Abstract
Background
Heart failure (HF) patients experience impaired functional status, diminished quality of life, high utilization of healthcare resources, and poor survival. Yet, the identification of patient‐centered factors that influence prognosis is lacking.
Methods and Results
We determined the association of 2 measures of self‐rated health with healthcare utilization and skilled nursing facility (SNF) admission in a community cohort of 417 HF patients prospectively enrolled between October 2007 and December 2010 from Olmsted County, MN. Patients completed a 12‐item Short Form Health Survey (SF‐12). Low self‐reported physical functioning was defined as a score ≤25 on the SF‐12 physical component. The first question of the SF‐12 was used as a measure of self‐rated general health. After 2 years, 1033 hospitalizations, 1407 emergency department (ED) visits, and 19,780 outpatient office visits were observed; 87 patients were admitted to a SNF. After adjustment for confounding factors, an increased risk of hospitalizations (1.52 [1.17 to 1.99]) and ED visits (1.48 [1.04 to 2.11]) was observed for those with low versus moderate‐high self‐reported physical functioning. Patients with poor and fair self‐rated general health also experienced an increased risk of hospitalizations (poor: 1.73 [1.29 to 2.32]; fair: 1.46 [1.14 to 1.87]) and ED visits (poor: 1.73 [1.16 to 2.56]; fair: 1.48 [1.13 to 1.93]) compared with good‐excellent self‐rated general health. No association between self‐reported physical functioning or self‐rated general health with outpatient visits and SNF admission was observed.
Conclusion
In community HF patients, self‐reported measures of physical functioning predict hospitalizations and ED visits, indicating that these patient‐reported measures may be useful in risk stratification and management in HF.
Keywords: healthcare utilization, heart failure, hospitalizations, physical functioning, self‐rated health
Introduction
Heart failure (HF) is a major public health problem, currently affecting nearly 6 million Americans with >550 000 new cases diagnosed each year.1–2 HF patients experience impaired functional status, diminished quality of life, high utilization of healthcare resources, and poor survival.3–4 Furthermore, HF is the leading cause of hospitalization in Medicare patients5 and nearly one‐fourth of hospitalized HF patients are discharged to skilled nursing facilities (SNF).6–8
The identification of patient‐centered factors that influence prognosis may be useful in the management of HF patients. Such factors may include self‐reported measures of physical health status, which have been shown to predict hospitalizations and mortality in some studies of HF patients.9 We have previously shown that among HF patients in the community, subjective measures of physical health, including the physical component 12‐item Short Form Health Survey (SF‐12) as well as the first question of the SF‐12 alone predicted all‐cause mortality as well as an objective measure of physical functioning, the 6‐minute walk test.10 In addition, associations of physical health status with hospitalizations have been reported in HF; however, limitations of these studies, including restricting to only HF‐related hospitalizations or utilization of a composite endpoint pooling mortality with hospitalizations,11–19 have limited the generalizability of these results. Few studies have assessed the relationship between self‐reported measures of physical health with all‐cause hospitalizations,20–22 which is particularly important to understand in HF because >80% of hospitalizations in HF patients are due to causes other than HF.23 Furthermore, data on the association of self‐reported physical health status measures with other adverse outcomes in HF patients, such as emergency department (ED) and outpatient visits, as well as admission to SNFs are lacking. To address these gaps in knowledge while optimizing clinical relevance, it is important to study these questions in a community population with comprehensive prospective capture of patient‐centered factors and complete capture of healthcare utilization. Thus, the goal of our study was to prospectively determine the association of 2 measures of self‐reported physical health with healthcare utilization and SNF admission in a cohort of HF patients from the southeastern Minnesota community.
Methods
Study Setting
This study was conducted in southeastern Minnesota. This area of Minnesota is relatively isolated from other urban centers and only a few providers, including Mayo Clinic, Olmsted Medical Center, and a few other practices, deliver most health care to local residents. The Rochester Epidemiology Project, a record‐linkage system, allows the indexing of medical records among residents in southeastern Minnesota, thus enabling the retrieval of all health care‐related events occurring in this geographic area.24–25 This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Identification of the Study Cohort
Potential HF events from October 2007 through December 2010 were identified among residents of Olmsted, Dodge, and Fillmore Counties in Minnesota using natural language processing of electronic medical records. Prompt ascertainment of events was possible as documentation from a clinical visit is transcribed and available in the medical record within 24 hours of the encounter. Trained nurse abstractors reviewed the medical records of potential HF events to verify that the Framingham criteria were met,26 and if so, patients were contacted to obtain consent for study participation.
Physical Health Status Measures
A 12‐item Short Form Health Survey (SF‐12) was completed by cohort participants as part of the study return visit, which occurred within 6 weeks of enrollment (median time from consent to survey completion: 23 days). Two different measures of self‐rated health status were obtained by the responses to the SF‐12. First, self‐reported physical functioning was assessed using the physical component of the SF‐12. Those scoring ≤25 were categorized as having low self‐reported physical functioning, whereas scores >25 indicated moderate‐high physical functioning. Second, the first question of the SF‐12 was used as a measure of self‐rated general health. Responses to this first question, “In general would you say your health is,” included poor, fair, good, very good, and excellent; those reporting good, very good, and excellent were pooled into one category and served as the reference group.
Clinical Data Collection
Nurse abstractors obtained information on demographics, risk factors, and comorbidities at the time of enrollment from manual review of the medical record. Body mass index (BMI) was calculated as weight (in kg) divided by height (in meters) squared. Current cigarette smoking status was defined as smoking within the past 6 months of HF index. A history of hypertension was defined as ≥2 ambulatory blood pressure readings of ≥140 mm Hg systolic and/or ≥90 mm Hg diastolic or a physician diagnosis of hypertension. Prevalent diabetes was defined according to the American Diabetes Association criteria.27 A clinical diagnosis documented in the medical record identified those with a previous myocardial infarction (MI). Finally, information on comorbid conditions was used to calculate a score using the Charlson comorbidity index.28
Glomerular filtration rate (GFR) was estimated using the closest serum creatinine value within 1 year of HF using the Modification of Diet in Renal Disease Study (MDRD) equation.29 Left ventricular ejection fraction (LVEF) (%) was determined using values collected from an echocardiogram performed within 6 months prior to or 2 months after study enrollment.
Ascertainment of Healthcare Utilization and Skilled Nursing Facility Admission
Hospitalizations, ED visits, outpatient office visits, and admissions to SNFs were obtained from study enrollment through September 30, 2011 from the Olmsted County Healthcare Expenditure and Utilization Database, which contains healthcare utilization occurring in Olmsted County, MN from 1987 to present. If the patient was hospitalized during the index HF event, only subsequent hospitalizations were included in the analysis. In‐hospital transfers or transfers between Olmsted Medical Center and Mayo Clinic were analyzed as a single hospitalization. ED visits that resulted in a hospital admission were counted as both an ED visit and a hospitalization. Outpatient visits included only office visits and excluded tests, imaging, or outpatient procedures. Individuals residing in a SNF at index were excluded from the SNF analysis. Both temporary stays for rehab and long‐term placement in SNFs were counted; however, assisted living and hospice care were not considered in the SNF analysis.
Statistical Analysis
Statistical analyses were performed using SAS statistical software, version 9.2 (SAS Institute Inc). Differences in participant baseline characteristics between low and moderate‐high self‐reported physical functioning were compared using 2‐sample t tests for normally distributed continuous variables, Wilcoxon rank‐sum tests for non‐normal continuous variables, and chi‐square and Fisher's exact tests for categorical variables. Follow‐up time was calculated from the index date of HF until death, last follow‐up, or September 30, 2011, whichever came first. The cumulative mean number of hospitalizations, ED visits, and outpatient visits over follow‐up time by levels of self‐reported physical functioning and self‐rated general health were plotted using a nonparametric estimator described by Nelson.30
Andersen‐Gill modeling, which allows for modeling of multiple outcome events, was used to estimate hazard ratios of hospitalizations and ED visits for both the self‐reported physical functioning and self‐rated general health exposure variables. The following Andersen‐Gill models were run for each comparison of physical functioning with hospitalizations and ED visits: unadjusted, age‐ and sex‐adjusted, and fully adjusted with adjustment for age, sex, BMI, eGFR, prevalent versus incident HF status, LVEF, and diabetes.
Because outpatient office visits during follow‐up may cluster together (eg, multiple outpatient visits on a given day or within a span of several days as part of the diagnostic process or for yearly physical examinations), a time‐to‐event analysis such as the Andersen‐Gill model was not appropriate. The number of outpatient visits was modeled with the natural logarithm of the duration of follow‐up as the offset using negative binomial regression since Poisson regression resulted in overdispersion. Rate ratios were estimated for the unadjusted, age‐ and sex‐adjusted, and fully adjusted models, adjusted for the same covariates as the models for hospitalizations and ED visits.
The Kaplan‐Meier (KM) method was used to visualize the cumulative probability of SNF admission over follow‐up time by levels of self‐reported physical functioning and self‐rated general health by plotting the 1‐KM curves. Cox proportional hazards regression was used to estimate the associations of self‐reported physical functioning and self‐rated general health with SNF admission in unadjusted, age‐ and sex‐adjusted, and fully adjusted models.
Results
Of the 519 HF patients enrolled in our study between October 2007 and December 2010, 91 did not complete the questionnaire and 11 did not have available healthcare utilization data, resulting in 417 patients included in the analysis. Compared with the 417 patients in our final cohort, those who did not complete the questionnaire were more likely to be a current smoker, less likely to have an LVEF <50%, and had a greater mean Charlson comorbidity index, although were similar in all other baseline characteristics.
The mean (standard deviation [SD]) age of the cohort was 73.3 (13.3) years and 178 (43%) were female. Sixty‐one (15%) patients had low self‐reported physical functioning, defined as scores ≤25 on the physical component of the SF‐12. In response to the first question of the SF‐12, 54 (13%) reported poor and 135 (32%) reported fair self‐rated general health. Of those with low self‐reported physical functioning, 46% reported poor, 39% fair, and 15% good‐excellent self‐rated general health. For those with moderate‐high self‐reported physical functioning, 7% reported poor, 31% fair, and 62% good‐excellent self‐rated general health. Those with low self‐reported physical functioning had higher BMI, lower eGFR, were more likely to have diabetes and had a greater number of comorbidities compared with those with moderate‐high physical functioning (Table 1).
Table 1.
Participant Baseline Characteristics by Levels of Self‐Reported Physical Functioning
| Low (N=61) | Moderate‐High (N=356) | P Value | |
|---|---|---|---|
| Age, y | 73.6±12.7 | 73.3±13.4 | 0.96 |
| Male | 30 (50.8) | 209 (58.7) | 0.16 |
| Non‐white | 0 | 12 (3.4) | 0.23 |
| Body mass index, kg/m2 | 33.0±8.7 | 30.4±7.3 | 0.033 |
| Current smoking status | 3 (4.9) | 30 (8.4) | 0.45 |
| Married | 33 (54.1) | 212 (59.6) | 0.42 |
| >High school education | 23 (38.3) | 169 (49.1) | 0.12 |
| Hyperlipidemia | 51 (83.6) | 290 (81.5) | 0.69 |
| Hypertension | 59 (96.7) | 319 (89.6) | 0.08 |
| Depression | 27 (44.3) | 140 (39.3) | 0.47 |
| Myocardial infarction | 21 (34.4) | 88 (24.8) | 0.11 |
| Diabetes | 34 (55.7) | 130 (36.6) | 0.005 |
| Chronic obstructive pulmonary disease | 21 (34.4) | 89 (25.0) | 0.12 |
| Charlson comorbidity index | 5.2±2.5 | 3.7±2.6 | <0.0001 |
| Estimated GFR, mL/min per 1.73 m2 | 45.1 (36.3, 72.0) | 57.8 (43.3, 71.5) | 0.04 |
| Prevalent heart failure | 42 (68.9) | 204 (57.3) | 0.09 |
| Left ventricular ejection fraction <50% | 27 (45.8) | 189 (53.9) | 0.25 |
| Beta blockers | 54 (88.5) | 297 (83.4) | 0.31 |
| Angiotensin converting enzyme inhibitors/angiotensin II receptor blockers | 38 (62.3) | 243 (68.3) | 0.36 |
| Statins | 35 (57.4) | 211 (59.3) | 0.78 |
Values are mean±standard deviation for normally distributed continuous variables, median (25th, 75th percentile) for non‐normally distributed continuous variables, and N (percent) for categorical variables. GFR indicates glomerular filtration rate.
Over a mean (SD) follow‐up of 2.1 (1.0) years, a total of 1033 hospitalizations, 1407 ED visits, and 19 780 outpatient office visits were observed. Seventy‐two percent of hospitalizations were preceded by an ED visit, whereas 53% of ED visits resulted in a hospitalization. The average number of hospitalizations and ED visits over time was higher for the lower levels of physical health (Figures 1A and 1B), although similar numbers of outpatient office visits were observed between categories of self‐reported physical functioning and self‐rated general health (Figure 1C).
Figure 1.
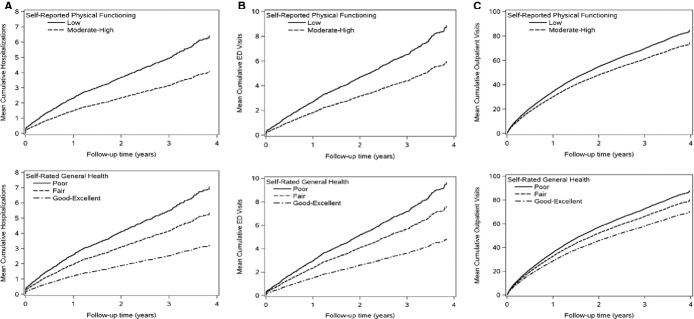
Mean cumulative number of hospitalizations (A), emergency department visits (B), and outpatient visits (C) over follow‐up by levels of self‐reported physical functioning and self‐rated general health. ED indicates emergency department.
After adjustment for important confounders, including age, sex, BMI, eGFR, diabetes, incident versus prevalent HF status, and EF, those with low self‐reported physical functioning exhibited a 50% increased risk of both hospitalizations and ED visits compared to those with moderate‐high physical functioning (hazard ratio (HR) 1.52, 95% confidence interval (CI) 1.17 to 1.99 for hospitalizations and 1.48, 1.04 to 2.11 for ED visits) (Table 2). Compared with those reporting good‐excellent self‐rated general health, those rating their general health as poor experienced a 70% increased risk of both hospitalizations and ED visits; fair self‐rated general health was associated with a 50% increased risk of both hospitalizations and ED visits. In contrast, the rates of outpatient office visits were consistent across levels of self‐reported physical functioning and self‐rated general health.
Table 2.
Rates, Hazard Ratios* (95% CI), and Rate Ratios* (95% CI) for Hospitalizations, Emergency Department Visits, and Outpatient Office Visits by Self‐Reported Physical Functioning and Self‐Rated General Health
| Self‐Reported Physical Functioning | Self‐Rated General Health | ||||
|---|---|---|---|---|---|
| Low (N=61) | Moderate‐High (N=356) | Poor (N=54) | Fair (N=135) | Good‐Excellent (N=228) | |
| Hospitalizations* | |||||
| Rate* | 1.8 | 1.1 | 2.0 | 1.5 | 0.9 |
| Crude | 1.57 (1.20 to 2.05) | 1.00 (ref) | 2.19 (1.67 to 2.87) | 1.66 (1.30 to 2.11) | 1.00 (ref) |
| Age‐ and sex‐adjusted | 1.59 (1.23 to 2.07) | 1.00 (ref) | 2.17 (1.66 to 2.85) | 1.65 (1.30 to 2.09) | 1.00 (ref) |
| Adjusted* | 1.52 (1.17 to 1.99) | 1.00 (ref) | 1.73 (1.29 to 2.32) | 1.46 (1.14 to 1.87) | 1.00 (ref) |
| Emergency department visits* | |||||
| Rate* | 2.3 | 1.5 | 2.6 | 2.0 | 1.2 |
| Crude | 1.49 (1.08 to 2.05) | 1.00 (ref) | 2.00 (1.44 to 2.78) | 1.58 (1.22 to 2.05) | 1.00 (ref) |
| Age‐ and sex‐adjusted | 1.49 (1.08 to 2.06) | 1.00 (ref) | 2.00 (1.43 to 2.78) | 1.58 (1.23 to 2.04) | 1.00 (ref) |
| Adjusted* | 1.48 (1.04 to 2.11) | 1.00 (ref) | 1.73 (1.16 to 2.56) | 1.48 (1.13 to 1.93) | 1.00 (ref) |
| Outpatient office visits* | |||||
| Rate* | 27.4 | 22.2 | 27.7 | 24.1 | 21.1 |
| Crude | 1.28 (1.07 to 1.54) | 1.00 (ref) | 1.41 (1.16 to 1.71) | 1.19 (1.04 to 1.38) | 1.00 (ref) |
| Age‐ and sex‐adjusted | 1.27 (1.06 to 1.53) | 1.00 (ref) | 1.38 (1.13 to 1.68) | 1.18 (1.03 to 1.36) | 1.00 (ref) |
| Adjusted* | 1.14 (0.94 to 1.38) | 1.00 (ref) | 1.14 (0.92 to 1.41) | 1.12 (0.96 to 1.31) | 1.00 (ref) |
Estimates are hazard ratios for hospitalizations and emergency department visits.
Estimates are rate ratios for outpatient office visits.
Crude event rate per person year.
Adjusted for age, sex, body mass index, estimated glomerular filtration rate, prevalent vs incident heart failure status, ejection fraction, and diabetes.
Nine patients residing in an SNF at the time of study enrollment were excluded from the SNF analysis. Among the 408 patients remaining, 87 were admitted to an SNF over a mean follow‐up of 1.9 years. Seventy‐five (86%) of the SNF admissions were immediately preceded by a hospitalization. The cumulative probability of SNF admission was higher for low versus moderate‐high self‐reported physical functioning, although differences between levels of self‐rated general health were not as apparent (Figure 2). In unadjusted models, low self‐reported physical functioning and poor self‐rated general health were associated with increased risk of SNF admission (Table 3). After adjustment for important confounders, similar associations for SNF admission as those for hospitalizations and ED visits were observed; however, these associations were not significant, likely due, in part, to the small number of SNF admissions.
Figure 2.
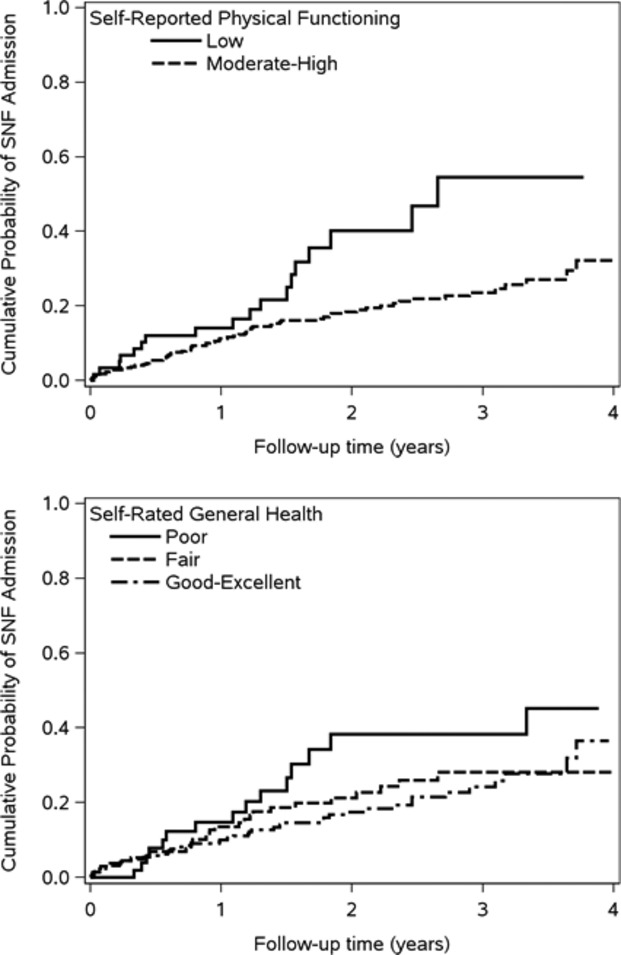
Cumulative probability of skilled nursing facility admission by levels of self‐reported physical functioning and self‐rated general health. SNF indicates skilled nursing facility.
Table 3.
Hazard Ratios (95%CI) for Admission to a Skilled Nursing Facility by Self‐Reported Physical Functioning and Self‐Rated General Health
| Self‐Reported Physical Functioning | Self‐Rated General Health | ||||
|---|---|---|---|---|---|
| Low (N=61) | Moderate‐High (N=356) | Poor (N=54) | Fair (N=135) | Good‐Excellent (N=228) | |
| Skilled nursing facility admission | |||||
| Rate* | 21.7 | 10.1 | 18.2 | 11.5 | 10.0 |
| Crude | 2.07 (1.23 to 3.50) | 1.00 (ref) | 1.79 (1.00 to 3.23) | 1.14 (0.71 to 1.83) | 1.00 (ref) |
| Age‐ and sex‐adjusted | 1.93 (1.15 to 3.26) | 1.00 (ref) | 1.99 (1.10 to 3.59) | 1.32 (0.82 to 2.12) | 1.00 (ref) |
| Adjusted* | 1.46 (0.80 to 2.67) | 1.00 (ref) | 1.49 (0.77 to 2.86) | 1.09 (0.64 to 1.87) | 1.00 (ref) |
Crude rate of nursing home placement per 100 person‐years.
Adjusted for age, sex, body mass index, estimated glomerular filtration rate, prevalent vs incident heart failure status, ejection fraction, and diabetes.
Discussion
In the community, HF patients with low self‐reported physical functioning (scores ≤25 on the physical component of the SF‐12) and poor and fair self‐rated general health experienced an increased risk of ED visits and hospitalizations. However, no association was observed between self‐reported physical functioning or self‐rated health with outpatient office visits or SNF admission.
Self‐ratings of one's health have been shown to be sensitive to declines in physical health and are reflective of trajectories in health over time.31 In addition, a single question asking a patient to rate their health in general may be as effective as longer questionnaires. In a large population of veteran patients (8% had HF), a single question asking about self‐rated general health was predictive of death and hospitalization equally well as the full SF‐36 physical component score.32 In a prior community study of HF patients, the first question of the SF‐12 equally discriminated those who would and would not die when compared to the physical component of the SF‐12 and even an objective measure, the 6‐minute walk test.10
While few studies have investigated self‐reported measures of physical health status with healthcare utilization and SNF admission in HF patients, our results corroborate a few prior studies that have found independent associations of self‐reported physical health status measures with hospitalizations after adjustment for important confounders. Among 394 HF patients admitted to 4 Spanish hospitals, scores below the median on the SF‐36 physical functioning and general health scales, as well as the physical component summary score, were each associated with >50% increased risk of hospital readmission after adjusting for demographics, comorbidities, biomedical, and treatment variables.22 In addition, worse scores on the physical dimension of the Minnesota Living with Heart Failure (MLWHF) questionnaire were predictive of rehospitalization over 2 years of follow‐up in 225 survivors of an HF admission attending a disease management program.21 Finally, in 208 hospitalized HF patients from the Optimizing Congestive Heart Failure Outpatient Clinic Project (OPTIMAL) study, worse physical mobility quality of life scores (measured by the Nottingham Health Profile) were observed among those who were readmitted compared with those not readmitted over a mean follow‐up of 3 years.20
Clinical Implications
Our results indicate that self‐reported measures of health status, including responses to the first question of the SF‐12 as well as the physical component score of the SF‐12, predict hospitalizations and ED visits in HF patients. However, an association of self‐reported physical functioning and self‐rated health with outpatient office visits and SNF admission was not observed. Nevertheless, given that hospital readmission rates after HF have not improved over recent years,23,33 self‐reported measures of health status may be a useful addition in the evaluation and management of HF patients to help identify those at risk of hospitalization. In particular, the administration of one simple question asking a patient about his/her general health may provide valuable information about the patient's current state of health above and beyond traditional assessments. This single question may help to identify patients who may not normally be considered at high risk of hospitalization based on standard assessments and, thus, may provide useful information for targeting those HF patients who may benefit from closer monitoring or stricter management.
Limitations and Strengths
We acknowledge the following limitations. First, our cohort was small, which may have limited our ability to detect modest associations and to identify effect modifiers of the associations between self‐reported physical functioning and self‐rated general health with healthcare utilization and skilled nursing facility admission. Second, our study results may have been impacted by differences in patients who completed the questionnaire compared to those who did not complete the questionnaire. Third, we did not have information on changes in confounders over time and were thus unable to adjust for time‐dependent covariates in our models. Fourth, some healthcare utilization and SNF admissions occurring outside of Olmsted County may have been missed. However, because Olmsted County is relatively isolated from other urban centers and the majority of health care for local residents is provided by the few medical centers within the county, we expect missing data to have been minimal. Finally, the participants in our cohort were primarily white, and thus, our results may not be generalizable to individuals of other race groups or ethnicities.
However, our study also has strengths that deserve mention. Our prospective cohort is a community cohort representing the full spectrum of HF, consisting of both inpatients and outpatients with either incident or prevalent HF and includes those with both preserved and reduced EF. In addition, we employed rigorous methods to validate each HF event and manually collected comprehensive data on risk factors, comorbidities, and patient characteristics, allowing us to minimize the amount of residual confounding present in our fully adjusted models. This approach helps delineate with clarity the role of patient‐centered and patient‐reported measures in the care of HF.
Conclusions
In community HF patients, self‐reported measures of physical functioning, from both the first question and the physical component of the SF‐12, predict hospitalizations and ED visits, but are not associated with outpatient visits or SNF admissions. These data indicate that self‐reported measures of physical health may be useful in the management of HF.
Acknowledgment
We thank Kay A. Traverse, RN for assistance in data collection, Jill M. Killian for assistance with statistical analysis, and Deborah S. Russell for secretarial assistance.
Sources of Funding
This work was supported by grants from the National Institutes of Health (R01 HL72435) and the National Institute on Aging (R01 AG034676). Dr Roger is an Established Investigator of the American Heart Association. The funding sources played no role in the design, conduct, or reporting of this study.
Disclosures
None.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005; 112:e154-e235. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009; 119:e391-e479. [DOI] [PubMed] [Google Scholar]
- 3.Juenger J, Schellberg D, Kraemer S, Haunstetter A, Zugck C, Herzog W, Haass M. Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002; 87:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012; 125:e2-e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009; 360:1418-1428. [DOI] [PubMed] [Google Scholar]
- 6.Allen LA, Hernandez AF, Peterson ED, Curtis LH, Dai D, Masoudi FA, Bhatt DL, Heidenreich PA, Fonarow GC. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail. 2011; 4:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolansky MA, Xu F, Zullo M, Shishehbor M, Moore SM, Rimm AA. Post‐acute care services received by older adults following a cardiac event: a population‐based analysis. J Cardiovasc Nurs. 2010; 25:342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Ross JS, Carlson MD, Lin Z, Normand SL, Bernheim SM, Drye EE, Ling SM, Han LF, Rapp MT, Krumholz HM. Skilled nursing facility referral and hospital readmission rates after heart failure or myocardial infarction. Am J Med. 2012; 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mommersteeg PMC, Denollet J, Spertus JA, Pedersen SS. Health status as a risk factor in cardiovascular disease: a systematic review of current evidence. Am Heart J. 2009; 157:208-218. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain AM, McNallan SM, Dunlay SM, Spertus JA, Redfield MM, Moser DK, Kane RL, Weston SA, Roger VL. Physical health status measures predict all‐cause mortality in patients with heart failure. Circ Heart Fail. 2013; 6:669-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alla F, Briançon S, Guillemin F, Juillière Y, Mertès P‐M, Villemot J‐P, Zannad Flfor the EPICAL Investigators. Self‐rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail. 2002; 4:337-343. [DOI] [PubMed] [Google Scholar]
- 12.Carson P, Tam SW, Ghali JK, Archambault WT, Taylor A, Cohn JN, Braman VM, Worcel M, Anand IS. Relationship of quality of life scores with baseline characteristics and outcomes in the African‐American Heart Failure Trial. J Card Fail. 2009; 15:835-842. [DOI] [PubMed] [Google Scholar]
- 13.Hulsmann M, Berger R, Sturm B, Bojic A, Woloszczuk W, Bergler‐Klein J, Pacher R. Prediction of outcome by neurohumoral activation, the six‐minute walk test and the Minnesota Living with Heart Failure Questionnaire in an outpatient cohort with congestive heart failure. Eur Heart J. 2002; 23:886-891. [DOI] [PubMed] [Google Scholar]
- 14.Kato N, Kinugawa K, Seki S, Shiga T, Hatano M, Yao A, Hirata Y, Kazuma K, Nagai R. Quality of life as an independent predictor for cardiac events and death in patients with heart failure. Circ J. 2011; 75:1661-1669. [DOI] [PubMed] [Google Scholar]
- 15.Konstam V, Salem D, Pouleur H, Kostis J, Gorkin L, Shumaker S, Mottard I, Woods P, Konstam MA, Yusuf S. Baseline quality of life as a predictor of mortality and hospitalization in 5,025 patients with congestive heart failure. Am J Cardiol. 1996; 78:890-895. [DOI] [PubMed] [Google Scholar]
- 16.Moser DK, Yamokoski L, Sun JL, Conway GA, Hartman KA, Graziano JA, Binanay C, Stevenson LW. Improvement in health‐related quality of life after hospitalization predicts event‐free survival in patients with advanced heart failure. J Card Fail. 2009; 15:763-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piotrowicz K, Noyes K, Lyness JM, McNitt S, Andrews ML, Dick A, Hall WJ, Moss AJ, Zareba W. Physical functioning and mental well‐being in association with health outcome in patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial II. Eur Heart J. 2007; 28:601-607. [DOI] [PubMed] [Google Scholar]
- 18.Stull DE, Clough LA, Van Dussen D. Self‐report quality of life as a predictor of hospitalization for patients with LV dysfunction: a life course approach. Res Nurs Health. 2001; 24:460-469. [DOI] [PubMed] [Google Scholar]
- 19.Havranek EP, Lapuerta P, Simon TA, L'Italien G, Block AJ, Rouleau JL. A health perception score predicts cardiac events in patients with heart failure: results from the IMPRESS trial. J Card Fail. 2001; 7:153-157. [DOI] [PubMed] [Google Scholar]
- 20.Mejhert M, Kahan T, Persson H, Edner M. Predicting readmissions and cardiovascular events in heart failure patients. Int J Cardiol. 2006; 109:108-113. [DOI] [PubMed] [Google Scholar]
- 21.O'Loughlin C, Murphy NF, Conlon C, O'Donovan A, Ledwidge M, McDonald K. Quality of life predicts outcome in a heart failure disease management program. Int J Cardiol. 2010; 139:60-67. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez‐Artalejo F, Guallar‐Castillon P, Pascual CR, Otero CM, Montes AO, Garcia AN, Conthe P, Chiva MO, Banegas JR, Herrera MC. Health‐related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005; 165:1274-1279. [DOI] [PubMed] [Google Scholar]
- 23.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009; 54:1695-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melton LJ., III History of the rochester epidemiology project. Mayo Clin Proc. 1996; 71:266-274. [DOI] [PubMed] [Google Scholar]
- 25.St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011; 173:1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993; 88:107-115. [DOI] [PubMed] [Google Scholar]
- 27. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997; 20:1183-1197. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373-383. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145:247-254. [DOI] [PubMed] [Google Scholar]
- 30.Nelson WB. Recurrent events data analysis for product repairs, disease recurrence, and other applications. ASA‐SIAM Series on Statistics and Applied Probability. 2003:151 [Google Scholar]
- 31.Ferraro KF, Kelley‐Moore JA. Self‐rated health and mortality among black and white adults: examining the dynamic evaluation thesis. J Gerontol B Psychol Sci Soc Sci. 2001; 56:S195-S205. [DOI] [PubMed] [Google Scholar]
- 32.DeSalvo KB, Fan VS, McDonell MB, Fihn SD. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005; 40:1234-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand S‐LT, Schreiner G, Spertus JA, Vidán MT, Wang Y, Wang Y, Krumholz HM. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010; 3:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]


