | (1.27 to 2.36) | <0.001 | 1.76 | (1.17 to 2.66) | 0.007 |
| hsCRP, μg/mL | 5.3 (2.6 to 7.5) | 2.0 (1.0 to 4.9) | 1.44 | (1.15 to 1.80) | 0.001 | 1.39 | (1.08 to 1.78) | 0.01 |
| IL‐6 and D‐dimer score | 0.94 (0.37 to 1.57) | 0.18 (−0.25 to 0.72) | 1.88 | (1.39 to 2.55) | <0.001 | 2.01 | (1.35 to 3.01) | <0.001 |
CVD indicates cardiovascular disease; hsCRP, high‐sensitivity C‐reactive protein; HR, hazard ratio.
Covariates include: study indicators, log‐transformed time to event, age, gender, race, body mass index, HIV‐RNA at baseline and proximal to nonfatal CVD event, CD4+ cell count at baseline and proximal to nonfatal CVD event, and earlier AIDS at baseline.
Number of deaths after a nonfatal CVD event=23, including deaths attributed to CVD and unwitnessed deaths not resulting from violence, suicide, or drug abuse.
Discussion
Based on our previous work and studies in the general population, we formulated 2 related hypotheses on the association of inflammatory and coagulation markers and the severity of future CVD events in HIV‐positive patients. In analyses that adjusted for HIV‐ and CVD‐related factors, we found that among HIV‐positive patients who developed CVD: (1) those with higher levels of IL‐6, D‐dimer, and an IL‐6 and D‐dimer score, but not hsCRP, measured several years earlier were significantly more likely to have a fatal CVD event and (2) the risk of death after an MI, stroke, or CAD event was significantly increased among HIV‐positive patients with higher baseline levels of IL‐6, hsCRP, and an IL‐6 and D‐dimer score, but not D‐dimer. In this latter analysis, the biomarkers were measured a median of 2.6 years before the nonfatal event. To our knowledge, the prognostic importance of these markers for fatal, as compared to nonfatal, CVD events has not been studied in the setting of HIV infection.
Considering the results based on the first hypothesis, our findings are consistent with studies in the general population that show an increased risk of more fatal CVD events for patients with higher inflammatory markers. In MRFIT, higher CRP levels at baseline were significantly associated with CHD death, all of which occurred 11 to 17 years after the CRP measurement (P<0.001). No association (P=0.78) was found between baseline CRP and nonfatal MIs, which occurred 6 to 7 years after CRP measurement.1 In the PROSPER study, IL‐6 and CRP, measured at baseline in over 5000 patients, were both more strongly related to fatal CVD events than nonfatal CVD events.3 Engström et al. measured 5 other inflammation‐sensitive plasma proteins (ISPs)—fibrinogen, orosomucoid, α1‐antirypsin, haptoglobin, and ceruloplasmin—6075 healthy men and found that, for men who subsequently developed a coronary event, fatal events were related to the number of ISPs measured at the baseline examination, which was an average of 12.9 years before the events.2
In addition to IL‐6, D‐dimer, a fibrin degradation product and marker of ongoing coagulation, was significantly associated with severity of CVD disease. We have previously shown that D‐dimer is elevated among patients with HIV infection.5 The significant association of baseline D‐dimer with severity of CVD outcomes may reflect activation of coagulation systems in response to low‐grade inflammation associated with HIV infection. It has been shown that HIV replication alters the composition of extrinsic pathway coagulation factors,23 and this cycle of inflammation and coagulation resulting from infection may increase the risk of progressive organ dysfunction and death.24–25
Higher levels of inflammatory markers and D‐dimer for those with fatal CVD may also reflect greater underlying disease. In cross‐sectional studies, higher D‐dimer levels have been associated with severity of peripheral atherosclerosis.26–27 In an overview of 3 studies, higher levels of IL‐6 measured at the time of stroke diagnosis were associated with an increased risk of a poor outcome, including death,28 and concluded that IL‐6 may be a general marker of disease severity. Although we excluded HIV‐positive patients with a history of CVD, recent cross‐sectional comparisons of HIV‐positive men and women without a history of CVD with HIV‐negative controls have found differences in vascular abnormalities.29–31 In 2 studies by the same group, those with HIV were found to have more noncalcified coronary plaque by CT angiography.29–30 Furthermore, in one of these studies, correlations between soluble CD163 and increased amount of noncalcified plaque suggests that monocyte and macrophage activation may contribute to formation of vulnerable plaque in HIV‐positive patients.30 Thus, it is possible that those with higher inflammatory markers had more vulnerable plaque than those who did not. In another study,31 HIV‐positive patients without a history of CVD had more structural and functional abnormalities based on cardiac MRI than HIV‐negative patients.
Studies in the general population have reported that higher levels of markers of inflammation measured at the time of an acute coronary event are related to subsequent mortality, but not to recurrent coronary events.32–34 These observations may be related to the findings of our second hypothesis, that mortality after nonfatal CVD events would be higher among HIV‐positive patients with higher, as compared to lower, levels of IL‐6, hsCRP, and D‐dimer. For patients in the general population, De Servi et al. speculated that the highest inflammatory markers observed during an acute coronary syndrome are more likely to be observed among patients who had high levels before the event.35 If HIV patients who are already in a state of ongoing immune activation with chronic low‐grade inflammation are at increased risk of an exaggerated inflammatory response after their CVD event, as has been suggested based on findings in the general population,3 this could explain the findings based on our second hypothesis. However, we cannot directly address this because biomarkers were not measured at the time of the nonfatal CVD event.
There are several limitations to our findings. Cause of death was uncertain for many of the deaths. However, because sudden cardiac deaths account for most cardiac and many non‐AIDS deaths among HIV‐positive individuals,36 we found it reasonable to consider unwitnessed deaths not attributable to suicide, drug abuse, or violence as fatal CVD. In addition, we showed, in a sensitivity analysis, that results did not differ when unwitnessed deaths were excluded from the logistic models. Another limitation was that important CVD risk factors at baseline were not fully assessed and could only be partially adjusted for. ORs were reduced when only SMART patients for whom smoking and blood lipids were measured at baseline were considered. Although IL‐6 levels were higher among smokers than nonsmokers in SMART, the percentages of patients with fatal and nonfatal events who smoked were similar (Table 2), and ORs from unadjusted and smoking‐adjusted models were also similar, suggesting that smoking was not an important confounding factor in studying severity of CVD events. We also did not measure the biomarkers in the time period immediately before events occurred or at the time of the event. Correlations among measurements taken more remotely with those proximal to the event may be informative. Finally, power for both hypotheses was limited, particularly for the analyses restricted to the subgroup of patients who participated in SMART, as well as for the patients in the control arms of the 3 studies, and, as a consequence, CIs are wide for those analyses. Strengths include the central measurement of the biomarkers, excellent follow‐up, and the uniqueness of this investigation in HIV‐positive patients.
Conclusion
In conclusion, we sought to assess the prognostic value of inflammatory and coagulation markers for fatal outcomes among patients with HIV who experience CVD events. We found that activated inflammatory and coagulation pathways, as demonstrated by higher IL‐6 and D‐dimer plasma levels, are associated with a greater risk of fatal, as compared to nonfatal, CVD and a greater risk of death after a nonfatal CVD event. These findings suggest that chronic inflammation and activated coagulation associated with HIV leads to a poor outcome when a CVD event occurs.
Supplementary Material
Table S1. Summary of biomarker measurements made on 20 samples.
Appendix. List of investigators - SMART Study Group.
Sources of Funding
This study was funded by the National Institutes of Health (Grant No.: U01AI46957 and U01AI068641 [ESPRIT and SMART]; U01AI042170 and U01AI46362 [SMART]). SILCAAT was supported by grants from Chiron and Novartis. The funding sources had no role in data collection, data analysis, or decisions to publish the results.
Disclosures
None.
Acknowledgments
The authors acknowledge the SMART, ESPRIT, and SILCAAT patients. See N Engl J Med 2006;355:2283‐2296 for the complete list of SMART investigators and N Engl J Med 2009;361:1548‐1559 for the complete list of ESPRIT and SILCAAT investigators.
References
- 1.Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C‐reactive protein and coronary heart disease in the MRFIT nested case‐control study. Am J Epidemiol. 1996; 144:537-547. [DOI] [PubMed] [Google Scholar]
- 2.Engström G, Hedblad B, Stavenow L, Tydén P, Lind P, Janzon L, Lindgärde F. Fatality of future coronary events is related to inflammation‐sensitive plasma proteins a population‐based prospective cohort study. Circulation. 2004; 110:27-31. [DOI] [PubMed] [Google Scholar]
- 3.Sattar N, Murrary HM, Welsh P, Blauw GJ, Buckley BM, Cobbe S, de Craen AJM, Lowe GD, Jukema JW, Macfarlane PW, Murphy MB, Stott DJ, Westendorp RGJ, Shepherd J, Ford I, Packard CJfor the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Study Group. Are markers of inflammation more strongly associated with risk for fatal than for non‐fatal vascular events? PLoS Med. 2009; 6:e1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Wijk DF, Boekholdt SM, Wareham NJ, Ahmadi‐Abhari S, Kastelein JJ, Stroes ES, Khaw KT. C‐reactive protein, fatal and nonfatal coronary artery disease, stroke, and peripheral artery disease in the prospective EPIC‐Norfolk cohort study. Arterioscler Thromb Vasc Biol. 2013; 33:2888-2894. [DOI] [PubMed] [Google Scholar]
- 5.Neuhaus J, Jacobs DR, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010; 201:1788-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalfamo M, Le Saout C, Lane HC. The role of cytokines in the pathogenesis and treatment of HIV infection. Cytokine Growth Factor Rev. 2012; 23:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic hiv infection. Immunity. 2013; 39:633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007; 92:2506-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang S, Mary‐Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D. Increased risk of myocardial infarction in HIV‐infected patients in France, relative to the general population. AIDS. 2010; 24:1228-1230. [DOI] [PubMed] [Google Scholar]
- 10.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sørensen HT, Gerstoft J. Ischemic heart disease in HIV‐infected and HIV‐uninfected individuals: a population‐based cohort study. Clin Infect Dis. 2007; 44:1625-1631. [DOI] [PubMed] [Google Scholar]
- 11.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S. Coronary heart disease in HIV‐infected individuals. J Acquir Immune Defic Syndr. 1999; 33:506-512. [DOI] [PubMed] [Google Scholar]
- 12.Freiberg MS, Chang CCH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Goetz MB, Leaf D, Oursler KA, Rimland D, Barradas MR, Brown S, Gilbert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De WitS, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon D, Paton NI, Prineas RJ, Neaton JDfor the INSIGHT SMART Study Group. Inflammation, coagulation and cardiovascular disease in HIV‐infected patients. PLoS One. 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SMART Study Group. CD4+ count‐guided interruption of antiretroviral treatment. N Engl J Med. 2006; 355:2283-2296. [DOI] [PubMed] [Google Scholar]
- 15.SMART Study Group. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy, a randomized trial. Ann Intern Med. 2008; 149:289-299. [DOI] [PubMed] [Google Scholar]
- 16.Emery S, Abrams DI, Cooper DA, Darbyshire JH, Lane HC, Lundgren JD, Neaton JDthe ESPRIT Study Group. The evaluation of subcutaneous proleukin (R) (interleukin‐2) in a randomized international trial: rationale, design, and methods of ESPRIT. Control Clin Trials. 2002; 23:198-220. [DOI] [PubMed] [Google Scholar]
- 17.The INSIGHT‐ESPRIT Study Group and SILCAAT Scientific Committee. Interleukin‐2 therapy in patients with HIV infection. N Engl J Med. 2009; 361:1548-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lifson ARINSIGHT Endpoint Review Committee Writing Group, Belloso WH, Davey RT, Duprez D, Gatell JM, Hoy JF, Krum EA, Nelson R, Pederson C, Perez G, Price RW, Prineas RJ, Rhame FS, Sampson JH, Worley Jfor the INSIGHT Study Group. Development of diagnostic criteria for serious non‐AIDS events in HIV clinical trials. HIV Clinical Trials. 2010; 11.4:205-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007; 50:2173-2195. [DOI] [PubMed] [Google Scholar]
- 20.Kowalska JD, Friis‐Møller N, Kirk O, Bannister W, Mocroft A, Sabin C, Reiss P, Gill J, Lewden C, Phillips A, D‐Arminio Monforte A, Law M, Sterne J, DeWit S, Lundgren JDfor the CoDe Working Group, the D: A: D Study Group. The Coding Causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology. 2011; 22:516-523. [DOI] [PubMed] [Google Scholar]
- 21.Grund B, Baker J, Deeks S, Wolfson J, Wentworth D, Cozzi‐Lepri A, Cohen C, Phillips A, Lundgren J, Neaton J; for the INSIGHT SMART/ESPRIT/SILCAAT Study Groups. Combined effect of interleukinx20106 and Dx2010dimer on the risk of serious nonx2010AIDS conditions: data from 3 prospective cohorts (Abstract 60). In: Program and abstracts of the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA; March 2013. [Google Scholar]
- 22.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV‐1‐Infected Adults and Adolescents. Department of Health and Human Services; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentsgl.pdf. Accessed May 15, 2014. [Google Scholar]
- 23.Baker JV, Brummel‐Ziedins K, Neuhaus J, Duprez D, Cummins N, Dalmau D, DeHovitz J, Lehmann C, Sullivan A, Woolley I, Kuller L, Neaton JD, Tracy RP. HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation. J Am Heart Assoc. 2013; 2:e000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellinger RP. Cardiovascular management of septic shock. Crit Care Med. 2003; 31:946-955. [DOI] [PubMed] [Google Scholar]
- 25.Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004; 109:2698-2704. [DOI] [PubMed] [Google Scholar]
- 26.Lassila R, Peltonen S, Lepäntalo M, Saarinen O, Kauhanen P, Manninen V. Severity of peripheral atherosclerosis is associated with fibrinogen and degradation of cross‐linked fibrin. Arterioscler Thromb Vasc Biol. 1993; 13:1738-1742. [DOI] [PubMed] [Google Scholar]
- 27.Lee AJ, Fowkes FG, Lowe GD, Rumley A. Fibrin D‐dimer, haemostatic factors and peripheral arterial disease. Thromb Haemost. 1995; 74:828-832. [PubMed] [Google Scholar]
- 28.Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, Wardlaw J, Dennis M, Sudlow C. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin‐6. PLoS Med. 2009; 6:e1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, Grinspoon SK. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV‐infected men. AIDS. 2013; 27:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, Lo J, Grinspoon SK. Noncalcified coronary atherosclerotic plaque and immune activation in HIV‐infected women. J Infect Dis. 2013; 208:1737-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L, Neubauer S. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013; 128:814-822. [DOI] [PubMed] [Google Scholar]
- 32.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long‐term mortality in unstable coronary artery disease. N Engl J Med. 2000; 343:1139-1147. [DOI] [PubMed] [Google Scholar]
- 33.James SK, Armstrong P, Barnathan E, Califf R, Lindahl B, Siegbahn A, Simoons ML, Topol EJ, Venge P, Wallentin L. Troponin and C‐reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndromea GUSTO‐IV substudy. J Am Coll Cardiol. 2003; 41:916-924. [DOI] [PubMed] [Google Scholar]
- 34.Zamani P, Schwartz GG, Olsson AG, Rifai N, Bao W, Libby P, Ganz P, Kinlay S. Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the MIRACL study. J Am Heart Assoc. 2013; 2:e003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Servi S, Mariani M, Mariani G, Mazzone A. C‐reactive protein increase in unstable coronary disease cause or effect? J Am Coll Cardiol. 2005; 46:1496-1502. [DOI] [PubMed] [Google Scholar]
- 36.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012; 59:1891-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of biomarker measurements made on 20 samples.
Appendix. List of investigators - SMART Study Group.


