Abstract
Background
Progenitor cells (PCs) are mobilized in response to vascular injury to effect regeneration and repair. Recruitment of PCs requires intact nitric oxide (NO) synthesis by endothelial cells, and their number and activity correlate with cardiovascular disease risk burden and future outcomes. Whereas cardiovascular vulnerability exhibits a robust circadian rhythm, the 24‐hour variation of PCs and their inter‐relation with vascular function remain unknown. We investigated the circadian variation of PCs and vascular function with the hypothesis that this will parallel the pattern observed for cardiovascular events (CVEs).
Methods and Results
In 15 healthy subjects (9 men, 37±16 years), circulating PCs and vascular function were measured at 8 am, noon, 4 pm, 8 pm, midnight, 4 am (only PCs counts), and 8 am the following day. Circulating PCs were enumerated as mononuclear cells (MNCs; CD45med) that express CD34 as well as CD133, and their activity was assessed as the number of colonies formed by culturing MNCs. Vascular function was evaluated by measurement of endothelium‐dependent, flow‐mediated vasodilation (FMD) of the brachial artery and tonometry‐derived indices of arterial stiffness. Higher CD34+ and CD34+/CD133+ cell counts were observed at 8 pm than any other time of the day (P‐ANOVA=0.038 and <0.001; respectively) and were lowest at 8 am. PC colony formation was highest at midnight (P‐ANOVA=0.045) and lowest in the morning hours. FMD was highest at midnight and lowest at 8 am and 8 pm, and systemic arterial stiffness was greatest at 8 am and lowest at 4 pm and midnight (P‐ANOVA=0.03 and 0.01; respectively).
Conclusion
A robust circadian variation in PC counts and vascular function occurs in healthy humans and both exhibit an unfavorable profile in the morning hours that parallels the preponderance of CVEs at these times. Whether these changes are precipitated by awakening and time‐dependent physical activity or governed by the endogenous circadian clock needs to be further investigated.
Keywords: arterial stiffness, circadian variation, endothelial function, progenitor cells
Introduction
Vulnerability to adverse cardiovascular disease (CVD) events, such as myocardial ischemia, infarction, sudden cardiac death, and cerebrovascular accidents, are subject to an intrinsic circadian rhythm with greatest prevalence during the morning hours.1–4 A similar circadian variability is observed in likely physiological precipitants, including heart rate and blood pressure (BP), vasoconstrictor tone, blood viscosity and fibrinolytic activity, platelet aggregability, epinephrine and norepinephrine levels, in addition to cortisol levels and plasma renin activity.5–12
Other precipitants of CVD events include impaired endothelial cell (EC) function and increased systemic arterial stiffness, which precede and contribute to the development of CVD and are long‐term predictors of morbidity and mortality.13 Indeed, vascular dysfunction and reduced nitric oxide (NO) bioavailability promote a proinflammatory and ‐coagulatory milieu that also triggers acute vascular events.13–14 Whereas several studies confirm the presence of significant changes in vascular endothelial function by the time of day, which may differ between patient populations, others demonstrate no significant circadian or diurnal variation.15–21
Recently, bone‐marrow (BM)‐derived progenitor cells (PCs) have been identified and quantitated in the circulation. PCs are released into the bloodstream in response to vascular injury, homing to areas of ischemia, and actively participate in tissue repair and regeneration.22 In experimental models, mobilization of PCs is dependent on endothelial nitric oxide synthase (eNOS) activity and bioavailability of NO.23 In humans, recruitment of PCs into the circulation is modulated by the burden of CVD and its risk factors, and lower counts predict adverse outcomes.24–29Significant circadian variation of circulating PCs in hospitalized patients and diurnal changes over 2 or 3 time points have been observed30–31 however, their detailed 24‐hour variability and relationship to changes in vascular function remain unknown.26
The aim of our study was to investigate the circadian variation of circulating PC counts and their in vitro proangiogenic activity, measured using a colony formation assay, in relation to changes in endothelium‐dependent and ‐independent measures of vascular function and arterial stiffness in healthy subjects. We hypothesized that PC counts and activity, in addition to endothelial function and arterial stiffness, have significant circadian variation that parallels the risk of CVD events.
Methods
Subjects
Fifteen healthy nonsmoking subjects were recruited after careful screening to include only normotensive (systolic arterial pressure<140 or diastolic pressure<90 mm Hg), normolipidemic (total cholesterol level<200 and low‐density lipoprotein<120 mg/dL), normoglycemic (fasting glucose<100 mg/dL), and nonobese subjects (body mass index≤25) subjects. Other exclusion criteria included pregnancy, history of CVD or chronic illnesses, or intake of medications over 4 weeks preceding screening. This study was approved by the Emory University Investigational Review Board and subjects gave informed consent.
Subjects were instructed to adhere to a regular wake‐sleep cycle (no later than 7 am and 11 pm, respectively) for at least 2 weeks before the study. On the evening preceding testing, subjects were admitted to the Atlanta Clinical and Translational Science Institute (ACTSI) at Emory University Hospital, and an intravenous catheter was placed and maintained in the nondominant arm for the duration of the study. Lights were turned off at 11 pm, and testing started at 7:30 am the following morning. Blood draws and vascular measurements were repeated at 4‐hour intervals (except for vascular testing at 4 am) and took approximately 1 hour to complete. Standardized, low‐fat meals prepared by the ACTSI food services were provided to participants after study measurements at the 8:00 am, 12:00 pm, and 6:00 pm time points, with a minimum of 4 hours separating consecutive meals. Subjects abstained from caffeinated beverages and were allowed to engage only in sedentary activities.
Circulating Progenitor Cell Counts and Proangiogenic Activity
Peripheral blood mononuclear cell subsets that are considered to be enriched for hematopoietic and endothelial progenitors were measured in CD45med cells expressing CD34+, CD133+, and vascular endothelial growth factor receptor 2 (VEGFR2)+ surface markers, either singly or in combination. The CD34+ population represents hematopoietic progenitors, and the CD34+/CD133+ subset are immature or early progenitors, because the CD133 epitope disappears as PCs mature.32 The VEGFR2+ cell population exhibits an intact VEGFR2 receptor, indicating these cells' capability to participate in angiogenesis and vasculogensis. We measured circulating numbers of CD34+, dual‐positive CD34+/CD133+ and CD34+/VEGFR2+, and triple‐positive CD34+/CD133+/VEGFR2+ cell populations.
Flow cytometry
Peripheral blood PCs were analyzed for the expression of surface antigens using direct flow cytometry (FCM; BD FACS Canto II Flow Cytometer; BD Biosciences, San Jose, CA). Three hundred microliters of venous blood (anticoagulant: EDTA) was incubated with fluorochrome‐labeled monoclonal mouse antihuman antibodies, namely, FITC‐CD34 (BD Biosciences), PE‐VEGF2R (also known as kinase insert domain receptor; R&D Systems, Minneapolis, MN), and APC‐CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 minutes. Red blood cells were removed by lysis in 1.5 mL of ammonium chloride lysing buffer, which was added to the sample and incubated for an additional 10 minutes. The lysis process was stopped by adding 1.5 mL of staining medium (PBS with 3% heat‐inactivated serum and 0.1% sodium azide). Five million events were acquired from the cytometer with FlowJo software (Treestar, Inc., Ashland, OR) used for subsequent analysis of accumulated data. Absolute numbers of each cell subset per milliliter were determined by multiplying the counts with the number of monocytes per milliliter of blood.
Reproducibility testing
Twenty samples were repeatedly analyzed on 2 occasions by 2 technicians to assess for reproducibility. The percent repeatability coefficients (%) were calculated as SD of differences between pairs of measurements/mean of measurements×100. The repeatability coefficients for the various cell types were: CD34+, 7.4%; CD133+, 7.0%; CD34+/CD133+, 4.4%; CD34+/VEGFR2+, 16.3%; and CD34+/CD133+/VEGFR2+, 19.2%.
Colony‐forming assay
To assess proangiogenic activity of circulating PCs, we performed a culture assay that quantifies colony formation units (CFUs) from mononuclear cells (MNCs), as detailed previously.33–34 This assay measures BM‐derived precursor cells that mature into both proangiogenic hematopoietic‐ and endothelial‐like cells, serving as a biomarker of a “combined” proangiogenic cell activity.
In brief, MNCs were isolated by density‐gradient centrifugation from a 20‐mL sample of blood using CPT (Becton Dickinson, Franklin Lakes, NJ) tubes, suspended in growth medium (DMEM supplemented with 20% FBS and 6.5% EC growth supplement) and plated on dishes coated with human fibronectin (Biocoat; Becton Dickinson). To eliminate mature circulating ECs, cells adherent after 24 hours were discarded and nonadherent cells were replated onto new fibronectin‐coated six‐well plates (1 million cells per well). Growth medium was changed every 2 days. Colonies, identified as multiple thin, flat cells emanating from central clusters of rounded cells, were counted 7 days after plating by a blinded observer and reported as the number of CFUs per 1 million cells.
Reproducibility was tested by measuring CFUs in 15 samples drawn 1 week apart from the same individuals. The overall correlation between the repeated assays was 0.84, and the mean number of colonies in each of the duplicates was 48±4 versus 50±3.
Proliferation assay
To determine whether the colonies were derived from mononuclear precursor cells by proliferation, 5‐bromo‐2′‐deoxyuridine (BrdU; 1 μg/mL; Sigma‐Aldrich, St. Louis, MO) was used to label cells for 24 hour on day 3 or 6 of growth. Subsequently, immunostaining with anti‐BrdU (Dako, Carpinteria, CA) was used to detect proliferating cells within the colonies.
Reverse‐transcriptase polymerase chain reaction
RNA was isolated from freshly isolated MNCs and from 7‐day colonies using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was prepared from RNA samples, and reverse‐transcriptase polymerase chain reaction (RT‐PCR) was performed in an ABI 7900 (Applied Biosystems, Foster City, CA). RT‐PCR was performed in an ABI 7900 (Applied Biosystems) instrument using pathway‐focused gene expression PCR arrays from Super Array (human endothelial biology, angiogenesis). mRNA expression levels were determined using SYBR Green–based real‐time PCR. Results were analyzed using the PCR Array data analysis Web portal to convert threshold cycles into fold changes.
Immunocytochemistry
We performed immunocytochemistry (ICC) for leukocyte and endothelial antigens. Seven colonies were fixed in 2% paraformaldehyde for 1 hour, washed in PBS, and blocked with 5% normal serum in 2% BSA in PBS for 30 minutes. Cells were incubated with the primary antibody (mouse anti‐CD31, 1:300; eBioscience, San Diego, CA), mouse anti‐CD45 (1:5000; eBioscience), mouse anti‐CD3 (1:300; eBioscience), mouse anti‐CD14 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti–vascular endothelial (VE) cadherin (1:50; Abcam, Cambridge, UK), rabbit polyclonal anti‐eNOS (1:1000; Santa Cruz Biotechnology), and rabbit polyclonal anti–von Willibrand's factor (vWF; 1:1000; Chemicon, Temecula, CA) in 2% BSA for 1 hour and then with biotinylated horse anti‐mouse immunoglobulin G (IgG) for monoclonal primary antibodies and biotinylated goat anti‐rabbit IgG for polyclonal antibodies (1:200; Vector Laboratories, Burlingame, CA) for 30 minutes. After another PBS wash, cells were stained with streptavidin‐conjugated quantum dots (QDot 605; 1:100; Invitrogen, Carlsbad, CA) for 1 hour and counterstained with Hoechst nuclear stain. Images were acquired on a Zeiss LSM 510 confocal microscope (Carl Zeiss, Jena, Germany).
To detect the surface expression of endothelial and hematopoietic lineage marker proteins, harvested colonies from 24‐well plates were suspended in PBS after washing and incubated for 1 hour in the presence of the anti‐CD14, ‐CD3, ‐CD45, ‐eNOS, ‐vWF, or ‐VE cadherin and then conjugated with biotinylated secondary antibodies. After washing with PBS, cells were stained with streptavidin‐conjugated quantum dots (QDot 605; Invitrogen), 1:100, and analyzed by FCM (FACSCalibur; Becton Dickinson).
ICC and FCM demonstrated cells expressing both leukocyte and endothelial antigens (80% of cells expressing CD45 and 20% of cells expressing eNOS and vWF), whereas reverse transcriptase demonstrated high levels of expression of proangiogenic factors as well as capillary‐like tubes formed in culture.
Assessment of Vascular Function
After blood draw and an initial rest period of 10 minutes and with subjects in a supine position in a quiet, temperature‐controlled room, BP was measured 3 times at 5‐minute intervals by an automatic device (Omron, Kyoto, Japan) from the dominant arm and documented as the mean value of the final 2 measurements.
Arterial stiffness
We utilized the augmentation index (AIX) and carotid‐femoral pulse wave velocity (PWV) for assessment of arterial wave reflections and stiffness, derived using the SphygmoCor device (Atcor Medical, Sydney, Australia), as previously described.35 Briefly, high‐fidelity sequential pressure waveforms were from the radial artery using a tonometer. Using a transfer function, central (aortic) pressure and the degree of pressure augmentation secondary to reflected waves from the periphery was estimated. AIX was then derived as augmented pressure/total central pulse pressure and was considered a composite marker of wave reflections and arterial stiffening. Because of its sensitivity to heart rate, a standardized value to 75 bpm was calculated and used for AIX for the purpose of this study.
PWV was determined by acquiring waveforms at the carotid and femoral arterial sites using EKG gating. Velocity (distance/time in m/s) was calculated by measuring the time interval between EKG R‐waves and the recorded waveforms at each site, whereas distance between sites was measured manually. Quality control indices were evaluated at the time of study and nonacceptable readings discarded and tests were repeated. Reproducibility studies in our laboratory on 9 subjects on consecutive days have demonstrated a coefficient of variation of 3.8%, 20.3%, and 13.8% for PWV, AIX, and subendocardial viability ratio, respectively.
Brachial artery flow‐mediated dilatation
Endothelium‐dependent brachial artery flow‐mediated dilatation (FMD) was measured to evaluate endothelium‐dependent vasodilation, as previously described.35 Briefly, sonographic images showing a clear intima‐media/adventitia interface, in addition to pulse Doppler velocities, were obtained before and after suprasystolic cuff occlusion at the forearm. FMD was calculated as the percent dilation observed at 1 minute after cuff release. In our laboratory, the mean difference in FMD between assessments performed in 11 subjects on consecutive days was 1.26±0.76%, with a correlation coefficient of 0.75. The mean difference in FMD between 2 readings of the same 11 measurements was 0.82±0.48% (r=0.97).
Statistical Considerations
Study variables are described as the mean±SD for continuous variables, or as counts or proportions for categorical variables. To account for interindividual differences, mean values of study variables were calculated for each subject and used to determine percent deviation (%Δ) from the individual mean at every time point. A cubic (natural) spline function was used to smooth the interpolation line connecting different time points. Analyses were performed using SPSS (version 21.0; SPSS, Inc., Chicago, IL).
Age, BP, heart rate, FMD, AIX, PCs, and CFUs were treated as continuous variables. Gender and time points were categorical variables, and group differences were determined by independent or paired t tests, as appropriate. One‐way ANOVA was utilized to examine differences in study variables across times points. Repeated‐measures ANOVA was used to determine within‐subject differences. For each study measure, Greenhouse‐Geisser's correction was applied because Mauchly's test of sphericity was significant in all variables. The P values on Figure 3 were derived from paired t tests between 2 time points.
Figure 3.
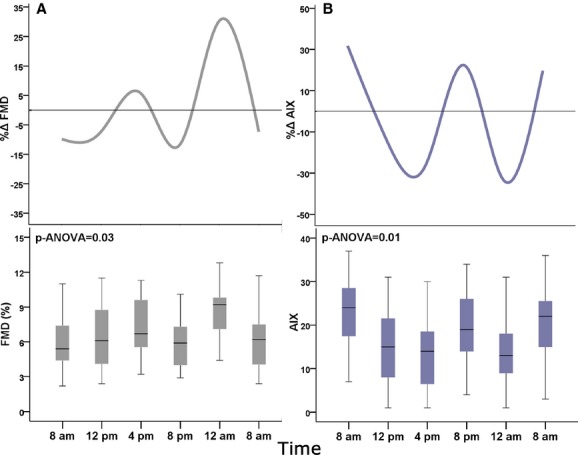
Circadian variation in (A) brachial artery FMD and (B) AIX. FMD, brachial artery flow‐mediated dilation (%). AIX, augmentation index. Bottom box plots: middle band represents median, bottom and top of the box represent lower and upper quartiles, and whiskers represent highest and lowest values that are not outliers. Top figures show average percent deviation (Δ%) from the individual mean. P values are for ANOVA, N=15.
Data presented in Table 2 were derived from Tukey's post‐hoc honest significant differences (HSD) between the time point at which peak values were observed and other times of the day.
Table 2.
Tukey's Post‐Hoc Honest Significant Differences (HSD) Between Peak and Values Observed at Other Times of the Day
| Time Point of Highest Value | Other Time Points | Mean Difference | Significance | |
|---|---|---|---|---|
| CD34+, cells/mL | 8 pm | 8 am | 551.49* | 0.04 |
| 12 pm | 313.07 | 0.56 | ||
| 4 pm | 414.08 | 0.23 | ||
| 12 am | 362.91 | 0.38 | ||
| 4 am | 506.53 | 0.07 | ||
| 8 am | 522.3 | 0.06 | ||
| CD34+CD133+, cells/mL | 8 pm | 8 am | 412.28* | <0.001 |
| 12 pm | 300.89* | 0.01 | ||
| 4 pm | 163.2 | 0.48 | ||
| 12 am | 161.53 | 0.49 | ||
| 4 am | 347.34* | <0.001 | ||
| 8 am | 315.56* | 0.01 | ||
| CFU, Δ% | 12 am | 8 am | 68.19* | 0.005 |
| 12 pm | 44.34 | 0.16 | ||
| 4 pm | 58.31* | 0.02 | ||
| 8 pm | 64.85* | 0.008 | ||
| 8 am | 90.93* | <0.001 | ||
| FMD, Δ% | 12 am | 8 am | 40.21* | 0.014 |
| 12 pm | 37.65* | 0.02 | ||
| 4 pm | 24.57 | 0.31 | ||
| 8 pm | 42.04* | 0.01 | ||
| 8 am | 37.69* | 0.017 | ||
| AIX, Δ% | 8 am | 12 pm | 45.80* | 0.04 |
| 4 pm | 57.84* | 0.003 | ||
| 8 pm | 9.57 | 0.99 | ||
| 12 am | 65.28* | <0.001 | ||
| 8 am | 12.03 | 0.97 |
Δ %=percent deviation from the individual mean. AIX indicates augmentation index; CFU, colony formation units; FMD, flow‐mediated vasodilation.
Denotes statistically significant difference between the time point of highest value and other individual time points, based on the 2‐tailed P values (significance) for the studentized range statistic.
Results
Baseline characteristics of study subjects are summarized in Table 1. The mean age of participants was 37 years, ranging from 21 to 67 years, 6 (40%) women, and 4 (27%) African‐American subjects. Subjects were free of CVD risk factors.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Age, y | 36.9±16 |
| Gender | |
| Women | 6 (40%) |
| Men | 9 (60%) |
| Race | |
| White | 9 (60%) |
| Black | 4 (27%) |
| Height, m | 1.71±0.08 |
| Weight, kg | 68.9±10.5 |
| Body mass index, kg/m2 | 23.9±2.2 |
| Heart rate, bpm | 60±9.8 |
| Systolic blood pressure, mm Hg | 117±9 |
| Diastolic blood pressure, mm Hg | 72±10 |
| Mean arterial pressure, mm Hg | 86.7±7.8 |
| Low‐density lipoprotein, mg/dL | 92±22 |
| Triglycerides, mg/dL | 60±27 |
| Glucose, mg/dL | 81±7 |
| White blood count, cells/mL | 4912±689 |
| Hemoglobin, g/dL | 14.4±1.6 |
Values are expressed as mean±SD, N=15.
Circadian Variation in Hemodynamic Measurements
Arterial BP and heart rate measurements did not vary significantly by the time of day. Mean arterial pressure ranged between 87.4±7.1 and 90.4±11 mm Hg, and mean resting heart rate was between 58.6±11 and 61.6±10.5 bpm (Figure 1).
Figure 1.
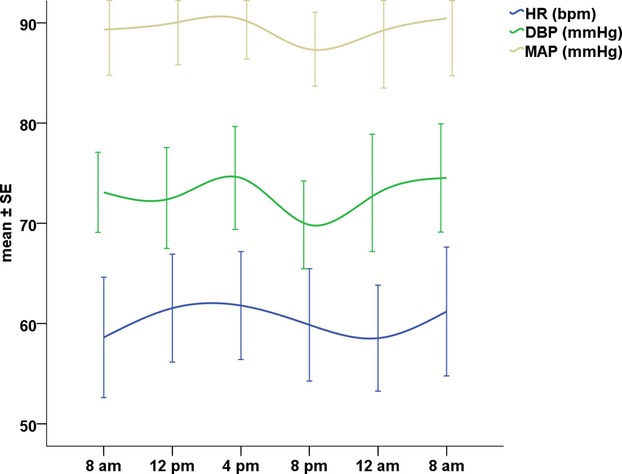
Circadian variation in hemodynamic parameters. Mean±standard error (SE) of heart rate (HR), diastolic blood pressure (DBP), and systolic blood pressure (SBP). P‐ANOVA: nonsignificant (>0.05) for all, N=15. MAP indicates mean arterial pressure.
Circadian Variation in PC Count
There were significant circadian variations in circulating CD34+ (P=0.038) and dual‐positive CD34+/CD133+ (P<0.001) counts by ANOVA, where counts were lowest at 8 am and highest at 8 pm, with a difference of 551±175 and 412±85 cells/mL, respectively (Figure 1A,1B; Table 2). To account for interindividual differences, percent change from the individual mean was calculated for each subject. The percent change from the individual mean for CD34+ and CD34+/CD133+ PCs was lowest at 8 am (−11±27% and −30±31%, respectively) and greatest at 8 pm (24±26%, and 44±40%, respectively; (Figure 2A,2B).
Figure 2.
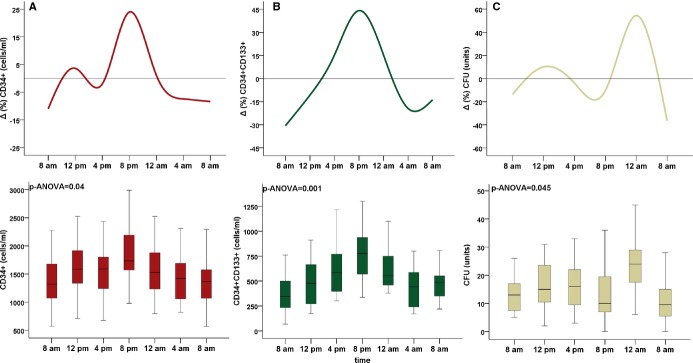
Circadian variation in (A) CD34+ and (B) CD34+/CD133+ (C) colony forming unit (CFU) counts. Bottom box plots: middle band represents median, bottom and top of the box represent lower and upper quartiles, and whiskers represent highest and lowest values that are not outliers. Top: average percent deviation (Δ%) from the individual mean. P values are for ANOVA, N=15 for A and B, N=11 for C.
Similarly, within‐subject differences were evaluated by repeated‐measures ANOVA with Greenhouse‐Geisser's correction revealing significant differences in CD34+ and CD34+/CD133+ counts by the time of day (F=2.73 and 8.09; P=0.046 and <0.001, respectively).
Although a similar trend was observed in CD34+/VEGFR2+ and CD34+/CD133+/VEGFR2+ cell counts, these changes were not statistically different (P‐ANOVA=0.09 and 0.1, respectively).
Circadian Variation in CFUs
There was a significant circadian variation in CFU counts across the 24‐hour period (P‐ANOVA=0.045; n=11), with the highest mean count (25.2±14.5) observed at midnight, compared to the nadir at 8 am (13.2±14.5; Figure 2C). Within‐subject differences were evaluated by repeated‐measures ANOVA with Greenhouse‐Geisser's correction revealing significant differences by the time of day (F=3.96; P=0.014).
The precent change from individual means was highest at midnight and lowest at 8 am (54±36% and −36±38%, respectively; Table 2).
Circadian Variation in Endothelial Function
There was a significant circadian variation in endothelial function (P‐ANOVA=0.028), where brachial artery FMD peaked at midnight (5.9±2.2%). The percent change from the individual mean was greatest at midnight (30.4±34) and lowest at 8 am and 8 pm (−9.9±31% and −11±28.8%, respectively; Figure 3A). Repeated‐measures ANOVA with Greenhouse‐Geisser's correction revealed significant within‐subject differences by the time of day (F=3.38; P=0.047).
Circadian Variation in Arterial Wave Reflections
There was a significant circadian variation in AIX (P‐ANOVA=0.014), with it being highest at 8 am (22.2±8.4; Figure 3B). The percent change from the individual mean was highest at 8 am (31.8±39.3%) and lowest at midnight (33.5±32.8%; Figure 3B). Differences evaluated by repeated‐measures ANOVA with Greenhouse‐Geisser's correction confirmed significant within‐subject differences by the time of day (F=7.32; P=0.001).
There was no significant circadian variation in PWV measurements, and the peak‐to‐nadir deviation did not exceed 30% in any of the subjects.
Post‐Hoc Analysis
Table 2 shows Tukey's post‐hoc HSD between the time point at which peak values were observed and other times of the day. There were significant within‐subject differences in PC counts, endothelial FMD, and AIX between values observed at 8 am and the time point at which the highest CD34+ and CD34+/CD133+ cell counts (8 pm) and at which peak FMD and lowest AIX (12 am) were observed (P<0.01 for all; Figure 4).
Figure 4.
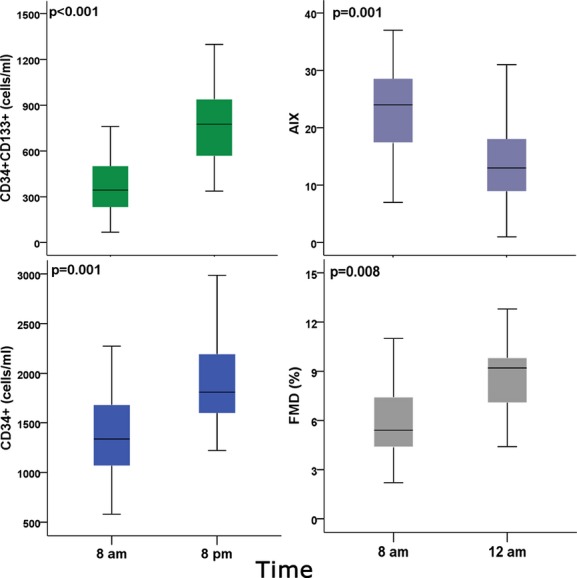
Time points at which peak and nadir values of progenitor cell counts, AIX, and brachial artery FMD were observed. AIX indicates augmentation index; FMD, brachial artery flow‐mediated dilation (%). P values are for paired t tests, N=15.
Discussion
In this study of healthy subjects, we assessed the circadian variation of PC number and activity and their temporal relationship with the circadian variation in vascular function, measured as both endothelium‐dependent vasodilation and arterial wave reflections/stiffness. We observed that the number of circulating PCs, measured as CD34+ and CD34+/CD133+ cell counts, and their proangiogenic activity, measured as CFUs in culture, were lowest in the early morning hours (8 am). This coincided with the time when endothelial NO activity was lowest and arterial stiffness, measured as AIX, was greatest. We also found that PC counts were highest at 8 pm, and their proangiogenic activity was highest at midnight. In parallel, FMD was highest, and AIX was lowest, at midnight.
For the first time, to our knowledge, we have demonstrated a simultaneous and parallel 24‐hour circadian variation in vascular function and PC numbers and activity. Our findings suggest that endogenous reparative or regenerative capacity varies by the time of day that may be related to, or reflected by, changes in vascular function. Whether these changes are the result of morning increases in physical activities after awakening (e.g., postural changes, mental activities, food intake, etc.) or are determined by an intrinsic circadian rhythm that is entrained over longer time periods (weeks or months) needs to be further investigated.
We and others have previously shown circadian changes in vascular function that are similar to those observed in our subjects.5,15 In only 2 previous studies, diurnal variation over 3 time points in PC counts in selected subjects have been investigated. The circulating CD34+/CD133+ cell count was higher at midnight than at noon or 6 pm in elderly inpatients,30 and higher PCs counts were observed at 10 pm, compared to 8 am and 3 pm, in healthy men.31 These results are in line with our findings that detail the full 24‐hour variation in PC counts in this study involving healthy men and women and further investigates circadian changes in their proangiogenic activity during culture.
Circulating PCs exhibited a single clear peak at 8 pm, and nadir values were observed in the morning hours. These pronounced differences (approximately 60% for CD34+/CD133+ cells) are not surprising in light of circadian changes of a similar magnitude in cytokines that mobilize BM progenitors, including granulocyte‐colony stimulating factor and erythropoietin. The circadian pattern of these cytokines also parallels that of PCs, with marked evening increases, and is similarly observed in other peripheral blood components, such as the total circulating granulocyte or lymphocyte counts.36–40 PC mobilization is partly dependent on NO availability, which also exhibits significant circadian changes.41–42
Several mechanisms may dictate the circadian pattern of PC egress from the BM, their circulating counts, and translocation into peripheral tissues,40,43 but the concomitant morning worsening of vascular function measurements and decrease in PC counts can be best explained by the surge of sympathetic nervous system activity observed in the morning hours. Indeed, sympathetic activity has been shown to blunt NO‐mediated vasodilation and increase vascular tone, as well as regulate trafficking of PCs by adrenergic receptor activation.23
Although some studies propose a counter‐regulatory effect of enhanced endothelial function that may mitigate circadian increases in vascular tone,21,44 our findings demonstrate simultaneous worsening of brachial artery FMD and systemic arterial stiffness. Furthermore, AIX exhibited a lesser peak at 8 pm, alongside a significant decline in FMD at that same time point (Table 2). However, the extent of improvements or worsening in arterial stiffness or wave reflections did not correlate with those in FMD at each time point. Nonetheless, both vascular function and PC counts exhibited an unfavorable morning profile with depressed PC counts and greater endothelial dysfunction, as well as increased arterial stiffness. This is also in agreement with recent findings of a possible lower hemostatic “set point” for vascular function parameters after a period of sleep.45
Limitations
Our study size may have been too small to detect circadian changes in rare cell populations (CD34+/VEGFR2+/CD133+ cells). Although both men and women across a wide age range were included, this study was designed to elucidate circadian changes in healthy individuals, thus limiting the generalizability of our findings. Similar to several other physiologic parameters, circadian variation in PC counts or vascular function may be blunted or accentuated in specific disease conditions, and this requires further investigation.
Whereas continuous wall motion tracking might have identified circadian variability in the postdeflation timing of the maximal dilatory responses, which may be vary by age,46 we used the 1‐minute time point to determine brachial artery flow‐mediated dilation because of this technique's high reproducibility and short duration.
This present study was not designed to detect BP or heart rate changes; however, we did not expect the absence of significant circadian changes. Although these measurements were performed before vascular testing, which required walking to the vascular lab and interrupted an otherwise sedentary day, subjects underwent an initial rest period of at least 10 minutes. Hemodynamic measurements were also performed after blood draws and, together with vascular testing, might have exerted a “white coat effect” on these measurements.
The study included 6 women (mean age of 28; range, 23 to 38 years), and all were premenopausal. Thus, differences that may occur after menopause cannot be addressed in this study. We studied these female participants a few days after cessation of menstruation to reduce known variability during the menstrual cycle.47
Subjects were instructed to maintain a regular sleep‐wake cycle over 2 weeks preceding the study, but compliance could only be ascertained by self‐report. Nevertheless, subjects were admitted the night before commencement of the study and lights were turned off by 11 pm at the latest. Moreover, all subjects were awakened by 7 am and standardized meals were served at a fixed time for all participants. Last, because sedentary activities (reading, watching television) were allowed, unidentified factors related to these activities might have affected our results.
Conclusion
In summary, our findings highlight the robust circadian variations in potential underlying precipitants of CVD events, which parallel their morning preponderance. This is evidenced by the paucity of peripheral blood PCs, as well as worsened NO‐dependent vasodilation and increased vascular stiffness.
Sources of Funding
This work was supported by the Marcus and Woodruff Foundations (Atlanta, GA) and the Georgia Tech/Emory University Predictive Health Institute, as well as PHS Grant UL1 TR000454 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resources.
Disclosures
None.
References
- 1.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, Sobel BE, Willerson JT, Braunwald E. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985; 313:1315-1322. [DOI] [PubMed] [Google Scholar]
- 2.Mulcahy D, Keegan J, Cunningham D, Quyyumi A, Crean P, Park A, Wright C, Fox K. Circadian variation of total ischaemic burden and its alteration with anti‐anginal agents. Lancet. 1988; 2:755-759. [DOI] [PubMed] [Google Scholar]
- 3.Marler JR, Price TR, Clark GL, Muller JE, Robertson T, Mohr JP, Hier DB, Wolf PA, Caplan LR, Foulkes MA. Morning increase in onset of ischemic stroke. Stroke. 1989; 20:473-476. [DOI] [PubMed] [Google Scholar]
- 4.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham heart study population. Am J Cardiol. 1987; 60:801-806. [DOI] [PubMed] [Google Scholar]
- 5.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha‐sympathetic vasoconstrictor activity. N Engl J Med. 1991; 325:986-990. [DOI] [PubMed] [Google Scholar]
- 6.Millar‐Craig MW, Bishop CN, Raftery EB. Circadian variation of blood‐pressure. Lancet. 1978; 1:795-797. [DOI] [PubMed] [Google Scholar]
- 7.Ehrly AM, Jung G. Circadian rhythm of human blood viscosity. Biorheology. 1973; 10:577-583. [DOI] [PubMed] [Google Scholar]
- 8.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987; 316:1514-1518. [DOI] [PubMed] [Google Scholar]
- 9.Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab. 1985; 60:1210-1215. [DOI] [PubMed] [Google Scholar]
- 10.Gordon RD, Wolfe LK, Island DP, Liddle GW. A diurnal rhythm in plasma renin activity in man. J Clin Investig. 1966; 45:1587-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreotti F, Davies GJ, Hackett DR, Khan MI, De Bart AC, Aber VR, Maseri A, Kluft C. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol. 1988; 62:635-637. [DOI] [PubMed] [Google Scholar]
- 12.Quyyumi AA, Panza JA, Diodati JG, Lakatos E, Epstein SE. Circadian variation in ischemic threshold. A mechanism underlying the circadian variation in ischemic events. Circulation. 1992; 86:22-28. [DOI] [PubMed] [Google Scholar]
- 13.Quyyumi AA, Patel RS. Endothelial dysfunction and hypertension: cause or effect? Hypertension. 2010; 55:1092-1094. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker‐Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006; 27:2588-2605. [DOI] [PubMed] [Google Scholar]
- 15.Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation. 2004; 109:2507-2510. [DOI] [PubMed] [Google Scholar]
- 16.Ringqvist A, Caidahl K, Petersson AS, Wennmalm A. Diurnal variation of flow‐mediated vasodilation in healthy premenopausal women. Am J Physiol. 2000; 279:H2720-H2725. [DOI] [PubMed] [Google Scholar]
- 17.Walters JF, Hampton SM, Deanfield JE, Donald AE, Skene DJ, Ferns GA. Circadian variation in endothelial function is attenuated in postmenopausal women. Maturitas. 2006; 54:294-303. [DOI] [PubMed] [Google Scholar]
- 18.Maruo T, Nakatani S, Kanzaki H, Kakuchi H, Yamagishi M, Kitakaze M, Ohe T, Miyatake K. Circadian variation of endothelial function in idiopathic dilated cardiomyopathy. Am J Cardiol. 2006; 97:699-702. [DOI] [PubMed] [Google Scholar]
- 19.Oflaz H, Cuhadaroglu C, Pamukcu B, Meric M, Ece T, Kasikcioglu E, Koylan N. Endothelial function in patients with obstructive sleep apnea syndrome but without hypertension. Respiration. 2006; 73:751-756. [DOI] [PubMed] [Google Scholar]
- 20.Kawano H, Motoyama T, Yasue H, Hirai N, Waly HM, Kugiyama K, Ogawa H. Endothelial function fluctuates with diurnal variation in the frequency of ischemic episodes in patients with variant angina. J Am Coll Cardiol. 2002; 40:266-270. [DOI] [PubMed] [Google Scholar]
- 21.Shaw JA, Chin‐Dusting JP, Kingwell BA, Dart AM. Diurnal variation in endothelium‐dependent vasodilatation is not apparent in coronary artery disease. Circulation. 2001; 103:806-812. [DOI] [PubMed] [Google Scholar]
- 22.Kwon SM, Lee YK, Yokoyama A, Jung SY, Masuda H, Kawamoto A, Lee YM, Asahara T. Differential activity of bone marrow hematopoietic stem cell subpopulations for epc development and ischemic neovascularization. J Mol Cell Cardiol. 2011; 51:308-317. [DOI] [PubMed] [Google Scholar]
- 23.Aicher A, Heeschen C, Mildner‐Rihm C, Urbich C, Ihling C, Technau‐Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003; 9:1370-1376. [DOI] [PubMed] [Google Scholar]
- 24.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999; 85:221-228. [DOI] [PubMed] [Google Scholar]
- 25.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997; 275:964-967. [DOI] [PubMed] [Google Scholar]
- 26.Mavromatis K, Aznaouridis K, Al Mheid I, Veledar E, Dhawan S, Murrow JR, Forghani Z, Sutcliffe DJ, Ghasemzadeh N, Alexander RW, Taylor WR, Quyyumi AA. Circulating proangiogenic cell activity is associated with cardiovascular disease risk. J Biomol Screen. 2012; 17:1163-1170. [DOI] [PubMed] [Google Scholar]
- 27.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007; 49:741-752. [DOI] [PubMed] [Google Scholar]
- 28.Al Mheid I, Quyyumi AA. Cell therapy in peripheral arterial disease. Angiology. 2008; 59:705-716. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt‐Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005; 111:2981-2987. [DOI] [PubMed] [Google Scholar]
- 30.Watson T, Shantsila E, Karthikeyan VJ, Jessani S, Goon PK, Lip GY. The effects of exercise stress testing, diurnal variation and temporal decline on circulating progenitor cells. Thromb Haemost. 2010; 103:419-425. [DOI] [PubMed] [Google Scholar]
- 31.Thomas HE, Redgrave R, Cunnington MS, Avery P, Keavney BD, Arthur HM. Circulating endothelial progenitor cells exhibit diurnal variation. Arterioscler Thromb Vasc Biol. 2008; 28:e21-e22. [DOI] [PubMed] [Google Scholar]
- 32.Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci. 2011; 120:263-283. [DOI] [PubMed] [Google Scholar]
- 33.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348:593-600. [DOI] [PubMed] [Google Scholar]
- 34.Mavromatis K, Sutcliffe DJ, Joseph G, Alexander RW, Waller EK, Quyyumi AA, Taylor WR. Proangiogenic cell colonies grown in vitro from human peripheral blood mononuclear cells. J Biomol Screen. 2012; 17:1128-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, Kavtaradze N, Uphoff I, Hooper C, Tangpricha V, Alexander RW, Brigham K, Quyyumi AA. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011; 58:186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato S, Kigawa J, Irie T, Itamochi H, Kanamori Y, Kamazawa S, Akeshima R, Terakawa N. Timing of G‐CSF administration based on the circadian rhythm in patients with ovarian cancer. Am J Clin Oncol. 2002; 25:289-290. [DOI] [PubMed] [Google Scholar]
- 37.Biregegard G. Circadian variation of granulocyte colony‐stimulating factor levels in man. Br J Haematol. 2000; 108:661. [DOI] [PubMed] [Google Scholar]
- 38.Jilma B, Hergovich N, Stohlawetz P, Eichler HG, Bauer P, Wagner OF. Circadian variation of granulocyte colony stimulating factor levels in man. Br J Haematol. 1999; 106:368-370. [DOI] [PubMed] [Google Scholar]
- 39.Wide L, Bengtsson C, Birgegard G. Circadian rhythm of erythropoietin in human serum. Br J Haematol. 1989; 72:85-90. [DOI] [PubMed] [Google Scholar]
- 40.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012; 37:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cubbon RM, Murgatroyd SR, Ferguson C, Bowen TS, Rakobowchuk M, Baliga V, Cannon D, Rajwani A, Abbas A, Kahn M, Birch KM, Porter KE, Wheatcroft SB, Rossiter HB, Kearney MT. Human exercise‐induced circulating progenitor cell mobilization is nitric oxide‐dependent and is blunted in South Asian men. Arterioscler Thromb Vasc Biol. 2010; 30:878-884. [DOI] [PubMed] [Google Scholar]
- 42.Bode‐Boger SM, Boger RH, Kielstein JT, Loffler M, Schaffer J, Frolich JC. Role of endogenous nitric oxide in circadian blood pressure regulation in healthy humans and in patients with hypertension or atherosclerosis. J Investig Med. 2000; 48:125-132. [PubMed] [Google Scholar]
- 43.Recalde A, Richart A, Guerin C, Cochain C, Zouggari Y, Yin KH, Vilar J, Drouet I, Levy B, Varoquaux O, Silvestre JS. Sympathetic nervous system regulates bone marrow‐derived cell egress through endothelial nitric oxide synthase activation: role in postischemic tissue remodeling. Arterioscler Thromb Vasc Biol. 2012; 32:643-653. [DOI] [PubMed] [Google Scholar]
- 44.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011; 108:980-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones H, Lewis NC, Thompson A, Marrin K, Green DJ, Atkinson G. Diurnal variation in vascular function: role of sleep. Chronobiol Int. 2012; 29:271-277. [DOI] [PubMed] [Google Scholar]
- 46.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow‐mediated dilatation in humans. Hypertension. 2008; 51:203-210. [DOI] [PubMed] [Google Scholar]
- 47.Robb AO, Mills NL, Smith IB, Short A, Tura‐Ceide O, Barclay GR, Blomberg A, Critchley HO, Newby DE, Denison FC. Influence of menstrual cycle on circulating endothelial progenitor cells. Hum Reprod. 2009; 24:619-625. [DOI] [PubMed] [Google Scholar]


