Abstract
Background
Postextrasystolic blood pressure potentiation (PESP), the pulse wave augmentation after an extrasystolic beat, is typically enhanced in heart failure (HF) patients. This study prospectively tested the association of PESP and mortality in cardiac patients.
Methods and Results
Consecutive patients (n=941; mean age, 61 years; 19% female) presenting with acute myocardial infarction were enrolled between May 2000 and March 2005 and followed up until August 2010. The main study outcome was 5‐year all‐cause mortality. Patients underwent noninvasive 30‐minute recordings of ECG and continuous blood pressure. PESP presence was based on the ratio between the first postectopic pulse wave amplitude and the mean of the subsequent 9 pulse wave amplitudes. A ratio above 1 was prospectively defined as PESP present. Ventricular premature complexes (VPCs) suitable for PESP quantification were present in recordings of 220 patients. PESP was present in 62 of these patients. Patients without suitable VPCs were classified as PESP absent.
During the follow‐up, 72 patients died. Among the 220 patients in whom PESP was measurable, 27 died. Under univariable analysis, PESP was a significant predictor of death (P<0.001) as were GRACE score (P<0.001), left ventricular ejection fraction (LVEF) (P<0.001), and the number of recorded VPCs (P<0.001). Under multivariable analysis, PESP (P<0.001), GRACE score (P<0.001), and LVEF (P=0.001) were independently associated with outcome. The combination of PESP presence and LVEF ≤35% identified a subgroup of patients with a particularly high mortality of 46.7%. Separate validation reproduced the finding in an unrelated population of 146 HF patients.
Conclusions
PESP, which likely reflects abnormalities of myocardial calcium cycling, predicts the mortality risk in postinfarction patients.
Clinical Trial Registration
URL: ClinicalTrials.gov. Unique identifier: NCT00196274.
Keywords: calcium cycling, myocardial infarction, risk assessment
Introduction
Augmented contractility of postextrasystolic heartbeats has been described almost 130 years ago1–2 and later termed postextrasystolic potentiation (PESP).3–4 For almost 1 century, PESP was thought to reflect normal cardiac function.5 However, during the second half of the previous century, an increasing number of reports showed that, measured at the blood pressure (BP) level, PESP is a typical finding in heart failure (HF) patients.6–8 There have been attempts to use PESP as a diagnostic test to identify ischemic, but viable, myocardium and elicit PESP by paired pacing to augment cardiac contractility in HF,9 but these approaches have not led to broad clinical application.
Cardiac contractility is closely correlated with systolic rise in intracellular calcium. The magnitude of systolic calcium transient is primarily determined by the amount of calcium stored in the sarcoplasmic reticulum and by the availability of sarcoplasmic reticulum calcium release channels (ryanodine receptors). PESP and its augmentation in HF can be understood on the basis of the interplay of calcium‐handling processes in cardiomyocytes10–11: A premature beat leads to a cellular depolarization and influx of trigger calcium through L‐type channels at a time point when a relevant fraction of ryanodine receptor channels is still refractory. This results in a smaller‐than‐normal calcium release from the sarcoplasmic reticulum. Resequestration of cytoplasmic calcium into the sarcoplasmic reticulum takes place during the postectopic pause, resulting in a higher‐than‐normal sarcoplasmic reticulum calcium content to be released during the first postectopic beat. Thus, the contractility of the first postectopic beat is augmented.
In the failing myocardium, the sarcoplasmic reticulum contains less calcium, mostly because of leaky ryanodine receptors and a reduced capacity of the calcium uptake mechanism and partly compensated by up‐regulation of the plasma membrane sodium calcium exchanger.12 Starting from this lower steady state, the relative increase in sarcoplasmic reticulum calcium content during the postectopic pause is even more pronounced, resulting in a greater amount of PESP than in a normal heart. This association between abnormal intracellular calcium cycling and increased PESP in HF is supported by both animal experiments10 and modeling studies.13
We hypothesized that PESP is a surrogate marker for HF at the cellular level that bears prognostic information in cardiac patients. The present study was designed to test PESP as a mortality predictor in myocardial infarction (MI) survivors. The study was a prospectively defined substudy of the Autonomic Regulation Trial (ART).14
Methods
Study Cohort
Between May 2000 and March 2005, consecutive patients were enrolled in the ART study (NCT00196274 registration at ClinicalTrials.gov) at the German Heart Center and the Klinikum rechts der Isar (both in Munich, Germany). Eligible patients survived the acute phase of MI, were aged ≤80 years, in sinus rhythm, and not eligible for implantable cardioverter‐defibrillator (ICD) for secondary prevention before hospital discharge. MI diagnosis required 2 or more of the following findings: typical chest pain for ≥20 minutes, creatine kinase above twice the upper normal limit of the respective laboratory, and admission ST‐segment elevation ≥0.1 mV in at least 2 limb leads or ≥0.2 mV in at least 2 contiguous precordial leads. Patients were followed for a median of 6.3 years (interquartile range [IQR], 5.5 to 7.0) with final follow‐up in August 2010. All‐cause mortality at 5 years after the index MI was prospectively defined as the primary study endpoint. The study protocol was approved by the local ethics committee and all participants gave informed written consent.
An independent validation cohort comprised 146 patients suffering from HF and presenting in sinus rhythm at the Glasgow Royal Infirmary (Glasgow, UK) between 1995 and 1998. These patients had an average age of 59 years, and 61 presented with New York Heart Association (NYHA) class III or IV. Detailed clinical characteristics have been reported elsewhere.15 Patients were followed up for 2.7±1.1 years.
ECG and Systolic Arterial BP Recordings
Patients of the ART cohort underwent simultaneous 30‐minute recordings of high‐resolution ECG (1.6‐kHz sampling of orthogonal XYZ leads; TMS International, Enschede, The Netherlands), and noninvasive arterial BP monitoring using a finger photoplethysmographic device (FMS, Amsterdam, The Netherlands). Recordings were obtained in supine resting position after routine morning medications and performed after patients were transferred from the intensive care unit to the ward (median day 7; IQR, 5 to 9 days after the index MI). Raw signals were reviewed to eliminate artifacts and classify premature beats as sinus or ventricular premature complexes (VPCs). These reviews were made by technicians blinded to the clinical outcome data.
Patients of the Glasgow cohort underwent simultaneous 10‐minute recordings of ECG and continuous BP. Signal recording and processing was identical to that used in the ART cohort.
PESP Assessment
A VPC qualified for PESP assessment if ≥20% premature, compared to the mean of preceding 5 sinus rhythm intervals, and if followed by ≥10 consecutive sinus rhythm beats. Postectopic sequences interrupted by artifacts or further ectopic beats were excluded. Post‐VPC BP changes were quantified as the relationship of the first post‐VPC pulse wave amplitude and the mean of the subsequent 9 pulse wave amplitudes. PESP was defined as present if the first post‐VPC pulse pressure wave exceeded the mean of the subsequent pulse pressure amplitudes (Figure 1). In order to eliminate the influence of fluctuations in BP recordings, a technical threshold was introduced: Taking into account that the mean standard deviation of systolic BP (SBP) in 10‐heartbeat sequences was 2.8% in our data, we defined PESP as present if the post‐VPC pulse pressure wave exceeded the mean of the subsequent pulse pressure amplitudes by more than 3%. If more VPCs in the same 30‐minute recording qualified for PESP evaluation, the ratios between the post‐VPC pulse pressure wave and the mean of the subsequent pulse pressure amplitudes were averaged. We also calculated the numerical ratio of PESP, that is, the relationship between post‐VPC pulse pressure and the mean pulse pressure of the subsequent sinus beats.
Figure 1.
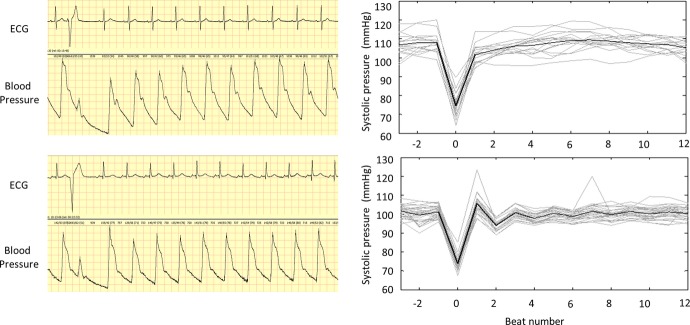
Determination of PESP based on ECG and BP signals. Left panel shows ECG and blood pressure signals of a patient surviving the follow‐up period (upper tracing) and a patient who died 16 months after the index MI (lower tracing). The systolic pressure of the first postectopic beat in the upper tracing is clearly lower than that of the following 9 beats, indicating that PESP is not present. Conversely, the systolic pressure of the corresponding beat in the lower tracing is noticeably higher than that of the subsequent beats (PESP is present). Right panels show peak systolic pressure of consecutive heart beats before, during, and after ectopic beats in these patients. Individual episodes are shown in gray, their average in black. MI indicates myocardial infarction; PESP, postextrasystolic potentiation.
Other Risk Predictors
VPC count was calculated from standard Holter recordings as the number of VPCs per hour. GRACE score was calculated according to Eagle et al.16 The elements of the GRACE score included age, history of HF, history of MI, serum creatinine at admission, cardiac biomarker status at admission, SBP at admission, pulse frequency at admission, ST deviation at admission, and in‐hospital percutaneous coronary intervention. Left ventricular (LV) ejection fraction (LVEF) was assessed by LV angiography (n=445; 47.3%) or biplane echocardiography according to Simpson's method (n=496; 52.7%; Sonos 5500; Hewlett Packard, Palo Alto, CA), on median day 7 (IQR, 5 to 9 days) after the index MI. GRACE score and LVEF were dichotomized at predefined cut‐off values of ≥120% and ≤35%, respectively.17 VPC count was dichotomized at ≥10 per hour.18 Incomplete revascularization at the time of PESP measurement was defined as remaining stenosis ≥75% in any of the main coronary vessels after percutaneous coronary intervention (PCI).
Follow‐up and Endpoints
Clinical follow‐up appointments were scheduled every 6 months. In August 2010, all patients who had less than 4 years of follow‐up were contacted by mail, telephone, or through the attending general practitioner. If none of these channels were successful, the local population registry was contacted to either provide a new address of the patient or confirm that the patient had deceased. Patients who could not be reached by any of these channels were considered lost to follow‐up and censored at the time of last contact. The prospectively defined study endpoint was death from any cause within 5 years of the index MI.
Statistical Analysis
The distribution of continuous variables is presented by median and IQR. Categorical data are expressed as absolute frequencies and percentages. We first calculated Cox's proportional hazards models with single predictors to evaluate their association with mortality. Subsequently, a multivariable Cox proportional hazards model was used with all 4 variables to simultaneously assess the independent prognostic value of each mortality predictor. Survival curves were estimated by Kaplan‐Meier's method and compared by the log‐rank test. Continuous variables were compared using Mann‐Whitney's U test. Qualitative data were compared using the chi‐square (χ2) test. The improvement of goodness of fit, measured by the Cox model's likelihood, upon the addition of PESP to the Cox model fixed to LVEF, GRACE score, and VPC counts was assessed in a permutation test framework.19 Collinearity was assessed by variance inflation factor (VIF). Values beyond 4 indicate collinearity. Linear effect estimates were used in the Cox model as a comparison to nonlinear effects by application of fractional polynomials showed no significant improvement in model fit (ie, likelihood). The improvement of risk prediction by inclusion of PESP in the Cox model is presented by the integrated discrimination improvement index (IDI).20–21 IDI values are presented separately for survivors and nonsurvivors. Three‐fold cross‐validation was repeated 25 times for an averaged assessment and internal validation of IDI values to be expected for external data. In a retrospective analysis, optimal cut‐off values for the proportion between the first post‐VPC pulse wave amplitude and the mean of the subsequent 9 pulse wave amplitudes (termed further the numerical ratio of PESP) were determined from the receiver‐operator characteristic (ROC) curve at the maximum of the Youden index J=Sensitivity+Specificity–1.22–23 The corresponding 95% confidence intervals (CIs) were determined by standard bootstrap with 5000 repetitions.24 ROC analysis at 5 years was performed to assess corresponding areas under the receiver‐operating characteristics curves (AUROCs).25
All statistical tests were performed on a 2‐sided 5% significance level. Accordingly, 95% CIs are given for effect measures. All statistical analyses were done using IBM SPSS Statistics 20.0 and R 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study cohort consisted of 941 patients. Table 1 shows the clinical and demographic characteristics. Thirteen patients (1.4%) were lost to follow‐up, and 45 additional patients were censored between 4 and 5 years of follow‐up. At 5 years, 72 patients (7.7%) had died.
Table 1.
Clinical Characteristics and Therapy Data
| Variable | ART Population n=941 | PESP Calculable n=220 | PESP Not Calculable n=721 | P Value |
|---|---|---|---|---|
| Clinical data | ||||
| Age (y), median (IQR) | 61 (52 to 69) | 65 (58 to 72) | 59 (50 to 68) | <0.0001 |
| Females, n (%) | 182 (19.3) | 38 (17.3) | 144 (20.0) | 0.375 |
| History of previous MI, n (%) | 90 (9.6) | 42 (19.1) | 48 (6.7) | <0.0001 |
| STEMI, n (%) | 703 (74.7) | 162 (73.6) | 541 (75.0) | 0.676 |
| Localization of infarction | 0.003 | |||
| Anterior, n (%) | 391 (41.6) | 95 (43.2) | 296 (41.1) | |
| Inferior, n (%) | 435 (46.2) | 92 (41.8) | 343 (47.6) | |
| Lateral, n (%) | 102 (10.8) | 25 (11.4) | 77 (10.7) | |
| Unclassified, n (%) | 12 (1.3) | 8 (3.6) | 4 (0.6) | |
| LVEF (%), median (IQR) | 53 (45 to 60) | 49 (40 to 58) | 54 (46 to 61) | <0.0001 |
| Incomplete revascularization (%) | 173 (18.4) | 82 (37.3) | 91 (12.6) | <0.0001 |
| GRACE score, median (IQR) | 110 (93 to 126) | 119 (103 to 135) | 107 (90 to 124) | <0.0001 |
| NYHA ≥3, n (%) | 40 (4.3) | 11 (5.0) | 29 (4.0) | 0.529 |
| Diabetes mellitus, n (%) | 184 (19.6) | 42 (19.1) | 142 (19.7) | 0.843 |
| CK max (U/L), median (IQR) | 1302 (647 to 2465) | 1309 (652 to 2594) | 1302 (644 to 2440) | 0.850 |
| Creatinine (mg/dL), median (IQR) | 1.1 (0.9 to 1.3) | 1.1 (1.0 to 1.3) | 1.1 (1.0 to 1.3) | 0.125 |
| Acute nephropathy, n (%) | 12 (1.3) | 5 (2.3) | 7 (1.0) | 0.132 |
| Therapy data | ||||
| PCI, n (%) | 878 (93.3) | 204 (92.7) | 674 (93.5) | 0.695 |
| Thrombolysis, n (%) | 14 (1.5) | 1 (0.5) | 13 (1.8) | 0,148 |
| CABG, n (%) | 6 (0.6) | 1 (0.5) | 5 (0.7) | 0.697 |
| No intervention | 43 (4.6) | 14 (6.4) | 29 (4.0) | 0.145 |
| Aspirin, n (%) | 913 (97.0) | 215 (97.7) | 698 (96.8) | 0.483 |
| Clopidogrel, n (%) | 920 (97.8) | 214 (97.3) | 706 (97.9) | 0.570 |
| Beta‐blockers, n (%) | 897 (95.3) | 211 (95.9) | 686 (95.1) | 0.639 |
| ACE inhibitors, n (%) | 885 (94.0) | 212 (96.4) | 673 (93.3) | 0.097 |
| Statins, n (%) | 879 (93.4) | 205 (93.2) | 674 (93.5) | 0.875 |
| Ca antagonists, n (%) | 14 (1.5) | 4 (1.8) | 10 (1.4) | 0.644 |
| Diuretics, n (%) | 415 (44.1) | 118 (53.6) | 297 (41.2) | 0.001 |
| Mortality data | ||||
| All‐cause mortality, n (%) | 72 (7.7) | 27 (12.3) | 45 (6.2) | 0.003 |
| Cardiac mortality, n (%) | 38 (4.0) | 15(6.8) | 23 (3.2) | 0.017 |
| Sudden cardiac death, n (%) | 14 (1.5) | 7 (3.2) | 7 (1.0) | 0.018 |
ACE indicates angiotensin‐converting enzyme; ART, Autonomic Regulation Trial; CABG, coronary artery bypass grafting; GRACE score, composite of age of the patient, serum creatinine, past myocardial infarction, past heart failure, in‐hospital percutaneous coronary intervention, heart rate, systolic blood pressure, ST segment deviation, and positive enzymes; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association functional class; PCI, percutaneous coronary intervention; STEMI, ST‐elevation myocardial infarction.
A patient flow chart is shown in Figure 2. A total of 220 (23.4%) patients exhibited 1 or more VPCs during the 30‐minute recording suitable for PESP assessment. PESP was present in 62 and absent in 158 of these patients. Eighteen of the former and 9 of the latter patients died during follow‐up.
Figure 2.
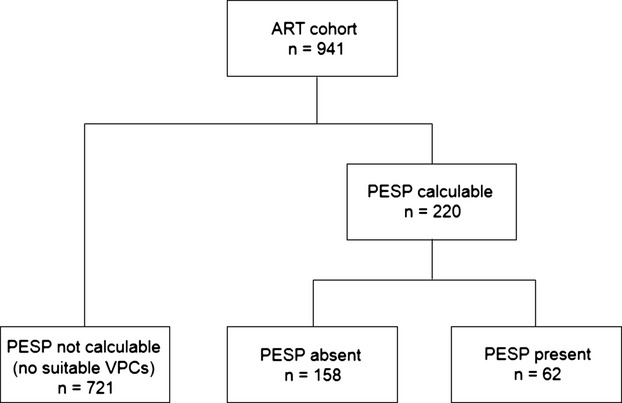
Flow chart of patient selection. ART indicates Autonomic Regulation Trial; PESP, postextrasystolic potentiation; VPC, ventricular premature complex.
Figure 3A shows cumulative mortality curves for patients classified as PESP present, PESP absent, and PESP not measurable. The probability of death at 5 years was 29.4% in patients with PESP present and 5.8% in patients with PESP absent. The probability of death of patients in whom PESP could not be measured was similar to that of patients with PESP absent (6.3%). For the following analyses, we consequently merged the categories of PESP absent and PESP not measurable. Merging these patient categories parallels the classification of heart rate turbulence, the assessment of which also considers patients without suitable VPCs to present with a negative result.26–27
Figure 3.
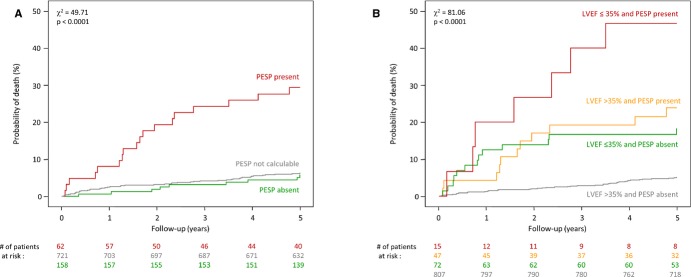
Kaplan‐Meier's curves for the association of PESP and mortality. A, Mortality probability over 5 years in patients stratified according to the presence (red curve) and absence of PESP (green curve). The mortality probability of patients whose recordings lacked VPCs suitable for the quantification of PESP is also shown (gray curve). Because there was no significant difference in patients with PESP absent and without suitable VPCs, both subgroups were merged for additional analyses. B, Probability of death in patients stratified by the combination of PESP (absent and present) and LVEF (≤35% and >35%). The numbers of patients at risk in the individual groups at 0, 1, 2, 3, 4, and 5 years are shown below the graphs in the same color coding. P values for the overall comparison at 5 years are indicated. LVEF indicates left ventricular ejection fraction; PESP, postextrasystolic potentiation; VPC, ventricular premature complex.
In univariable Cox regression analysis, all 4 considered risk factors (ie, PESP, LVEF, VPC count, and GRACE score) were significantly associated with outcome. The presence of PESP was associated with a hazard ratio (HR) of 5.5 (95% CI, 3.2 to 9.4; P<0.001; left column of Table 2). In multivariable Cox regression analysis, PESP, LVEF, and GRACE score were significant risk predictors with HRs of 3.2 (95% CI, 1.8 to 5.7; P<0.001), 2.6 (95% CI, 1.5 to 4.6; P=0.001), and 4.7 (95% CI, 2.7 to 8.0; P<0.001), respectively (right column of Table 2).
Table 2.
Uni‐ and Multivariable Cox Regression Analysis
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | χ2 | P Value | HR | χ2 | P Value | |
| PESP present | 5.51 (3.23 to 9.40) | 39.2 | <0.0001 | 3.21 (1.82 to 5.66) | 16.3 | <0.0001 |
| LVEF ≤35% | 4.29 (2.56 to 7.19) | 30.6 | <0.0001 | 2.60 (1.49 to 4.55) | 11.2 | 0.001 |
| VPC ≥10 per 30 hours | 2.75 (1.68 to 4.49) | 16.4 | <0.0001 | 1.25 (0.72 to 2.17) | 0.6 | 0.420 |
| GRACE score ≥120 points | 5.82 (3.41 to 9.92) | 41.9 | <0.0001 | 4.65 (2.70 to 8.01) | 30.6 | <0.0001 |
GRACE score indicates composite of age of the patient, serum creatinine, past myocardial infarction, past heart failure, in‐hospital percutaneous coronary intervention, heart rate, systolic blood pressure, ST segment deviation and positive enzymes; HR, hazard ratio with 95% confidence intervals in parentheses; LVEF, left ventricular ejection fraction; PESP, postextrasystolic potentiation; VPC, ventricular premature complex.
The goodness of fit improved significantly when PESP was added to the Cox model fixed to LVEF, GRACE score, and VPC counts (P<0.001). The VIF for PESP was 1.098, which is way below the critical value 4, which indicates collinearity.
Combined Use of LVEF and PESP
Figure 3B shows cumulative mortality curves for patients classified by PESP and LVEF. Patients with both LVEF >35% and PESP absent had a 5‐year mortality risk of 5.1%. Patients with both LVEF ≤35% and PESP present had a mortality risk of 46.7%. Patients in whom only 1 variable was abnormal had mortality risks of 18.2% (LVEF ≤35% and PESP absent) or 23.8% (LVEF >35% and PESP present).
When PESP was added to LVEF, the AUROC curve increased significantly from 0.61 to 0.75 (P<0.001). The IDI index was 0.003 in survivors and 0.034 in nonsurvivors (P=0.05 and 0.009, respectively). An internal validation by 3‐fold cross‐validation, repeated 25 times, resulted in average IDI values of 0.003 and 0.034, stressing the robustness of the results.
When a high‐risk group was defined by use of LVEF alone, positive predictive value was 23.0% at a sensitivity level of 27.8% (Table 3). The definition of a high‐risk group was more precise if LVEF and PESP were used in combination: Positive predictive value (PPV) remained stable at 23.1%, whereas sensitivity significantly increased to 43.1% (P<0.001).
Table 3.
Group Sizes and Numbers of Primary Endpoints, Sensitivities, Specificities, and Positive and Negative Predictive Values for Selected Subroups Defined by LVEF and PESP
| Risk‐Stratification Criterion | e/n | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| LVEF ≤35% alone | 20/87 | 27.8 | 92.3 | 23.0 | 93.9 |
| LVEF ≤35% or LVEF >35% and PESP present | 31/134 | 43.1 | 88.1 | 23.1 | 94.9 |
e indicates number of primary endpoints; LVEF, left ventricular ejetion fraction; n, size of subgroup; NPV, negative predictive value; PESP, postextrasystolic potentiation; PPV, positive predictive value.
Analysis of Possible Confounding Factors
Because the baseline variables presented in Table 1 might represent confounders, especially if associated both with PESP and mortality, we tested whether inclusion of these factors in the multivariable Cox regression model consisting of PESP, LVEF, VPC count, and GRACE score would reduce the prognostic power of PESP. This was not the case (Figure 4). None of the tested parameters led to a loss of significant association of PESP with mortality when included in the analysis. We conclude that the association of PESP and mortality is not a result of confounding by any of these parameters.
Figure 4.
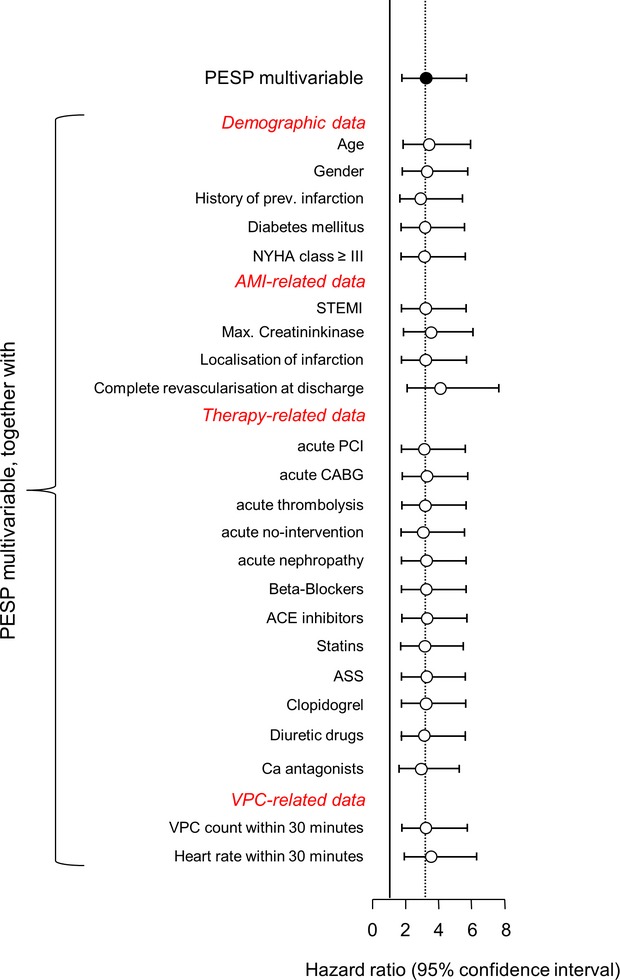
Analysis for possible confounders. Hazard ratios for PESP, together with 95% confidence intervals, are shown for multivariable models in which singular additional baseline variables (as indicated) were included in the Cox analysis in addition to the baseline model consisting of PESP, LVEF, VPC count, and GRACE score. ASS indicates acetylsalicylic acid; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; PCI, percutaneous coronary intervention; PESP, postextrasystolic potentiation; STEMI, ST‐elevation myocardial infarction; VPC, ventricular premature complex.
Validation in an Independent Cohort of HF Patients
The novelty of the concept of PESP as a post‐MI risk predictor requires independent validation. To our best knowledge, there is only 1 other data set of simultaneous ECGs and continuous BP measurements suitable for both PESP quantification and risk assessment: a Scottish cohort of 146 HF patients.15 During a follow‐up period of 2.7±1.1 years, 42 of these patients died (28.8% mortality). PESP could be measured in 105 of these patients; data in 41 were not suitable for assessment because of the lack of ventricular ectopy during the 10‐minute recording. Figure 5 shows survival analysis of patients with PESP present and absent, including patients in whom PESP was not measurable. The same principal result as in the main study cohort is seen. This was confirmed by uni‐ and multivariable Cox regression analysis, indicating that presence of PESP was a strong predictor of mortality independent of other established risk predictors (data not shown). PESP was associated with mortality regardless if applied as a dichotomized or continuous variable. Figure 6 depicts the mortality probability of patients from both cohorts as a function of numerical ratio of PESP. The optimum cut‐off values for numerical ratio of PESP were 1.037 (95% CI, 0.994 to 1.045) and 1.093 (0.985 to 1.124) in the ART and Scottish cohorts, respectively. Thus, the prospective definition of PESP present and absent was well within the 95% CI of both ART and Scottish cohorts.
Figure 5.
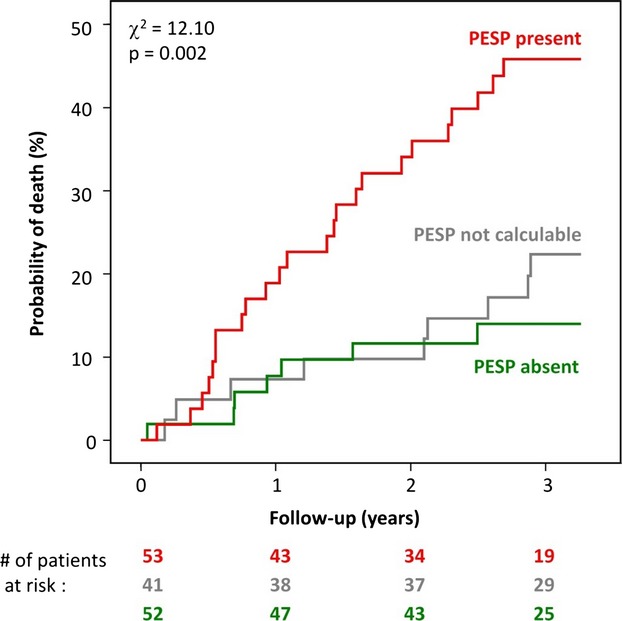
Validation cohort: Kaplan‐Meier curves for the association of PESP and mortality. Mortality probability over the follow‐up period in patients stratified according to PESP presence (red curve) and absence (green curve). Mortality probability of patients in whom PESP was not evaluable is also shown (gray curve). The numbers of patients of the 2 groups involved in the analysis at 0, 1, 2, and 3 years are shown below the graph in the same color coding. P values for the overall comparison at 3 years are indicated. PESP indicates postextrasystolic potentiation.
Figure 6.
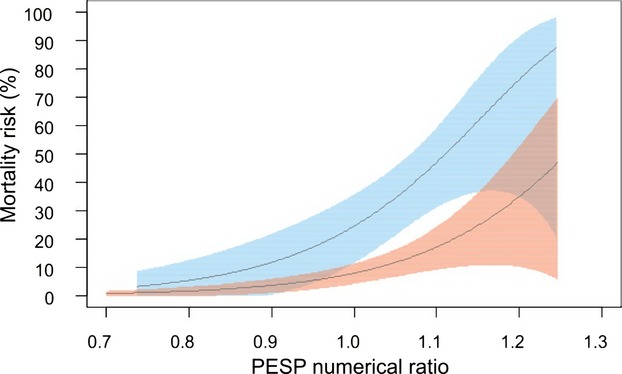
Continuous association of PESP numerical ratio and mortality. Mortality probabilities and their 95% confidence intervals on a continuous scale of a PESP numerical ratio. Data from the postinfarction patients included in the ART study are depicted in pink, and data from the heart failure patients from the validation study are shown in cyan. The horizontal axis shows the proportion of the first post‐VPC pulse wave amplitude to the mean of the subsequent 9 pulse wave amplitudes. ART indicates Autonomic Regulation Trial; PESP indicates postextrasystolic potentiation; VPC, ventricular premature complex.
Discussion
This is the first study that prospectively investigated postextrasystolic BP potentiation as a risk predictor in cardiac patients. The main finding in our data is that PESP was a strong predictor of mortality in post‐MI and HF patients: In patients with PESP present in the ART cohort, mortality was 5‐fold higher than in the remaining patients. In the multivariable model, which included the GRACE score, arrhythmia count, and LVEF, the HR for PESP still exceeded 3 (see Table 2).
The high‐risk subgroup identified by PESP was small (only 6.6% of the enrolled patients). Naturally, our study does not provide data on whether any intervention can reduce this risk. However, because of the small size of the high‐risk group identified by PESP, such an intervention would have to be targeted only at a small subgroup of postinfarction patients.
The contribution to risk stratification provided by PESP and LVEF was complementary: Whereas the large subgroup of patients with both PESP and LVEF normal was at very low risk of subsequent death (5‐year mortality risk, 5.1%), this risk was substantial for patients with 1 abnormal parameter, regardless of whether this was LVEF (18.2%) or PESP (23.8%). The highest mortality risk was observed in the subgroup of patients with both PESP and LVEF abnormal (46.7%).
The association of PESP with mortality was consistently observed also in the independent Scottish cohort of HF patients. In both cohorts, there was a continuous association of numerical PESP ratio and mortality.
The concept that PESP represents a surrogate for clinical or subclinical HF might be counterintuitive at first sight. It is, however, supported not only by clinical observations,6 but also by experimental studies10 as well as by mathematical modeling of calcium cycling processes.13 For example, a mouse model of depressed LV function induced by overexpression of phospholamban (the major negative regulator of sarcoplasmic reticulum calcium uptake)28 showed a greater magnitude of PESP, compared to isogenic control mice.10
If PESP is invasively measured as LV dP/dtmax of the first postectopic beat, a potentiation is observed in both healthy subjects and HF patients, but augmented in the latter.6–8 The beat‐to‐beat BP changes are determined not only by the contractility of the heart, but also by a vascular component. If PESP is measured as SBP of the first postectopic beat, a potentiation is typically not observed in healthy subjects, but in HF patients.6 In both of our patient cohorts, a PESP BP pattern consistently signified a substantially increased mortality risk.
The calcium cycling abnormalities underlying PESP may facilitate the generation of both early and delayed afterdepolarizations.29–30 Consequently, PESP does not only indicate HF‐related abnormalities at the cellular level, but also a propensity to potentially lethal arrhythmias.
Potential Clinical Applications
PESP can be observed either after spontaneous VPCs—as it was done in this study—or after instrumentally induced VPCs.6 Programmed ventricular stimulation might thus indirectly evaluate also abnormalities of myocardial calcium cycling. We foresee a scale of potential clinical applications, including risk assessment in various cardiac conditions, improved patient selection for ICD and cardiac resynchronization therapy, and a contribution to the optimization of HF therapy. Because the pharmacological modulation of the pathways involved in myocardial calcium homeostasis is a promising approach for the treatment of both systolic and diastolic HF,31–32 PESP might become helpful either in selecting patients who will most likely benefit from such treatment or in providing a measurable surrogate of treatment effects.
Limitations
The prespecified primary endpoint of the study was all‐cause mortality. Because of the limited number of deaths (especially in the group of patients with VPCs suitable for PESP assessment), the power to assess the association with more‐specific endpoints is limited. When we repeated the analyses for the association with cardiac mortality (as adjudicated by an event committee), we obtained practically the same results (not shown here). Because of even smaller numbers of sudden cardiac deaths, we cannot make any confident statement on the prediction of this endpoint.
Because no independent cohort of MI patients was available to validate our results from the ART trial, we used the Scottish cohort of advanced HF patients for the validation study. MI was not an inclusion criterion for this cohort, even though 86% had ischemic heart disease. The disparities between the 2 cohorts might be interpreted as a weakness, because the 2 cohorts are not necessarily comparable both in terms of clinical variables and pathophysiology. However, the disparities may be also considered a strong point, because they permit to extend the results of our study to patients with HF of different origin.
Postinfarction patients with severely reduced LVEF are potential candidates for primary prophylactic ICD implantation. Because we did not systematically assess subsequent ICD implantations, we cannot provide reliable data on how many of the patients received this therapy during follow‐up. This might have lead to underestimation of the predictive power of PESP in case patients predicted to be at high risk had adequate ICD shocks that prevented sudden cardiac death.
To make the calculation less noise sensitive, we prospectively decided to relate the first post‐VPC pulse wave amplitude to the mean of the subsequent 9 pulse wave amplitudes, rather than to the pre‐VPC pulse wave amplitude. When retrospectively using the ratio between the post‐ and pre‐VPC pulse wave amplitudes, the results were practically the same, although reaching slightly lesser statistical significances (likely the result of noise influence).
Conclusion
A PESP BP pattern is characteristic of a disturbed contractile state of the heart. In this study, PESP was a strong, independent predictor of adverse outcome.
Sources of Funding
This work was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (German Federal Ministry of Education and Research: 13N/7073/7) and by the Deutsche Forschungsgemeinschaft (German Research Foundation: Si 1747/1‐1). The sponsors had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosures
None.
References
- 1.Langendorff O. Ueber elektrische Reizung des Herzens. Du‐Bois‐Reymond's Archiv f. Physiologie. 1885; 8:284-287. [Google Scholar]
- 2.Langendorff O. Untersuchungen am überlebenden Säugetierherzen III. Abhandlung: Unregelmäßigkeiten des Herzschlages und ihre Ausgleichung. Pflügers Archiv für die gesamte Physiologie. 1898; 70:473-486. [Google Scholar]
- 3.Bornstein A. Die Postextrasystole. Zbl Physiol. 1906; 20:401-405. [Google Scholar]
- 4.Hoffman BF, Bindler E, Suckling EE. Postextrasystolic potentiation of contraction in cardiac muscle. Am J Physiol. 1956; 185:95-102. [DOI] [PubMed] [Google Scholar]
- 5.Wiggers CJ. The Henry Jackson memorial lecture: dynamics of ventricular contraction under abnormal conditions. Circulation. 1952; 5:321-348. [DOI] [PubMed] [Google Scholar]
- 6.Beck W, Chesler E, Schrire V. Postextrasystolic ventricular pressure responses. Circulation. 1971; 44:523-533. [DOI] [PubMed] [Google Scholar]
- 7.Merillon JP, Motte G, Aumont MC, Masquet C, Lecarpentier Y, Gourgon R. Postextrasystolic left ventricular peak pressure with and without left ventricular failure. Cardiovasc Res. 1979; 13:338-344. [DOI] [PubMed] [Google Scholar]
- 8.Seed WA, Noble MI, Walker JM, Miller GA, Pidgeon J, Redwood D, Wanless R, Franz MR, Schoettler M, Schaefer J. Relationships between beat‐to‐beat interval and the strength of contraction in the healthy and diseased human heart. Circulation. 1984; 70:799-805. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MW. Postextrasystolic potentiation. Do we really know what it means and how to use it? Circulation. 1993; 88:2962-2971. [DOI] [PubMed] [Google Scholar]
- 10.Hoit BD, Tramuta DA, Kadambi VJ, Dash R, Ball N, Kranias EG, Walsh RA. Influence of transgenic overexpression of phospholamban on postextrasystolic potentiation. J Mol Cell Cardiol. 1999; 31:2007-2015. [DOI] [PubMed] [Google Scholar]
- 11.Zaugg CE, Kojima S, Wu ST, Wikman‐Coffelt J, Parmley WW, Buser PT. Intracellular calcium transients underlying interval‐force relationship in whole rat hearts: effects of calcium antagonists. Cardiovasc Res. 1995; 30:212-221. [PubMed] [Google Scholar]
- 12.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002; 34:951-969. [DOI] [PubMed] [Google Scholar]
- 13.Iribe G, Kohl P, Noble D. Modulatory effects of calmodulin‐dependent kinase II (CaMKII) on sarcoplasmic reticulum Ca2+ handling and interval‐force relations: a modelling study. Philos Trans A Math Phys Eng Sci. 2006; 364:107-133. [DOI] [PubMed] [Google Scholar]
- 14.Barthel P, Bauer A, Müller A, Huster KM, Kanters JK, Paruchuri V, Yang X, Ulm K, Malik M, Schmidt G. Spontaneous baroreflex sensitivity: prospective validation trial of a novel technique in survivors of acute myocardial infarction. Heart Rhythm. 2012; 8:1288-1294. [DOI] [PubMed] [Google Scholar]
- 15.Bauer A, Morley‐Davies A, Barthel P, Müller A, Ulm K, Malik M, Schmidt G. Bivariate phase‐rectified signal averaging for assessment of spontaneous baroreflex sensitivity: pilot study of the technology. J Electrocardiol. 2010; 43:649-653. [DOI] [PubMed] [Google Scholar]
- 16.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KAGRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6‐month postdischarge death in an international registry. J Am Med Assoc. 2004; 291:2727-2733. [DOI] [PubMed] [Google Scholar]
- 17.Barthel P, Wensel R, Bauer A, Müller A, Wolf P, Ulm K, Huster KM, Francis DP, Malik M. Respiratory rate predicts outcome after acute myocardial infarction: a prospective cohort study. Eur Heart J. 2013; 34:1644-1650. [DOI] [PubMed] [Google Scholar]
- 18.The Multicenter Postinfarction Research Group. Risk stratification and survival after myocardial infarction. N Engl J Med. 1983; 309:331-336. [DOI] [PubMed] [Google Scholar]
- 19.Boulesteix AL, Hothorn T. Testing the additional predictive value of high‐dimensional data. BMC Bioinformatics. 2010; 10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013; 32:2430-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uno H, Cai T. survIDINRI: IDI and NRI for comparing competing risk prediction models with censored survival data. 2013. R package version 1.1x20101. Availalbe at: http://CRAN.R-project.org/package=survIDINRI. Accessed April 2, 2014.
- 22.Youden WJ. Index for rating diagnostic tests. Cancer. 1950; 3:32-35. [DOI] [PubMed] [Google Scholar]
- 23.Böhning D, Böhning W, Holling H. Revisiting Youden's index as a useful measure of the misclassification error in meta‐analysis of diagnostic studies. Stat Methods Med Res. 2008; 17:543-554. [DOI] [PubMed] [Google Scholar]
- 24.DiCiccio TJ, Efron B. Bootstrap confidence intervals. Stat Sci. 1996; 11:189-228. [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristics curves: a nonparametric approach. Biometrics. 1988; 44:837-845. [PubMed] [Google Scholar]
- 26.Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, Camm AJ, Bigger JT, Jr, Schömig A. Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999; 353:1390-1396. [DOI] [PubMed] [Google Scholar]
- 27.Bauer A, Malik M, Schmidt G, Barthel P, Bonnemeier H, Cygankiewicz I, Guzik P, Lombardi F, Müller A, Oto A, Schneider R, Watanabe M, Wichterle D, Zareba W. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology consensus. J Am Coll Cardiol. 2008; 52:1353-1365. [DOI] [PubMed] [Google Scholar]
- 28.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, Walsh RA, Kranias EG. Cardiac‐specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocytes mechanics in transgenic mice. J Clin Invest. 1996; 97:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schillinger W, Fiolet JW, Schlotthauer K, Hasenfuss G. Relevance of Na+‐Ca2+ exchange in heart failure. Cardiovasc Res. 2003; 57:921-933. [DOI] [PubMed] [Google Scholar]
- 30.Pogwizd SM, Bers DM. Calcium cycling in heart failure: the arrhythmia connection. J Cardiovasc Electrophysiol. 2002; 13:88-91. [DOI] [PubMed] [Google Scholar]
- 31.Jacobshagen C, Belardinelli L, Hasenfuss G, Maier LS. Ranolazine for the treatment of heart failure with preserved ejection fraction: background, aims, and design of the RALI‐DHF study. Clin Cardiol. 2011; 34:426-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks AR. Caclium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013; 123:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]


