Abstract
Background
During the past decade, survival after in‐hospital cardiac arrest has improved markedly. It remains unknown whether the improvement in survival has occurred uniformly at all hospitals or was driven by large improvements at only a few hospitals.
Methods and Results
We identified 93 342 adults with an in‐hospital cardiac arrest at 231 hospitals in the Get With The Guidelines®‐Resuscitation registry during 2000–2010. Using hierarchical regression models, we evaluated hospital‐level trends in survival to discharge. Mean age was 66 years, 59% were men, and 21% were black. Between 2000 and 2010, there was a significant decrease in age, prevalence of heart failure and myocardial infarction, and cardiac arrests due to shockable rhythms (P<0.001 for all) and an increase in prevalence of sepsis, respiratory insufficiency, renal insufficiency, intensive care unit location, and mechanical ventilation before arrest (P<0.001 for all). After adjustment for temporal trends in baseline characteristics, hospital rates of in‐hospital cardiac arrest survival improved by 7% per year (odds ratio [OR] 1.07, 95% CI 1.06 to 1.08, P<0.001). Improvement in survival varied markedly and ranged from 3% in the bottom hospital quartile to 11% in the top hospital quartile. Compared with minor teaching hospitals (OR 1.04, 95% CI 1.02 to 1.06), hospital rate of survival improvement was greater at major teaching (OR 1.08, 95% CI 1.06 to 1.10) and nonteaching hospitals (OR 1.07, 95% CI 1.05 to 1.09, P value for interaction=0.03).
Conclusion
Although in‐hospital cardiac arrest survival has improved during the past decade, the magnitude of improvement varied across hospitals. Future studies are needed to identify hospital processes that have led to the largest improvement in survival.
Keywords: cardiac arrest, cardiopulmonary resuscitation, health services research, survival
Introduction
A recent study found that survival after in‐hospital cardiac arrest has improved markedly over the past decade, from 13.7% in 2000 to 22.3% in 2009.1 While encouraging, it remains unknown whether this survival trend has occurred uniformly across most hospitals or driven by large improvements at a smaller number of hospitals. It is possible that some hospitals have achieved larger gains in survival over time compared with other hospitals. Identifying top‐performing sites that have achieved large gains in in‐hospital cardiac arrest survival and the associated hospital factors is the critical next step to inform ongoing quality improvement efforts for in‐hospital resuscitation.
To address these gaps in knowledge, we used data from Get With The Guidelines® (GWTG)‐Resuscitation, a large national registry of in‐hospital cardiac arrests, to characterize hospital‐level trends in in‐hospital cardiac arrest survival over the past decade. Additionally, we determined whether some hospitals have achieved greater improvements in survival compared with others, and evaluated hospital factors associated with the extent of hospital rates of survival improvement.
Methods
Study Design
GWTG‐Resuscitation, formerly known as the National Registry for Cardiopulmonary Resuscitation, is a large, prospective, hospital‐based clinical registry of patients with in‐hospital cardiac arrest in the United States. The design of the registry has been previously described in detail.2 Briefly, cardiac arrest in the registry is defined as the absence of a palpable central pulse, apnea, and unresponsiveness. Consecutive patients with a cardiac arrest, without do‐not‐resuscitate orders, and who received cardiopulmonary resuscitation (CPR) are identified and enrolled by specially trained personnel using an online, interactive case report form.
Multiple case finding methods are used to ensure that all cases within a hospital are captured. These include centralized collection of cardiac arrest flow sheets, review of hospital page system logs, and routine checks of code carts, pharmacy tracer drug records, and hospital billing charges for use of resuscitation medications. Data are collected according to the Utstein‐style definitions, which are a template of uniform reporting guidelines developed by international experts.3–4 Prior to data collection and entry, hospital staff undergo a rigorous training and certification process. There is a periodic re‐abstraction process to ensure that data submitted are accurate. Moreover, the data submission software performs internal data checks to ensure data completeness and to alert the data entry person for outlier responses.
Study Population
Within GWTG‐Resuscitation, we identified 122 746 patients at 590 hospitals during 2000–2010 who were 18 years of age or older and had an index cardiac arrest with an identifiable initial rhythm (asystole, pulseless electrical activity, ventricular fibrillation, or pulseless ventricular tachycardia) (Figure 1). From this sample, we excluded patients who were missing information on survival (n=197) and calendar year of the arrest (n=55). We also excluded 43 hospitals (5348 patients with cardiac arrest) that were missing information on hospital characteristics. Finally, given that the estimate of survival improvement from hospitals with low cardiac arrest volume or few years of available data would be unreliable, we restricted our sample to only those hospitals that participated in the registry for ≥5 years and had a mean annual case volume of ≥10 cases in accordance with previous studies.5 As a result, 313 hospitals with 23 804 patients were excluded. Our final sample consisted of 93 342 patients from 231 hospitals.
Figure 1.
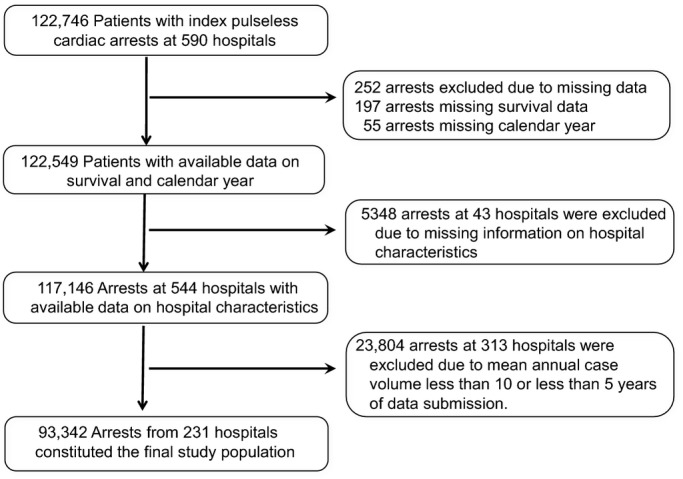
Study cohort.
Hospital and Patient Variables
In an effort to better understand which hospital characteristics are associated with survival improvement, we merged data from the 2008 American Hospital Association annual survey with GWTG‐Resuscitation to obtain information on hospital characteristics. These included information on a hospital's geographic region (North Mid‐Atlantic, South Atlantic, North Central, South Central, and Mountain/Pacific), location (rural, urban), ownership (nonprofit, public, private), teaching status (fellowship program, residency program, or nonteaching), and bed number (≤250, 250 to 499, ≥500).
Patient‐level data available from GWTG‐Resuscitation included demographics (age, sex, race), initial cardiac arrest rhythm (asystole, pulseless electrical activity, ventricular fibrillation, pulseless ventricular tachycardia), location of cardiac arrest (intensive care unit, telemetry unit, nonmonitored unit), time of day (work hours: 7 am to 10:59 pm versus after hours: 11 pm to 6:59 am) and day of week (weekday versus weekend) of cardiac arrest, and use of a hospital‐wide cardiopulmonary arrest alert (ie, “Code Blue”). Moreover, we used information on comorbid conditions (congestive heart failure; myocardial infarction; diabetes mellitus; renal, hepatic, or respiratory insufficiency; neurological status prearrest [as determined by admission Cerebral Performance Category {CPC} scores]6; baseline evidence of motor; cognitive, or functional deficits [central nervous system depression]; acute stroke; pneumonia; hypotension; arrhythmia; sepsis; trauma; metabolic or electrolyte abnormality; cancer), and therapeutic interventions in place at the time of cardiac arrest (use of mechanical ventilation, antiarrhythmic drugs, intravenous vasopressors, dialysis, pulmonary artery catheter, intra‐aortic balloon pump).
Statistical Analyses
The main independent variable was calendar year, and the primary outcome was hospital rate of survival to discharge.
We first conducted bivariate analyses to evaluate unadjusted trends in patient and hospital characteristics over time by using the Maentel‐Haenszel test for categorical variables and linear regression for continuous variables. To evaluate temporal trends in hospital‐level survival rates, we created a 2‐level (patient and hospital) hierarchical logistic regression model. Such models account for clustering of patients within a hospital and avoid overestimation of significance of statistical associations.7 The initial model included hospital‐level random intercepts and slopes by calendar year, which was modeled as a continuous variable. From this model, empirical Bayesian estimates of hospital‐specific intercepts and slopes were obtained, which are “shrunken” toward the mean for small‐volume hospitals, reflecting the lack of information available for those hospitals and thus mitigating issues of outliers. Slopes estimates were exponentiated to represent the relative change in odds of survival per year for each hospital. Hospitals with a slope >0 (odds ratio [OR] >1) were considered to have improved trends in survival, with the magnitude of the slope (and OR) quantifying the extent of annual survival improvement. Finally, we repeated these analyses including adjustment for patient and hospital characteristics, to obtain adjusted annual trends in hospital rates of survival.
We examined hospital variation in survival trends using a cumulative frequency plot of the hospital‐specific OR. Next, we categorized hospitals into quartiles using the hospital‐specific estimates of survival improvement from the model just described. Finally, to explore which hospital characteristics were associated with a higher rate of survival improvement, we included interaction terms between each hospital characteristic (as described earlier in the Hospital and Patient Variables section) and calendar year in our multivariable model.
Data were complete for all covariates, except race (6.5%), admission CPC score (15%), hospital location of arrest (2.1%), and time of cardiac arrest (1.1%). Missing data on covariates were assumed to be missing at random and were imputed using multiple imputation.8
Sensitivity Analyses
First, to exclude the possibility that hospitals with the largest temporal improvement in survival to discharge were not confounded by higher rates of neurological disability, we examined whether hospitals with the greatest survival gains were also the same hospitals with the largest temporal improvement in favorable neurological survival. Information on discharge neurological status in GWTG‐Resuscitation was collected using CPC scores.6 Because data on discharge CPC scores were missing in 2241 (12%) survivors, we used multiple imputation to assign discharge CPC scores for survivors for whom that information was missing. We defined favorable neurological survival as survival to discharge with a CPC of 1 (ie, none or mild neurological disability) and calculated adjusted hospital‐level rates of favorable neurological survival using the hierarchical model just described. We then examined Pearson's correlation between hospital trends in favorable neurological survival and survival to discharge. Second, to ensure that our findings were not influenced by temporal changes in hospital discharge practice patterns (eg, early discharge to hospice of patients with poor likelihood of survival), we repeated our analyses of hospital‐level trends after classifying patients who were discharged to hospice as having died. We then examined whether this substantially altered our classification of hospitals with the greatest improvement in survival.
All statistical analyses were prespecified and conducted using SAS Version 9.1.3 (SAS Institute), IVEWARE (University of Michigan), and R Version 2.6.0 (Free Software Foundation). All hypothesis tests were 2‐sided with a significance level of 0.05. The Institutional Review Board at University of Iowa approved the study and waived the requirement for informed consent.
Results
Table 1 describes characteristics of the study population. The mean age (SD) was 65.9 (15.9) years. Approximately 59% patients were men, and 21% were black. The initial cardiac arrest rhythm was asystole or pulseless electrical activity in nearly 4 in 5 patients. More than 80% of in‐hospital cardiac arrests occurred in a monitored setting (intensive care unit [48.0%], telemetry unit [15.7%], or other closely monitored hospital locations [17.6%]), and >30% of arrests each occurred during nighttime and on the weekend. Approximately, 80% of all patients had a witnessed arrest, and this was substantially higher for cardiac arrests in the intensive care unit (94.5%) compared with those in monitored units (68.7%) and nonmonitored units (48.3%). In general, patients had a high burden of comorbidities—heart failure (18.1%), respiratory insufficiency (42.5%), renal insufficiency (33.4%), diabetes mellitus (30.4%), septicemia (15.9%), as well as use of mechanical ventilation (31.0%) and intravenous vasopressors (27.4%) before the cardiac arrest.
Table 1.
Characteristics of Study Patients*
| Characteristic | Total Cohort N=93 342 | Year Groups* | P Value for Trend | ||
|---|---|---|---|---|---|
| 2000–2003 (n=20 518) | 2004–2006 (n=33 771) | 2007–2010 (n=39 053) | |||
| Demographics | |||||
| Age, mean±SD y | 65.9±15.9 | 66.6±15.6 | 66.0±15.8 | 65.5±16.1 | <0.001 |
| Male sex | 54 695 (58.6) | 11 884 (57.9) | 19 855 (58.8) | 22 956 (58.8) | 0.21 |
| Black race* | 18 509 (21.2) | 3880 (20.7) | 6689 (21.0) | 7940 (21.6) | <0.001 |
| Cardiac arrest characteristics | |||||
| Initial cardiac arrest rhythm | <0.001 | ||||
| Asystole and PEA | 73 048 (78.3) | 15 299 (74.6) | 26 231 (77.7) | 31 518 (80.7) | |
| VF and pulseless VT | 20 294 (21.7) | 5219 (25.6) | 7540 (22.3) | 7535 (19.3) | |
| Arrest at night (11 pm to 7 am)* | 30 031 (32.5) | 6755 (33.4) | 10 834 (32.4) | 12 442 (32.2) | 0.01 |
| Arrest on weekend | 28 817 (30.9) | 6308 (30.7) | 10 441 (30.9) | 12 068 (30.9) | 0.58 |
| Hospital location* | 0.004 | ||||
| Intensive care unit | 43 822 (48.0) | 9328 (47.5) | 15 716 (48.1) | 18 778 (48.1) | |
| Telemetry unit | 14 333 (15.7) | 2938 (15.0) | 5185 (15.9) | 6210 (15.9) | |
| Nonmonitored unit | 17 063 (18.7) | 4102 (20.9) | 6263 (19.2) | 6698 (17.2) | |
| Other (ED, procedural areas, etc) | 16 143 (17.6) | 3082 (15.0) | 5496 (16.3) | 7365 (18.9) | |
| Witnessed arrest* | 75 789 (81.2) | 15 820 (77.1) | 27 430 (81.2) | 32 539 (83.3) | <0.001 |
| Hospital‐wide response activated | 71 920 (77.1) | 16 393 (79.9) | 26 321 (77.9) | 29 206 (74.8) | <0.001 |
| CPC category on admission* | 0.002 | ||||
| 1 | 41 357 (52.1) | 8297 (51.3) | 15 120 (49.5) | 17 940 (54.9) | |
| 2 | 22 076 (27.8) | 4792 (29.6) | 9108 (29.8) | 8176 (25.0) | |
| 3 | 10 388 (13.1) | 2071 (12.8) | 4264 (14.0) | 4053 (12.4) | |
| 4 or 5 | 5552 (7.0) | 1029 (6.4) | 2039 (6.7) | 2484 (7.6) | |
| Preexisting conditions | |||||
| Heart failure this admission | 16 894 (18.1) | 3835 (18.7) | 6419 (19.0) | 6640 (17.0) | <0.001 |
| Prior heart failure | 19 864 (21.3) | 4996 (24.3) | 7232 (21.4) | 7636 (19.6) | <0.001 |
| Myocardial infarction this admission | 17 240 (18.5) | 4218 (20.6) | 6588 (19.5) | 6434 (16.5) | <0.001 |
| Prior myocardial infarction | 15 833 (17.0) | 4041 (19.7) | 5966 (17.7) | 5826 (14.9) | <0.001 |
| Arrhythmia | 31 518 (33.8) | 6403 (31.2) | 12 785 (37.9) | 12 330 (31.6) | 0.015 |
| Hypotension | 26 554 (28.4) | 5421 (26.4) | 10 656 (31.6) | 10 477 (26.8) | 0.315 |
| Respiratory insufficiency | 39 680 (42.5) | 8016 (39.1) | 15 203 (45.0) | 16 461 (42.2) | <0.001 |
| Renal insufficiency | 31 157 (33.4) | 6373 (31.1) | 11 673 (34.6) | 13 111 (33.6) | <0.001 |
| Hepatic insufficiency | 7096 (7.6) | 1370 (6.7) | 2730 (8.1) | 2996 (7.7) | <0.001 |
| Metabolic or electrolyte abnormality | 16 023 (17.2) | 3715 (18.1) | 6481 (19.2) | 5827 (14.9) | <0.001 |
| Diabetes mellitus | 28 389 (30.4) | 5930 (28.9) | 10 549 (31.2) | 11 910 (30.5) | <0.001 |
| Baseline CNS depression | 11 981 (12.8) | 2414 (11.8) | 4929 (14.6) | 4638 (11.9) | 0.08 |
| Acute stroke | 3695 (4.0) | 797 (3.9) | 1427 (4.2) | 1471 (3.8) | 0.05 |
| Pneumonia | 12 558 (13.5) | 2623 (12.8) | 4649 (13.8) | 5286 (13.5) | 0.02 |
| Septicemia | 14 820 (15.9) | 2548 (12.4) | 5588 (16.5) | 6684 (17.1) | <0.001 |
| Major trauma | 4020 (4.3) | 751 (3.7) | 1477 (4.4) | 1792 (4.6) | <0.001 |
| Metastatic or hematologic malignancy | 11 325 (12.1) | 2207 (10.8) | 4325 (12.8) | 4793 (12.3) | <0.001 |
| Interventions in place prior to the arrest | |||||
| Mechanical ventilation | 28 943 (31.0) | 5353 (26.1) | 10 589 (31.4) | 13 001 (33.3) | <0.001 |
| Intravenous antiarrhythmic therapy | 5427 (5.8) | 1078 (5.3) | 1876 (5.6) | 2473 (6.3) | <0.001 |
| Intravenous vasopressors | 25 541 (27.4) | 5328 (26.0) | 8690 (25.7) | 11 523 (29.5) | <0.001 |
| Dialysis | 3564 (3.8) | 703 (3.4) | 1446 (4.3) | 1415 (3.6) | 0.54 |
| Intra‐aortic balloon pump | 1437 (1.5) | 323 (1.6) | 538 (1.6) | 576 (1.5) | 0.13 |
| Pulmonary artery catheter | 3543 (3.8) | 1106 (5.4) | 1404 (4.2) | 1033 (2.6) | <0.001 |
CNS indicates central nervous system; CPC, cerebral performance category; ED, emergency department; PEA, pulseless electrical activity, VF, ventricular fibrillation; VT, ventricular tachycardia.
Values are expressed as number (percent) unless otherwise specified.
For illustrative purposes, trends in baseline characteristics are presented as 3 time periods (2000–2003, 2004–2006, and 2007–2010). However, the P value for trend is for temporal changes in these characteristics by calendar year.
Data were missing for the following variables: race 6023 (6.5%), time of cardiac arrest 1031 (1.1%), hospital location 1981 (2.1%), witnessed arrest 13 (0.01%), and CPC category on admission 13 969 (14.9%).
Temporal trends in patient characteristics are also described in Table 1. Between 2000 and 2010, there was a significant decline in cardiac arrests due to ventricular fibrillation and pulseless ventricular tachycardia (P<0.001). Significant temporal trends were also noted in demographics (decrease in age, P<0.001), comorbidities (decrease in prevalence of heart failure and myocardial infarction and increase in prevalence of respiratory insufficiency, renal insufficiency, diabetes, septicemia, and malignancy; P<0.001 for all comparisons), and acute illness severity (increase in intensive care unit location, in patients receiving mechanical ventilators and vasopressors before the cardiac arrest; P<0.001 for all comparisons).
The median duration of hospital participation in GWTG‐Resuscitation was 7 years, and 73 (31.6%) hospitals participated for ≥8 years. Characteristics of the study hospitals are summarized in Table 2. Nearly 90% of study hospitals were located in urban areas. Most hospitals were either nonprofit (70.1%) or government owned (16.9%) with few for‐profit hospitals (13%). Hospitals were distributed relatively uniformly across geographic census regions. Most hospitals (44.2%) were intermediate in size (200 to 499 beds), whereas 35.5% were small hospitals (<250 beds) and 20.3% were large hospitals (≥500 beds). A majority of hospitals were academic teaching hospitals with a residency (30.3%) or a residency and fellowship (23.8%) program.
Table 2.
Characteristics of Study Hospitals (N=231)*
| Hospital Characteristics | No. (%) |
|---|---|
| Geographic region | |
| North Mid‐Atlantic | 33 (14.3) |
| South Atlantic | 59 (25.5) |
| North Central | 52 (22.5) |
| South Central | 41 (17.7) |
| Mountain/Pacific | 46 (19.9) |
| Urban location | 207 (89.6) |
| Ownership | |
| Private | 30 (13.0) |
| Public | 39 (16.9) |
| Nonprofit | 162 (70.1) |
| Hospital bed size | |
| <250 | 82 (35.5) |
| 250 to 499 | 102 (44.2) |
| ≥500 | 47 (20.3) |
| Academic hospital | |
| Residency and fellowship program (major) | 55 (23.8) |
| Residency program only (minor) | 70 (30.3) |
| No training program (nonteaching) | 106 (45.9) |
Hospital‐Level Trends in Survival
At baseline (ie, during the first year of participation in GWTG‐Resuscitation), the mean unadjusted hospital survival rate for in‐hospital cardiac arrest was 18.2%. Survival rates improved at 218 (94%) hospitals (ie, hospitals with estimate of slope >0), with a mean 4% improvement in survival per year (OR 1.04, 95% CI 1.03 to 1.05, P<0.001; Figure 2A and Table 3). Notably, the magnitude of improvement varied widely. Hospitals in the top quartile had a mean year‐over‐year survival improvement of 7%, while hospitals in the second and third hospital quartile had a mean year‐over‐year survival improvement of 5% and 3%, respectively. The mean year‐over‐year change in survival for hospitals in the lowest hospital quartile was 1%, suggesting little to no improvement in survival over time.
Figure 2.
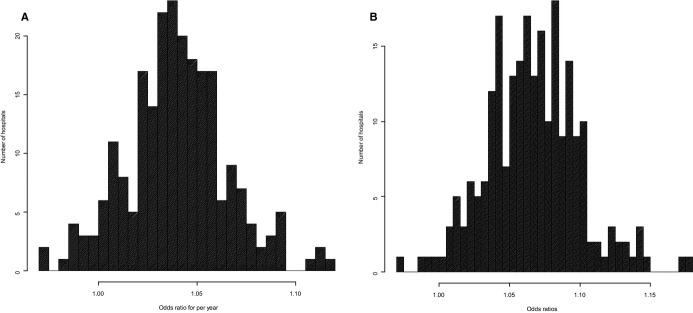
Distribution of (A) unadjusted and (B) adjusted hospital‐level survival trends. The odds ratio (OR) represents the rate of change in survival year‐over‐year. Hospitals with OR>1.00 had an improvement in survival over time.
Table 3.
Unadjusted and Adjusted Relative Rate of Annual Survival Improvement, by Hospital Quartiles*
| Overall | First Quartile (Top) | Second Quartile | Third Quartile | Fourth Quartile (Bottom) | |
|---|---|---|---|---|---|
| Unadjusted | |||||
| Mean annual improvement | 1.04 | 1.07 | 1.05 | 1.03 | 1.01 |
| Hospital range | 0.97 to 1.12 | 1.05 to 1.12 | 1.04 to 1.05 | 1.02 to 1.04 | 0.97 to 1.02 |
| Adjusted | |||||
| Mean annual improvement | 1.07 | 1.11 | 1.08 | 1.06 | 1.03 |
| Hospital range | 0.97 to 1.18 | 1.09 to 1.18 | 1.07 to 1.09 | 1.04 to 1.07 | 0.97 to 1.04 |
Hospitals were divided into quartiles using hospital‐specific odds ratios for annual survival improvement obtained from the multivariable hierarchical regression model. Values in the table are the mean and the range of the hospital‐specific odds ratio for annual survival improvement.
After adjustment for patient and hospital characteristics, the mean relative improvement in in‐hospital cardiac arrest survival rates was 7% per year (adjusted OR 1.07, 95% CI 1.06 to 1.08, P<0.001; Table 3). Compared with a mean adjusted hospital survival rate of 18.1% during the 2000–2003 period, hospital survival rate increased to 21.4% in 2007–2010, which translated into a 3.3% absolute improvement in survival during this period (Figure 3). Notably, there was marked variation in annual survival improvement across sites (Figure 2B). Hospitals in the top quartile achieved a mean year‐over‐year adjusted survival increase of 11%, whereas the hospitals in the bottom quartile experienced only a mean annual improvement of survival of 3% (Table 3).
Figure 3.
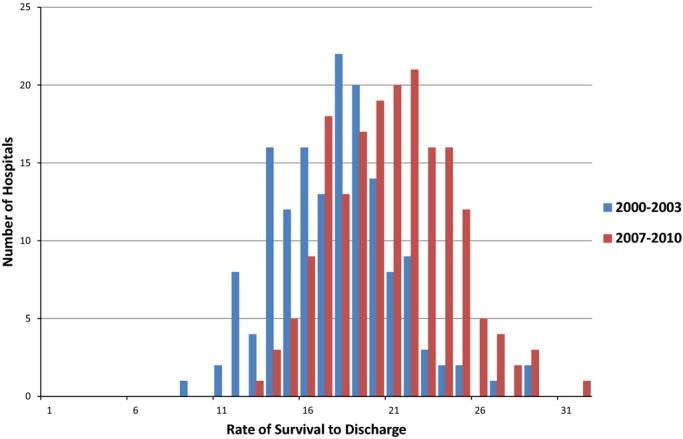
Change in in‐hospital cardiac arrest survival rates from 2000–2003 to 2007–2010. The mean adjusted hospital survival rate increased from 18.1% in 2000–2003 (range 9.1% to 29.8%) to 21.4% in 2007–2010 (range 13.9% to 32.2%).
There was a significant interaction between academic status and rate of survival improvement across hospitals. Compared with minor teaching hospitals (OR 1.04, 95% CI 1.02 to 1.06), hospital rate of survival improvement was greater at major teaching (OR 1.08, 95% CI 1.06 to 1.10) and nonteaching hospitals (OR 1.07, 95% CI 1.05 to 1.09, P value for interaction=0.03; Table 4). However, other hospital characteristics, including bed size, geographic status, ownership status, and rural versus urban location, were not associated with a hospital's rate of survival improvement for in‐hospital cardiac arrest.
Table 4.
Association Between Hospital Structural Characteristics and Improvement in Cardiac Arrest Survival
| Hospital Main Effects Odds Ratio (95% CI) | P value for Interaction With Calendar Year | |
|---|---|---|
| Hospital Characteristics, % | ||
| Geographic region | 0.81 | |
| Northeast | 1.06 (1.03 to 1.10) | |
| Southeast | 1.07 (1.05 to 1.10) | |
| Midwest | 1.06 (1.04 to 1.08) | |
| Southwest | 1.08 (1.05 to 1.11) | |
| West | 1.06 (1.03 to 1.09) | |
| Location | 0.57 | |
| Rural | 1.05 (1.01 to 1.10) | |
| Urban | 1.07 (1.05 to 1.08) | |
| Ownership | 0.55 | |
| Private | 1.07 (1.05 to 1.08) | |
| Government | 1.08 (1.05 to 1.11) | |
| Nonprofit | 1.05 (1.02 to 1.09) | |
| Hospital bed size | 0.73 | |
| <250 | 1.07 (1.05 to 1.10) | |
| 250 to 499 | 1.06 (1.04 to 1.08) | |
| ≥500 | 1.07 (1.04 to 1.09) | |
| Academic hospital | 0.03 | |
| Residency and fellowship program (major) | 1.08 (1.06 to 1.10) | |
| Residency program only (minor) | 1.04 (1.02 to 1.06) | |
| Nonteaching hospital | 1.07 (1.05 to 1.09) | |
In sensitivity analyses, there was a moderately strong positive correlation between hospital trends in favorable neurological survival and survival to discharge (Pearson's correlation coefficient=0.62; P<0.0001), which confirms that hospitals with the highest survival gains were largely the same hospitals with the greatest improvements in favorable neurological survival. Moreover, after reclassifying 833 patients (4.5% of survivors) who were discharged to hospice as having died, we still found an average 6% per‐year improvement in survival. There was excellent agreement when we compared hospital classification in quartiles of survival improvement by using this approach with our primary analysis (κ=0.80, 95% CI 0.74 to 0.87), which suggests that our findings were robust to hospital discharge patterns.
Discussion
Among hospitals participating in a large national quality improvement registry, we found hospital‐level survival rates for in‐hospital cardiac arrest have significantly improved during the past decade. Importantly, there was marked variation in the extent of survival improvement at participating hospitals. We found that a quarter of hospitals achieved an average 11% relative improvement in survival per year, while another quarter of hospitals experienced little to no improvement. Many of the individual hospital structural characteristics were unrelated to the extent of variation in hospital‐level trends, which suggests that unmeasured hospital processes of care are likely driving the survival improvement at top‐performing sites. A number of our findings merit further discussion.
Recently, we reported that in‐hospital cardiac arrest survival has improved during the past decade at the patient level.1 While it is possible that the improved survival trends that we observed are limited to hospitals committed to quality improvement due to participation in GWTG‐Resuscitation, 2 subsequent studies have confirmed similar trends in other nationally representative databases.9–10 Here, we extend the findings of our previous work by showing that survival improvement varied markedly across sites even after accounting for differences in patient characteristics between sites. Given that treatment of in‐hospital cardiac arrest is time sensitive and requires a coordinated effort among a diverse group of providers, marked variation in survival improvement across sites likely reflects differences in how individual hospitals approach resuscitation care and organize quality improvement efforts.11
Delivering high‐quality resuscitation care in hospitals is a challenging task. It requires identification of “at‐risk” patients and their appropriate triage to telemetry or intensive care units, timely recognition of cardiac arrest, prompt mobilization of a resuscitation team to the patient bedside, rapid evaluation of the patient and initiation of resuscitative efforts, conduct of an organized and coordinated acute resuscitation response, and high‐quality postresuscitation care. Hospitals that consider cardiac arrest to be a priority, examine their performance through ongoing data collection and feedback, identify areas of weakness, and invest resources and personnel to improve processes of care are likely to have achieved greater gains in cardiac arrest survival. In prior work in acute myocardial infarction, a number of institutional attributes—organizational values and goals, senior leadership, staff engagement, communication, coordination, problem solving, and learning from previous mistakes—were also found to distinguish top‐performing hospitals from low‐performing hospitals.12 It is likely that similar organizational factors underlie hospitals' ability to improve systems of care for resuscitation.
Among hospital structural characteristics, only teaching status was found to be significantly associated with extent of survival improvement. Survival improvement was comparable at major teaching hospitals (hospitals with residency and fellowship programs) and nonteaching hospitals but was lower at minor teaching hospitals (hospitals with only residency programs). This relationship may be due to the presence of less‐experienced trainees (eg, residents) who may be first responders during acute resuscitation and primary providers during postresuscitation care. The presence of advanced trainees (eg, fellows) at major teaching hospitals appears to mitigate this relationship, as survival improvement at major teaching hospitals was similar to that of nonteaching hospitals where experienced physicians provide patient care. Although this may be a plausible explanation, we did not have data on composition of code teams or inpatient staffing patterns to confirm whether this is accurate.
The general lack of association between hospital structural characteristics and survival improvement is not entirely unexpected. Chan et al found wide variation (2% to 55%) in rates of delayed defibrillation for cardiac arrest due to ventricular fibrillation and pulseless ventricular tachycardia. In that study, hospital performance on defibrillation times was unrelated to measured structural characteristics (except bed size).13 Based on these findings, we posit that hospital processes of care, rather than structure, are more important in achieving improved in‐hospital cardiac arrest survival over time. In fact, single‐center studies have demonstrated the value of innovative process redesign such as debriefing after a cardiac arrest event,14 use of simulation or routine mock codes,15 and implementation of efforts to improve quality of CPR, as well as devices capable of providing audiovisual feedback during resuscitation,16 in improving resuscitation performance and outcomes. It is possible that top‐performing hospitals have implemented similar and other novel strategies to realize their cardiac arrest survival gains. Evaluating important resuscitation‐related processes of care as potential drivers of in‐hospital cardiac arrest survival improvement is a critical next step.
Currently, neither GWTG‐Resuscitation nor other existing hospital databases collect information on these innovative resuscitation processes of care and treatment strategies. Therefore, to better understand the organizational and process variables that underlie hospital resuscitation performance, detailed site surveys within large registries like GWTG‐Resuscitation or studies using mixed methods (qualitative and quantitative) may be needed. Such studies would be instrumental in identifying “best practices” at high‐performing institutions. In fact, Bradley and colleagues used a similar approach to identify best practices associated with shorter door‐to‐balloon times for primary percutaneous coronary intervention for ST‐elevation myocardial infarction—another time‐sensitive condition.17–18 This approach has been widely successful in significantly reducing door‐to‐balloon times nationally and serves as powerful example to guide future quality improvement work for other time‐sensitive conditions such as in‐hospital cardiac arrest.19
The following issues also merit discussion. First, it is possible that hospital cardiac arrest survival rates have improved because of a decrease in overall risk among patients who undergo resuscitation. However, this is not likely to be the case. Although mean patient age has decreased over time, there has been a temporal increase in the prevalence of comorbidities (sepsis, renal insufficiency, respiratory insufficiency, and malignancy), mechanical ventilation, and vasopressor use before arrest, suggesting that illness severity has increased over time. This finding was also noted in another study, which found an increase in the mean Charlson comorbidity score among patients with an in‐hospital cardiac arrest (2.5 in 2000–2001 to 2.7 in 2008–2009; P<0.001).10 Second, although clinical practice guidelines support the use of therapeutic hypothermia for preventing neurological damage and improving survival following an out‐of‐hospital cardiac arrest, 2 recent studies from GWTG‐Resuscitation found therapeutic hypothermia to be rarely used after in‐hospital cardiac arrest (<3%). Therefore, it is unlikely that the observed variation in survival improvement between hospitals is explained by differences in use of therapeutic hypothermia. Third, we assumed that improvement in survival occurred in a linear fashion in this study. Although we did not test this assumption, our prior work using data from GWTG‐Resuscitation was most consistent with a linear temporal trend in survival.1
Our study should be interpreted in light of the following limitations. First, we had limited information regarding hospital characteristics. Important processes of care (eg, quality of CPR, innovative resuscitation strategies, quality improvement programs, etc) that may be associated with survival improvement were not available. Second, we had only a single year of American Hospital Association data to define hospital‐level characteristics, and it is possible that “structural” characteristics of hospitals changed over time. Third, although we adjusted for a number of patient and hospital factors in our multivariable models, the possibility of residual confounding remains. Fourth, hospitals began and ended participation in GWTG‐Resuscitation at different times. We addressed this by requiring that study hospitals be restricted to those that participated in GWTG‐Resuscitation for at least 5 years. Finally, hospitals participating in GWTG‐Resuscitation are more likely to be committed hospitals that are engaged in quality improvement. Moreover, we also excluded hospitals that were low volume (annual cardiac arrest volume <10) or participated infrequently (<5 years) in the registry. For both these reasons, our findings may not be generalizable to all US hospitals.
Conclusion
During the past decade, survival after in‐hospital cardiac arrest survival has improved at nearly all hospitals participating in a large national quality improvement registry. However, marked differences in the extent of survival improvement were observed. Most structural hospital characteristics were not associated with the extent of hospital improvements in cardiac arrest survival. Future studies are needed to identify hospital process of care (“best practices”) that have achieved the largest improvement in in‐hospital cardiac arrest survival.
Role of the Sponsor
The American Heart Association, which sponsors the Get With The Guidelines®‐Resuscitation, had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Appendix
In addition to study authors Saket Girotra and Paul S. Chan, the American Heart Association Get With The Guidelines – Resuscitation Adult Trask Force include Comilla Sasson, MD, MS and Steven Bradley, MD, MPH, University of Colorado; Michael W. Donnino, MD, Beth Israel Deaconess Medical Center; Dana P. Edelson, MD, MS, University of Chicago; Robert T. Faillace, MD, ScM, Geisinger Healthcare System; Romergryko Geocadin, MD, Johns Hopkins University School of Medicine; Raina Merchant, MD, MSHP, University of Pennsylvania School of Medicine; Vincent N. Mosesso, Jr., MD, University of Pittsburgh School of Medicine; Joseph P. Ornato, MD and Mary Ann Peberdy, MD, Virginia Commonwealth University.
Supplementary Material
Appendix Hospital Variation in Survival Trends For In-hospital Cardiac Arrest.
Sources of Funding
This study is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under awards K08HL122527 (Dr Girotra) and K23HL102224 (Dr Chan).
Disclosures
None.
References
- 1.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012; 367:1912-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane‐Trultt T. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation. 2003; 58:297-308. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D'Este K, Finn J, Halperin H, Handley A, Herlitz J, Hickey R, Idris A, Kloeck W, Larkin GL, Mancini ME, Mason P, Mears G, Monsieurs K, Montgomery W, Morley P, Nichol G, Nolan J, Okada K, Perlman J, Shuster M, Steen PA, Sterz F, Tibballs J, Timerman S, Truitt T, Zideman D. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, Interamerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004; 110:3385-3397. [DOI] [PubMed] [Google Scholar]
- 4.Cummins RO, Chamberlain D, Hazinski MF, Nadkarni V, Kloeck W, Kramer E, Becker L, Robertson C, Koster R, Zaritsky A, Bossaert L, Ornato JP, Callanan V, Allen M, Steen P, Connolly B, Sanders A, Idris A, Cobbe S. Recommended guidelines for reviewing, reporting, and conducting research on in‐hospital resuscitation: the in‐hospital ‘Utstein style.’ American Heart Association. Circulation. 1997; 95:2213-2239. [DOI] [PubMed] [Google Scholar]
- 5.Chan PS, Berg RA, Spertus JA, Schwamm LH, Bhatt DL, Fonarow GC, Heidenreich PA, Nallamothu BK, Tang F, Merchant RMInvestigators AG‐R. Risk‐standardizing survival for in‐hospital cardiac arrest to facilitate hospital comparisons. J Am Coll Cardiol. 2013; 62:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1:480-484. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein H. Multilevel Statistical Models. 1995London, England: Edward Arnold [Google Scholar]
- 8.Raghunathan T, Solenberger P, Van Hoeyk J. Iveware: Imputation and Variance Estimation Software—User Guide. 2002Michigan: Survey Research Center, Institute for Social Research University of Michigan [Google Scholar]
- 9.Fugate JE, Brinjikji W, Mandrekar JN, Cloft HJ, White RD, Wijdicks EF, Rabinstein AA. Post‐cardiac arrest mortality is declining: a study of the us national inpatient sample 2001 to 2009. Circulation. 2012; 126:546-550. [DOI] [PubMed] [Google Scholar]
- 10.Kazaure HS, Roman SA, Sosa JA. Epidemiology and outcomes of in‐hospital cardiopulmonary resuscitation in the United States, 2000–2009. Resuscitation. 2013; 84:1255-1260. [DOI] [PubMed] [Google Scholar]
- 11.Chan PS, Nallamothu BK. Improving outcomes following in‐hospital cardiac arrest: life after death. JAMA. 2012; 307:1917-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry LA, Spatz E, Cherlin E, Thompson JW, Berg D, Ting HH, Decker C, Krumholz HM, Bradley EH. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? A qualitative study. Ann Intern Med. 2011; 154:384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK. Hospital variation in time to defibrillation after in‐hospital cardiac arrest. Arch Intern Med. 2009; 169:1265-1273. [DOI] [PubMed] [Google Scholar]
- 14.Edelson DP, Litzinger B, Arora V, Walsh D, Kim S, Lauderdale DS, Vanden Hoek TL, Becker LB, Abella BS. Improving in‐hospital cardiac arrest process and outcomes with performance debriefing. Arch Intern Med. 2008; 168:1063-1069. [DOI] [PubMed] [Google Scholar]
- 15.Hunt EA, Shilkofski NA, Stavroudis TA, Nelson KL. Simulation: translation to improved team performance. Anesthesiol Clin. 2007; 25:301-319. [DOI] [PubMed] [Google Scholar]
- 16.Abella BS, Edelson DP, Kim S, Retzer E, Myklebust H, Barry AM, O'Hearn N, Hoek TL, Becker LB. CPR quality improvement during in‐hospital cardiac arrest using a real‐time audiovisual feedback system. Resuscitation. 2007; 73:54-61. [DOI] [PubMed] [Google Scholar]
- 17.Bradley EH, Curry LA, Webster TR, Mattera JA, Roumanis SA, Radford MJ, McNamara RL, Barton BA, Berg DN, Krumholz HM. Achieving rapid door‐to‐balloon times: how top hospitals improve complex clinical systems. Circulation. 2006; 113:1079-1085. [DOI] [PubMed] [Google Scholar]
- 18.Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, McNamara RL, Parkosewich J, Loeb JM, Krumholz HM. Strategies for reducing the door‐to‐balloon time in acute myocardial infarction. N Engl J Med. 2006; 355:2308-2320. [DOI] [PubMed] [Google Scholar]
- 19.Krumholz HM, Herrin J, Miller LE, Drye EE, Ling SM, Han LF, Rapp MT, Bradley EH, Nallamothu BK, Nsa W, Bratzler DW, Curtis JP. Improvements in door‐to‐balloon time in the united states, 2005 to 2010/clinical perspective. Circulation. 2011; 124:1038-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Hospital Variation in Survival Trends For In-hospital Cardiac Arrest.


