Abstract
Background
The 4‐variable risk score from University of California, Los Angeles (UCLA) demonstrated superior discrimination in advanced heart failure, compared to established risk scores. However, the model has not been externally validated, and its suitability as a selection tool for heart transplantation (HT) and left ventricular assist device (LVAD) is unknown.
Methods and Results
We calculated the UCLA risk score (based on B‐type natriuretic peptide, peak VO2, New York Heart Association class, and use of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker) in 180 patients referred for HT. The outcome was survival free from urgent transplantation or LVAD. The model‐predicted survival was compared to Kaplan‐Meier's estimated survival at 1, 2, and 3 years. Model discrimination and calibration were assessed. During a mean follow‐up of 2.1 years, 37 (21%) events occurred. One‐, 2‐ and 3‐year observed event‐free survival was 88%, 81%, and 75%, and the observed/predicted ratio was 0.97, 0.96, and 0.97, respectively. Time‐dependent receiver operating characteristic curve analyses demonstrated good discrimination overall (1‐year area under curve, 0.801; 2‐year, 0.774; 3‐year, 0.837), but discrimination between the 2 highest risk groups was poor. The difference between observed and predicted survival ranged from −14 to +17 percentage points, suggesting poor model calibration. Fairly similar results were found when the analyses were repeated in 715 patients after multivariate imputation of missing data.
Conclusions
The UCLA 4‐variable risk model calibration was inconsistent and high‐risk discrimination was poor in an external validation cohort. Further model assessment is warranted before widespread use.
Keywords: heart failure, heart transplantation, prognostic risk models
Introduction
Objective risk assessment is critical in allocating scarce or expensive resources, such as heart transplantation (HT) or left ventricular assist devices (LVADs). Standard selection tools include the peak VO2, the Heart Failure Survival Score (HFSS), and the Seattle Heart Failure Model (SHFM). Recently, a 4‐variable risk prediction model for patients with advanced heart failure (HF) was reported from University of California, Los Angeles (UCLA).1 Model discrimination (distinction between risk strata) was better than for the HFSS and the SHFM. The investigators performed internal validation by splitting their data set into 2 subsets (a derivation cohort and a validation cohort) and further by reporting the bootstrap‐adjusted performance. However, the performance of a risk prediction model cannot be assessed by internal validation alone. It is essential to evaluate the performance in a different and independent patient population, known as external validation.2 Furthermore, for clinical utility, a model must also have good calibration (similar observed vs. predicted risk) for all risk strata. Therefore, we performed external validation of discrimination and assessed calibration of the UCLA model in patients with severe HF referred for HT.
Methods
The local human investigations committee approved chart review. Individual patient consent was not required. From a population of 715 consecutive HF patients referred to the Columbia University Medical Center for HT evaluation, 180 patients with complete information regarding all 4‐variable UCLA risk score variables were included. The risk score was derived in each patient from the 4 variables: B‐type natriuretic peptide (BNP), peak oxygen consumption (pVO2), New York Heart Association (NYHA) class, and use of angiotensin‐converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB). We also categorized the patients into the same 4 risk groups based on the risk score as described in the UCLA publication.1 In a supplementary analysis, we calculated the 4‐variable risk score in the total population of 715 patients, enabled by a multivariable missing data imputation strategy. Outcome events were defined as death, urgent transplantation (United Network of Organ Sharing [UNOS] Status 1), or LVAD implantation. Patients who were transplanted as nonurgent (UNOS Status 2) were censored alive on the date of transplant. Vital status of patients lost to clinical follow‐up was assessed using the Social Security Death Index.
Statistical Methods
Kaplan‐Meier's method was used to calculate observed survival and 95% confidence intervals (95% CIs). For overall discrimination, we used Cox's model, with the calculated risk score as the only independent variable, and calculated the C index. Discrimination was also assessed by plotting the cumulative survival over 3 years for patients classified in 4 risk groups as in the original model derivation.1 Time‐dependent receiver operating characteristic (ROC) curves were computed by using the risk score and the 1‐, 2‐, and 3‐year Kaplan‐Meier estimated survival, and the area under the curve (AUC) was calculated. For overall calibration, we calculated the ratio of observed/risk model‐predicted survival at 1, 2, and 3 years, according to the equation from the UCLA publication.1
In the supplementary analysis, including 715 patients, data were missing regarding BNP (74%), pVO2 (0.1%), NYHA (0.2%), and ACEI/ARB (2%). We used multiple imputation by chained equations to impute missing values.3 Multiple imputation by chained equations is a flexible, efficient technique for handling missing data, even in large data sets. The imputation procedure consists of a series of regression models (chained equations) where each variable with missing data is modeled conditional upon the other variables in the data. This means that each variable can be modeled according to its own distribution. Twenty‐four clinical variables, the event indicator, and the Nelson‐Aalen estimator of the cumulative baseline hazard were included in the imputation model. We generated and combined estimates from 50 multiply imputed data sets.
Data management and statistical analyses were performed using Stata 13.1 (StataCorp LP, College Station, TX) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical characteristics of the study population are shown in the 1. Overall, baseline characteristics in our study population were similar to the original UCLA cohort. During a mean follow‐up of 2.1 years, 37 (21%) events occurred. Overall, event‐free survival (EFS) was 88% at 1 year, 81% at 2 years, and 75% at 3 years. In Kaplan‐Meier's EFS analysis, there was lack of discrimination between the 2 highest risk groups (P=0.692) (Figure 1). Cox's model, with the risk score as a continuous independent variable, had better discrimination (C‐index, 0.781), compared to the risk group Cox model (C‐index, 0.757). The time‐dependent ROC curve analyses demonstrated good overall discrimination; AUCs at 1, 2, and 3 years, by continuous risk score: 0.801 (95% CI, 0.722 to 0.891), 0.774 (95% CI, 0.691 to 0.857), and 0.837 (95% CI, 0.751 to 0.922), respectively; by risk groups: 0.776 (95% CI, 0.676 to 0.876), 0.748 (95% CI, 0.658 to 0.837), and 0.798 (95% CI, 0.709 to 0.887), respectively. The overall observed/predicted ratios were 0.97, 0.96, and 0.97, respectively. The observed and predicted EFS in the 4 risk groups are shown in Figure 2. The difference between observed and predicted survival ranged from −14 to +17 percentage points.
Table 1.
Baseline Characteristics in 180 Patients With Advanced Heart Failure Referred for Heart Transplantation
| Characteristic | All Patients (n=180) | Patients Without Events (n=143) | Patients With Events (n=37) |
|---|---|---|---|
| Clinical | |||
| Age, y | 52.7 (13.3) | 52.2 (13.1) | 54.4 (14.3) |
| Females, n | 47 (26%) | 35 (24%) | 12 (32%) |
| NYHA class | 2.7 (0.8) | 2.6 (0.8) | 3.3 (0.7) |
| Weight, kg | 88 (21) | 90 (20) | 80 (21) |
| Resting sBP, mm Hg | 112 (19) | 114 (18) | 101 (17) |
| Peak VO2, mL/min per kg | 13.1 (4.81) | 13.7 (4.89) | 10.9 (3.76) |
| LVEF, % | 21 (7.7) | 22 (7.7) | 18 (6.6) |
| Ischemic etiology | 59 (33%) | 45 (31%) | 14 (38%) |
| Medications | |||
| ACEI | 136 (76%) | 109 (76%) | 27 (73%) |
| Beta‐blockers | 157 (87%) | 122 (85%) | 35 (95%) |
| Aldosterone blockers | 72 (40%) | 48 (34%) | 24 (65%) |
| Statins | 75 (42%) | 62 (43%) | 13 (35%) |
| Allopurinol | 9 (5%) | 9 (6%) | 0 |
| ARB | 10 (6%) | 10 (7%) | 0 |
| Loop diuretic equivalent, mg/kg | 0.84 (0.95) | 0.75 (0.81) | 1.2 (1.3) |
| Laboratory data | |||
| Hemoglobin, g/dL | 13.7 (1.7) | 14.0 (1.6) | 12.7 (1.7) |
| Lymphocytes percentages | 26 (9.5) | 27 (9.3) | 22 (9.6) |
| Total cholesterol, mg/dL | 183 (53) | 188 (52) | 162 (52) |
| Uric acid, mg/dL | 7.7 (2.5) | 7.6 (2.3) | 8.1 (2.9) |
| Sodium, mEq/L | 137 (3.2) | 138 (2.8) | 135 (3.6) |
| Device | |||
| CRT | 7 (4%) | 5 (4%) | 2 (5%) |
| ICD | 66 (37%) | 47 (33%) | 19 (51%) |
| CRT‐D | 52 (29%) | 40 (28%) | 12 (32%) |
Data are presented as mean (standard deviation) for continuous variables or n (%) for categorical variables. ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; sBP, systolic blood pressure; VO2, oxygen uptake; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; CRT‐D, CRT+ICD.
Figure 1.
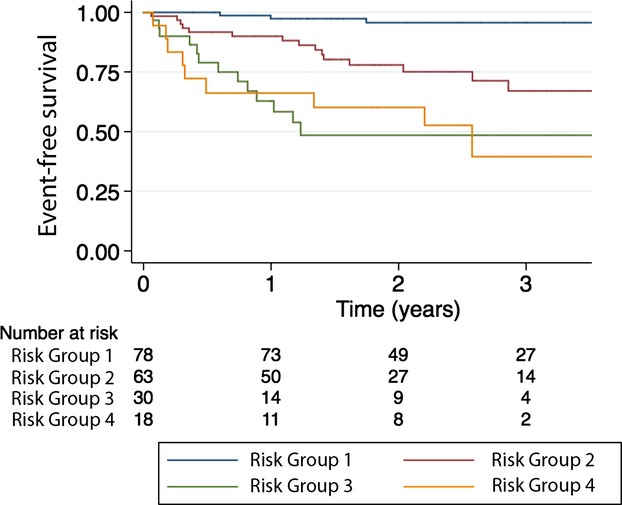
Kaplan‐Meier's estimated event‐free survival in 180 patients with advanced heart failure referred for heart transplantation assigned into 4 risk groups based on the UCLA risk model.
Figure 2.
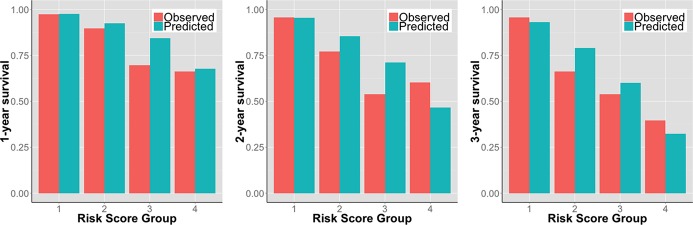
Observed versus the UCLA risk model‐predicted event‐free survival at 1, 2, and 3 years in 180 patients with advanced heart failure referred for heart transplantation assigned into 4 risk groups based on the UCLA risk score.
Supplementary Analyses in 715 Patients After Multivariate Imputation of Missing Data
Clinical characteristics of the total study population (n=715) have been reported in previous assessments of the HFSS and SHFM.4 During a mean follow‐up of 2.6 years, 354 (49.5%) events occurred. One‐, 2‐, and 3‐year observed EFS was 79%, 66%, and 55%, respectively. There was lack of discrimination between the 2 highest risk groups (P=0.695) (Figure 3). Cox's model, with the risk score as a continuous independent variable, had better discrimination (C‐index, 0.740), compared to the risk group Cox model (C‐index, 0.719). Overall discrimination was good; AUCs at 1, 2, and 3 years, by continuous risk score: 0.784 (95% CI, 0.746 to 0.821), 0.782 (95% CI, 0.745 to 0.819), and 0.808 (95% CI, 0.770 to 0.846), respectively; by risk groups: 0.753 (95% CI, 0.713 to 0.792), 0.758 (95% CI, 0.721 to 0.795), and 0.772 (95% CI, 0.733 to 0.810), respectively. The overall observed/predicted ratio was 0.87, 0.79, and 0.73, at 1, 2, and 3 years, respectively (Figure 4). Except for the lowest risk group, there were large differences between observed and predicted survival. The difference ranged from 1 to 34 absolute percentage points, depending on risk strata and time of follow‐up, and there was a consistent overestimation of EFS and thus underestimation of risk.
Figure 3.
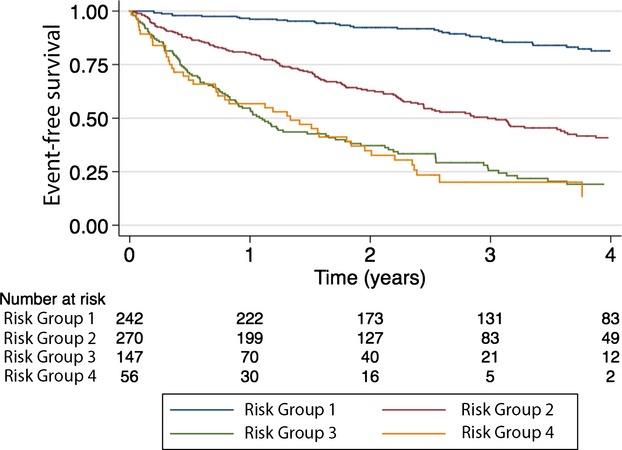
Kaplan‐Meier's estimated event‐free survival in 715 patients with advanced heart failure referred for heart transplantation assigned into 4 risk groups based on the UCLA risk model. UCLA indicates University of California, Los Angeles.
Figure 4.
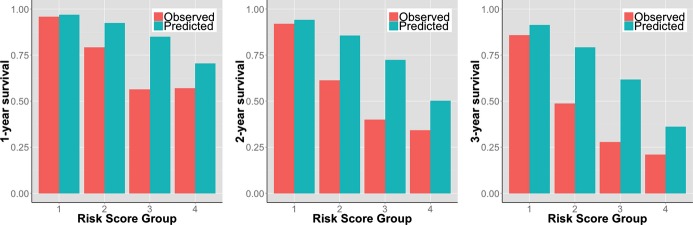
Observed versus UCLA risk model‐predicted event‐free survival at 1, 2, and 3 years in 715 patients with advanced heart failure referred for heart transplantation assigned into 4 risk groups based on the UCLA risk score.
Discussion
Accurate risk assessment in severe HF is critical for proper selection for HT or LVAD. The peak VO2 is a strong single variable,5 but is outperformed by the HFSS5 and SHFM.4 These have been extensively validated for risk assessment and HT selection in different populations.4 However, the HFSS and SHFM are cumbersome and inadequately used in practice. The new simple 4‐variable risk model from UCLA is promising in its simplicity and greater likelihood of widespread use. We assessed its performance by external validation in patients referred for HT. We found that the UCLA model had good discrimination overall, but was seriously limited by the inability to separate (discriminate between) patients in the highest risk groups into different risk strata. Furthermore, calibration (ie, the similarity between observed and predicted risk) was unsatisfactory, with a difference between predicted and observed EFS of up to 17 absolute percentage points. Additionally, calibration deteriorated and we found a consistent underestimation of risk in the supplementary analyses, which included 715 patients. This underestimation of risk, particularly in the highest risk groups, has been observed also for the SHFM,6 suggesting that the most ill patients have risk in addition to what is captured by the known and standard risk markers included in these models.
The performance of the HFSS and SHFM has previously been evaluated by our group4–5 in the same study cohort that was used in the current external validation of the UCLA 4‐variable risk model. The ROC curve analyses for 1‐year EFS were 0.72 and 0.73 for HFSS and SHFM, respectively, compared to 0.78 for the UCLA 4‐variable risk model. Similarly, the ROC curve analyses for 2‐year EFS were 0.70 and 0.74 for HFSS and SHFM, respectively, compared to 0.78 for the UCLA 4‐variable risk model. These results suggest potentially a better overall discrimination for the UCLA 4‐variable risk model in this study population. However, with regard to calibration, the SHFM has been suggested to perform poorly, with overestimation of EFS in transplant referred patients.6–8 Likewise, in the current work, the UCLA 4‐variable risk model also underestimated risk. We did not proceed with further comparisons of the performance between the UCLA risk model and, for example, the SHFM or HFSS. Such comparisons could be performed by using reclassification measures (Net Reclassification Improvement/Index and Integrated Discrimination Improvement).9–10 However, these methods may not be appropriate if there is suspicion of poor model calibration11–12; they are not suitable for time‐to‐event data; and for HF risk prediction, there is no widely adopted single baseline model with which to compare.
The widespread use of the HFSS is somewhat limited by the need for peak VO2, but the SHFM is well suited for prognostication in general HF because of its overall accuracy, and the use is facilitated by the web application at www.SeattleHeartFailureModel.org. Admittedly, the SHFM depends on lymphocyte count, uric acid, and total cholesterol, which sometimes are not readily available. In contrast, the recently derived13 and externally validated14 MAGGIC project HF risk score includes 13 universally available variables. An easy‐to‐use online calculator is accessible at www.heartfailurerisk.org. The UCLA 4‐variable risk model is indeed simple, but shares the shortcomings of both the HFSS and the SHFM, including the need for peak VO2 for the HFSS as well as the underestimation of risk among high‐risk patients for the SHFM. Technically, the UCLA risk model score can be calculated in patients with missing data on peak VO2. However, the point of the 4‐variable UCLA risk score is to include specifically the 4 variables, and what distinguishes it from simple informal clinical judgment based on symptoms (NYHA class), natriuretic peptide levels, and drug treatment is the presence of the extensively validated, highly objective, and strongly prognostic peak VO2.
The implications of our findings are that: (1) The performance of a new risk model needs to be evaluated in another patient cohort apart from the one it was developed in (ie, the training set); (2) the UCLA model should not replace any existing risk model without further validation; and (3) the UCLA model has good potential for clinical utility because of its simplicity, but may benefit from recalibration. Furthermore, the tendency for underestimation of risk among high‐risk patients, which seems to affect both the UCLA model as well as the SHFM, should be taken into account by clinicians when they assess overall risk or counsel patients regarding advanced treatment options, such as LVAD placement or HT. In general, these risk scores (UCLA, HFSS, and SHFM) apply to ambulatory or noninotrope, non‐LVAD‐dependent patients, which constitute a shrinking proportion of overall transplants,15 at least in the United States.
Limitations
Our study was limited by a small sample size, but our results were replicated in a larger population after accounting for missing data by multiple imputation. Both analytic strategies generated fairly similar findings, which add strength to our conclusions. Another limitation was the single‐center design.
Sources of Funding
This work was supported by the Swedish Heart Lung Foundation (grants 20080409 and 20100419 to Lund), the Stockholm County Council (grants 20090556 and 20110120 to Lund), and the Foundation for Cardiac Therapies (FACT Fund to Mancini) and the Altman Fund (to Mancini).
Disclosures
Lund reports research funding to author's institution and consulting and lecture honoraria from Thoratec and HeartWare, manufacturers of LVADs.
References
- 1.Chyu J, Fonarow GC, Tseng CH, Horwich TB. Four‐variable risk model in men and women with heart failure. Circ Heart Fail. 2014; 7:88-95. [DOI] [PubMed] [Google Scholar]
- 2.Moons KG, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman DG, Woodward M. Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012; 98:691-698. [DOI] [PubMed] [Google Scholar]
- 3.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011; 30:377-399. [DOI] [PubMed] [Google Scholar]
- 4.Goda A, Williams P, Mancini D, Lund LH. Selecting patients for heart transplantation: comparison of the Heart Failure Survival Score (HFSS) and the Seattle Heart Failure Model (SHFM). J Heart Lung Transplant. 2011; 30:1236-1243. [DOI] [PubMed] [Google Scholar]
- 5.Goda A, Lund LH, Mancini D. The Heart Failure Survival Score outperforms the peak oxygen consumption for heart transplantation selection in the era of device therapy. J Heart Lung Transplant. 2011; 30:315-325. [DOI] [PubMed] [Google Scholar]
- 6.Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail. 2010; 3:706-714. [DOI] [PubMed] [Google Scholar]
- 7.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, Laskar S, Puskas J, Dunbar S, Vega D, Levy WC, Butler J. Utility of the Seattle Heart Failure Model in patients with advanced heart failure. J Am Coll Cardiol. 2009; 53:334-342. [DOI] [PubMed] [Google Scholar]
- 8.Sartipy U, Goda A, Yuzefpolskaya M, Mancini DM, Lund LH. Utility of the Seattle Heart Failure Model in patients with cardiac resynchronization therapy and implantable cardioverter defibrillator referred for heart transplantation. Am Heart J. 2014 [DOI] [PubMed] [Google Scholar]
- 9.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014; 160:122-131. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009; 150:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilden J, Gerds TA. A note on the evaluation of novel biomarkers: do not rely on integrated discrimination improvement and net reclassification index. Stat Med. 2013 [DOI] [PubMed] [Google Scholar]
- 12.Vickers AJ, Pepe M. Does the net reclassification improvement help us evaluate models and markers? Ann Intern Med. 2014; 160:136-137. [DOI] [PubMed] [Google Scholar]
- 13.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RNMeta‐Analysis Global Group in Chronic Heart Failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013; 34:1404-1413. [DOI] [PubMed] [Google Scholar]
- 14.Sartipy U, Dahlstrom U, Edner M, Lund LH. Predicting survival in heart failure: validation of the MAGGIC heart failure risk score in 51 043 patients from the Swedish heart failure registry. Eur J Heart Fail. 2014; 16:173-179. [DOI] [PubMed] [Google Scholar]
- 15.Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Yusen RD, Stehlik J. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013; 32:951-964. [DOI] [PubMed] [Google Scholar]


